Abstract
Tropical hilsa shad (Tenualosa ilisha) contributes significantly to the society and economy of Bangladesh, India and Myanmar, but little is known about their habitats across the life cycle and their relationship with environmental drivers. This study describes spatial and temporal variability of productivity in the Bay of Bengal (BoB) relating to hilsa fishery. Decadal data on net primary productivity, nutrients (i.e. nitrate, phosphate and silicate) and zooplankton were collected from Aqua MODIS, world ocean database and COPEPOD respectively with spatial resolution 1°×1°. Moreover, monthly abundance of phytoplankton, hilsa catch and long-term catch dynamics were analyzed to determine the associations between variables. The present study was extended over 3.568 million km2 area, of which 0.131–0.213 million km2 area characterized as the most productive with net primary production of >2,000 mg C/m2/day, 0.373–0.861 million km2 area as moderately productive with 500–2,000 mg C/m2/day, and 2.517–3.040 million km2 area as the least productive with <500 mg C/m2/day which were consistent with field verification data. In case of nutrients, the Ganges-Brahmaputra-Meghna (GBM) delta was rich in nitrate and phosphate than that of the Ayeyarwady delta, while silicate concentration persisted high all over the northern BoB including the deltas. A peak abundance of phytoplankton was observed in GBM delta during the months of August-November, when ~80% of total hilsa are harvested in Bangladesh annually. Variations in seasonal productivity linked with nutrients and phytoplankton abundance are important factors for predicting hilsa habitat and their migration patterns in the deltaic regions and shelf waters of BoB. These results can be useful in forecasting potential responses of the hilsa in BoB ecosystem to changing global ocean productivity.
Similar content being viewed by others
Introduction
The Bay of Bengal (BoB), which shares many characteristics of the Indian Ocean, is a distinctive system characterized by shallow oceanic arm, deposition of sediment, freshwater plume, seasonal reversal of ocean currents, semidiurnal tides, oxygen-rich surface waters, and abundant biodiversity and fisheries resources1. The northern BoB has the widest shallow shelf region, extending more than 100 nautical miles (=185 km), which is 3–4 times wider in Bangladesh than that in the Myanmar, eastern coast of India and a global average of 65 km. Nutrient is the major driver of primary productivity in BoB2,3. The coupled climate-oceanographic processes (e.g. atmospheric depressions and tropical cyclone, storm surge, internal wave, eddy pumping, and river inputs) are injecting nutrients to the shallow zone and thereby promoting the primary production in upper layers of coastal waters4,5,6, although productivity of an area or ecosystem is dependent on many other bio-physico-chemical parameters such as light7,8.
Phytoplankton is the base of marine food web and thus primary productivity is a key driver of zooplankton and ichthyoplankton dynamics influencing the planktivorous and predatory organisms, mammals, and seabirds9. It accounts for ~50% of global primary productivity10,11, and is important to ecological, biological and biogeochemical processes in oceans12,13. Moreover, marine phytoplankton annually fixes 30–50 billion metric tons of carbon equivalent to 40–50% of the global total14. The majority of phytoplankton species require nitrate, phosphate, iron, molybdenum and copper for growth and reproduction, while diatoms have special requirement of silicate for cell structure and metabolism. Thus, changes in nutrient types and concentrations may change phytoplankton species composition and growth rate, and the resultant net primary production15,16. Zooplankton and herbivore fishes are directly dependent on phytoplankton for food and thus effect primary production through top-down control17. Moreover, productivity (=15–30% of total primary production) is necessary for sustaining the pelagic fish communities18,19. Overall, primary productivity is reported to control the abundance, recruitment, migration pattern and yields of pelagic and migratory fisheries20,21,22, which are important sources of human food, protein, employment and livelihoods17.
The anadromous hilsa shad (Tenualosa ilisha), which feeds on phytoplankton, zooplankton, ichthyoplankton, protozoa, small crustacean and molluscs larvae23,24,25, moves into and out of deltaic ecosystems depending on foraging density and water flow patterns22,26,27. Hilsa acquire energy reserves in the offshore waters until reaching maturity, after which they start upstream spawning migration28, appear to protect reproductive value29,30 with similar behavior in other anadromous fishes, like salmonids and gobiids31,32. Hilsa is mainly harvested from coastal and marine waters (72%), and a quarter from freshwater rivers33. Bangladesh (76%), Myanmar (15%) and India (4%) contribute 95% of the global hilsa catches; while the remaining 5% shared by Iraq, Iran, Kuwait, Thailand and Pakistan34,35. Over the past three decades hilsa production has been increased in Bangladesh with annual harvest reaching 0.5 million tonnes (Fig. 1) that supports livelihoods of 0.5 million fishermen directly, 2.5 million people in the value chain and distribution, and a business valued at US$2 billion33. While peak hilsa fishing takes place from July to November, little is known about their spawning, feeding and/or transboundary migration as well as their spatial and temporal distribution in response to primary production. This study aims to map the productivity zones in the Bay of Bengal and explore their relationship with hilsa fishery.
Materials and Methods
Study area
The present study was carried out in the Bay of Bengal covering an area of 3.6 × 106 km2 between latitude 5° and 24°N, and longitude 79° and 100°E (Fig. 2) covering marine waters of Sri Lanka, India, Bangladesh, Myanmar, Thailand and Indonesia. Major river systems in the study area were Ganges-Brahmaputra-Meghna (GBM) and Ayeyarwady including their tributaries. The BoB has been governed by southwest monsoon winds from May to October and northeast monsoon winds from November to April36,37. Depth of the BoB varies between 10 m in the shelf area of Bangladesh to more than 4500 m as it approaches the Equator38,39. The GBM and Ayeyarwady river systems discharge about 1400 km3/year freshwater, while precipitation (i.e. precipitation over BoB) contributing 5,900 km3/year into the northern BoB40, making it fresher as “river in the sea”41,42. This freshening signal concentrate within the upper 40 m and spreads southward as a narrow strip (~100 km wide) along the western boundary of the bay and reaches the Indian Ocean after 3–5 months journey43,44. Also, freshwater flows southward along the eastern boundary of the basin and gradually mixes with the underlying oceanic layer by vertical exchanges45,46,47. In contrary, the deep canyon in GBM delta (i.e. Swatch of No Ground) has no evidence for sinking freshwater but supports sediment transport to the submarine fan38.
