Abstract
Ongoing climate change results in increasing temperatures throughout the seasons. The effects of climate change on insect performance are less studied during the winter season than during the growing season. Here, we investigated the effects of various winter temperature regimes (warm, normal and cold) on the winter performance of the invasive ladybird Harmonia axyridis (Coleoptera: Coccinellidae). Winter survival, body mass loss and post-winter starvation resistance were measured for a laboratory-reared population as well as three populations collected from the field prior to overwintering. The warm winter regime increased the survival rate and body mass loss and reduced post-winter starvation resistance compared to those of the ladybirds in the cold winter regime. The effects of the temperature regime were qualitatively similar for the laboratory-reared and field-collected beetles; however, there were significant quantitative differences in all measured overwintering parameters between the laboratory-reared and field-collected populations. The winter survival of the laboratory-reared beetles was much lower than that of the field-collected beetles. The laboratory-reared beetles also lost a larger proportion of their body mass and had reduced post-winter starvation resistance. Winter survival was similar between the females and males, but compared to the males, the females lost a smaller proportion of their body mass and had better post-winter starvation resistance. The pre-overwintering body mass positively affected winter survival and post-winter starvation resistance in both the laboratory-reared and field-collected ladybirds. The significant differences between the laboratory-reared and field-collected individuals indicate that quantitative conclusions derived from studies investigating solely laboratory-reared individuals cannot be directly extrapolated to field situations.
Similar content being viewed by others
Introduction
An increasing body of literature shows that temperature is one of the most important factors determining the distribution of organisms on the globe1,2,3. Also the distribution of insect species is strongly affected by temperature, and this relationship is especially apparent for winter temperatures4,5. Overwintering insects have to overcome stressful environmental conditions, e.g., unavailable food resources, low temperatures or water deficits, and thus, it is unsurprising that many insect species suffer from substantial mortality during the winter period5. In response, insects have adopted complex strategies to overcome stressful winter conditions6,7.
The low temperatures experienced during overwintering can result in the mortality of insects due to severe tissue damage caused by ice crystallization within cells or due to accumulated chill injuries resulting in metabolic disruptions, even at non-freezing temperatures7. In general, insects may adopt one of two strategies to mitigate the danger of internal ice formation: either tolerate freezing (i.e., withstand the formation of ice) or avoid freezing (i.e., reduce the freezing point to a very low temperature8). The temperature of water crystallization is close to 0 °C, and ice is usually restricted to the extracellular compartments in freeze-tolerant species. Osmotic dehydration ensures that water does not freeze inside cells9. In freeze-avoiding species, removing ice nucleators and accumulating antifreezing substances results in liquid bodily fluids at temperatures well below the melting point of water10,11,12. However, the majority of insects, including the harlequin ladybird Harmonia axyridis (Coleoptera: Coccinellidae) investigated in this study, are chill-susceptible species, for which even low temperatures well above the melting point cause chill injuries and can induce a reversible state of immobility called “chill coma”13,14.
As food resources are commonly very limited during the winter period, especially in temperate zones, the management of energy reserves is crucial for insects to successfully overwinter. A great majority of insect species overwinter in a dormant state (quiescence or diapause) in which metabolic rates are suppressed6,15, but they are still temperature dependent5. Quiescence is a reversible state of very low activity with suppressed metabolism, but insects remain highly responsive to environmental cues, and their activity, e.g., feeding or reproduction, can be renewed quickly16. In contrast, diapause is a hormonally determined state, including the complex “diapause syndrome”, i.e., the modification of insect physiology (e.g., the investment in body fat growth and slowdown of ovariole development) as well as behaviour (e.g., the selection of protected microhabitats6,15,17). Some insects are able to evaluate their energy reserves during diapause and to terminate diapause prematurely. However, the termination of diapause is a gradual process that can take several weeks; thus, insects exposed to suboptimal conditions during diapause can be significantly negatively affected15. In some species, high winter temperatures linked to a relatively high energy drain can result in enhanced winter mortality, reduced spring longevity or limited fecundity during the following growing season18,19.
Insects have a limited ability to regulate their body temperature, and thus, ongoing climate change is expected to have serious fitness consequences5,20. However, the majority of climate change research is focused on the effects taking place during the growing season, whereas far less is known about the effects of the increased temperatures experienced by insects during the winter period4,5. The link between the winter air temperatures and the temperatures experienced by overwintering insects is not always clear. As an example, reduced snow cover can result in relatively low winter temperatures for insects overwintering in the upper soil layer4. Moreover, species-specific responses to temperature preclude any generalization of the effects of relatively high winter temperatures across species. The positive effects of increased winter temperatures have been reported, e.g., for Drosophila suzukii, Nezara viridula and Halyomorpha halys20,21,22, but negative effects are as common, e.g., for Erebia medusa, Osmia lignaria and Chilo suppressalis23,24,25. As climatic changes seem to be linked to shifts in species distribution26,27, knowledge of the effects of relatively high winter temperatures can be especially important for predicting potential distribution changes in invasive species.
In general, the investigation of the effects of climate change on overwintering insects is bound to several potential methodological problems. The temperature treatment should be set to realistically mimic ongoing climate change: a very large temperature increase will surely induce a significant response in the experimental insects but is improbable in nature. The commonly employed practice of exposing insects to unrealistic constant temperatures would not provide reliable results, as temperature fluctuations naturally occur in the field28,29. Not mean but extreme temperatures, i.e., daily minima or maxima, can drive the observed effects of temperature on insects30. In many cases, laboratory-reared insects are employed in overwintering studies, and insects that experienced unnatural environmental conditions prior to overwintering may respond differently than wild insects to the elevated overwintering temperatures (e.g., lower winter survival in the laboratory-reared H. axyridis individuals compared to the field-collected ones31). In this study, we did our best to address all these methodological challenges.
