Abstract
A new eco-friendly approach for the preparation of sustainable heterogeneous palladium catalysts from rice husk-derived biogenic silica (RHP-Si and RHU-Si). The designed heterogeneously supported palladium species (RHP-Si-NH2-Pd and RHU-Si-NH2-Pd) were fully characterized and successfully employed as catalysts for various chemical transformations (C–C bond-forming reactions, aerobic oxidations and carbocyclizations). Suzuki-Miyaura transformations were highly efficient in a green solvent system (H2O:EtOH (1:1) with excellent recyclability, providing the cross-coupling products with a wide range of functionalities in high isolated yields (up to 99%). Palladium species (Pd(0)-nanoparticles or Pd(II)) were also efficient catalysts in the green aerobic oxidation of an allylic alcohol and a co-catalytic stereoselective cascade carbocyclization transformation. In the latter case, a quaternary stereocenter was formed with excellent stereoselectivity (up to 27:1 dr).
Similar content being viewed by others
Introduction
Rice husk (RH) is a major waste product from the rice industry with high content of silica. This abundant material is a sustainable and cheap raw material and therefore within the context of sustainability1. De facto, rice husk ash (RHA) is known to contain 94% silica2. Here, silica is a very important component for various industrial and biomedical applications3, being high surface area silicates highly desirable and good candidates as catalyst support4. For these reasons the development of efficient methods for the preparation of silica and silica based materials are of high interest. In general, the production of silica from RH is energy intensive i.e. obtained by burning RH in a muffle furnace at high temperature (500, 600 or 700 °C)5,6. Nevertheless, even though there are some reports on simple and energy-efficient method for the generation of silica from RH, drawbacks such as risk for mineral contamination7, several acid and based extraction steps or tedious approaches8 are encountered9. In this context, novel methods for the extraction of silica in an eco-friendly, cost-efficient, scalable and facile approach can be highly attractive. This report discloses the preparation of palladium based multifunctional heterogenous catalysts from RH-derived silica as support. There are reports on the immobilization of various metals on RH and their use as heterogeneous catalysts for chemical syntheses10,11,12,13,14,15,16. In this context, Chang and co-workers developed [Pd(NH3)4]2+-modified nanopore silica, derived from RH and further demonstrated their use in the solvent-free Suzuki-Miyaura cross-coupling reaction17. However, some examples of the coupling reactions resulted in very poor reactivity (~4% yield). Within this theme, Gogoi and co-workers also developed a highly efficient RH based Pd(II)-Schiff base complex heterogenous catalyst for the Suzuki- Miyaura coupling reaction in water18. Even though the catalyst was recyclable and reusable up to 6 cycles, it successively lost its activity (1st cycle 98% and 6th cycle 90% yield). The same reaction was employed by Boruah et al. by using recyclable Pd(OAc)2 in neat Water Extract of Rice Straw Ash (WERSA), nevertheless, moderate to high yields (45–90% yields) and low enduring recyclability was observed (1st cycle 88% and 6th cycle 65% yield)19. Additionally, Liu et al. demonstrated the preparation of porous silica derived from acid leached RH after calcination as support for palladium and cerium (IV) oxide (CeO2). The catalyst was employed for the catalytic methane combustions20. Moreover, Esmaeilpour et al. disclosed the preparation of dendrimer-encapsulated Pd(0) nanoparticles immobilized on nanosilica (nSiO2-dendrimer-Pd(0)) and their application in the Sonogashira-Hagihara reactions in the absence of any copper and phosphorous ligand in water under aerobic conditions21. Despite these notable advances, there is a need to find novel, facile and green approaches to obtain high purity and surface area silica rice husk (RH-Si) for further catalyst design into multifunctional, recyclable and highly efficient heterogenous catalytic systems. Heterogenous catalysts offers several advantages such as allowing the facile and practical recycling of the catalyst, reuse in several cycles without any loss of efficiency and avoiding the leaching of expensive and toxic metals22. All of these features characterize a sustainable and eco-friendly technology. Based on the above challenges and our previous experience in developing palladium based heterogenous catalysts for various green chemical transformations23,24,25,26,27,28,29, we designed an eco-friendly approach for the preparation of heterogeneous and multifunctional palladium catalysts suitable for a wide spectrum of chemical transformations. The activity and versatility of the designed catalysts was demonstrated in the Suzuki-Miyaura cross-coupling reaction, aerobic oxidation approach of allylic alcohols and stereoselective cascade carbocyclization reactions (Fig. 1a)30.