Data and software
Monthly gridded data during 1998–2018 on net primary productivity (NPP) were collected from Aqua MODIS from NOAA (https://coastwatch.pfeg.noaa.gov) in the area between 5°N–24°N and 79°E–100°E corresponding to the Bay of Bengal. Nutrients data (i.e. silicate, nitrate and phosphate) were collected from the world ocean database (https://www.nodc.noaa.gov/) for the same study domain. Zooplankton data were collected from the Coastal & Oceanic Plankton Ecology, Production, & Observation Database (COPEPOD) (https://www.st.nmfs.noaa.gov/copepod/). The NetCDF format data, which were level 3 products (e.g. SeaWiFS, MODIS and MERIS) with spatial resolution 1° latitude × 1° longitude grid48 (Kodama et al. 2017), were downloaded and geospatially analyzed in ArcGIS software. Marine regions’ data of International Hydrographic Organization (IHO) Sea Areas (version 3) for the Bay of Bengal were collected from http://www.marineregions.org/ and used as base map. The monthly net primary productivity was rated in terms of significance for hilsa habitat modeling (Table 1). The spatial extension module was used for surface interpolation in ArcGIS. All maps and data were transformed into decimal degrees projection. Monthly phytoplankton abundance in the GBM delta was collected from Zafar49. Long term hilsa catch data of 1983–2018 was collected from the Department of Fisheries (DoF), Government of Bangladesh and used for the analysis of hilsa catch dynamics. Besides, monthly hilsa catch data were generated through focus group discussions (FGD) and key informant interviews (KII) with experienced fishers, fish traders and distributors in the coastal fishing villages50.
Data integration
The shape file of study area was converted to grid with cell size of 0.0085 degree, equivalent to 4 km to prepare the mask layer with values 1 inside the mask and 0 outside. The geospatial tabular data of monthly net primary productivity were converted to grids by interpolation, maintaining the same geographic extent and cell size as the mask51. In map calculation, individual parameter grid layers multiplying with the mask layer thus resulted in masked grid layers of corresponding months. These parameter grid layers were then reclassified into three classes with <500, 500–2,000 and > 2,000 mg C/m2/day as most productive, moderately productive and least productive zones (Table 1) respectively, and then evaluated by adding respective months. The two seasons i.e. southwest monsoon (SWM) and northeast monsoon (NEM) were calculated by Eqs. (1 and 2) to develop productivity classification map for hilsa migration in the Bay of Bengal.
Validation
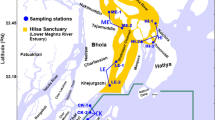
Aqua MODIS results of spatial patterns of NPP in the Bay of Bengal were verified after field observation2,6,52,53,54,55,56,57,58,59,60,61. For this, a stratified simple random sampling was used in different habitats (e.g. estuary and delta, shelf water, and high sea) to identify 32 sites for subsequent assessment. Such an approach is appropriate to verify individual locations after the Aqua MODIS have been employed to assess spatial patterns of NPP in the Bay of Bengal. The productivity maps were verified by comparison between predicted productive zones and habitats of hilsa. For this purpose, geographical distribution map of hilsa by FAO, FishBase and GBIF (Global Biodiversity Information Facility), shads distribution by the Shad Foundation, and marine fishing zones of Bangladesh were used. In addition, participatory field visits, 120 semi-structured interviews and 60 focus group discussions were conducted with professional fishers in the coastal fishing villages of Cox’s Bazar, Chittagong, Noakhali, Laxmipur, Bhola and Patuakhali51. In addition, direct observations of hilsa in 55 landing centers were useful and meaningful way to confirm hilsa yields across space and time. The purpose of verification was to find out whether the existing hilsa habitats are in line with productivity classes or not. In addition, members of the academia, researcher and extension manager were consulted to verify the results of geo-spatial models and the distribution of productivity classes of this study.
Results
Net primary productivity (NPP)
The most productive zone is designated with higher NPP and the productivity ranking is presented in Table 2. Monthly and seasonal NPP classification for the study area are illustrated in geo-spatial model (Fig. 3) and summarized in Table 3. After integration of respective months, 0.168 and 0.145 million km2 area were identified as the most productive zone with >2,000 mg C/m2/day during the southwest monsoon (May-Oct) and northeast monsoon (Nov-Apr) seasons respectively. There were 0.636 and 0.489 million km2 moderately productive zone with 500–2,000 mg C/m2/day, and 2.763 and 2.934 million km2 least productive zone with <500 mg C/m2/day (Table 3). The most productive zone was extended over 520 × 90–170 km (lying between 87–92°E and 21–23°N) in GBM delta, and 380 × 40–190 km (lying between 94–97°E and 15–17°N) in the Ayeyarwady delta. Moderately productive zone was confined to 1000 × 30–190 km in GBM delta and 980 × 70–180 km in the Ayeyarwady delta. While, 1540 × 40–130 km along the western boundary (i.e. Indian east coast) and 2130 × 50–180 km along the eastern boundary (i.e. Bangladesh-Myanmar-Thailand coast) of BoB basin were moderately productive. The offshore deep waters and high seas were the least productive zones.
Nutrients
Geo-spatial distribution of nitrate in the upper 10 m indicated that northern and eastern BoB including GBM and the Ayeyarwady deltas were devoid of nitrate (<0.20 μmol/L) during northeast monsoon, but improved situation was evident with >0.60 μmol/L in western BoB along the Indian coast and Sri Lanka during southwest monsoon (Fig. 4). During northeast monsoon the western part of the GBM delta became enrich with nitrate (~0.40 μmol/L) than the east (>0.10 μmol/L), but the Ayeyarwady delta remained with minimum nitrate. The amount of phosphate was >0.4 μmol/L in GBM delta and in western BoB, while higher concentration of >0.6 μmol/L was recorded in the coastal waters (80–120 km wide) of eastern India during southwest monsoon. There were 0.2–0.3 μmol/L phosphate in GBM and Ayeyarwady deltas during northeast monsoon, while lower level (<0.2 μmol/L, a level same as the open ocean) observed in central and southern BoB. Irrespective of season, silicate distribution was higher (>2.5 μmol/L) in northern, western and eastern BoB and also in the deltas (i.e. GBM and Ayeyarwady) possibly due to freshwater influx and residual flow. Lower level of silicate (<2 μmol/L), which is typical for open ocean, was common in southern BoB.
Zooplankton
Spatial distribution of zooplankton biomass demonstrated that GBM and Ayeyarwady deltas had high zooplankton biomass (> 40 mgC/m3), while reduction of biomass was observed in the western BoB along the Indian coast and eastern BoB along the Myanmar coast (25–40 mgC/m3; Fig. 5). Lower zooplankton biomass (<10 mgC/m3) observed in central and southern BoB. However, the nearshore waters of Sri Lanka, Andaman-Nicobar and Sumatra indicated 15–25 mgC/m3. Spatial distribution of NPP and zooplankton biomass showed that NPP was significantly related with the zooplankton biomass (r = 0.65, p < 0.001). Temporal distribution of zooplankton biomass had minimum variation among the months, i.e. 2.60–2.70 mgC/m3 in January–February, followed by 2.49–2.51 mgC/m3 in June–August and 1.58–1.70 mgC/m3 in October–November (Fig. 6).
