Abstract
The prevalence of dry eye disease is high worldwide and poses a great burden on patients’ daily lives. Accurate diagnosis of the disease is important, and it requires application of various methods. Hyperosmolarity is believed to be the disease marker and thus measuring it provides useful information. In this study we investigated utility of tear osmolarity measured with TearLab osmometer, along with other diagnostic tests (Ocular Surface Disease Index questionnaire, Tear film break-up time, Ocular Protection Index, Ocular Surface Staining, Schirmer I test, Meibomian gland functionality in 757 patients (1514 eyes) with dry eye disease and 29 healthy controls (58 eyes). Statistical differences between the patient group and the control group were observed for all the tests apart from tear osmolarity, regardless of cut-off value (>308 mOsm/L, >316 mOsm/L, and inter-eye difference >8 mOsm/L). Moreover, in the receiver operating characteristics curve analyses tear osmolarity measurement could not discriminate dry eye disease pathological scores. Therefore, our study suggests that tear osmolarity measured with TearLab osmometer cannot be used as a key indicator of DED.
Similar content being viewed by others
Introduction
The International Dry Eye Workshop II 2017 defines dry eye disease (DED) as “… a multifactorial disease of the ocular surface characterized by a loss of homeostasis of the tear film, and accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiological roles”1. The symptoms of DED include ocular burning, foreign body sensation, soreness, stinging, irritation, reduced visual acuity, photophobia, and ocular pain1. The burden of DED can vary from mild discomfort to severe complaints that impact daily activities, reduce quality of life, and have significant socioeconomic implications2. The prevalence of DED increases with age and ranges from 5% to 50%2.
Accurate diagnosis of DED is complex and requires the application of a battery of tests, including questionnaires of patient-reported symptoms3, tear film break-up time (TFBUT)4, the Schirmer test5, ocular surface staining6, and meibomian gland functionality7. However, most of the tests lack consistency and reliability for diagnosing DED; therefore, they are subject to clinical interpretation based on experience8. The lack of a strong association between the signs and symptoms of DED is another challenge clinicians face in diagnosing and following-up patients with the disease9. Numerous ancillary diagnostic tests have been developed with the purpose of overcoming these challenges, including several patient-reported DED-specific questionnaires and new tools enabling the quantification of tear film characteristics. One of these tools is the measurement of tear osmolarity. Previous studies highlight tear hyperosmolarity as a significant pathophysiological factor in the development and the clinical course of DED10,11,12,13,14,15. Patients with DED present with elevated tear osmolarity compared to healthy controls11,16 and the hyperosmolarity increases with dry eye severity level (DESL)16,17,18.
In vivo measurement of tear osmolarity was not widely available for clinical practitioners until the TearLab osmometer (TearLab, San Diego, CA, USA) was approved by the US Food and Drug Administration in 2008. Since then, several studies have demonstrated that it is a reliable test with good performance for complementing DED diagnosis16,19,20. In situ analyses have also shown the inherent accuracy and precision of the TearLab osmometer21. Some studies have concluded that the TearLab osmometer is the best single marker for diagnosing and classifying DED levels of severity11 and for distinguishing DESL18. On the other hand, several clinical studies have raised questions on the diagnostic ability of the tear osmolarity measured with the TearLab osmometer in DED13,22,23,24. The varying results in the aforementioned studies might be due to the limitations of small sample size, studies conducted at multiple centers, and different diagnostic criteria for DED. Therefore, we conducted a single-center study with a large group of patients with DED (n = 757). Here, we explore whether tear osmolarity measurement with the TearLab system can be used as a diagnostic test along with clinical examination diagnostic methods for distinguishing patients with DED from healthy subjects.
Methods
Participants
This single-center, retrospective study was carried out at the Norwegian Dry Eye Clinic in Oslo, Norway. The study was conducted in accordance with the Declaration of Helsinki. The use of the data for the study from the Norwegian Dry Eye Clinic has been reviewed and approved by The Regional Committee for Medical & Health Research Ethics, Section C, South East Norway (REC). All participants gave a written informed consent for the data to be used for research purposes.