(a) Catalytic applications of the versatile Pd heterogeneous catalyst into various green chemical transformation. (b) Novel, low-cost and energy-efficient environmentally friendly approach for the preparation of rice husk (RH) based silica (RHU-Si and RHP-Si) through a simple process involving grinding, microwave assisted extraction, washing and calcination. (c) Synthetic strategy for further modification of the RH-derived silica (RHU-Si and RHP-Si) into a palladium based heterogenous catalyst ((RHP-Si-NH2-Pd(II), RHU-Si-NH2-Pd(II), RHP-Si-NH2-Pd(0) and RHU-Si-NH2-Pd(0)).
Results and Discussion
Initially, RH derived silica was obtained through a previously reported facile and novel approach (Fig. 1b)31. RH was grinded, underwent microwave-assisted extraction and then further purification (washing and calcination) to afford the silica products. Two type of silica materials were prepared, one unrefined biosilicate (RHU-Si, 90% purity, grey color) and the second one, pure biosilicate (RHP-Si, >98% purity, white color). Subsequently, the homogenous palladium catalyst was incorporated onto the RHU-Si and RHP-Si via conjugation with amino groups through a silylation step (RHU-Si-NH2 and RHp-Si-NH2), followed by treatment with the palladium precursor (Li2PdCl4) providing the heterogenous palladium catalysts (RHU-Si-NH2-Pd(II) and RHp-Si-NH2-Pd(II)). These two heterogenous palladium (II) catalysts were simply converted to the corresponding RHU-Si-NH2-Pd(0) and RHp-Si-NH2-Pd(0) by a reduction step (Fig. 1c). The fabricated materials were thoroughly characterized (Fig. 2). Firstly, the porosity and pore size of the RH based materials (RHP-Si, RHU-Si-NH2-Pd(II)/Pd(0) and RHP-Si-NH2-Pd(II)/Pd(0)) were determined by nitrogen physisorption experiments (Figs. 2a and S3−10). Unmodified RHP-Si displayed a Brunauer-Emmet-Teller (BET) with a surface area of ca. 352 m2/g−1 and with mesoporous characteristics (8.0 nm) and pore volume of 0.56 cm3/g−1 (Fig. 2b). However, both surface area and pore volume decreased after palladium incorporation, whilst the pore size distribution changed to macropores (e.g. RHU-Si-NH2-Pd(II) = 163 m2/g−1, 89.4 nm and 0.36 cm3/g−1). Such is a normal behaviour observed in our previous reports, indicating the binding and incorporation of palladium into the material23,27,32. The elemental analysis confirmed the palladium content of the various heterogenous catalysts as 20.30 wt% for RHU-Si-NH2-Pd(II), RHP-Si-NH2-Pd(II) = 19.11 wt%, RHU-Si-NH2-Pd(0) = 19.05 wt% and RHP-Si-NH2-Pd(0) = 16.90 wt%, respectively.
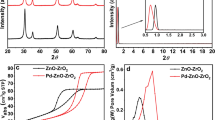
(a) N sorption isotherms of the silica materials derived from rice husk (RHP-Si). (b) Textural properties of RHP-Si and corresponding palladium based heterogenous catalysts (RHP-Si-NH2-Pd(II), RHU-Si-NH2-Pd(II), RHP-Si-NH2-Pd(0) and RHU-Si-NH2-Pd(0)). Definitions: SBET: specific surface area calculated by the Brunauer-Emmett-Teller (BET) equation. DBJH: mean pore size diameter calculated by the Barret-Joyner-Halenda (BJH) equation. VBJH: pore volumes calculated by the Barret-Joyner-Halenda (BJH) equation. (c) Powder X-ray diffraction (XRD) patterns of RHP-Si. (d) Transmission Electron Microscopy (TEM) of RHP-Si at different magnifications, at (i) 500 nm and (ii) 200 nm. TEM of the various palladium heterogenous catalysts (e) RHU-Si-NH2-Pd(II) (f) RHU-Si-NH2-Pd(0) (g) RHP-Si-NH2-Pd(II) (h) RHP-Si-NH2-Pd(0).
Moreover, the surface area decreased and pore size increased when the Pd(II) was reduced to Pd(0) (e.g. RHP-Si-NH2-Pd(II) = 153 m2/g−1 and 63.6 nm; RHP-Si-NH2-Pd(0) = 144 m2/g−1 and 70.5 nm). X-ray diffraction (XRD) patterns of the RHP-Si displayed a strong broad peak at about 22° 2θ angle indicating its amorphous structure (Fig. 2c). This is also consistent with previous reports on amorphous silica derived from rice husk33. The amorphous characteristics of the RHP-Si could also be confirmed by Scanning electron microscopy (SEM) micrographs (Figure S11). Additionally, Transmission electron microscopy (TEM) of the RHP-Si at different magnification are presented in Fig. 2d, which further demonstrated the amorphous and porous structure. After palladium incorporation, a clear difference could be observed in the TEM, where well dispersed and spherical palladium nanoparticles could be visualized (Fig. 2e–h).