We aimed to investigate the effects of warm, normal and cold winter temperatures, based on real long-term meteorological data, on overwintering survival, body mass loss during the winter and post-winter starvation resistance in the invasive ladybird H. axyridis. Harmonia axyridis is an interesting model species as it has been considered to be one of the most invasive insect species in Europe and North America32,33. Harmonia axyridis is native to Asia, particularly to areas with a temperate and subtropical climate34, but has recently spread to many European countries, North and South America and some African countries35,36,37. The speed of their range expansion in a novel environment has been extremely fast because in 15 years (2000–2015), the species spread over almost the whole of Europe34,38. However, its spread seems to be partially limited by the environmental conditions, especially high summer temperatures, as its thermal optimum is lower than that of many other ladybird species39. In general, in Europe, H. axyridis has not spread into relatively high altitudes (above ca. 2000 m a.s.l.), indicating a possible problem with overwintering there35. However, note that during the summer season, H. axyridis adults were recorded at much relatively high altitudes (ca. 3500 m a.s.l.) in the Andes40.
Harmonia axyridis overwinters as an adult, which is very common in beetles, especially in ladybirds41,42. In the autumn, during warm and sunny days, mass flights to overwintering sites can be observed35. Harmonia axyridis commonly overwinters in aggregations (from tens to several thousands of individuals) and prefers indoor sites or shelters that buffer the low winter temperatures (e.g., window frames, old buildings or caves43). The adults have only a weak winter diapause, commonly terminated in January and followed by a quiescent state44. The total time spent in diapause can be variable. Such flexibility in diapause behaviour may be an important factor that contributes to the invasive success of H. axyridis44,45. In the spring, commonly from March to April, the beetles disperse slowly from their hibernation sites (after quiescence termination) towards their feeding and breeding sites35,42. It is a common phenomenon that ladybirds suffer from starvation during the early spring as their preferred food resources (aphids) are rare at that time42.
We hypothesize that higher winter temperatures will result in increased survival rates due to decreased chill injuries14, but at the cost of reduced post-overwintering conditions, i.e., a greater body mass reduction during the winter and reduced post-overwintering survival due to the higher energy consumption at higher winter temperatures19. We also compared the results obtained for the laboratory-reared and field-collected populations to reveal the potential biases linked to the use of laboratory insects in ecophysiological studies and investigated the effects of sex and pre-winter body mass on overwintering survival, body mass loss during winter and post-winter starvation resistance in H. axyridis.
Results
Effects of the temperature regime
We found qualitatively consistent effects of the winter temperature on survival, body mass loss and post-winter starvation resistance across all the investigated populations (one laboratory-reared and three field-collected; Tables 1 and 2). Exposure to low winter temperatures (cold regime) resulted in a significantly reduced winter survival (Fig. 1), a reduced body mass loss (Fig. 2) and improved post-winter starvation resistance (Fig. 3) compared to those in the exposure to high winter temperatures (warm regime). For detailed comparisons of the differences among all three temperature regimes for the laboratory-reared animals, see the post hoc test values in Table 1.
Winter survival of adult Harmonia axyridis ladybirds under different temperature regimes. Cold, normal and warm winter conditions are represented by the blue, grey and red colours, respectively. Laboratory-reared (Lab) and field-collected (Nat) beetles are represented separately, as there were significant differences in their survival probabilities. The data for the females and males were pooled, as there were no significant differences in their survival. For the same reason, we also pooled the data from all three different natural populations.
Relative body mass loss of Harmonia axyridis during overwintering under the different temperature regimes. Laboratory-reared and field-collected beetles (Nature 1–3) are represented separately. The females (grey columns) and males (black columns) are shown separately. The mean values + SEMs are shown. The numbers in the brackets represent the sample sizes.
Post-winter starvation resistance of Harmonia axyridis after overwintering under the different temperature regimes. Laboratory-reared and field-collected beetles (Nature 1–3) are represented separately. The female (grey columns) and male (black columns) post-winter starvation resistance (longevity without food in days) is shown separately. The mean values + SEMs are shown. The numbers in the brackets represent the sample sizes.
Differences between the laboratory-reared and field-collected populations
The qualitatively similar effects of the winter temperature regime on winter survival, body mass loss, and post-winter starvation resistance were observed across the investigated populations, but the absolute values differed significantly between the populations (mainly, the laboratory-reared beetles differed from all the field-collected populations). The laboratory-reared beetles had significantly lower winter survival compared to that of the field-collected ladybirds (Fig. 1; Table 2). Additionally, the laboratory-reared beetles suffered a significantly higher body mass loss during overwintering compared to that of the field-collected ladybirds (Fig. 2; Table 2). Two out of three field-collected ladybird populations (‘Nature 1’ and ‘Nature 2’) outperformed the laboratory-reared beetles in post-winter starvation resistance (Fig. 3; Table 2).
Differences between the sexes
There were no significant differences in winter survival between the males and females (Tables 1 and 2). However, there were significant differences between the sexes in relative body mass loss during winter and in post-winter starvation resistance (Tables 1 and 2). The females lost a smaller proportion of their body mass during overwintering compared to that of the males (Fig. 2). Note that also the absolute body mass loss differed between the sexes, while body mass was reduced more in males compared to females (P < 0.001). Apart from the ‘Nature 3’ population, the females outperformed the males in post-winter starvation resistance (Fig. 3).
Effects of pre-overwintering body mass
Pre-overwintering body mass significantly affected winter survival in H. axyridis. The relatively heavy individuals, both laboratory-reared and field-collected, had increased survival probabilities, and this effect was stronger for the males compared to the females (see the significant interaction between sex and pre-overwintering mass in Tables 1 and 2; Supplementary Material Fig. S1). Pre-overwintering mass had no effect on body mass loss during the winter, indicating that individuals with a high pre-winter body mass lost a proportion of their live mass similar to that of the individuals with a low pre-winter mass (Tables 1 and 2). High pre-overwintering mass had a significant positive effect on post-winter starvation resistance, and this effect was stronger for females compared to males (see the significant interaction between sex and pre-overwintering mass in Tables 1 and 2). Interestingly, the significantly higher pre-overwintering mass achieved by the laboratory-reared beetles (Supplementary Material Fig. S2) was not able to ensure a winter performance comparable to that of the field-collected beetles (Figs. 1–3), and thus, the positive effect of pre-overwintering body mass is significant mainly at the intra-population level, i.e., when the performance of individuals originating from the same population is compared.