Productivity and hilsa fishery
Concurrent with the nutrient distribution, phytoplankton abundance in GBM delta had an enhanced concentration that also coincided with hilsa catch in the northern BoB at Bangladesh coast (Fig. 7). There were two peaks of phytoplankton in GBM delta of the northern BoB. The first peak of phytoplankton abundance with 3,085 cells/L was recorded in October, while the second peak with 2,470 cells/L was found in March. The peak period of plankton production in August-November was clearly linked to the highest hilsa catch (=~80% of 0.5 million tonnes annual catch), while the second peak in January-March can enhance growth and survival of hilsa juvenile that need scientific investigation. The model outputs for the productivity classes were accurately coincided with available maps of hilsa distribution from various sources such as FAO, FishBase, GBIF, IUCN, Shad Foundation and Discover life (Fig. 8). Acording to the fishermen, estuaries/deltas and nearshore waters are the most suitable zones for hilsa fishing. For that reason they operate hilsa gears within 80 m depth and a distance about 200 km from the coast. This fact suggests that suitable hilsa habitats are distributed in areas where primary productivity is typically high, varifying the model outputs for the productivity classes of this study.
Among 32 locations (i.e. 4 locations in the estuary and delta, 12 locations in the shelf water and 16 locations in the high sea) selected to verify the patterns of in-situ NPP distribution, the results of 27 locations were comparable to those Aqua MODIS data. Whereas, three locations in the estuary and delta (ID # 1, 2 and 4) and one location in the shelf water (ID # 15) were overestimated, and one location in the shelf water (ID # 9) was underestimated for NPP distribution. Thus, 84.4% of Aqua MODIS output did corroborate with field data (Table 4). The varification error matrix for Aqua MODIS imagery shows the incorrectly classified locations, based on 32 field verification sites (Table 5). MODIS accuracy (MA) and field accuracy (FA) for each of the ranking classes showed that high sea had the highest value of MA and FA (1.0). The shelf water was well discriminated from the rest of the classes (MA = 0.83 and FA = 0.83). The estuary and delta was poorly represented in the sampling (4/32) with MA and FA of 0.05 and 0.25, respectively. The Kappa Index of Agreement (KIA) was generated to determine the degree of agreement between the two outputs. Its value ranges from −1 to +1 after adjustment for chance agreement. A value of 1 indicates that the two outputs are in perfect agreement (no change has occurred), whereas if the two outputs are completely different from one another, then Kappa value is −1. The Kappa (K) and Kendall’s tau (T) coefficients had the value of 0.73 and 0.77 at 95% confidence, indicating that there is good agreement between field reference and Aqua MODIS data for NPP in the Bay of Bengal. We concluded that a high percentage of the pixels was classified correctly, better than would be expected by a completely random classification.
Discussion
Geo-spatial distribution of nutrients in northern BoB is linked with runoff from GBM and the Ayeyarwady river systems signifying an upward pumping of nutrients from subsurface zone that overlaps with Madhupratap et al.6. Muraleedharan et al.52 recorded <0.1 μM nitrate, 0.4 μM phosphate and >4 μM silicate in the open waters of BoB which is almost similar to present findings. In contrast, Choudhury and Pal53 recorded 11.13–24.19 μM nitrate, 2.23–9.41 μM phosphate and 19.97–127.32 μM silicate along the southeastern coast of India, higher than the present study. Rao et al.62 and De Sousa et al.63 mentioned that rivers flowing into BoB might not contribute much to the inorganic nutrient pool as substantial part of the terrigenous materials are lost at its confluence due to oceanographic processes64. However, river discharges are associated with greater lithogenic fluxes in BoB65, where biogenic matters may rapidly scavenged along with terrigeneous origin and ballasts the materials in faster sedimentation to the deeper ocean66,67. Rivers and atmosphere can supply 20% nitrogenous inputs to BoB, while 80% nitrate comes to the surface from deeper waters by the cyclones, tidal surges, depressions or high speed winds occurring frequently in BoB during Oct-Nov and Mar-Apr68. In general, GBM and the Ayeyarwady deltas are categorized as eutrophic as levels of inorganic nutrients remain high throughout the year compared to the oligotrophic reference values (e.g. phosphate 0.011–0.077 µM, nitrate 0.087–1.900 µM, primary productivity 0.135–0.143 mg Cm−3 h−1) of Karydis69 and Ignatiades70. Continental water flow, nutrients and organic matters originated from the upstream rivers maintain ecosystem functions in the deltas and supply food to the resident species including hilsa71. For instance, the crisscrossed rivers/tributaries of the GBM and Ayeyarwady deltas have an intimate relationship with surrounding land-based activities, such as agriculture, forest, wet meadow, human settlement, industrial development, port operation and tourism activities that play important roles on aquatic habitats. Some areas, such as the offshore deep waters and high seas, are not known as suitable hilsa habitats, but the reasons behind the fact need to uncover with scientific interpretation. Moreover, geo-spatial models can assess suitable habitats of hilsa across the life cycle51, which requires data on bathymetry, oceanographic processes, water quality, primary productivity and habitat characteristics specific to life stages. Some other methods including single nucleotide polymorphism technology (SNP), 87Sr/86Sr isotope ratios in otoliths72, allozymes and morphometric analysis73, and distinctive trait of the parasite fauna74, can provide information about the seasonal movements and residency of hilsa.