We included a total of 1514 eyes of 757 patients (mean age, 52.1 ± 16.1 years, range 8–89 years) diagnosed with symptomatic DED of different etiologies in at least one eye, with osmolarity measured using the TearLab osmometer in both eyes. The examinations took place from 2014 to 2018. The inclusion criteria were presence of clinically diagnosed DED of different severity levels (I–IV) according to the classification recommended by the 2007 Dry Eye Workshop (DEWS) developed by the Tear Film and Ocular Surface Society (TFOS)25 based on subjective feeling of ocular dryness (e.g. discomfort, pain), negative effect on visual ability (e.g. annoying or activity-limiting, OSDI > 12), conjunctival injection, conjunctival and corneal staining, signs of DED on cornea and tear film, presence of meibomian gland dysfunction, abnormal tear film break-up time and Schirmer test values. It is important to note that these signs and symptoms vary based on severity levels. For the control group, we recruited 29 healthy participants (58 eyes). These participants were recruited from another ongoing study in collaboration with the Faculty of Dentistry, University of Oslo, Oslo (REK 2015/363). The participants were deemed healthy if the Ocular Surface Disease Index (OSDI)3 score was <12 and TFBUT ≥ 5 seconds. Control participants were excluded if they had any symptoms of DED (controlled with OSDI), seasonal allergy, used contact lenses or lubricating drops, had a history of refractive surgery, used of medications or had a systemic disease affecting tear production. Initially we had access to 65 control subjects who didn’t report symptoms of DED. However, clinical examinations revealed positive dry eye signs in some of them, and they were therefore excluded. We wanted to ascertain that controls had neither symptoms nor signs of DED. This rather strict inclusion criteria resulted in a smaller sample size of the control group.
Dry eye examination
The participants underwent comprehensive dry eye assessment in the following order: subjective dry eye symptom evaluation using the OSDI questionnaire3 (Allergan, Irvine, CA, USA), tear osmolarity measurement using the TearLab osmometer11, inter-blink interval (IBI)26, TFBUT4, ocular surface staining (OSS)6, Schirmer I test5, meibum expressibility and meibum quality evaluation7, and meibography27. The examinations were carried out between 9 AM and 4 PM, and the participants were instructed not to use any eye drops within 2 hours prior to examination. Table 1 summarizes the ophthalmological work-up. All patients were examined between 9 am and 3 pm at the same clinic, where temperature and lighting were stable. Upon arrival to the appointment at the clinic, the patients were seated in a waiting area for at least 10 minutes. This was to ensure that the patients adjusted to indoor climate and did not have reflexive tearing after coming from outdoors. The tear osmolarity measurement was performed as described in the user manual28. The patient was seated with the chin tilted upwards and eyes directing towards the ceiling. The tip of the osmometer was positioned just above the lower lid in the lateral canthal area. The osmometer was gently lowered until the bottom of the tip touched the thin line of the tear film. Successful tear collection was confirmed by a beeping sound and disappearance of the green light. The osmometer was docked into the reader immediately (5–10 seconds), the test card code was selected, the OK button was pressed, and the test result was obtained. TFBUT was evaluated after instillation of 5 μl 2% fluorescein sodium, and an average of three readings was recorded. The ocular protection index (OPI) was calculated as the TFBUT divided by the IBI29. Ocular surface staining was graded according to the Oxford grading scheme30. Finally, meibomian gland expressibility and meibum quality were assessed by application of light pressure to the lower eyelids using a cotton swab7. Based on symptoms, ocular surface staining, TFBUT, Schirmer test, and meibomian gland functionality, the DESL in each eye was determined according to the TFOS DEWS dry eye severity grading system25.