Discussion
Main findings
In the present study, we demonstrated contrasting effects of the winter temperature regime on the winter survival, body mass loss and post-winter starvation resistance of the invasive ladybird H. axyridis. Lower winter temperatures significantly decreased the survival probability, reduced body mass loss and enhanced post-winter starvation resistance compared to those of higher winter temperatures. Interestingly, there were also significant differences in the majority of the investigated parameters between the laboratory-reared and field-collected beetles, indicating that the results of the ecophysiological measurements performed solely on laboratory-reared animals need to be interpreted with caution.
Effects of the temperature regime
Our temperature regimes did not represent very extreme winter conditions for H. axyridis, as they were far from the physiological limits of the species (the lower lethal temperature is approximately −16 °C in the European population of H. axyridis31). Applied temperatures are also completely within the range of the winter temperatures experienced by naturally overwintering H. axyridis adults in Central Europe (Řeřicha, unpublished temperature measurements at various overwintering sites). Despite this fact, the cold temperature regime (lower winter temperatures) caused significantly higher ladybird mortality than did the warm winter temperature regime. A possible explanation could be the long-term accumulation of chill injuries that can take place even at temperatures much higher than the lower lethal temperature (measured in short-term laboratory assay4). Energy exhaustion is unlikely to be the cause of death in our overwintering beetles, as the energy reserves (body mass) of the surviving beetles were higher in the cold regime than in the warm regime. In general, the energy consumption in overwintering insects increases with temperature within the range of ecologically relevant temperatures. This pattern is driven by the temperature dependence of metabolic rates5. This pattern was also confirmed for H. axyridis by our post-winter starvation resistance experiment. The ladybirds exposed to the cold regime survived under starvation conditions for a longer time than the individuals exposed to the warm regime.
Such a complex effect of the winter temperature regime complicates predictions of the effects of climate change on overwintering insects. With ongoing climate change, many insect species benefit from increased minimum winter temperatures during the coldest months in terms of reduced mortality due to chill injuries21,46. On the other hand, a substantial proportion of species suffer from enhanced energy demands due to higher autumn and early spring temperatures47. The relative importance of chill injury reduction and increased energy exhaustion might be species-specific, as species differ substantially in their traits, such as cold tolerance, energy acquisition abilities, diapause duration, and energy demands in the spring5,47. Research on different insect species have shown that the effects of climate change on insect overwintering success range from highly positive to highly negative. For example, higher winter temperatures increased survival of Nezara viridula, Drosophila suzukii and Halyomorpha halys20,21,22, but reduced survival of Osmia lignaria, Erebia medusa and Chilo suppressalis23,24,25. To reveal the overall effects of winter temperature, more complex experiments tracking insects throughout the complete life cycle are needed20, as post-overwintering performance, e.g., reproductive success, is crucial5. Based on the published literature, it is unfeasible to predict whether increased temperatures during overwintering will result in enhanced fitness in H. axyridis. Therefore, future studies investigating the performance of ladybirds that have experienced various winter temperature regimes during the following spring and summer seasons are needed. Moreover, ongoing climate change could result in an increased variability of environmental conditions during winter in many regions. Repeated switching between detrimental high and low temperatures can be exceptionally challenging for many insect species4.
Differences between the laboratory-reared and field-collected populations
Differences observed in the overwintering performance of the three field-collected H. axyridis populations can be caused by different genetic background or environmental conditions experienced in nature, i.e., phenotypic differences, both representing a common source of intraspecific variation in insects4,21,25,48. Lower post-winter starvation resistance recorded by survival Population originating from České Budějovice could be linked to exposition to elevated temperatures during autumn transportation to Prague. However, we have no simple explanation for the difference between populations Nature 1 and Nature 2, both originating from Prague. Much more pronounced differences were observed between the field-collected and laboratory-reared individuals for all measured parameters. Even larger differences in H. axyridis winter survival between the laboratory-reared and field-collected individuals were observed by Berkvens et al.31; however, their laboratory beetles probably did not enter diapause at all. Our experimental ladybirds seemed to be diapausing, as exposure to room temperature did not result in higher movement activity in November and December. Despite our effort to mimic autumnal conditions in the laboratory, some natural cues remain difficult to mimic under laboratory conditions. For example, continuous changes in food quality and quantity during autumn or a long flight to an overwintering site may have resulted in better physiological readiness for overwintering among the field-collected ladybirds. The differences in insect performance between the laboratory-reared and field-collected individuals are not limited only to ladybirds and overwintering conditions22,49. Thus, we recommend conducting physiological and fitness measurements on both populations simultaneously if possible, as the laboratory populations could not provide reliable estimates of the field performance. It should also be noted that our laboratory beetles were relatively young (up to 30 days old) when the overwintering experiment started, whereas the field-collected beetles were of unknown age but probably much older on average (adults of the relatively large ladybird species can live for more than one year under field conditions42). Differences in ladybird age can significantly affect their winter performance, as physiological traits, including those related to overwintering, vary significantly, especially across the life of young adults13,50.