High productivity in the nearshore and deltaic waters of BoB integrates with high ambient nutrients. The distribution of NPP positively correlated with phytoplankton concentration (r = 0.75, p < 0.001) that implies that pelagic-neritic fishes like hilsa is expected to maintain their positions within productive areas for sustaining their growth and maturity. Hilsa fed on algae, diatoms, copepods, cladocerans, protozoa, rotifers and the larvae of molluscs24,75. The relationship between hilsa and the abundance of their diets suggests that habitat of hilsa is restricted up to the mixed layer depth (e.g. 20–40 m), which is possibly above the thermocline having maximum level of NPP. At this depth, phytoplankton, copepods, cladocerans and protozoa may be more intense76 and more competently obtained. The present study indicate two peaks of phytoplankton in August-November and in January-March with the lowest abundance in April-July, and this data corresponded well with Choudhury and Pal53 who reported maximum phytoplankton (1,611 cells/mL) in December and minimum (494 cells/mL) in July along the eastern Indian coast of the Bay of Bengal. Incidentally, spawning grounds of hilsa are located in GBM delta77 and Ayeyarwaddy delta78,79, suggesting that these productive water bodies are suitable for hilsa to retain the larvae and juveniles, as illustrated in Fig. 9. In this connection, hilsa migrates to GBM delta for spawning in September-October77 when the levels of NPP and abundance of phytoplankton are high in the system. In addition, coastal rivers and nearshore shallow waters in GBM deltaic region are also suitable nursery grounds for hilsa juveniles until March80. April onwards juveniles start seaward migration as the second phase of anadromous behavior and moves up to 250 km from the coast81 with daily travels of about 71 km82. Moreover, Day83 and Milton84 mentioned that hilsa spends part of life in the sea but not far from the shallow coastal belt. However, operational limitations of gears made difficulties to determine the abundance, extent of seaward migration and fishing potential of hilsa in offshore and high seas due to lack of data and observations. Consequently, Hossain et al.33 recommend for comprehensive study in determining the range of hilsa migration with spatial and temporal distribution across the life cycle. It is evident from the present study that being plankton feeder, hilsa tend to follow plankton rich areas and keep moving from one place to another in search of productive zones and continue to grow. For example, sardine larvae requires 5.7–9.6 mg C/day of primary productivity85 while maximum consumption rates for 1, 2 and 3 years old sardine are 0.042, 0.012 and 0.0049 g-prey g-fish−1 day−1, respectively22. Thus, nursery ground of sardine larvae is located in high productivity zone, where adult sardine (1–3 years old) can live in relatively low productive zone, similar information are not available for hilsa that need to examine. Hilsa prefers zooplankton in early stages and shift towards phytoplankton in adult stage86,87. Diatom, green algae, and blue green algae represent phytoplankton menu of hilsa, while zooplankton menu comprises copepod, cladocera, rotifer, and ostracod23,88,89,90,91. Adult hilsa comprised 97–98% phytoplankton with only 2–3% zooplankton24,92,93 (Fig. 10).
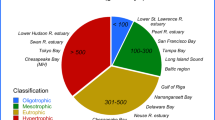
The relationship between hilsa yield and NPP could be interesting to forecast how hilsa population in BoB might respond to future changes in productivity. The Galathea Expedition in 1950–195254 measured NPP 0.1–0.3 mg C m−2 d−1 in the deep sea and 0.01–2.16 g C m−2 d−1 in the shelf region of BoB6. Data of the subsequent studies from different regions of BoB are given in Table 6. Thaw et al.55 proposed 300–500 g C m−2 year−1 as standard reference value of NPP for eutrophic region. The present study found NPP of >2,000 mg C m−2 day−1 in GBM and the Ayeyarwady deltas that coincided with Thaw et al.55, i.e. 2,590 ± 1,569 mg C m−2 day−1 at the Ayeyarwady delta. Conversely, a drop in primary productivity ranging 500–2,000 mg C m−2 day−1 was noted in the area below 22°N along the western and eastern boundary of BoB basin. The least productivity of <500 mg C m−2 day−1 was found in deeper part of BoB and the Andaman Sea. Though no specific trend was observed in seasonal variations, August-October represented with higher productivity in GBM delta, and lower productivity found in April-June that agrees with the findings of Routray and Patra94, Mohanty et al.13, Kumar et al.2 and Radhakrishna et al.95. Phytoplankton production also follows similar pattern, i.e. phytoplankton abundance is high during August-October in GBM delta. Thus, deltas, estuaries and shelf regions of the northern BoB have higher NPP that may support rich neritic and pelagic fisheries. For instance, Sarmiento et al.95 used coupled climate models to forecast responses of NPP in 2040–2060 and predicted that NPP in the sub-polar North Pacific could increase by 10–20% that could result 20% increase in carrying capacity of pelagic species, such as herring. In this context, distribution of NPP and nutrients in the northern BoB including mega deltas and shelf regions can explain and predict the distribution of pelagic fishes such as hilsa fishery in Bangladesh.
Data suggests that total hilsa catch in Bangladesh exceeds nine million tonnes since 1983–84. Specifically, hilsa catch has increased to 517,198 tonnes in 2017–18 from 146,082 tonnes in 1983–84, an annual growth of 7% in the past three decades. The marine waters of northern BoB share the major catch (65%), where the remaining portion fished from the Meghna estuarine system (33%) and from several rivers/tributaries (2%). As the instance in GBM delta, spatial variations in hilsa catch (Fig. 11) interpret habitat suitability and movement routes that can enhance conservation and management initiatives of hilsa fishery. The geographical distribution of hilsa along the coast of Bay of Bengal has been documented by FAO (Fischer and Whitehead 197496; Whitehead97), IUCN98, shad foundation99, FishBase100, Global Biodiversity Information Facility (GBIF)101 and Discover life102 (Fig. 8). Moreover, their occurrences in the adjacent rivers were reported by Hora103, Motwani et al.104 and Quereshi105. Interestingly, hilsa distribution maps with modelled year 2100 native range map based on IPCC A2 emissions scenario (Aquamaps)106 has endorsed similar range for hilsa. Indeed, major efforts of hilsa fishing in Bangladesh have been concentrated within about 100 km from the coast107.
The majority of global oceanic data have been collected through cruises for specific periods which is expensive and not many research institutions and country can afford, especially the developing countries like Bangladesh. As a proxy, satellite imagery is useful for specific temporal resolution of any area during the routine observation schedule. For example, MODIS captures data in 36 spectral bands (0.4–14.4 μm wavelength) for 250–1000 m spatial resolutions with viewing swath of 2330 km and the revisit cycle one to two days. MODIS utilizes four on-board calibrators to provide in-flight calibration, whereas vicarious calibration enhances by using marine optical buoy. Thus, the shortwave infrared (SWIR) bands enable more robust atmospheric corrections in turbid coastal waters (Wang et al.)108, while the band around 685 nm is important for detection of phytoplankton fluorescence109,110 (Gower et al., 1999; Hu et al., 2005). Thus, a comparison among the observation/cruises can enhance scientific understanding and interpret the validation process that we applied in this study. Nevertheless, primary productivity is important for pelagic fisheries recruitment, growth and yield, and found to largely controlled by variation in seasonal temperature gradient56, freshwater plume and haline stratification40,43,44,57, vertical transfer of nutrients from the subsurface levels into the euphotic zone111 and dissolved oxygen, which were out of scope of this study. Therefore, further studies are necessary to investigate the multicriteria evaluation of above mentioned parameters to clarify the relationships of hilsa and its habitat conditions.
References
Hossain, M. S. et al. Background paper for preparation of the 7th Five Year Plan 2016-2020 on opportunities and strategies for ocean and river resources management. Economic Relation Department, Ministry of Planning, The Government of Bangladesh, 67 pp (2014a).
