Abstract
It is challenging to develop a low-cost household water treatment (HWT) that simultaneously deliver an effective and robust way for safe and reliable water supply. Here, we report a simple flow-through filter made by zeolite-cotton packing in a tube (ZCT) as low-cost HWT device to remove heavy metal ions from contaminated water. The zeolite-cotton is fabricated by an on-site template-free growth route that tightly binds mesoporous single-crystal chabazite zeolite onto the surface of cotton fibers. As a result, the ZCT set-up with optimized diameter achieves both high adsorption efficiency, proper flow rate, reliable supply and strong stability at the same time. After flowed through the set up packed with 10 g of zeolite-cotton, 65 mL 1000 ppm Cu2+ solution was purified down to its safety limit (<1 ppm). Notably, their efficiency remains unaltered when filtering several ions simultaneously. In a simulated purification process, 8 L of water contaminated by Cu2+, Cd2+ and Pb2+ could be transformed into drinking water and it enables the removal of heavy metals to concentrations of below 5 ppb (μg L−1). We also show that the ZCT can be used for disinfection by introducing Ag-exchanged zeolite-cotton without contaminating the water with Ag ions (<0.05 ppm).
Similar content being viewed by others
Introduction
Drinking water safety is one of the most serious health problems throughout the world, especially for those people in relatively undeveloped districts. Health risks may arise from consumption of water contaminated with numerous pollutants such as heavy metal ions, persistent organic pollutants, pharmaceutical waste, virus and microbial pathegens1. As reported by World Health Organization (WHO), nearly 2 billion people are using either un-improved drinking water source or faecally-contaminated water. Close to half a million diarrheal deaths in low- and middle-income countries are attributed to unsafe drinking-water, and the vast majority of these deaths occur among children under five2. Household water treatment (HWT) is an important public health intervention to improve the quality of drinking-water, particularly among those who rely on water from unimproved sources. Further, safe drinking-water is an immediate priority in most emergencies, and HWT can be an effective emergency response intervention3,4, for example, the project solar disinfection (SODIS) of drinking water5,6,7,8, which is awarded the 2020 UNESCO Prizes, is an important HWT technique approved by WHO.
Unlike organic pollutants, heavy metal ions in water is hard to notice and avoid but pose a long-term threat to human. Heavy metal contaminated water usually has no visible difference as compared to safe drinking water, however, every little intake will not degrade but accumulate in human body. Even if the heavy metal-polluted water is not directly taken by human, it can also accumulate in algae, shellfish and fish, then concentrate through food chain and finally result in serious disease such as encephalopathy, hemolytic anemia or even cancer9,10,11. Those global issues have reminded people to search for more proper ways to de-contaminate water against heavy metal ions.
Cost is priority for undeveloped districts12. Traditional water treatment methods include ion exchange resin/zeolite13,14,15,16, chemical precipitation17,18, electro-chemical treatment19,20,21 and reverse osmosis22,23, et al. The latter three treatment methods are often chemically, energetically and operationally intensive, focused on large systems, and thus require considerable infusion of capital, engineering expertise and infrastructure24, all of which precludes their use in much of the world. The cost of ion-exchange resins or zeolite is only about 1/3~1/10 of other metioned treatments25, making it the most economic technique to remove heavy metal ions and other pollutants as HWT. However, due to slow ion diffusion, it generally suffers from low efficiencies (60–90%)26,27 which needs a large mass loading to satisfy high treatment rate demands28. It is of great importance to develop an effective, lower-cost, robust methods to decontaminate waters from source to point-of-use, without further stressing the environment or endangering human health by the treatment itself.
Herein, we develop a simple HWT filter device that is made by zeolite cotton packed in a tube (ZCT). Zeolite cotton (ZC) is prepared by growth of mesoporous zeolite CHA (mCHA) onto the surface of cotton fiber. ZC hybrid material exhibits superior ion-exchange activity and outperforms the conventionally zeolite granules or zeolite powder impregnated cotton cloth in terms of high heavy ion removal capacity and short flow-through time, easy operation and low cost. It achieved not only an ionic adsorption capacity for over 6.5 mg g−1 (Cu2+) but also a treatment efficiency of 84 mL day−1 g−1, enabling the removal of heavy metals to the concentrations below 5 ppb (μg L−1). The performance of the flow-through filter is enabled by the ability of the zeolite nanocrystals to selectively absorb heavy metal pollutants from solutions. We also showed that the ZCT can be used for disinfection by introducing Ag-exchanged ZC without contaminating the water with excessive Ag ions (<0.05 ppm). These performance parameters demonstrate that this simple flow-through filter not only has high efficiency, capacity, and water cleaning power, but also requires no energy input and is easy to set up. We envision that ZCT could be an economically viable process for a daily drinking water purification for undeveloped regions.