Differences between the sexes and effects of pre-overwintering body mass
In general, females are the larger sex in H. axyridis51, and this was also true for the beetles investigated in this study. Our body mass loss data revealed that both the absolute and the relative body mass loss were higher in males compared to females. Thus, a smaller relative body mass loss in females cannot be linked solely to the existence of sexual size dimorphism, but a sex-specific management of energy reserves probably exists in H. axyridis. The larger female body mass may be responsible for the enhanced post-winter starvation resistance in the H. axyridis females, as a larger body size can be linked to the advantages of relatively larger energy stores and lower mass-specific metabolic rates52. However, based on our data, it is not possible to reject the possibility that H. axyridis females have also a sex-specific physiological adaptation enabling them to survive longer in the spring under starvation conditions, as was shown for the carabid beetle Anchomenus dorsalis53. On the other hand, our results clearly indicate that even within the sexes, a larger pre-overwintering body mass was linked to enhanced winter survival and post-winter starvation resistance. A greater pre-overwintering body mass can represent both individuals with a larger structural body size, e.g., a longer body length, and individuals in better body condition, e.g., higher energy reserves54. There is evidence that both structural size and body condition can affect starvation resistance53,55 as well as winter survival56,57 in insects. Without measurements of the structural body size in this study, we are not able to clearly distinguish whether heavier H. axyridis individuals were only larger, i.e., with a larger structural body size, or in better condition, i.e., with higher energy reserves.
Conclusions
In conclusion, our results indicate that even small changes in winter temperatures can significantly affect winter mortality, winter body mass loss and post-winter starvation resistance in H. axyridis. While elevated temperature has contrasting effects on winter survival (positive) and post-overwintering energy reserves (negative), its overall effects on the population growth of H. axyridis remain unknown and should be investigated in a future study. The significant differences in overwintering performance between the laboratory-reared and field-collected individuals indicate that the quantitative conclusions derived from studies investigating solely laboratory-reared individuals cannot be directly extrapolated to field situations. We recommend employing both field-collected and laboratory-reared individuals in studies investigating physiological traits in insects if this approach is feasible.
Materials and methods
Experimental insects
Ladybirds originating from two sources were employed in our experiment: 1) laboratory-reared ladybirds and 2) field-collected ladybirds. The parents of our laboratory-reared ladybirds were collected in August 2015 from shrubs and lime trees on the university campus of the Czech University of Life Sciences Prague, Czech Republic (GPS: 50°8′N, 14°21′E; 300 m a.s.l.). After transportation to the laboratory, the ladybirds were sexed, and 20 parental pairs were formed at random (for details see13). Each couple was placed in a separate Petri dish (9 cm in diameter) containing crumpled filter paper strips, which provided a suitable substrate for egg laying. The Petri dishes were placed into a computer-controlled climatic chamber (made on order by the AVIKO-PRAHA company, Czech Republic) set to a 16 L:8 D photoperiod, 70% humidity and a temperature of 26 °C. From the reproducing pairs, 10 pairs were selected at random, and their offspring became our laboratory-reared beetles. Laboratory-reared beetles were also exposed to the standardized laboratory conditions (16 L:8 D photoperiod, 70% humidity, 26 °C) throughout their preimaginal development. The selected temperature is optimal for rearing H. axyridis42,51. The newly hatched larvae were fed ad libitum with the eggs of Ephestia kuehniella (Zeller, 1879) (Lepidoptera: Pyralidae) and provided water in cotton wool. The adult laboratory-reared ladybirds (emerged between September 15th and 25th, 2015) were sexed, placed individually into Petri dishes, provided food and water ad libitum and exposed to the same standardized conditions as the larvae until October 7th, when the ladybirds were transferred to an 18 °C and 12 L:12 D photoperiod regime to initiate winter diapause. Thirty beetles (15 males + 15 females) from each parental pair were employed in our overwintering experiment, i.e., 300 laboratory-reared ladybirds in total.
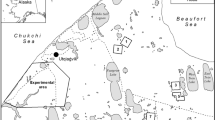
In addition to the laboratory-reared ladybirds, adult ladybirds collected in the field in late autumn 2015 (mid-October) were employed in our overwintering experiment (field-collected beetles). We collected three geographically distinct H. axyridis populations when the beetles were aggregating at overwintering sites in Prague–Farkáň (Nature 1: 26 males + 26 females; aggregation on a building), Prague–Prokopské údolí (Nature 2: 20 males + 20 females; aggregation on pine trees) and České Budějovice (Nature 3: 19 males + 20 females; aggregation on a building). These field-collected ladybirds were then stored in groups in glass jars under natural outdoor conditions for 2–3 days until the beginning of the overwintering experiment. Ladybirds originating from České Budějovice also spent one additional day at higher temperature due to their transportation to Prague.
Experimental setup and measurements
The overwintering experiment was started on October 15th, when all the ladybirds (both laboratory-reared and field-collected) were weighed for live mass (pre-overwintering mass) using a Sartorius balance with a precision of 10−4 g. At the same time, field-collected beetles were individually accommodated in Petri dishes, and all the ladybirds were transferred to computer-controlled climatic chambers set to a pre-overwintering regime with a low fluctuating temperature and a short photoperiod [8 L (12 °C):16 D (6 °C)] to mimic the outdoor conditions in late autumn. Six days later, the overwintering phase started, and the beetles were assigned to three winter temperature regimes: (1) warm winter, (2) normal winter and (3) cold winter. These temperature regimes mimicked the real temperatures experienced in outdoor shelters in Prague, i.e., the temperature 1 cm below the soil surface. Based on long-term meteorological data (November to March temperatures from 1995 to 2015) from the meteorological station situated in the Crop Research Institute, Praha-Ruzyně, extreme years with cold and warm temperatures were selected. The temperature course for our normal thermal regime was computed as the mean winter temperature course for the non-extreme years (from 1995 to 2015). The original meteorological data represented hourly mean temperatures (measured 1 cm below the soil surface). The data used as the normal thermal regime in our experiment thus represent the mean hourly temperature, averaged across multiple years. Three computer-controlled climatic chambers, one for each temperature regime (i.e., warm, normal or cold), were adjusted every hour with a new temperature value following the data in Supplementary Material File Table S1. The mean temperature (November to March) for the warm regime was 2.8 °C (the minimum temperature reached was −2.0 °C), the mean temperature for the normal regime was 0.7 °C (the minimum temperature reached was −4.1 °C) and the mean temperature for the cold regime was −1.6 °C (the minimum temperature reached was −8.1 °C). The laboratory-reared ladybirds were exposed to all three temperature regimes (five males and five females per parental pair per treatment, i.e., 100 beetles per treatment in total). The field-collected ladybirds were exposed only to the extreme temperature regimes (warm and cold) due to the limited number of individuals available. Field-collected ladybirds were assigned to particular temperature regime at random. This setting resulted in even distribution of pre-overwintering body masses (see below) across temperature regimes for laboratory-reared ladybirds and for two out of three field-collected populations (Supplementary Material File Table S2). For the third population (Nature 3) slightly higher pre-overwintering body mass was observed in the cold treatment (mean mass = 36.13 mg) than in the warm treatment (mean mass = 32.46 mg). Note that variation between individuals within temperature treatments is very high (cold: 26.1–48.9 mg; warm: 24.2–42.8 mg), allowing us to easily analyse the effects of pre-overwintering mass independently of temperature regime.