Statistics
Statistical analyses were performed with commercial software SPSS for Windows, version 23 (IBM, Chicago, IL, USA). The normal distribution of variables was verified by the Kolmogorov-Smirnov test. Intergroup analyses were carried out with Kruskal Wallis test. The Mann-Whitney U test was used to compare two groups. The ability of tear osmolarity measurement for predicting the pathological scores of other clinical dry eye tests was assessed using receiver operating characteristics (ROC) curve analysis. Pathological TFBUT, Schirmer I test, OSS, meibum expressibility, and meibum quality scores were defined as <5 sec, <10 mm/5 min, <1, >1, and >1, respectively. Values from the right and left eyes were analyzed separately. In addition, we calculated the average values from the right and left eyes. A p-value of <0.05 was considered statistically significant throughout the study. The analyses results are presented as the mean ± standard deviation (SD).
Results
Demographics
Among the patients, 629 (83.1%) were female and 128 (16.9%) (4.9:1) were male; the control group comprised 19 female (65.5%) and 10 male subjects (34.5%) (1.9:1). The mean age of the patient group was 52.2 ± 16.0 years; that of the control group was 44.9 ± 17.7 years (p = 0.201).
Tear osmolarity in the patient group was not significantly different from the control group
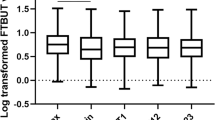
Using the average values of both eyes the patient group showed significantly worse values for all clinical dry eye tests compared to the control group, except for tear osmolarity (OsmAvg), (310.6 ± 16.2 vs. 310.3 ± 19.4 mOsm/L, p = 0.754), (Table 2).
The patient group presented with worse subjective symptoms, shorter TFBUT, higher OSS, shorter Schirmer I test results and higher values of meibomian gland functionality. Apart from tear osmolarity, none of the mean test values in the control group reached pathological levels. In the patient group, the range of OsmAvg was from 278.5 to 374.5 mOsm/L (95% confidence interval [95% CI] 309.5–311.8). Osmolarity levels in the right and left eye were 275–398 mOsm/L (95% CI 311.9–314.7) and 272–346 mOsm/L (95% CI 296–315.8), respectively. In the control group, the OsmAvg ranged between 281.5 and 347.5 mOsm/L (95% confidence interval [95% CI] 301.9–318.7). Osmolarity levels in the right and left eyes were 281–369 mOsm/L (95% CI 304.7–323.7) and 275–398 mOsm/L (95% CI 306.6–309.3), respectively. Comparison of the parameters from the right and left eyes separately produced results that were consistent with those in Table 2.
Tear osmolarity cut-off values could not distinguish DED participants from healthy participants
Analyses of the right eye revealed that 58% of the patients and 50% of the control subjects had osmolarity levels exceeding the suggested cut-off of 308 mOsm/L. The respective levels in the left eye were 42% and 39%. When the cut-off value of 316 mOsm/L was employed, 38% of right eyes and 27% of left eyes in the patient group had osmolarity levels exceeding the cut-off value. Similar results were obtained for the control group: 38% of the right eyes and 24% of the left eyes had osmolarity >316 mOsm/L. Whilst 62% of patients had inter-eye difference of >8 mOsm/L (range, 8–94) mOsm/L, the same was true for 74% of the controls (range, 8–66 mOsm/L).
Among the patients, about 10% had DESL 1. The vast majority of the patients had DESL 2 (72.5% of right eyes and 71.3% of left eyes), followed by DESL 3 in 14.4% of right eyes and 16.6% in left eyes. Only 1.3% of patients had DESL 4. We combined the participants based on DESL and created new groups: non-DED = DESL 0; mild DED = DESL 1 + DESL 2; severe DED = DESL 3 + DESL 4. Comparison of the groups based on DESL (Table 3) revealed that dry eye signs and symptoms were worsened in the mild DED group compared to the non-DED group and in the severe DED group compared to both the mild and the non DED group. However, there was not a significant difference (p = 0.123) in tear osmolarity values in the right eyes; non-DED 310.8 ± 21.9 mOsm/L, mild DED 312.7 ± 18.3 mOsm/L and severe DED 316.4 ± 23.5 mOsm/L. Similar to findings in the right eyes, subjective and objective parameters in the left eyes worsened as DESL increased. Severe DED group had tear osmolarity values of 312.9 ± 21.9 mOsm/L while mild DED had 306.9 ± 17.9 mOsm/L. The non-DED group showed tear osmolarity of 307.4 ± 21.1 mOsm/L.