Upon characterization, the catalytic performance of the designed heterogenous palladium catalysts were further evaluated. The Suzuki cross-coupling reaction was selected as model reaction. The initial reaction between iodobenzene 1a and phenylboronic acid 2a, in the presence of potassium carbonate (K2CO3)29 and catalytic amounts of RHU-Si-NH2-Pd(II) (5 mol%) in water (H2O) provided the corresponding diphenyl product 3a in 54% isolated yield after 3 h at 100 °C (Table 1, entry 1). Further screening of the solvents showed that dimethylformamide (DMF) displayed the best efficiency among the investigated (toluene (85%) and ethanol (EtOH) (95%)) to afford 3a in 98% yield (Table 1, entries 2–4). However, enduring our vision in designing an eco-friendly process, we decided to mix H2O and ethanol (EtOH) (1:1) as reaction medium, and to our delight, the reaction provided the product 3a in 99% yield (Table 1, entry 5). This improvement could be due to the improved solubility of the substrates with the addition of ethanol than having solely H2O as solvent. It is well-known that silicate is not soluble or show very low solubility in water34. No differences in catalytic activity between the various palladium catalysts were observed (Table 1, entries 5–8), and moreover, a decrease in catalyst amount (from 5.0 to 0.25 mol%) did not negatively impact the reaction efficiency (Table 1, entries 8–11). However, when the amount of the Pd-catalyst was decreased to 0.10 mol% the efficiency was decreased and provided the product 3a with 81% yield (Table 1, entry 12). Enduring the fine-tuning of the reaction, a decrease in reaction temperature and time were further investigated, where 1 h reaction time at 70 °C worked well (Table 1, entries 13–15). However, further decrease of the temperature to 50 °C decreased the efficiency of the reaction (72% yield, Table 1, entry 16). Delighted by these findings, the substrate scope of the reaction was further explored, which showed that the reaction tolerated a wide range of functionalities with both aryl iodide 1a and aryl bromide 1b and various boronic acids 2 (Table 2). Nevertheless, a slight decrease in yields was observed for the reaction between bromobenzene 1b and phenylboronic acid 2a and between the iodobenzene 1a and 4-(trifluoromethyl) phenylboronic acid 2e providing products 3a and 3 f (85 and 88% yields, Table 2, entries 2 and 8). Overall, the coupling reaction showed high efficiency and provided the coupling products in high yields (up to 99%). Since the recyclability of a heterogenous catalyst is an eminence feature both in the economic and environmental aspects, the recyclability and reusability of the devised palladium heterogenous catalysts were further studied. The heterogenous systems could be recycled for 6 consecutive cycles without losing any efficiency (Fig. 3a). Notable all the four heterogenous palladium catalysts (RHU-Si-NH2-Pd(II), RHP-Si-NH2-Pd(II only), RHU-Si-NH2-Pd(0), RHP-Si-NH2-Pd(0) were recycled at least one cycle. However RHP-Si-NH2-Pd(0) catalyst was selected for further recycling study. Moreover, no leaching was observed as determined by elemental analysis performed on the filtrate after the hot filtration and after the completion of the reaction.
(a) Table demonstrating the recyclability study of RHP-Si-NH2-Pd(0) catalyst in the catalytic Suzuki-Miyaura reaction. (b) Expanding the reaction scope of the devised catalyst RHP-Si-NH2-Pd(0) for the oxidation of the cinnamyl alcohol 4 to the corresponding cinnamic aldehyde 5. (c) Expanding the reaction scope for the combined heterogeneous palladium and amine catalyzed stereoselective carbocylization reaction between the cinnamic aldehyde 5 and propargylcyanomalonate 6. Definitions: n.d; not determined, d.r.; diastereomeric ratio and e.r.; enantiomeric ratio.