Over the course of the overwintering experiment, the beetles were checked at monthly intervals (November 16th, December 15th, January 14th, February 17th and March 17th, 2016–when the overwintering experiment was terminated). During all the inspections, the Petri dishes with beetles were moved to outdoor conditions to minimize the unwanted warming of the experimental ladybirds, the survival of each ladybirds was recorded, and the watered cotton wool in each Petri dish was replaced with a new piece. Live diapausing ladybirds were commonly tightly attached to the substrate, whereas dead ladybirds dropped off and laid freely on their dorsum. Dead iladybirds were not removed from the experiment to minimize the small risk of false death records (as described in13). After the completion of the overwintering stage (on March 17th), the ladybirds in the Petri dishes were transported to a room temperature (22 °C ± 1 °C) setting. The next day, the live weight (post-overwintering mass) of all the surviving ladybirds was collected. In the following days, the survival of the ladybirds was checked daily to measure their post-winter starvation resistance (water was provided ad libitum).
Statistical analyses
Two datasets were created prior to the data analysis. The first dataset (“laboratory”) consisted of the laboratory-reared ladybirds, and this dataset allowed us to investigate the effects of all three winter temperature regimes (warm, normal and cold) as well as to account for the genetic background of the investigated ladybirds (the parents of the investigated ladybirds were known, and their identity could be incorporated as a random effect into the analyses). The raw data serving as the input for the respective analyses are available in the Supplementary Material File (Table S3). The second dataset (“both”) consisted of all the field-collected ladybirds and a subset of the laboratory-reared ladybirds that were exposed to the extreme (warm and cold) winter temperature regimes. This dataset allowed us to compare the effects of the extreme temperature regimes between the laboratory-reared ladybirds and field-collected ladybirds. The raw data serving as input for the respective analyses are available in the Supplementary Material File (Table S4).
To investigate the winter survival of the laboratory-reared ladybirds (employing the “laboratory” dataset) under different winter temperature regimes, a mixed-effects Cox model was run using the “coxme” function implemented in the “coxme” package58 in R59. Winter survival in months (one to five months) was used as the response variable, parental pair identity was used as a random effect and winter temperature regime (warm, normal or cold), sex (male or female), pre-overwintering mass and all possible interactions between temperature regime, sex and pre-overwintering mass were used as the independent variables in the model. The significance of the differences between particular temperature regimes was tested by Tukey’s HSD post hoc tests using the “glht” function as implemented in the “multcomp” package60.
To investigate the effects of the extreme winter temperature regimes on the survival of both the laboratory-reared and field-collected ladybirds (employing the “both” dataset), a Cox proportional-hazards model (Cox-PH) was run using the “coxph” function implemented in the “survival” package58 in R59. Winter survival in months (one to five months) was used as the response variable, temperature regime (warm or cold), sex (male or female), population identity (Laboratory, Nature 1, Nature 2 and Nature 3), pre-overwintering mass and all the possible interactions between temperature regime, sex, population identity and pre-overwintering mass were used as the independent variables in the model. The survival data were right-censored because some beetles were still alive at the end of the overwintering stage. Tukey’s HSD post hoc tests were employed to reveal the significant differences in the winter survival between beetles originating from the different populations.
To analyse the relative body mass loss during overwintering in the laboratory-reared ladybirds, a linear mixed-effects model (LME) was run using the “lme” function implemented in the “nlme” package61 in R59. Relative body mass loss was calculated as the proportion of pre-overwintering mass lost during overwintering, i.e., the difference between the pre-overwintering mass and post-overwintering mass divided by the pre-overwintering mass of a given individual. Parental pair identity was used as a random effect in our analysis. Winter temperature regime (warm, normal or cold), sex (male or female), pre-overwintering mass and all the possible interactions between temperature regime, sex and pre-overwintering mass were used as the independent variables in the model. The significance of the differences between the particular temperature regimes was tested by Tukey’s HSD post hoc tests.
To compare the effects of the extreme winter temperature regimes on the relative body mass loss between the laboratory-reared and field-collected ladybirds, analysis of covariance (ANCOVA) was run using the “lm” function in R59. Relative body mass loss was used as the response variable, and temperature regime (warm or cold), sex (male or female), population identity (Laboratory, Nature 1, Nature 2 and Nature 3), pre-overwintering mass and all the possible interactions between temperature regime, sex, population identity and pre-overwintering mass were used as the independent variables in the model. Tukey’s HSD post hoc tests were employed to reveal the significant differences in the relative body mass loss between beetles originating from different populations. Analogous model was fitted also for absolute body mass loss data to check whether the difference between sexes can be explained by the existence of sexual size dimorphism (females are the larger sex in H. axyridis51).