We attempted to determine whether tear osmolarity levels can discriminate DED pathological values employing ROC analyses. The results are shown in Table 4. The area under curve (AUC) was only significant for Schirmer I test in the right eyes (AUC = 0.544, p = 0.038) and left eyes (AUC = 0.546, p = 0.033). The other parameters did not demonstrate significant values. When using OsmAvg, statistically significant differences for the AUC values for average Schirmer I test (Avg Schirmer ≤ 10) and average ocular surface staining (Avg OSS > 3) were obtained. The remaining parameters did not show statistically significant differences.
Moreover, we wanted to test whether there was a difference in osmolarity values between the two major forms of DED, aqueous deficiency dry eye (ADDE) and evaporative dry eye (EDE). In our dataset, 90.1% of the patients had a diagnosis of EDE due to MGD with various severity levels, while 9.9% of the patients were diagnosed with ADDE. Comparison of osmolarity levels between ADDE and EDE showed values of 326.8 ± 28.9 mOsm/L and 312 ± 18.2 mOsm/L (p = 0.123) in the right eye, respectively. Results for ADDE and EDE in the left eye were 304.5 ± 15.8 mOsm/L and 306.8 ± 17.5 mOsm/L, respectively, p = 0.809.
Discussion
This retrospective study focused on investigating tear osmolarity measurement as compared to other subjective and clinical parameters of DED, including the scores of the OSDI, TFBUT, OSS, Schirmer I, and meibomian gland functionality tests. Our data show that osmolarity was not significantly different between the healthy controls and the patients (Table 2) and that a significant percentage of the healthy controls had tear osmolarity levels exceeding the recommended cut-off values (>308 mOsm/L, >316 mOsm/L, and inter-eye difference >8 mOsm/L). On the other hand, a substantial proportion of the patients with clinically diagnosed DED had tear osmolarity levels below the above cut-off values, suggesting a wide overlap of osmolarity between the controls and the patients. Using the mean value of the two eyes, osmolarity could be used to discriminate pathological Schirmer test values (<10 mm/5 min) (p = 0.043, optimum balanced sensitivity and specificity of 54% and 51%, respectively) and average OSS value (>3) (p = 0.019), but not any other clinical tests.
Tear film hyperosmolarity is believed to be an etiological factor in DED, hence the measurement of tear osmolarity has been considered important for diagnostic purposes31. Several measurement techniques have been used in the past32,33, with the Clifton and vapor pressure osmometers being the most commonly used methods. Despite high accuracy, sensitivity, and specificity32,33, these methods are not available for use at the point-of-care and require special setups that would need a significant amount of time. Clinicians did not have point-of-care diagnostic tests for measuring tear osmolarity until 2008, when the TearLab osmometer became commercially available. This technology uses a microchip, requiring only a 0.2-μl tear sample obtained without direct contact with the ocular surface. Lemp et al.11 concluded that tear osmolarity is the best single metric for diagnosing and classifying DED and suggested 308 mOsm/L as the most sensitive threshold between normal and mild DED. Similarly, Jacobi et al.19 proposed that a cut-off value of 316 mOsm/L demonstrated superior accuracy to other single tests for diagnosing DED. However, other studies demonstrated high variability of tear osmolarity in DED diagnosis13,22,23,24, and suggested that the TearLab osmolarity results should be interpreted with caution and in the context of other established methods. Accordingly, our data show that tear osmolarity levels measured with the TearLab osmometer were not significantly different between the controls and the patients.