Results and discussion
Characterization of ZC
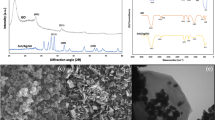
Figure 1a shows the X-ray diffraction (XRD) patterns of ZC. The peaks appearing at 12.56°, 17.64°, 20.44°, 28.12° and 35.56° can be readily indexed as chabazite (JCPDS: 34–0137) while the peaks located at 6.27°, 10.14°, 18.62°, 26.86°, 31.05° and 33.64° matches with the standard faujasite pattern (JCPDS: 12–0228). These two kinds of zeolite are also observed by Scanning Election Microscope (SEM). As shown in Fig. 1c, the majority of the zeolites have hemi-spherical morphology, corresponding to CHA, in good line with our previous report29. The plate-like particles, on the other hand, are FAU. Particle size of CHA and FAU are both around 10 μm. Interestingly, the spherical-like CHA particles are tightly bonded with rod-like cotton fibers, giving a strong connection between the zeolite and cotton, which makes the zeolite hard to fall off from the fiber (Fig. 1d). Thermal gravity analysis (TGA) result reveals that the average zeolite loading of ZC is 20.27 ± 0.7w% (Fig. 1b). Chemical formulation of the zeolite is determined by XRF to be (Na2O)0.43(SiO2)0.90(Al2O3)0.24.
ZCT set up
The procedure to set up ZCT is very simple. All steps are shown in Fig. 2. A piece of zeolite cotton was cut into small pieces and then put into a clean tube. Polluted water can be then poured into the tube and after several tens of minutes, the water flowed through would be transformed into qualified drinking water. No extra energy input, operating skills, spaces or complicate facilities are needed. Everyone can set up this kind of HWT at his/her home using any kind of tubes, bottles or cans available to him/her.
Heavy metal removal
A series of experiments were conducted to evaluate the heavy metal removal properties (Cu2+ as an example) of ZC and traditional zeolite. As suggested by WHO, the safety limit for Cu2+ in drinking water is 1 ppm. Here, the filtration system is shown in Fig. 3a. Four types of zeolite: granular zeolite, powder zeolite, impregnated zeolite and ZC were added into four plastic tubes (d = 10 mm) under the same filling height (50 cm). Pure cotton was set as a reference. The mass of zeolite was determined to be 20.9 g, 32.4 g, 2.0 g and 2.0 for granular zeolite, powder zeolite, impregnated zeolite and ZC, respectively. The treatment efficiency is defined as the volume of safe water (Cu2+ <1 ppm) purified by every 1 gram of material per day. As shown in Fig. 3b, the Cu2+ concentration after filtration was 386.1 ppm for granular zeolite, which is significantly higher than the safety limit. This poor adsorption performance was caused by the slow ionic diffusion rate among the large-particle zeolite and heavy metal ions. The water purified by powder zeolite was below Cu2+ safety limit but the flow rate was highly restricted by the dense stacking of zeolite powder so the treatment efficiency was only 1.6 mL day−1 g−1. In addition, large amount of powder zeolite consumed in this section is another reason for this low treatment efficiency whose unit is mL day−1 g−1. Impregnated zeolite has both safe water and optimal flow rate. Overall treatment efficiency of impregnated zeolite was 78.3 mL day−1 g−1. However, the impregnated zeolite has poor connection with cotton so considerable muddiness was observed in the water after purification (Fig. 3b, inset). Only ZC achieved qualified drinking water, high flow rate and strong stability at the same time. The Cu2+ concentration after filtration was only 0.3 ppm and the treatment efficiency was 84 mL day−1 g−1. Finally, 65 mL qualified water was collected before reaching a maximum capacity.