To investigate the post-winter starvation resistance in the laboratory-reared ladybirds, a generalized linear mixed model (GLMM) was run using the “glmmPQL” function implemented in the “MASS” package62 in R59. The post-winter starvation resistance (i.e., the longevity of adults under no food conditions) was used as the response variable in the model, and a quasi-Poisson distribution of errors was employed. The parental pair identity was used as a random effect in this analysis. The winter temperature regime (warm, normal or cold), sex (male or female), pre-overwintering mass and all possible interactions between temperature regime, sex and pre-overwintering mass were used as the independent variables in the model. The significance of the differences between the particular temperature regimes was tested by Tukey’s HSD post hoc tests.
To compare the effects of the extreme winter temperature regimes on the post-winter starvation resistance between the laboratory-reared and field-collected ladybirds, a generalized linear model (GLM) with a quasi-Poisson distribution of errors was run using the “glm” function in R59. Post-winter starvation resistance was used as the response variable, and temperature regime (warm or cold), sex (male or female), population identity (Laboratory, Nature 1, Nature 2 and Nature 3), pre-overwintering mass and all the possible interactions between temperature regime, sex, population identity and pre-overwintering mass were used as the independent variables in the model. Tukey’s HSD post hoc tests were employed to reveal the significant differences in winter survival between beetles originating from different populations.
References
Araujo, M. B. et al. Quaternary climate changes explain diversity among reptiles and amphibians. Ecography 31, 8–15, https://doi.org/10.1111/j.2007.0906-7590.05318.x (2008).
Morgan, E. R., Jefferies, R., Krajewski, M., Ward, P. & Shaw, S. E. Canine pulmonary angiostrongylosis: The influence of climate on parasite distribution. Parasitology International 58, 406–410, https://doi.org/10.1016/j.parint.2009.08.003 (2009).
Szentivanyi, T. et al. Climatic effects on the distribution of ant- and bat fly-associated fungal ectoparasites (Ascomycota, Laboulbeniales). Fungal Ecology 39, 371–379, https://doi.org/10.1016/j.funeco.2019.03.003 (2019).
Bale, J. S. & Hayward, S. A. L. Insect overwintering in a changing climate. Journal of Experimental Biology 213, 980–994, https://doi.org/10.1242/jeb.037911 (2010).
Williams, C. M., Henry, H. A. L. & Sinclair, B. J. Cold truths: How winter drives responses of terrestrial organisms to climate change. Biological Reviews 90, 214–235, https://doi.org/10.1111/brv.12105 (2015).
Hahn, D. A. & Denlinger, D. L. Meeting the energetic demands of insect diapause: Nutrient storage and utilization. Journal of Insect Physiology 53, 760–773, https://doi.org/10.1016/j.jinsphys.2007.03.018 (2007).
Turnock, W. J. & Fields, P. G. Winter climates and coldhardiness in terrestrial insects. European Journal of Entomology 102, 561–576, https://doi.org/10.14411/eje.2005.081 (2005).
Sinclair, B. J., Addo-Bediako, A. & Chown, S. L. Climatic variability and the evolution of insect freeze tolerance. Biological Reviews of the Cambridge Philosophical Society 78, 181–195, https://doi.org/10.1017/S1464793102006024 (2003).
Toxopeus, J. & Sinclair, B. J. Mechanisms underlying insect freeze tolerance. Biological Reviews 93, 1891–1914, https://doi.org/10.1111/brv.12425 (2018).
Duman, J. G. Antifreeze and Ice Nucleator Proteins in Terrestrial Arthropods. Annual Review of Physiology 63, 327–357, https://doi.org/10.1146/annurev.physiol.63.1.327 (2001).
Sinclair, B. J., Vernon, P., Klok, C. J. & Chown, S. L. Insects at low temperatures: An ecological perspective. Trends in Ecology and Evolution 18, 257–262, https://doi.org/10.1016/S0169-5347(03)00014-4 (2003).
Watanabe, M. Cold tolerance and myo-inositol accumulation in overwintering adults of a lady beetle, Harmonia axyridis (Coleoptera: Coccinellidae). European Journal of Entomology 99, 5–9, https://doi.org/10.14411/eje.2002.002 (2002).
Knapp, M., Vernon, P. & Renault, D. Studies on chill coma recovery in the ladybird, Harmonia axyridis: Ontogenetic profile, effect of repeated cold exposures, and capacity to predict winter survival. Journal of Thermal Biology 74, 275–280, https://doi.org/10.1016/j.jtherbio.2018.04.013 (2018).
Overgaard, J. & MacMillan, H. A. The Integrative Physiology of Insect Chill Tolerance. Annual Review of Physiology 79, 187–208, https://doi.org/10.1146/annurev-physiol-022516-034142 (2017).
Hahn, D. A. & Denlinger, D. L. Energetics of Insect Diapause. Annual Review of Entomology 56, 103–121, https://doi.org/10.1146/annurev-ento-112408-085436 (2011).
Tauber, M. J., Tauber, C. A. & Masaki, S. Seasonal adaptations of insects. (Oxford University Press, 1986).
Koštál, V. Eco-physiological phases of insect diapause. Journal of Insect Physiology 52, 113–127, https://doi.org/10.1016/j.jinsphys.2005.09.008 (2006).
Irwin, J. T. & Lee, R. E. Cold winter microenvironments conserve energy and improve overwintering survival and potential fecundity of the goldenrod gall fly, Eurosta solidaginis. Oikos 100, 71–78, https://doi.org/10.1034/j.1600-0706.2003.11738.x (2003).
Sinclair, B. J. Linking energetics and overwintering in temperate insects. Journal of Thermal Biology 54, 5–11, https://doi.org/10.1016/j.jtherbio.2014.07.007 (2015).
Musolin, D. L., Tougou, D. & Fujisaki, K. Too hot to handle? Phenological and life-history responses to simulated climate change of the southern green stink bug Nezara viridula (Heteroptera: Pentatomidae). Global Change Biology 16, 73–87, https://doi.org/10.1111/j.1365-2486.2009.01914.x (2010).