Kumar, S. P. et al. Is the biological productivity in the Bay of Bengal light limited? Cur. Sci. 98(10), 1331–1339 (2010).
Narvekar, J. & Kumar, S. P. Seasonal variability of the mixed layer in the central Bay of Bengal and associated changes in nutrients and chlorophyll. Deep-Sea Res.I 53, 820–835 (2006).
Akester, M. J. Productivity and coastal fisheries biomass yields of the northeast coastal waters of the Bay of Bengal Large Marine Ecosystem. Deep-Sea Res.II 163, 46–56 (2019).
Sarker, S. et al. From science to action: exploring the potentials of blue economy for enhancing economic sustainability in Bangladesh. Oce. Coast. Mag. 157, 180–192 (2018).
Madhupratap, M. et al. U.S. Biogeochemistry of the Bay of Bengal: physical, chemical and primary productivity characteristics of the central and western Bay of Bengal during summer monsoon 2001. Deep-Sea Res. II 50, 881–896 (2003).
Singh, A. K. & Singh, D. K. A Comparative study of the Phytoplanktonic primary production of river Ganga and Pond of Patna (Bihar). Ind.J. Env. Bio. 20, 263–270 (1999).
Moharana, M. & Patra, A. K. Studies on Primary Productivity of Bay of Bengal at Puri Sea-Shore in Orissa. Int. J. Sci. Res. Pub. 2(10), 1–6 (2013).
Capuzzo, E. et al. A decline in primary production in the North Sea over 25 years, associated with reductions in zooplankton abundance and fish stock recruitment. Glob. Change Biol. 24, e352–e364 (2018).
Field, C. B., Behrenfeld, M. J., Randerson, J. T. & Falkowski, P. Primary production of the biosphere: integrating terrestrial and oceanic components. Science 281, 237–240 (1998).
Gao, K., Zhang, Y. & Häder, D. Individual and interactive effects of ocean acidification, global warming, and UV radiation on phytoplankton. J. App. Phy. 30(2), 743–759 (2017).
Sarker, S. & Wiltshire, K. H. Phytoplankton carrying capacity: Is this a viable concept for coastal seas? Oce. & Coast. Mag. 148, 1–8 (2017).
Mohanty, S. S., Pramanik, D. S. & Dash, B. P. Primary Productivity of Bay of Bengal at Chandipur in Odisha, India. Int. J. Sci. Res. Pub. 4(10), 143–147 (2014).
Raymont, J. E. Plankton and Productivity in the Oceans: Volume 1: Phytoplankton. Southampton: Elsevier (2014).
Howarth, R. W. Nutrient Limitation of Net Primary Production in Marine Ecosystems. Ann.Rev.Eco.Sys. 19, 89–110 (1988).
Moore, C. M. et al. Processes and patterns of oceanic nutrient limitation. Nature Geo. 6, 701–710 (2013).
Chassot, E. et al. Global marine primary production constrains fisheries catches. Ecol.Let. 13, 495–505 (2010).
Pauly, D. & Christensen, V. Primary production required to sustain global fisheries. Nature 376, 279–279 (1995).
Jarre-Teichmann, A. & Christensen, V. Comparative modelling of trophic flows in four large upwelling ecosystems: global vs. local effects. In M.H. Durand, P. Cury, R. Mendelssohn, C. Roy, A. Bakun, & D. Pauly (Eds.), Global vs. Local Changes in Upwelling Ecosystems (pp. 423–443). Monterey, CA, USA (1998).
Reid, P. C., Borges, Md. F. & Svendsen, E. A regime shift in the North Sea circa 1988 linked to changes in the North Sea horse mackerel fishery. Fish. Res. 50, 163–171 (2001).
Corten, A. & Lindley, J. A. The use of CPR data in fisheries research. Prog. Ocean. 58, 285–300 (2003).
Okunishi, T., Yamanaka, Y. & Ito, S. A simulation model for Japanese sardine (Sardinops melanostictus) migrations in the western North Pacific. Ecol. mode. 220, 462–479 (2009).
Hora, S. L. A preliminary note on the spawning grounds and Bionomics of the so called Indian Shad, Hilsa ilisha (Hamilton) in the river Ganges. Rec. Indian Mus. 40, 147–158 (1938).
Hasan, K. M. M., Ahmed, Z. F., Wahab, M. A. & Mohammed, E. Y. Food and feeding ecology of hilsa (Tenualosa ilisha) in Bangladesh’s Meghna River basin. IIED Working Paper. IIED, London, 20 pp (2016).
Hossain, M. S., Sharifuzzaman, S. M., Chowdhury, S. R., Rouf, M. A. & Hossain, M. D. Hilsa (Clupeidae: Tenualosa ilisha) predators in the marine and riverine ecosystems of Bangladesh. Sci. Asia 45, 154–158 (2019a).
Chavez, F. P., Ryan, J., Lluch-Cota, S. E. & Ñiquen, C. M. From anchovies to sardines and back: multidecadal change in the Pacific. Ocean. Science 299, 217–221 (2003).
Vieira, J. P., Garcia, A. M. & Grimm, A. M. Evidences of El Niño Effects on the Mullet Fishery of the Patos Lagoon Estuary. Bra. Arc. Bio.Tech. 51, 433–440 (2008).
Shafi, M., Quddus, M. M. A. & Islam, N. Maturation and spawning of Hilsa ilisha (Hamilton-Buchanan) of the River Meghna. Dacca Univ. Stu. Ser. B 26, 63–71 (1978).
Fisher, R. A. The Genetical Theory of Natural Selection. Oxford: Oxford University Press (1930).
Jonsson, B. & Jonsson, N. A review of the likely effects of climate change on anadromous Atlantic salmon Salmo salar and brown trout Salmo trutta, with particular reference to water temperature and flow. J. Fish Biol. 75, 2381–2447 (2009).
Jonsson, N. & Jonsson, B. Energy density and content of Atlantic salmon: variation among developmental stages and types of spawners. Canadian J. Fish. Aqu. Sci. 60, 506–516 (2003).
Neira, F. J., Potter, I. C. & Bradley, J. S. Seasonal and spatial changes in the larval fish fauna within a large temperate Australian estuary. Mar. Biol. 112, 1–16 (1992).
Hossain, M. S. et al. Tropical Hilsa Shad (Tenualosa ilisha): Biology, Fishery and Management. Fish and Fish. 20, 44–65 (2019b).
BoBLME (Bay of Bengal Large Marine Ecosystem). Status of hilsa (Tenualosa ilisha) management in the Bay of Bengal. BoBLME-2010-Ecology-01, 70 pp (2010).
FAO (Food and Agricultural Organization). Fisheries statistics. FAO, Rome (accessed December 2016) (2015).