To further broaden the application of the synthesized Pd catalyst, the selected optimum system (RHP-Si-NH2-Pd(0) was employed in the aerobic oxidation of cinnamyl alcohol 4 to the corresponding aldehyde as model reaction32. The reaction proceeded efficiently to afford cinnamic aldehyde 5 (>99%) as only product after 48 h, in toluene at 70 °C, employing 5 mol% of the catalyst in the presence of oxygen gas (Fig. 3b). In addition to the cross-coupling and oxidation reactions, the reaction portfolio was further expanded for the application of the heterogenous palladium catalyst in amine/palladium co-catalyzed carbocyclization reactions (Fig. 3c). The reaction was conducted between cinnamic aldehyde 5 and propargylcyanomalonate 6 in the presence of catalytic amount of palladium heterogenous catalyst (RHU-Si-NH2-Pd(II) and RHP-Si-NH2-Pd(II) (5 mol%)) and the chiral amine catalyst 7 (20 mol%). The chemical transformation proceeds via formation of the enaminyne I intermediate35, and subsequent stereoselectivity nucleophilic enamine addition provided the carbocycle 8, in high yields (up to 82%) and diastereoselectivities (up to 27:1 dr determined through 1H-NMR analysis and the e.r. where not determined. However, the obtained optical rotation ([α]D25 = −6.31 (c = 1.0 CHCl3)) resembles to the previously reported ee of >97.5:2.5 er) (Fig. 3c)23. These all examples highlight the versatility and simplicity of the devised RH-based heterogenous palladium catalyst employed in various relevant chemical transformations.
Conclusion
A highly efficient Pd-heterogeneous catalyst from the biomass-based Rice husk waste was synthesized. The novel preparation approach integrating the valorization of renewable starting materials represents a green, facile and simple eco-friendly method for catalyst design. The devised heterogenous palladium catalysts were proved to be highly versatile, being successfully employed in Suzuki-Miyaura cross-couplings (high product yields, up to 99%, wide range of functionalities), the aerobic oxidation of cinnamoyl alcohol and the amine catalyzed stereoselective carbocylization reaction (cyclopentene derivatives obtained in high yields and stereoselectivities, up to 82%, 27:2 dr). Additionally, the Pd system was highly recyclable and could be reused after simple centrifugation in 6 cycles without any loss of efficiency. The disclosed protocol represents a green and sustainable chemical approach that may find relevant and suitable future applications in additional chemical transformations.
Methods
General and materials
Chemicals and solvents were either purchased puriss p. a. from commercial suppliers or were purified by standard techniques. Commercial reagents were used as purchased without any further purification. Aluminum sheet silica gel plates (Fluka 60 F254) were used for thin-layer chromatography (TLC), and the compounds were visualized by irradiation with UV light (254 nm) or by treatment with a solution of phosphomolybdic acid (25 g), Ce(SO4)2·H2O (10 g), conc. H2SO4 (60 mL), and H2O (940 mL), followed by heating. 1H NMR spectra were recorded on a Bruker Avance (500 MHz or 400 MHz) spectrometer. Chemical shifts are reported in ppm from tetramethylsilane with the solvent resonance resulting from incomplete deuterium incorporation as the internal standard (CDCl3: δ 7.26 ppm). Data are reported as follows: chemical shift, multiplicity (s = singlet, d = doublet, q = quartet, br = broad, m = multiplet), and coupling constants (Hz), integration. 13C NMR spectra were recorded on a Bruker Avance (125.8 MHz or 100 MHz) spectrometer with complete proton decoupling. Chemical shifts are reported in ppm from tetramethylsilane with the solvent resonance as the internal standard (CDCl3: δ 77.16 ppm). Gas sorption measurements were carried out on a Micrometrics ASAP2020 analyzer and recorded at 77 K. N2 adsorption measurements on the RH-Si were performed at 77 K by using a Micromeritics ASAP 2000 volumetric adsorption analyzer. The samples were degassed for 24 h at 130 °C under vacuum (Po < 10−2 Pa) and subsequently analyzed. Surface area of the RH-Si was calculated according to the Brunauer-Emmet-Teller (BET) equation. Mean pore size diameter and pore volumes were obtained from porosimetry data by using Barret-Joyner-Halenda (BJH) method. Wide-angle X-ray diffraction experiments were recorded on a Pan-Analytic/Philips X’pert MRD diffractometer (40 kV, 30 mA) with CuKα (λ = 0.15418 nm) radiation. Scans were performed over a 2θ = 10–80 °C at step size of 0.0188 with a counting time per step of 5 s. TEM image of the RH-Si was obtained on JEM 2010F (JEOL) and Phillips Analytical FEI Tecnai 30 microscopes. All other TEM experiments were carried out on a 200 kV JEOL JEM-2100F field-emission electron microscope equipped with an ultra-high-resolution pole piece. A Gatan ultra-high tilt tomography holder was used. TEM samples were prepared by crushing, and the tomography data was acquired between −60° and +60° with 1° increments. Each image per tilt angle was recorded with a Gatan Ultrascan 1000 camera. The data acquisition was assisted by a commercial tomography packed, TEMography (version 2.15.07) developed by JEOL System Technology Co. Ltd. SEM micrographs of the RH-Si was recorded in a JEOL-SEM JSM-6610 LV scanning electron microscope in backscattered electron mode at 3/15 kV. Elemental analyses were carried out by Medac LTD Analytical and chemical consultancy services (United Kingdom) by ICP-OES.