Dalton, D. T. et al. Laboratory survival of Drosophila suzukii under simulated winter conditions of the Pacific Northwest and seasonal field trapping in five primary regions of small and stone fruit production in the United States. Pest Management Science 67, 1368–1374, https://doi.org/10.1002/ps.2280 (2011).
Taylor, C. M., Coffey, P. L., Hamby, K. A. & Dively, G. P. Laboratory rearing of Halyomorpha halys: methods to optimize survival and fitness of adults during and after diapause. Journal of Pest Science 90, 1069–1077, https://doi.org/10.1007/s10340-017-0881-9 (2017).
Bosch, J. & Kemp, W. P. Effect of Wintering Duration and Temperature on Survival and Emergence Time in Males of the Orchard Pollinator Osmia lignaria (Hymenoptera: Megachilidae). Environmental Entomology 32, 711–716, https://doi.org/10.1603/0046-225X-32.4.711 (2003).
Stuhldreher, G., Hermann, G. & Fartmann, T. Cold-adapted species in a warming world - an explorative study on the impact of high winter temperatures on a continental butterfly. Entomologia Experimentalis et Applicata 151, 270–279, https://doi.org/10.1111/eea.12193 (2014).
Xiao, H., Chen, J., Chen, L., Chen, C. & Wu, S. Exposure to mild temperatures decreases overwintering larval survival and post-diapause reproductive potential in the rice stem borer Chilo suppressalis (J Pest Sci, 10.1007/s10340-016-0769-0). Journal of Pest Science 90, 127, https://doi.org/10.1007/s10340-016-0799-7 (2017).
Aukema, B. H. et al. Movement of outbreak populations of mountain pine beetle: Influences of spatiotemporal patterns and climate. Ecography 31, 348–358, https://doi.org/10.1111/j.0906-7590.2007.05453.x (2008).
Caminade, C. et al. Suitability of European climate for the Asian tiger mosquito Aedes albopictus: recent trends and future scenarios. Journal of The Royal Society Interface 9, 2708–2717, https://doi.org/10.1098/rsif.2012.0138 (2012).
Enriquez, T., Ruel, D., Charrier, M. & Colinet, H. Effects of fluctuating thermal regimes on cold survival and life history traits of the spotted wing Drosophila (Drosophila suzukii). Insect Science 27, 317–335, https://doi.org/10.1111/1744-7917.12649 (2020).
Xing, K., Hoffmann, A. A., Zhao, F. & Ma, C. S. Wide diurnal temperature variation inhibits larval development and adult reproduction in the diamondback moth. Journal of Thermal Biology 84, 8–15, https://doi.org/10.1016/j.jtherbio.2019.05.013 (2019).
Colinet, H., Sinclair, B. J., Vernon, P. & Renault, D. Insects in Fluctuating Thermal Environments. Annual Review of Entomology 60, 123–140, https://doi.org/10.1146/annurev-ento-010814-021017 (2015).
Berkvens, N., Bale, J. S., Berkvens, D., Tirry, L. & De Clercq, P. Cold tolerance of the harlequin ladybird Harmonia axyridis in Europe. Journal of Insect Physiology 56, 438–444, https://doi.org/10.1016/j.jinsphys.2009.11.019 (2010).
Brown, P. M. J. et al. The global spread of Harmonia axyridis (Coleoptera: Coccinellidae): Distribution, dispersal and routes of invasion. BioControl 56, 623–641, https://doi.org/10.1007/s10526-011-9379-1 (2011).
Lombaert, E. et al. Inferring the origin of populations introduced from a genetically structured native range by approximate Bayesian computation: Case study of the invasive ladybird Harmonia axyridis. Molecular Ecology 20, 4654–4670, https://doi.org/10.1111/j.1365-294X.2011.05322.x (2011).
Brown, P. M. J. et al. Harmonia axyridis in Europe: Spread and distribution of a non-native coccinellid. BioControl 53, 5–21, https://doi.org/10.1007/978-1-4020-6939-0_2 (2008).
Roy, H. E. et al. The harlequin ladybird, Harmonia axyridis: global perspectives on invasion history and ecology. Biological Invasions 18, 997–1044, https://doi.org/10.1007/s10530-016-1077-6 (2016).
Camacho-Cervantes, M., Ortega-Iturriaga, A. & Del-Val, E. From effective biocontrol agent to successful invader: the harlequin ladybird (Harmonia axyridis) as an example of good ideas that could go wrong. Peerj 5, https://doi.org/10.7717/peerj.3296 (2017).
Hiller, T. & Haelewaters, D. A case of silent invasion: Citizen science confirms the presence of Harmonia axyridis (Coleoptera, Coccinellidae) in Central America. Plos One 14, e0220082, https://doi.org/10.1371/journal.pone.0220082 (2019).
Ukrainsky, A. S. & Orlova-Bienkowskaja, M. J. Expansion of Harmonia axyridis Pallas (Coleoptera: Coccinellidae) to European Russia and adjacent regions. Biological Invasions 16, 1003–1008, https://doi.org/10.1007/s10530-013-0571-3 (2014).
Barahona-Segovia, R. M., Grez, A. A. & Bozinovic, F. Testing the hypothesis of greater eurythermality in invasive than in native ladybird species: From physiological performance to life-history strategies. Ecological Entomology 41, 182–191, https://doi.org/10.1111/een.12287 (2016).
Grez, A. A., Zaviezo, T., Roy, H. E., Brown, P. M. J. & Segura, B. In the shadow of the condor: invasive Harmonia axyridis found at very high altitude in the Chilean Andes. Insect Conservation and Diversity 10, 483–487, https://doi.org/10.1111/icad.12258 (2017).
Danks, H. V. Insect Dormancy: An Ecological Perspective. Biological Survey of Canada (Terrestrial Arthropods), 433 (1987).
Hodek, I., van Emden, H. F. & Honěk, A. Ecology and Behaviour of the Ladybird beetles (Coccinellidae). 561 (2012).