Comparison of granular zeolite, powder zeolite, impregnated zeolite and ZC. (a) Schematic of these four kinds of zeolite packing in the tube. (b) Cu2+ remaining concentration and removal efficiency of four zeolite and the optical images of the collected water (inset). (c) Residual weight of zeolite on impregnated zeolite and ZC after ultrasonic clean. (d) Cu2+ static adsorption performance of impregnated zeolite and ZC before and after hand-washing.
To further compare the stability of ZC and impregnated zeolite, ultrasonic clean was applied and the weight loss percentage of the zeolite was calculated. As shown in Fig. 3c, impregnated zeolite lost around 80% of total zeolite after 4 min ultrasonic clean while no weight loss was observed for the ZC after 10 min, corresponding to different binding strength between zeolite and cotton fiber. Besides, we further compared the static adsorption performance of ZC or impregnated zeolite before and after hand-washing. 1 g of ZC or impregnated zeolite was put into a beaker containing 20 mL of 1000 ppm CuCl2 solution, the change of concentration of Cu2+ was monitored during the purification process. As shown in Fig. 3d, for the impregnated zeolite sample, there is a significant change of the adsorption curve. The final concentration of Cu2+ increased from 532.1 ppm to 857.7 ppm, indicating a 70% loss of its original adsorption capability. On the other hand, ZC adsorption performance stayed unchanged before and after washing. These results clearly demonstrate the superior reliability of ZC over impregnated zeolite.
To summarize, ZC overcomes the drawbacks of granular zeolite and powder zeolite: granular zeolite usually gives very poor heavy metal adsorption performance due to its slow ionic diffusion rate and powder zeolite makes the water hard to flow through. ZC achieved a good adsorption performance and appropriate flow rate simultaneously. In addition, the strong binding of zeolite nanocrystals to the cotton fiber of ZC ensure its safe application during the water treatment, which excludes the re-contaminating water by leached zeolite powders observed in impregnated sample.
The tube diameter is a key parameter for this HWT filter device. At a given mass of ZC, the tube diameter determines the height of filter and therefore the pathway of the solution. In this study, four tubes with different diameter (20 mm, 15 mm, 10 mm and 8 mm, Fig. 4a) were evaluated. The Cu2+ concentration of the initial solution was 1000 ppm. According to Fig. 4b, a maximum of 25, 35, 65 and 85 ml qualified water could be obtained by 20 mm, 15 mm, 10 mm and 8 mm tubes, respectively. Thinner tubes turned more contaminated water into drinking water but the flow rate was slower as well. Increasing tube diameter leads to an obvious decrease of adsorption capacity but the flow rate is significantly enhanced. The flow rate is as low as 6 mL h−1 for 8 mm tube, which is too slow for daily use. 20 mm tube has the highest flow rate but its capacity is limited. Among these four tubes, 10 mm tube is the optimal one as it produces the most safety drinking water within 2 h (65 mL, Fig. 4c). Therefore, we selected the 10 mm diameter tube for further experiments.
Performance of four ZCT with different tube diameters (20 mm, 15 mm, 10 mm and 5 mm). (a) Schematic of 10 g ZC packing in those four tubes. (b) Volume of safety water obtained by four tubes, starting concentration: Cu2+ = 1000 ppm. (c) The volume of the safety water purified by four tubes within 2 h. (d) Remaining concentration for long-term flowing with three ions (Cu2+, Cd2+ and Pb2+) with a starting concentration: Cu2+ = 4.29 ppm, Cd2+ = 3.12 ppm and Pb2+ = 14.19 ppm. The flowed through water is below the safety limit for drinking water.