Preparation of the RHP-Si-Pd-heterogeneous catalysts Rice husk silica preparation (RHP-Si)
The particle size of the rice husk (RH) was reduced by grinding in a Retsch-PM-100 planetary ball mill using a 125 mL reaction chamber end eighteen stainless steel balls (10 nm diameter, 5 g weight). Milling was conducted at 350 rpm for 10 min. The obtained silica from RH was treated to microwave assisted extraction in ETHOS-ONE. The RH was then refluxed in a 0.29 M HCl solution at 300 W for 30 min. The silica solution was cooled to room temperature, filtered and washed with distilled water and then dried in oven at 100 °C for 24 h. The resulting solid was calcined in a furnace at 550 °C for 4 h to obtain pure silica (RHP-Si).
General preparation of the RHP-Si-NH2
The preparation of the RHP-Si-NH2-Pd-catalyst started with the amino functionalization of the RHP-Si. Dry toluene (20 mL) was added to the RHP-Si (1.38 g, 22.2 mmol mg, 1.0 equiv.), followed by addition of a solution of 3-aminopropyltrimethoxy silane (7.8 mL, 44.4 mmol, 2.0 equiv.) in toluene (10 mL). The mixture was stirred under nitrogen for 10 minutes, and then refluxed for 24 h. The mixture was allowed to cool to room temperature and the solid was collected by filtration and washed several times with toluene, ethanol, acetone and dichloromethane to remove any unreacted precursor. The material was further dried under vacuum giving RHP-Si-NH2 (1.405 g).
General preparation of the RHP-Si-NH2-Pd(II)
The amino-functionalized RHP-Si-NH2 (1.0 g) was suspended in deionized water (15 mL) and the solution was pH-adjusted to pH 9, by the use of 0.1 N LiOH. In parallel Li2PdCl4 (600 mg) was diluted in deionized water (10 mL) and the solution was pH-adjusted to pH 9, by the use of 0.1 N LiOH. This solution was transferred to the flask containing RHP-Si-NH2 solution. The reaction was stirred at room temperature for 24 h. Subsequently, the suspension was then centrifuged, and the solid material was further washed with water (3 × 40 mL) and acetone (3 × 40 mL) and dried overnight under vacuum to afford RHP-Si-NH2-Pd(II) (1.408 g). Elemental analysis on the Pd content were 20.30 wt.% for RHU-Si-NH2-Pd(II) and 19.11 wt.% for RHP-Si-NH2-Pd(II).
General preparation of the RHP-Si-NH2-Pd(0)
RHP-Si-NH2-Pd(II) (500 mg) was suspended in deionized water (15 mL), followed by slow addition of a solution of NaBH4 (310.2 mg, 8.2 mmol) in water (5 mL) at room temperature. The reaction was stirred at room temperature for 30 minutes. Afterwards the solution was centrifuged and the solid diluted with water (3×40 mL), acetone (3×40 mL) and centrifuged. The material was then dried overnight under vacuum providing RHP-Si-NH2-Pd(0). Elemental analysis on Pd content were 19.05 wt.% for the RHU-Si-NH2-Pd(0) and 16.90 wt.% for RHP-Si-NH2-Pd(0), respectively.
General procedure for Pd-catalyst catalyzed Suzuki-Miyaura reaction (Table 1)
A microwave vial equipped with a magnetic stir bar was charged with the Pd-catalyst (mol%), phenylboronic acid 2a (146.4 mg, 1.2 mmol, 1.2 equiv.), K2CO3 (414.6 mg, 3.0 mmol, 3.0 equiv.), followed by addition of solvent (3.0 mL). Subsequently, iodobenzene 1a (204.0 mg, 1.0 mmol, 1.0 equiv.) was added and the reaction mixture heated and run for the temperature and time stated at Table 1. Next, the reaction mixture was either centrifuged and the solid diluted with acetone (3×10 mL) and centrifuged and then concentrated before purification or directly subjected to flash chromatography on silica (petroleum ether/EtOAc 100−90%) affording the pure product 3a.
Procedure for RHP-Si-NH2-Pd (0) catalyst catalyzed Suzuki-Miyaura reaction (Table 2)
A microwave vial equipped with a magnetic stir bar was charged with RHP-Si-NH2-Pd(0) catalyst (1.6 mg, 0.0025 mmol, 0.25 mol%), arylboronic acid 2 (1.2 mmol, 1.2 equiv.), K2CO3 (414.6 mg, 3.0 mmol, 3.0 equiv.), followed by addition of H2O:EtOH (1:1, 3.0 mL). Subsequently, aryl halide 1 (1.0 mmol, 1.0 equiv.) was added and the reaction mixture heated to 70 °C and stirred for 1 h. Next, the reaction mixture was either centrifuged and the solid diluted with acetone (3 × 10 mL) and centrifuged and then concentrated before purification or directly subjected to flash chromatography on silica (petroleum ether/EtOAc, 100–90%) affording the pure products 3.