If, as was done in the present study, osmolarity measured with the TearLab osmometer were used as the only diagnostic criteria for DED, the recommended cut-off value of 308 mOsm/L would exclude a significant proportion of patients (58% of right eyes and 42% of left eyes) with otherwise clinically diagnosed DED in our cohort. On the contrary, around 50% of the controls could have been diagnosed with DED when the same cut-off value was used. Using the cut-off value of 316 mOsm/L yielded similar results. Furthermore, another reference value commonly used for diagnosing DED, i.e., inter-eye difference >8 mOsm/L, failed to distinguish clinical DED from healthy eyes in the present study. Our data show that 62% of the patients and 74% of the controls had inter-eye difference >8 mOsm/L. These findings imply that tear osmolarity measurement with the TearLab osmometer has high variability and that it failed to distinguish patients with clinically diagnosed DED from healthy controls, which is in line with a recently published review by Baenninger et al.24. Our data differ from that of Lemp et al.11. The inconsistency might be caused by differences in the study population and DED diagnostic criteria. For example, the 314 subjects in their study were recruited from 10 centers, whereas we included 757 participants from a single center. In the present study, all clinical tests were performed by one experienced ophthalmologist with one or two assistants, which could have minimized the inter-observer variation in performing dry eye tests. The larger sample size might also have contributed to the statistical power of the analyses.
Despite the finding of Lemp and associates11, indicating a high accuracy of the TearLab osmometer in classifying DESL, the low discriminating ability of the TearLab osmometer and the overlap in osmolarity values have been reported previously16. In the present study, comparison of eyes with different DESL showed that in the right eye, all tests except the tear osmolarity measurement demonstrated inter-group differences. In the left eye, the severe DED group had the highest tear osmolarity level compared to the non-DED group (312.9 ± 21.9 vs. 307.4 ± 21.1, p = 0.467) and mild DED group (312.9 ± 21.9 vs. 306.9 ± 17.9, p = 0.002), but no statistically significant difference was detected between the non-DED and mild DED groups (307.4 ± 21.1 vs. 306.9 ± 17.9, p = 1.00) (Table 3). Comparison of DESL 0–4 as recommended in the DEWS guidelines yielded similar results.
There are several possible reasons for the lack of association between TearLab-measured osmolarity and the other clinical parameters. The osmolarity of the precorneal tear film differs significantly from that measured in the tear meniscus13, where the tears were collected by the TearLab system. The precorneal tear film in DED apparently has higher osmolarity levels, even spiking up to 800–900 mOsm/L in areas of tear film break-up34. Direct measurement of the precorneal tear film might show a stronger correlation with other clinical dry eye tests. Moreover, with the TearLab system, we probably measured the reflex tear, while earlier studies have suggested that pathological changes would be best obtained in basal tears rather than in reflex tears13,35,36. Using the TearLab osmometer might have led to reflex tear production, resulting in varying values.
Strengths and limitations
The strength of the present study is the largest sample size in a published investigation of the utility of tear osmolarity measurement for diagnosing DED. Moreover, the data were collected at a single center. Well-trained ophthalmic assistant carried out standardized diagnostic examinations, and a senior ophthalmologist confirmed the examination results. One limitation of the present study is its retrospective design. The patients were not controlled for contact lens or systemic/ocular drug use, which might have affected tear osmolarity. Moreover, tear osmolarity levels were not measured repeatedly, thus it was not possible to detect the reliability or repeatability of the test. However, the patients were instructed not to use any eye drops two hours prior to their visit to the clinic, and the TearLab system was operated daily according to the manufacturer’s instructions before use. High prevalence of DED in age-matched population was another limiting factor in recruiting a large number of healthy controls with no DED signs and symptoms. Nevertheless, the number of control subjects was verified to be sufficient on statistical grounds. The study may appear to be underpowered due to a smaller sample size in the control group, as it is the smallest group is the one that mostly determines the study power. However, whether the study could possibly be under-powered also depends on the effect size. Since many highly significant results were found, it is evident that there are quite large effects in our data. Future prospective studies comparing a large number of DED-free controls are warranted.