Schott, F. & McCreary, J. P. The monsoon circulation of the Indian Ocean. Prog. Ocean. 51, 1–123 (2001).
Shankar, D., Vinayachandran, P. N., Unnikrishnan, A. S. & Shetye, S. R. The monsoon currents in the north Indian Ocean. Prog. Ocean. 52, 63–120 (2002).
Berner, U., Poggenburg, J., Faber, E., Quadfasel, D. & Frische, A. Methane in ocean waters of the Bay of Bengal: its sources and exchange with the atmosphere. Deep-Sea Res. II 50, 925–950 (2003).
Wyrtki, K. Physical oceanography of the Indian Ocean. In Zeitzschel, B. (Ed.), The Biology of the Indian Ocean (pp. 18–36), Springer, Berlin (1973).
Dai, A. & Trenberth, K. E. Estimates of freshwater discharge from continents: latitudinal and seasonal variations. J. Hydr. 3, 660–687 (2002).
Sengupta, D., Raj, B. G. N. & Shenoi, S. S. C. Surface freshwater from Bay of Bengal runoff and Indonesian throughflow in the tropical Indian Ocean. Geop. Res. Let. 33, L22609 (2006).
Keerthi, M. G., Lengaigne, M., Vialard, J. & Benshilla, R. Impact of horizontal salinity gradients on the Bay of Bengal circulation and mesoscale variability. Geop. Res. Abst. 20(EGU2018), 11757–1 (2018).
Chaitanya, A. V. S. et al Salinity measurements collected by fishermen reveal a “river in the sea” flowing along the Eastern coast of India. American Met. Soc. BAMS December, 1897–1908 (2014).
Benshila, R. et al. The upper Bay of Bengal salinity structure in a high-resolution model. Ocean Mode. 74, 36–52 (2014).
Han, W. & McCreary, J. P. Modelling salinity distributions in the Indian Ocean. J. Geop. Res. 106(C1), 859–877 (2001).
Jensen, T. G. Cross-equatorial pathways of salt and tracers from the north Indian Ocean: Modelling results. Deep-Sea Res.II 50(12–13), 2111–2127 (2003).
Jensen, T. G. Arabian Sea and Bay of Bengal exchange of salt and tracers in an ocean model. Geop. Res. Let. 28(20), 3967–3970 (2001).
Kodama, T. et al. Improvement in recruitment of Japanese sardine with delays of the spring phytoplankton bloom in the Sea of Japan. Fish. Oceano. 27, 289–301 (2017).
Zafar, M. Southwest monsoon effect on plankton occurrence and distribution in parts of Bay of Bengal. Asian Fish. Sci. 20, 81–94 (2007).
Sharifuzzaman, S. M. et al. Elements of fishing community resilience to climate change in the coastal zone of Bangladesh. J. Coast. Cons. 22(6), 1167–1176 (2018).
Hossain, M. S., Sharifuzzaman, S. M., Chowdhury, S. R. & Sarker, S. Habitats across the lifecycle of hilsa shad (Tenualosa ilisha) in aquatic ecosystem of Bangladesh. Fish. Man. Ecol. 23, 450–462 (2016).
Muraleedharan, K. R. et al. Influence of basin-scale and mesoscale physical processeson biological productivity in the Bay of Bengal duringthe summer monsoon. Prog. Ocean. 72, 364–383 (2007).
Choudhury, A. K. & Pal, R. Phytoplankton and nutrient dynamics of shallow coastal stations at Bay of Bengal, Eastern Indian coast. Aquat. Ecol. 44, 55–71 (2010).
Brunn, A. F., Mielche, G. H. & Sparck, R. The Galathea Deep Sea Expedition 1950–1952. George Allen Unwin Ltd, London, 304 pp (1956).
Thaw, M. S. H. et al. Seasonal dynamics influencing coastal primary production and phytoplankton communities along the southern Myanmar coast. J. Ocean. 73(3), 345–364 (2017).
Chowdhury, S. R., Hossain, M. S., Shamsuddoha, M. & Khan, S. M. M. H. Coastal Fishers’ Livelihood in Peril: Sea Surface Temperature and Tropical Cyclones in Bangladesh. Foreign and Commonwealth Office through British High Commission and Centre for Participatory Research and Development (CPRD), Dhaka, Bangladesh, 66 pp (2012).
Vinayachandran, P. N. & Kurian, J. Hydrographic observations and model simulation of the Bay of Bengal freshwater plume. Deep Sea Res. I 54, 471–486 (2007).
Madhu, N. V. et al. Lack of seasonality in phytoplankton standing stock (chlorophyll a) and production in the western Bay of Bengal. Cont. Shelf Res. 26, 1868–1883 (2006).
Odum, E. P. Ecology. Holt, Rinehart &Winston, New York and London, 152 pp (1963).
Perry, R. I. & Schweigert, J. F. Primary productivity and the carrying capacity for herring in NE Pacific marine ecosystems. Prog. Ocean. 77, 241–251 (2018).
Radhakrishna, K., Devassay, V. P., Bhargava, R. M. S. & Bhattathiri, P. M. A. Primary production in the northern Arabian Sea. Indian J. Mar. Sci. 7, 271–275 (1978).
Rao, C. K. et al. Hydrochemistry of the Bay of Bengal: possible reasons for a different water-column cycling of carbon and nitrogen from the Arabian Sea. Mar. Chem. 47, 279–290 (1994).
De Sousa, S. N., Naqvi, S. W. A. & Reddy, C. V. G. Distribution of nutrients in the western Bay of Bengal. Ind. J. Mar. Sci. 10, 327–331 (1981).
Gupta, R. S., De Sousa, S. N. & Joseph, T. On Nitrogen & Phosphorus in the Western Bay of Bengal. Ind.J. Mar. Sci. 6, 107–110 (1977).
Ittekkot, V. et al. Enhanced particle fluxes in Bay of Bengal induced by injection of fresh water. Nature 351, 385–387 (1991).
Ittekkot, V. B., Haake, B., Bartsch, M., Nair, R. R. & Ramaswamy, V. Organic carbon removal in the sea: the continental connection. Upwelling Systems: Evolution since the early Miocene. Geological Society of London, Special Publication No. 64, 167–176 (1992).
Kumar, M. D., Sarma, V. V. S. S., Ramaiah, N., Gauns, M. & De Sousa, S. N. Biogeochemical significance of transparent exopolymer particles in the Indian Ocean. Geop. Res. Let. 25, 81–84 (1998).
Kumar, S., Ramesh, R., Sardesai, S. & Sheshshayee, M. S. High new production in the Bay of Bengal: possible causes and implications. Geop. Res. Let. 31, L18304 (2004).