Procedure for the recycling of RHP-Si-NH2-Pd(0) catalyst
A microwave vial equipped with a magnetic stir bar was charged with RHP-Si-NH2-Pd(0) catalyst (6.3 mg, 0.01 mmol, 1.0 mol%), phenylboronic acid 2a (146.4 mg, 1.2 mmol, 1.2 equiv.), K2CO3 (414.6 mg, 3.0 mmol, 3.0 equiv.), followed by addition of H2O:EtOH (1:1, 3.0 mL). Subsequently, iodobenzene 1a (204.0 mg, 1.0 mmol, 1.0 equiv.) was added and the reaction mixture heated to 70 °C and stirred for 1 h. Next, the reaction mixture was centrifuged and the solid diluted with acetone (3×10 mL) and centrifuged. The collected liquid was concentrated and purified by flash chromatography on silica (petroleum ether/EtOAc 100−90%) affording the pure product 3a. The solid catalyst was diluted with water (10 mL) in order to remove remaining base and centrifuged. The solid catalyst was further diluted with acetone (2 × 10 mL) and centrifuged. Afterwards the solid heterogeneous catalyst was dried under vacuum and then further used in next cycle. Notable all the four heterogenous palladium catalysts (RHU-Si-NH2-Pd(II), RHU-Si-NH2-Pd(0), RHP-Si-NH2-Pd(II), RHP-Si-NH2-Pd(0)) were recycled at least one cycle. RHP-Si-NH2-P(0) was selected for further cycle studies.
Typical procedure for the hot-filtration test
A microwave vial equipped with a magnetic stir bar was charged with pure RHP-Si-NH2-Pd(0) catalyst (1.6 mg, 0.25 mol%), phenylboronic acid 2a (146.4 mg, 1.2 mmol, 1.2 equiv.), K2CO3 (414.6 mg, 3.0 mmol, 3.0 equiv.), followed by addition of solvent (3.0 mL). Subsequently, iodobenzene 1a (204.0 mg, 1.0 mmol, 1.0 equiv.) was added and the reaction mixture heated to 70 °C. RHP-Si-NH2-Pd(0) catalyst was removed through centrifugation after 30% conversion was reached and the solid free filtrate was allowed to stir for 24 h reaction conditions. Analysis of the reaction mixture showed that no further conversion of the substrate had occurred.
Procedure for the catalytic aerobic oxidation
To a suspension of RHP-Si-NH2-Pd(0) (5 mol% Pd to 4, 7.6 mg) in toluene (0.5 mL) placed in an oven-dried microwave vial equipped with a magnetic stir bar was charged with cinnamyl alcohol 4 (32.3 mg, 0.24 mmol, 1.2 equiv.). The vial was capped, evacuated and an O2-balloon was connected to the reaction vessel. The reaction mixture was stirred at 70 °C for 48 h affording the the corresponding cinnamic aldehyde 5.
General procedure for the combined transition metal/amine catalytic reaction using propargylcyanomalonate
An oven-dried microwave vial equipped with a magnetic stir bar was charged with propargylcyanomalonate 6 (16.1 mg, 0.12 mmol, 1.2 equiv.) and Pd-catalyst (5 mol%, 0.005 mmol), followed by addition of toluene (0.100 mL) and the resulting mixture was stirred at room temperature for 5 min. In parallel to the above procedure, an oven- dried vial was charged with the cinnamic aldehyde 5 (13 mg, 0.1 mmol, 1.0 equiv.), aminocatalyst 7 (6.5 mg, 0.02 mmol, 20 mol%) and followed by addition of toluene (0.15 mL), after stirring at room temperature for additional 5 min, the resulting mixture was transferred to the vial containing the mixture of palladium catalyst and propargylcyanomalonate via a syringe. (Total volume of Toluene = 0.25 mL, Final concentration = 0.8 M to aldehyde). The reaction was stirred for 24 h at room temperature. The conversions and diastereomeric ratio were monitored by 1H NMR analysis of the crude mixture. Upon completion, the mixture was directly subjected to flash chromatography on silica (pentane/EtOAc) affording the pure products in 8.
References
Mansaray, K. G. & Ghaly, A. E. Physical and Thermochemical Properties of Rice Husk. Energy Sources 19, 989–1004 (1997).