Labrie, G., Coderre, D. & Lucas, E. Overwintering Strategy of Multicolored Asian Lady Beetle (Coleoptera: Coccinellidae): Cold-Free Space as a Factor of Invasive Success. Annals of the Entomological Society of America 101, 860–866, https://doi.org/10.1093/aesa/101.5.860 (2008).
Raak-Van Den Berg, C. L., De Jong, P. W., Hemerik, L. & Van Lenteren, J. C. Diapause and post-diapause quiescence demonstrated in overwintering Harmonia axyridis (Coleoptera: Coccinellidae) in northwestern Europe. European Journal of Entomology 110, 585–591, https://doi.org/10.14411/eje.2013.079 (2013).
Reznik, S. Y., Dolgovskaya, M. Y., Ovchinnikov, A. N. & Belyakova, N. A. Weak photoperiodic response facilitates the biological invasion of the harlequin ladybird Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae). Journal of Applied Entomology 139, 241–249, https://doi.org/10.1111/jen.12158 (2015).
Falt-Nardmann, J. J. J. et al. The recent northward expansion of Lymantria monacha in relation to realised changes in temperatures of different seasons. Forest Ecology and Management 427, 96–105, https://doi.org/10.1016/j.foreco.2018.05.053 (2018).
Sinclair, B. J. & Marshall, K. E. The many roles of fats in overwintering insects. The Journal of Experimental Biology 221, jeb161836, https://doi.org/10.1242/jeb.161836 (2018).
Lidwien Raak-van den Berg, C., Stam, J. M., De Jong, P. W., Hemerik, L. & van Lenteren, J. C. Winter survival of Harmonia axyridis in The Netherlands. Biological Control 60, 68–76, https://doi.org/10.1016/j.biocontrol.2011.10.001 (2012).
Yang, X.-B., Zhang, Y.-M., Henne, D. C. & Liu, T.-X. Life Tables of Bactericera cockerelli (Hemiptera: Triozidae) on Tomato Under Laboratory and Field Conditions in Southern Texas. Florida Entomologist 96, 904–913, https://doi.org/10.1653/024.096.0326 (2013).
Řeřicha, M., Dobeš, P., Hyršl, P. & Knapp, M. Ontogeny of protein concentration, haemocyte concentration and antimicrobial activity against Escherichia coli in haemolymph of the invasive harlequin ladybird Harmonia axyridis (Coleoptera: Coccinellidae). Physiological Entomology 43, 51–59, https://doi.org/10.1111/phen.12224 (2018).
Knapp, M. & Nedvěd, O. Gender and Timing during Ontogeny Matter: Effects of a Temporary High Temperature on Survival, Body Size and Colouration in Harmonia axyridis. PLoS ONE 8, e74984, https://doi.org/10.1371/journal.pone.0074984 (2013).
Aggarwal, D. D. Physiological basis of starvation resistance in Drosophila leontia: analysis of sexual dimorphism. Journal of Experimental Biology 217, 1849–1859, https://doi.org/10.1242/jeb.096792 (2014).
Knapp, M. Relative importance of sex, pre-starvation body mass and structural body size in the determination of exceptional starvation resistance of Anchomenus dorsalis (Coleoptera: Carabidae). PLoS ONE 11, e151459, https://doi.org/10.1371/journal.pone.0151459 (2016).
Knapp, M. & Knappova, J. Measurement of body condition in a common carabid beetle, Poecilus cupreus: a comparison of fresh weight, dry weight, and fat content. Journal of Insect Science 13(article), 6, https://doi.org/10.1673/031.013.0601 (2013).
Gergs, A. & Jager, T. Body size-mediated starvation resistance in an insect predator. Journal of Animal Ecology 83, 758–768, https://doi.org/10.1111/1365-2656.12195 (2014).
Kovacs, J. L. & Goodisman, M. A. D. Effects of Size, Shape, Genotype, and Mating Status on Queen Overwintering Survival in the Social Wasp Vespula maculifrons. Environmental Entomology 41, 1612–1620, https://doi.org/10.1603/en12023 (2012).
Sgolastra, F. et al. The long summer: Pre-wintering temperatures affect metabolic expenditure and winter survival in a solitary bee. Journal of Insect Physiology 57, 1651–1659, https://doi.org/10.1016/j.jinsphys.2011.08.017 (2011).
Therneau, T. M. Package ‘coxme’: Mixed Effects Cox Models, version 2.2-10. (2018).
R Development Core Team. A language and environment for statistical computing. Available at http://www.R-project.org, (2018).
Hothorn, T., Bretz, F. & Westfall, P. Simultaneous inference in general parametric models. Biometrical Journal 50, 346–363, https://doi.org/10.1002/bimj.200810425 (2008).
Pinheiro, J., Bates, D., DebRoy, S., Sarkar, D. & Team, R. D. C. Nlme: linear and nonlinear mixed effects models. R package version 3.1-107. Available at https://cran.r-project.org/web/packages/nlme/nlme.pdf, (2018).
Ripley, B. et al. Package ‘MASS’, version 7.3-50. (2018).
Acknowledgements
We are grateful to Radek Svoboda for his help with the laboratory rearing of ladybirds and the overwintering experiment, to Jana Knappová for the creation of the figures and to Tiit Teder and an anonymous reviewer for insightful suggestions on a previous version of the manuscript. This study was supported by grant No. 42110/1312/3145 awarded by the Internal Grant Agency of the Faculty of Environmental Sciences, Czech University of Life Sciences Prague.
Author information
Authors and Affiliations
Contributions
M.K. and M.Ř. conceived the ideas and designed the methodology; both authors collected the data; M.K. analysed the data; M.K. led the writing of the manuscript. Both authors contributed critically to the drafts and gave final approval for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Knapp, M., Řeřicha, M. Effects of the winter temperature regime on survival, body mass loss and post-winter starvation resistance in laboratory-reared and field-collected ladybirds. Sci Rep 10, 4970 (2020). https://doi.org/10.1038/s41598-020-61820-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-61820-7
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.