Karydis, M. Eutrophication assessment of coastal waters based on indicators: a literature review. Glob. NEST J. 11, 373–390 (2009).
Ignatiades, L. The productive and optical status of the oligotrophic waters of the Southern Aegean Sea (Cretan Sea), Eastern Mediterranean. J. Plankton Res. 20, 985–995 (1998).
Hossain, M. S., Das, N. G. & Chowdhury, M. S. N. Fisheries Management of the Naaf River. Coastal and Ocean Research Group of Bangladesh, 268 pp (2007).
Milton, D. A. & Chenery, S. R. Movement patterns of the tropical shad (Tenualosa ilisha) inferred from transects of 87Sr/86Sr isotope ratios in their otoliths. Canadian J. Fish. Aqu. Sci. 60, 1376–1385 (2003).
Salini, J. P., Milton, D. A., Rahman, M. J. & Hussain, M. G. Allozyme and morphological variation throughout the geographic range of the tropical shad, Tenualosa ilisha. Fish. Res. 66, 53–69 (2003).
Alam, A. Comparison of the parasite fauna of Tenualosa ilisha from different locations in Bangladesh. In S. J. M. Blaber, D. J. Brewer, D. A. Milton & C. Biano (Eds.), International Terubok Conference Proceedings, Sarawak Development Corporation, Kuching, Malaysia, pp. 124–138 (2001).
Pillay, S. R. & Rao, K. V. Observations on the biology and fishery of hilsa, Hilsa ilisha (Hamilton), of river Godavari. Proc. Indo-Pacific Fish. Comm. 10, 37–61 (1963).
Lamb, J. & Peterson, W. T. Ecological zonation of zooplankton in the COAST study region off central Oregon in June and August 2001 with consideration of rention mechanisms. J. Geop. Res. 110(C10), C10515 (2005).
Hossain, M. S., Sarker, S., Sharifuzzaman, S. M. & Chowdhury, S. R. Discovering Spawning Ground of Hilsa Shad (Tenualosa ilisha) in the coastal waters of Bangladesh. Ecol. Mod. 282, 59–68 (2014b).
Tezzo, X. et al Fisheries Research Using Digital Tablets in Myanmar. Proceedings of the 9th Conference of the Asian Federation for Information Technology in Agriculture (AFITA), Perth, Western Australia, 498–504 (2014).
Than, T. & Lay, K. K. Pelagic fisheries management for sustainable development: Myanmar initiative. Fish for the People 6(2), 29–31 (2008).
Hossain, M. S., Sarker, S., Sharifuzzaman, S. M. & Chowdhury, S. R. Habitat Modelling of Juvenile Hilsa Tenualosa ilisha (Clupeiformes) in the Coastal Ecosystem of the Northern Bay of Bengal, Bangladesh. J. Icht. 54(2), 203–213 (2014c).
Haldar, G. C. & Islam, M. R. Hilsa fisheries conservation, development and management technique (in Bengali). Department of Fisheries, Matshya Bhaban, Dhaka, Bangladesh, 42 pp (2008).
BoBP (Bay of Bengal Programme). A review of the biology and fisheries of Hilsa ilisha in the upper Bay of Bengal. Marine Fisheries Resources Management in the Bay of Bengal, Colombo, Sri Lanka, BoBP/WP/37, 58 pp (1985).
Day, F. Report on the freshwater fish and fisheries of India and Burma. Calcutta. 35-36, 22–23 (1873).
Milton, D. A. Living in Two Worlds: Diadromous fishes and factors affecting population connectivity between tropical rivers and coasts. In I. Nagelkerken (Ed.), Ecological Connectivity among Tropical Coastal Ecosystems (pp. 325–355). Dordrecht, Netherlands: Springer Science+Business Media B.V (2009).
Watanabe, Y. & Saito, H. Feeding and growth of early juvenile Japanese sardines in the Pacific waters off central Japan. J. Fish Bio. 52, 519–533 (1998).
Bhaumik, U. Decadal studies on hilsa and its fishery in India – a review. J.Interacad 17(2), 377–405 (2013).
De, D., Anand, P. S. S., Subhasmita Sinha, S. & Suresh, V. R. Study on preferred food items of Hilsa (Tenualosa ilisha). Int. J. Agri.Food Sci.Tech. 4(7), 647–658 (2013).
Hora, S. I. & Nair, K. K. Further observations on the bionomics and fishery of the Indian Shad, Hilsa ilisha (Ham.) in Bengal waters. Rec. Indian Mus. 42, 35–50 (1940).
Nair, K. K. on some early stages in the development of the so-called Indian shad, Husa Uisha (Ham.). Rec. Indian Mus. 41(4), 409–418 (1939).
Lakshminarayana, M. Studies of the phytoplankton of the river Ganges, Varanasi, India. Pt. IV. Phytoplankton in relation to fish populations. Hydrobiologia 25, 171–175 (1965).
Rahman, M. A., Rahman, M. J., Moula, G. & Mazid, M. A. Observation on the food habits of Indian shad, Tenualosa (=Hilsa) ilisha (Ham.) in the Gangetic river system of Bangladesh. J. Zool. 7, 27–33 (1992).
Islam, A. K. M. N. Preliminary studies on the food of some fishes. The Dhaka Univ. Stud. Part. B 22, 47–51 (1974).
Akter, A., Rahman, M. A., Isaac, S. & Sarker, M. J. Zooplankton in the Gut Content of Indian Shad (Tenualosa ilisha): Case Study at the Meghna River Estuary, Bangladesh. J. Micro. Biot. 5(3), 1–6 (2016).
Routray, N. & Patra, A. K. Studies on seasonal variations in primary production of river Mahanadi, Banki, Odisha, India. Int. J. Bioassays 5(2), 4779–4781 (2016).
Sarmiento, J. L. et al. Response of ocean ecosystems to climate warming. Global Biog. Cyc. 18 GB3003, 23 (2004).
Fischer, W. & Whitehead, P. J. P. FAO species identification sheets for fishery purposes. Eastern Indian Ocean (fishing area 57) and Western Central Pacific (fishing area 71). Volume 1. FAO, Rome, 214 pp (1974).
Whitehead, P. J. P. FAO species catalogue. Volume 7, Clupeoid fishes of the world. An annotated and illustrated catalogue of the herrings, sardines, pilchards, sprats, anchovies and wolf-herrings. FAO Fisheries Synopsis No. 125, Vol. 7, Part 1. FAO, Rome, 303 pp (1985).
IUCN (International Union for Conservation of Nature). Tenualosa ilisha. The IUCN Red List of Threatened Species. Version 2019-2, https://www.iucnredlist.org/species/166442/1132697 (accessed September 2019) (2007).