A real contaminated water purification process needs to remove various kinds of heavy metal ions at the same time. Here, Cu2+, Cd2+ and Pb2+ containing water was used to simulate the real contaminated water. Numerous studies have been reported to remove these three metal ions in water9,30,31. Cu2+, Cd2+ and Pb2+ are commonly presented in industrial-polluted waters and all pose various threats to human body. Cd2+ accumulation results in lung cancer, proteinuria and osteomalacia after long-term absorption28,32. Excessive Pb2+ intake causes encephalopathy, inanemia, and nephropathy33,34. The toxicity of Cu2+ is not as severe as Cd2+ and Pb2+ but exceeding safe limits of copper can also induce hemolytic anemia and necrotizing hepatitis28,35. As suggested by WHO, the upper limit for Cu2+, Cd2+ and Pb2+ in drinking water is 1 ppm, 0.005 ppm and 0.010 ppm, respectively. After literature review of the current levels of heavy metal pollution in Africa14,36,37,38,39,40, the initial concentration of Cu2+, Cd2+ and Pb2+ were set to be 4.29 ppm, 3.12 ppm and 14.19 ppm, respectively, similar to that of the water in a south Africa river, reported by Yabe and coworkers38. The remaining metal concentration at 2000 mL interval is shown in Fig. 4d. All three metal ions were adsorbed down to their safety limit: Cu2+ to ~0.1 ppb, Cd2+ to ~3 ppb and Pb2+ to ~1 ppb, with consistent values over the entire volume of water. The removal mechanism of heavy metal in water using zeolite is a combination of adsorption and ionic exchange41,42. Na+ concentration, which is exchanged by Cu2+, Cd2+ and Pb2+, in the flowed through water was around 6~7.5 ppm, fairly less than the WHO suggested limit (200 ppm). Finally, around 8000 mL contaminated water was purified by 10 g ZC. Suppose an adult require 2000 mL drinking water every day and a cloth made of ZC, for example, a T-shirt is 200 g, then the water purified by this T-shirt would be at least 160 L, fairly enough for his/her need for 80 days.
We note that the real wastewater is more complicated than we have tested. Other components including alkali and alkaline earth metal ions available in water might affect the sorption characteristics of the zeolitic materials43. Fortunately, the impact of competing cations is considerable only if their concentration is significantly higher than that of heavy metal ions. Zamzow and co-workers44 tested the impact of competing cations, including Na+ and Ca2+, on the uptake of Pb2+. They found that the adsorption of 50 ppm of Pb2+ did not decrease by Na+ and Ca2+ until their concentration beyond 1500 ppm. Similar results were also suggested by Panayotova45. In addition, from the practicle point of view, it is also possible to increase the flow rate of thinnest tube (8 mm) by connecting to a tap, which allow us to achieve the proper flow rate and satisfied adsorption capacity at the same time.
Anti-bacterial test
Silver has been well known as an anti-bacterial agent for centuries46. After exchanged into the zeolite framework, silver-zeolite also possessed disinfection function. Many researches have explored the anti-bacterial function of silver-zeolite47,48,49,50. Kwakye-Awuah and co-workers48 examined the antimicrobial action and efficacy of 2.0 wt% Ag+ loaded zeolite X on Escherichia coli, Staphylococcus aureus and Pseudomonas aeruginosa. They found 1 g/L Ag+ loaded zeolite X could purify all three microbial solution from 106 CFU/ml down to 101 CFU/ml within 0.5 h. The silver-loaded zeolite X retained its antimicrobial action after three times of wash and re-try. However, after treated by Ag+ loaded zeolite X, the resulted solution contained Ag+ ions with a concentration of 1.8–2.5 ppm, which is two-order-of magnitude higher than safety Ag+ concentration for drinking water (<0.05 ppm). Excessive intake of Ag+ can lead to imbalance of intestinal flora and pose a threat to human health46. Therefore, this problem must be solved before applying silver exchanged zeolite into drinking water treatment.