In conclusion, tear osmolarity measured with the TearLab osmometer shows a large overlap between patients with DED and healthy individuals. Therefore, DED diagnosis relying on tear osmolarity measured with the TearLab osmometer can be inconclusive.
References
Craig, J. P. et al. TFOS DEWS II Definition and Classification Report. Ocul Surf 15, 276–283 (2017).
Stapleton, F. et al. TFOS DEWS II Epidemiology Report. Ocul Surf 15, 334–365 (2017).
Schiffman, R. M., Christianson, M. D., Jacobsen, G., Hirsch, J. D. & Reis, B. L. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol 118, 615–621 (2000).
Bron, A. J. Diagnosis of dry eye. Surv Ophthalmol 45(Suppl 2), S221–226 (2001).
Bron, A. J. et al. Methodologies to diagnose and monitor dry eye disease: report of the Diagnostic Methodology Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 5, 108–152 (2007).
Bron, A., Argüeso, P., Irkec, M. & Bright, F. Clinical staining of the ocular surface: mechanisms and interpretations. Prog Retin Eye Res. 44, 36–61 (2015).
Tomlinson, A. et al. The international workshop on meibomian gland dysfunction: report of the diagnosis subcommittee. J Investigative ophthalmology visual science 52, 2006–2049 (2011).
Nichols, K. K., Nichols, J. J. & Mitchell, G. L. The lack of association between signs and symptoms in patients with dry eye disease. J Cornea 23, 762–770 (2004).
Amparo, F., Jin, Y., Hamrah, P., Schaumberg, D. A. & Dana, R. What is the value of incorporating tear osmolarity measurement in assessing patient response to therapy in dry eye disease? Am J Ophthalmol. 157, 69–77. e62 (2014).
Nichols, J. J., Mitchell, G. L. & King-Smith, P. E. Thinning rate of the precorneal and prelens tear films. J Investigative ophthalmology visual science 46, 2353–2361 (2005).
Lemp, M. A. et al. Tear osmolarity in the diagnosis and management of dry eye disease. J American journal of ophthalmology 151, 792–798. e791 (2011).
Tomlinson, A., Khanal, S., Ramaesh, K., Diaper, C. & McFadyen, A. Tear film osmolarity: determination of a referent for dry eye diagnosis. J Investigative ophthalmology visual science 47, 4309–4315 (2006).
Messmer, E. M., Bulgen, M. & Kampik, A. In Research Projects in Dry Eye Syndrome Vol. 45 129–138 (Karger Publishers, 2010).
Li, D.-Q., Chen, Z., Song, X. J., Luo, L. & Pflugfelder, S. C. Stimulation of matrix metalloproteinases by hyperosmolarity via a JNK pathway in human corneal epithelial cells. J Investigative ophthalmology visual science 45, 4302–4311 (2004).
Sullivan, B. D. et al. Correlations between commonly used objective signs and symptoms for the diagnosis of dry eye disease: clinical implications. J Acta ophthalmologica 92, 161–166 (2014).
Versura, P., Profazio, V. & Campos, E. Performance of tear osmolarity compared to previous diagnostic tests for dry eye diseases. J Current eye research 35, 553–564 (2010).
Suzuki, M. et al. Tear osmolarity as a biomarker for dry eye disease severity. J Investigative ophthalmology visual science 51, 4557–4561 (2010).
Sullivan, B. D. et al. An objective approach to dry eye disease severity. J Investigative ophthalmology visual science 51, 6125–6130 (2010).
Jacobi, C., Jacobi, A., Kruse, F. E. & Cursiefen, C. Tear film osmolarity measurements in dry eye disease using electrical impedance technology. Cornea 30, 1289–1292 (2011).
Masmali, A., Alrabiah, S., Alharbi, A., El-Hiti, G. A. & Almubrad, T. Investigation of tear osmolarity using the TearLab Osmolarity System in normal adults in Saudi Arabia. J Eye contact lens 40, 74–78 (2014).