Shawkataly, O. B. et al. Ru-nanoparticle deposition on naturally available clay and rice husk biomass materials−Benzene hydrogenation catalysis and synthetic strategies for green catalyst development. Catal. Sci. Technol. 2, 538–546 (2012).
Vallet-Regí, M. & Balas, F. Silica Materials for Medical Applications. Open Biomed. Eng. J 2, 1–9 (2008).
Min, B. K., Santra, A. K. & Goodman, D. W. Understanding silica-supported metal catalysts: Pd/silica as a case study. Catal. Today 85, 113–124 (2003).
Liu, N., Huo, K., McDowell, M. T., Zhao, J. & Cui, Y. Rice husks as a sustainable source of nanostructured silicon for high performance Li-ion battery anodes. Sci. Rep 3, 1919 (2013).
Bogeshwaran, K., Kalaivani, R., Ashraf, S., Manikandan, G. N. & Prabhu, G. E. Production of silica from rice husk. Int. J. ChemTech Res. 6, 4337–4345 (2014).
Kalapathy, U., Proctor, A. & Shultz, J. A simple method for production of pure silica from rice hull ash. Bioresour. Technol. 73, 257–262 (2000).
An, D., Guo, Y., Zhu, Y. & Wang, Z. A green route to preparation of silica powders with rice husk ash and waste gas. Chem. Eng. J. 162, 509–514 (2010).
Bakar, R. A., Yahya, R. & Gan, S. N. Production of High Purity Amorphous Silica from Rice Husk. Procedia Chem. 19, 189–195 (2016).
Ahmed, A. E. & Adam, F. Indium incorporated silica from rice husk and its catalytic activity. Microporous and Mesoporous Mater. 103, 284–295 (2007).
Adam, F., Kandasamy, K. & Balakrishnan, S. Iron incorporated heterogeneous catalyst from rice husk ash. J. Colloid Interface Sci. 304, 137–143 (2006).
Adam, F., Balakrishnan, S. & Wong, P.-L. Rice husk ash silica as a support material for ruthenium based heterogeneous catalyst. J. Phys. Sci. 17, 1–13 (2006).
Gogoi, N., Begum, T., Dutta, S., Bora, U. & Gogoi, P. K. Rice husk derived nanosilica supported Cu(II) complex: an efficient heterogeneous catalyst for oxidation of alcohols using TBHP. RSC Adv. 5, 95344–95352 (2015).
Ahmed, A. E. & Adam, F. Microporous and Mesoporous Mater. 118, 35–43 (2009).
Adam, F., Andas, J. & Rahman, I. A. The Synthesis and Characterization of Cobolt-Rice Husk Silica Nanoparticles. The Open Colloid Sci. J. 4, 12–18 (2010).
Thabet, A., Basheer, C., Maung, T. H., Al-Muallem, H. A. & Kalanthoden, A. N. Rice Husk Supported Catalysts for Degradation of Chlorobenzenes in Capillary Microreactor. J. Nanomater. 2015, 1–9 (2015).
Chang, W., Shin, J., Oh, Y. & Ahn, B. J. A simple and efficient Suzuki reaction catalyzed by palladium-modified nanopore silica under solvent-free conditions. J. Ind. Eng. Chem. 14, 423–428 (2008).
Gogoi, N., Bora, U., Borah, G. & Gogoi, P. K. Nanosilica-anchored Pd(II)-Schiff base complex as efficient heterogeneous catalysts for activation of aryl halides in Suzuki-Miyaura cross-coupling reaction in water. Appl. Organomet. Chem. 31, e3686 (2017).
Boruah, P. R., Ali, A. A., Chetia, M., Saikia, B. & Sarma, D. Pd(OAc)2 in WERSA: a novel green catalytic system for Suzuki−Miyaura cross-coupling reactions at room temperature. Chem. Commun. 51, 11489–11492 (2015).
Liu, D. et al. Rice Husk Derived Porous Silica as Support for Pd and CeO2 for Low Temperature Catalytic Methane Combustion. Catalysts 9, 26 (2019).
Esmaeilpour, M., Sardarian, A. & Javidi, J. Dendrimer-encapsulated Pd(0) nanoparticles immobilized on nanosilica as a highly active and recyclable catalyst for the copper- and phosphine-free Sonogashira−Hagihara coupling reaction in water. Catal. Sci. Technol. 6, 4005–4019 (2016).
Liu, Y., Zhao, G., Wang, D. & Li, Y. Heterogeneous catalysis for green chemistry based on nanocrystals. Natl. Sci. Rev. 2, 150–166 (2015).