Shad Foundation. Distribution of shads around the world, http://www.cbr.washington.edu/shadfoundation/shad/distribution/distribution.html, (accessed February 2015) (2015).
Fishbase. Native distribution map for Tenualosa ilisha (Hilsa shad), with modelled year 2100 native range map based on IPCC A2 emissions scenario, https://www.fishbase.se/summary/Tenualosa-ilisha.html# (accessed September 2019) (2019).
GBIF (Global Biodiversity Information Facility). Occurrences of Tenualosa ilisha (Hamilton, 1822). GBIF Secretariat, Universitetsparken, Copenhagen, Denmark, https://www.gbif.org/occurrence/map?taxon_key=2413382 (accessed September 2019) (2019).
Discover life. Tenualosa ilisha (Hamilton, 1822) distribution map, https://www.discoverlife.org/20/q?search=Tenualosa+ilisha&b=FB1596 (accessed September 2019) (2019).
Hora, S. L. Life history and wanderings of hilsa in Bengal waters. J. Asiatic Soc. (Sci.) 6, 93–112 (1941).
Motwani, M. P., Jhingran, V. G. & Karamchandni, S. J. On the breeding of the Indian shad, Hilsa ilisha (Hamilton) in freshwaters. Sci. and Cult. 23, 47–48 (1957).
Quereshi, M. R. Hilsa fishery in East Pakistan. Pakistan J. Sci. and Ind. Res. 11, 95–103 (1968).
Aquamaps. Computer generated distribution maps for Tenualosa ilisha (Hilsa shad), with modelled year 2100 native range map based on IPCC A2 emissions scenario, www.aquamaps.org, (accessed September 2019) (2019).
Hossain, M. S., Chowdhury, S. R. & Sharifuzzaman, S. M. Blue Economic Development in Bangladesh: A policy guide for marine fisheries and aquaculture. Chittagong: Institute of Marine Sciences and Fisheries, University of Chittagong, Bangladesh, 32 pp (2017).
Wang, M., Son, S. & Shi, W. Evaluation of MODIS SWIR and NIR-SWIR atmospheric correction algorithms using SeaBASS data. Remote Sens. Environ. 113, 635–644 (2009).
Gower, J. F. R., Doerffer, R. & Borstad, G. A. Interpretation of the 685 nm peak in water-leaving radiance spectra in terms of fluorescence, absorption and scattering, and its observation by MERIS. Int. J. Remote Sens. 20, 1771–1786 (1999).
Hu, C. et al. Red tide detection and tracing using MODIS fluorescence data: a regional example in SW Florida coastal waters. Remote Sens. Environ. 97, 311–321 (2005).
Prasanna Kumar, S. et al. Why is the Bay of Bengal less productive during summer monsoon compared to the Arabian Sea? Geop. Res. Lett. 29, 2235 (2002).
Prasanna Kumar, S. et al. Eddy-mediated biological productivity in the Bay of Bengal during fall and spring intermonsoons. Deep-Sea Res. I 54, 1619–1640 (2007).
Gauns, M. et al. Comparative accounts of biological productivity characteristics and estimates of carbon fluxes in the Arabian Sea and the Bay of Bengal. Deep-Sea Res. II 52, 2003–2017 (2005).
Gomes, H. R., Goes, J. I. & Saino, T. Infuence of physical processes and freshwater discharge on the seasonality of phytoplankton regime in the Bay of Bengal. Cont. Shelf Res. 20, 313–330 (2000).
Bhattathiri, P. M. A., Devassy, V. P. & Radhakrishna, K. Primary production in the Bay of Bengal during southwest monsoon of 1978. Mahasagar-Bull. Nat. Inst. Ocean. 13, 315–323 (1980).
Acknowledgements
The Standard NPP datasets is collected from Aqua MODIS of NOAA (https://coastwatch.pfeg.noaa.gov). Nutrients data is collected from the world ocean database (https://www.nodc.noaa.gov/). Zooplankton data were collected from the Coastal & Oceanic Plankton Ecology, Production, & Observation Database (COPEPOD) (https://www.st.nmfs.noaa.gov/copepod/). Marine regions’ data of IHO Sea Areas for the Bay of Bengal is collected from http://www.marineregions.org/. Hilsa fishery catch data is collected from the Department of Fisheries (DoF), Bangladesh (http://www.fisheries.gov.bd) and FAO (http://www.fao.org/fishery/statistics/en). Geographical distribution map of hilsa is collected from FAO (http://www.fao.org/3/ac482e/ac482e00.htm), FishBase (https://www.fishbase.se/summary/1596), the European Nucleotide Archive (EMBL-EBI) Global Biodiversity Information Facility (https://www.gbif.org/species/2413382), Shad Foundation (http://www.cbr.washington.edu/shadfoundation/shad/distribution/), Discover life (https://www.discoverlife.org/20/q?search=Tenualosa+ilisha&b=FB1596) and IPCC A2 emissions scenario Aquamaps (www.aquamaps.org). We are grateful to the NOAA, IHO, DoF, FAO, FishBase, GBIF, Shad Foundation Discover life and Aquamaps for access to these data.
Author information
Authors and Affiliations
Contributions
M.S.H. and S.R.C. conceived and designed the research. S.S. led collection of the data. M.S.H. contributed to data collation. S.S. led data analyses and M.S.H. contributed to data analyses. S.R.C. led illustrations and M.S.H. contributed to illustrations. M.S.H., S.S. and S.M.S. wrote the manuscript. S.R.C. and S.M.S. revised the manuscript. All the authors edited and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hossain, M.S., Sarker, S., Sharifuzzaman, S.M. et al. Primary productivity connects hilsa fishery in the Bay of Bengal. Sci Rep 10, 5659 (2020). https://doi.org/10.1038/s41598-020-62616-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-62616-5
This article is cited by
-
Epipelagic mesozooplankton communities in the northeastern Indian Ocean off Myanmar during the winter monsoon
Acta Oceanologica Sinica (2023)
-
The impact of climate variables on marine fish production: an empirical evidence from Bangladesh based on autoregressive distributed lag (ARDL) approach
Environmental Science and Pollution Research (2022)
-
DNA barcoding confirms a new record of flyingfish Cheilopogon spilonotopterus (Beloniformes: Exocoetidae) from the northern Bay of Bengal
Conservation Genetics Resources (2021)
-
Seasonal Surface and Bottom Temperature-salinity Anomaly of a Subtropical River in Response to Sea Surface Elevation
Thalassas: An International Journal of Marine Sciences (2021)
-
Remote sensing of fish-processing in the Sundarbans Reserve Forest, Bangladesh: an insight into the modern slavery-environment nexus in the coastal fringe
Maritime Studies (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.