Yoon, D., Gadaria-Rathod, N., Oh, C. & Asbell, P. A. Precision and accuracy of TearLab osmometer in measuring osmolarity of salt solutions. J Current eye research 39, 1247–1250 (2014).
Bunya, V. Y. et al. Variability of tear osmolarity in patients with dry eye. JAMA Ophthalmol 133, 662–667 (2015).
Szalai, E., Berta, A., Szekanecz, Z., Szûcs, G. & Módis, L. Jr. Evaluation of tear osmolarity in non-Sjögren and Sjögren syndrome dry eye patients with the TearLab system. J. Cornea 31, 867–871 (2012).
Baenninger, P. B. et al. Variability of tear osmolarity measurements with a point-of-care system in healthy subjects—systematic review. Cornea. 37, 938–945 (2018).
The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf 5, 75–92 (2007).
Methodologies to diagnose and monitor dry eye disease: report of the Diagnostic Methodology Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf 5, 108–152 (2007).
Arita, R., Itoh, K., Inoue, K. & Amano, S. Noncontact infrared meibography to document age-related changes of the meibomian glands in a normal population. Ophthalmology 115, 911–915 (2008).
Corporation, T. In Tear Collection Procedure (TearLab, tearlab.com, 2017).
Johnston, P. R., Rodriguez, J., Lane, K. J., Ousler, G. & Abelson, M. B. The interblink interval in normal and dry eye subjects. J Clinical ophthalmology 7, 253 (2013).
Bron, A., Evans, V. & Smith, J. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea 22, 640–650 (2003).
Potvin, R., Makari, S. & Rapuano, C. J. Tear film osmolarity and dry eye disease: a review of the literature. J Clinical ophthalmology 9, 2039 (2015).
Tomlinson, A., McCann, L. C. & Pearce, E. I. Comparison of human tear film osmolarity measured by electrical impedance and freezing point depression techniques. J Cornea 29, 1036–1041 (2010).
Gokhale, M., Stahl, U. & Jalbert, I. In situ osmometry: validation and effect of sample collection technique. Optom Vis Sci. 90, 359–365 (2013).
Liu, H. B. et al. A link between tear instability and hyperosmolarity in dry eye. Invest Ophthalmol Vis Sci 50, 3671–3679 (2009).
Balik, J. The lacrimal fluid in keratoconjunctivitis sicca; a quantitative and qualitative investigation. Am J Ophthalmol 35, 1773–1782 (1952).
Gilbard, J. P., Farris, R. L. & Santamaria, J. Osmolarity of tear microvolumes in keratoconjunctivitis sicca. Arch Ophthalmol 96, 677–681 (1978).
Author information
Authors and Affiliations
Contributions
Study concept and design: Bezhod Tashbayev (B.T.), Tor Paaske Utheim (T.P.U.), Øygunn Aass Utheim (Ø.A.U.), Sten Ræder (S.R.), Janicke Liaaen Jensen (J.L.J.), Mazyar Yazdani (M.Y.), Neil Lagali (N.L.), Valeria Vitelli (V.V.), Darlene A Dartt (D.A.D.), Xiangjun Chen (X.C.). Patients and controls recruitment: S.R., Ø.A.U. and J.L.J. Clinical data collection: B.T., T.P.U., Ø.A.U., S.R., J.L.J. and X.C. Analysis and interpretation of data: B.T., T.P.U., M.Y., N.L., V.V., D.A.D. and X.C. Writing the manuscript: B.T., X.C. and T.P.U. Statistical expertise: V.V. and N.L. Supervision: T.P.U., J.L.J., D.A.D. and X.C.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tashbayev, B., Utheim, T.P., Utheim, Ø.A. et al. Utility of Tear Osmolarity Measurement in Diagnosis of Dry Eye Disease. Sci Rep 10, 5542 (2020). https://doi.org/10.1038/s41598-020-62583-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-62583-x
This article is cited by
-
Different perception of dry eye symptoms between patients with and without primary Sjogren’s syndrome
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.