Deiana, L. et al. Highly Enantioselective Cascade Transformations by Merging Heterogeneous Transition Metal Catalysis with Asymmetric Aminocatalysis. Sci. Rep 2, 851 (2012).
Deiana, L. et al. Combined heterogeneous metal/chiral amine: multiple relay catalysis for versatile eco-friendly synthesis. Angew. Chem. Int. Ed. Engl. 53, 3447–51 (2014).
Deiana, L. et al. Efficient and Highly Enantioselective Aerobic Oxidation−Michael−Carbocyclization Cascade Transformations by Integrated Pd(0)-CPG Nanoparticle/Chiral Amine Relay Catalysis. Synthesis 46, 1303–1310 (2014).
Deiana, L. et al. Enantioselective Heterogeneous Synergistic Catalysis for Asymmetric Cascade Transformations. Adv. Synth. Catal. 356, 2485–2492 (2014).
Xu, C. et al. The Use of Porous Palladium(II)-polyimine in Cooperatively-catalyzed Highly Enantioselective Cascade Transformations. Adv. Synth. Catal. 357, 2150–2156 (2015).
Palo-Nieto, C. et al. Integrated Heterogeneous Metal/Enzymatic Multiple Relay Catalysis for Eco-Friendly and Asymmetric Synthesis. ACS Catal. 6, 3932–3940 (2016).
Xu, C., Afewerki, S., Tai, C. W., Córdova, A. & Hedin, N. Cyclopalladated Azo−linked Porous Polymers in C−C Bond Forming Reactions. ChemistrySelect 1, 5801–5804 (2016).
Afewerki, S. & Córdova, A. Combinations of Aminocatalysts and Metal Catalysts: A Powerful Cooperative Approach in Selective Organic Synthesis. Chem. Rev. 116, 13512 (2016).
Franco, A., De, S., Balu, A. M., Romero, A. A. & Luque, R. Integrated Mechanochemical/Microwave-Assisted Approach for the Synthesis of Biogenic Silica-Based Catalysts from Rice Husk Waste. ACS Sustainable Chem. Eng. 6, 11555–11562 (2018).
Johnston, E. V. et al. Highly Dispersed Palladium Nanoparticles on Mesocellular Foam: An Efficient and Recyclable Heterogeneous Catalyst for Alcohol Oxidation. Chem. – Eur. J. 18, 12202–12206 (2012).
Kalapathy, U., Proctor, A. & Shultz, J. An improved method for the production of silica from rice hull ash. Bioresour. Technol. 85, 285–9 (2002).
Tateda, M., Sekifuji, R., Yasui, M. & Yamazaki, A. A Proposal for Measuring Solubility of the Silica in Rice Husk Ash. J. Sci. Res. Rep. 11, 1–11 (2016).
Santoro, S. et al. Mechanism of Palladium/Amine Cocatalyzed Carbocyclization of Aldehydes with Alkynes and Its Merging with “Pd Oxidase Catalysis”. ACS Catal. 4, 4474–4484 (2014).
Acknowledgements
We gratefully acknowledge financial support from Mid Sweden University and Swedish National Research Council. The Berzelii Center EXSELENT is financially supported by VR and the Swedish Governmental Agency for Innovation systems (VINNOVA). Funding from MINECO is gratefully acknowledged under project CTQ2016-78289-P, co-financed with FEDER funds. Ana Franco gratefully acknowledges MINECO for the provision of an FPI contract (BES-2017-081560) associated to the MINECO CTQ2016-78289-P. The publication has been prepared with support from RUDN University Program 5-100.
Author information
Authors and Affiliations
Contributions
A.C., R.L., S.A. and A.M.B. designed the research; S.A, A.F. and A.M.B. performed the research and C.-W. Tai performed the TEM experiments and analysis. A.C., R.L., S.A. and A.M.B. wrote the paper. All the authors analyzed the data.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Afewerki, S., Franco, A., Balu, A.M. et al. Sustainable and recyclable heterogenous palladium catalysts from rice husk-derived biosilicates for Suzuki-Miyaura cross-couplings, aerobic oxidations and stereoselective cascade carbocyclizations. Sci Rep 10, 6407 (2020). https://doi.org/10.1038/s41598-020-63083-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-63083-8
This article is cited by
-
Nickel Supported MCM-Functionalized 1,2,3-Triazol-4-ylmethanamine: An Efficient Nano-particle-Heterogeneous Catalyst Activate for Suzuki Reaction
Catalysis Letters (2022)
-
Fabrication of sodium hydrogen sulfate onto silica from waste for biomass energy
Chemistry Africa (2021)
-
Copper nanoparticles on controlled pore glass (CPG) as highly efficient heterogeneous catalysts for “click reactions”
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.