In this work, we propose a dual-bed filter configuration to solve this problem, in which 5 g of Ag exchanged ZC (AgZC) was set at the top of ZC bed. The mass loading of silver is quite low (1 mg/g) which would not significantly affect the performance of ZC for heavy metal ions. Considering the low cost of silver-exchanged ZC (extra $0.0009/g), it is disposable and does not require a complicated and costly regeneration process. Figure 5a demonstrates the main idea: bacterial-polluted water would be first purified by AgZC then excessive Ag+ released from the cotton above would be re-adsorbed by those un-exchanged ZC and finally the water would contain neither bacterial nor overdose Ag+. During the experiment, the original bacterial solution had a Escherichia coli (E. coli) concentration of 0.5~1 × 106 CFU/100 mL. E. coli is a traditionally applied indicator to monitor the microbiological water quality since it provide a direct evidence of recent faecal pollution51. WHO guideline suggests that no detection of E. coli in 100 mL water sample is required to identify a water source safe to drink. As shown in Fig. 5b, the tube packed with 10 g of ZC is unable to kill E. coli and final bacterial colony was 0.97 × 106 CFU/100 mL. However, the tube with 5 g of AgZC had an obvious anti-bacterial function. No viable colony was obtained in the flowed through solution but the remained Ag+ concentration was 0.310 ppm, 6 times higher than the safety limit. The dual-bed tube packed with 5 g AgZC + 5 g ZC gave a sufficient anti-bacterial activity while Ag+ was reduced down to 0.025 ppm which is lower than the safety concentration. Therefore, this kind of device could be used to remove bacterial in water without re-contaminating the water with excess Ag ions, suitable for drinking water.
Conclusion
Filters composed of cotton and cloths have been used to filter water and other beverages since ancient time, which is only used to reduce water turbidity. In this work, by combining the zeolite together with cotton fibers, we endure the zeolite cotton with the function of heavy metal removal and disinfection: 8 L of simulated waste water could be transformaned into safety drinking water using 10 g ZC packing in a diameter optimized tube. Overall treatment efficiency was 84 mL day−1 g−1. Silver exchanged ZC was proved to show anti-bacterial property, E.Colo. contaminated water could flow through the device with neither remaining E. Coli nor excessive Ag+. We believe this simple ZCT filter is practical and affordable household water treatment throughout much of the world, and is available for widespread.
Methods
Materials
Ludox HS-30 colloidal silica (30 wt% suspension in H2O) was purchased from Sigma-Aldrich (Shanghai, China). Sodium hydroxide (NaOH), aluminium hydroxide (Al(OH)3), copper chloride (CuCl2), cadmium chloride (CdCl2), lead nitrate (Pb(NO3)2) and silver nitrate (AgNO3) were obtained from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). Two complexing agents: ethylenediaminetetraacetic acid (EDTA) and Diethyldithiocarbamic acid sodium salt (DDTC), were purchased from Aladdin (Shanghai, China). The Cotton, which is consisted of 100% cotton cellulose and was pretreated with alkaline boiling, was bought from Xiamen Xinhong Extension Trade Co., Ltd. E. coli (CMCC(B)44102), together with R2A medium, were obtained from Shanghai Zhiqiao Biological Engineering Co., Ltd. Commercial zeolite faujausite (CAS: 12173-28-3, (Na2O)43(SiO2)106(Al2O3)43) and chabazite (CAS: 12251-32-0, (Na2O)4(SiO2)16(Al2O3)4) was purchased from Meryer (Shanghai) Chemical Technology Co., Ltd.
Synthesis of zeolite cotton
The synthesis procedure is similar to the method reported in literature29. A gel precursor with molar ratio 9 Na2O:0.7 Al2O3:10 SiO2:331 H2O was prepared from solution A and B. Solution A was prepared by dissolving 400 g sodium hydroxide and 109 g aluminum hydroxide into 3200 mL deionized water. Solution B was a mixture from 320 g sodium hydroxide, 1360 mL deionized water and 2000 g colloidal silica (30 wt%). Both A and B was heated until the solution turned clear. A piece of cotton (200 g) was then added into solution B and transferred into ice-water mixture. Solution A was mixed into solution B dropwise with vigorous stirring. The precursor was then placed into a Teflon-lined autoclave and kept at 100 °C for 24 h. The sample was then washed with deionized water for several times and ultrasonic cleaned for 5 min to removal those loosely-bonded zeolite, and finally dried at 65 °C.
Cu2+ filtration comparison
Granular zeolite, powder zeolite, impregnated zeolite and ZC were separately added into four plastic tubes (d = 10 mm) until reaching the same height (h = 50 cm). The weight ratio for granular zeolite, powder zeolite and impregnated zeolite is CHA: FAU = 1:1. CuCl2 solution with a Cu2+ concentration of 1000 ppm was poured into those tubes and the water flowed down was collected. Remained Cu2+ concentration was determined by Ultraviolet-visible Spectrophotometer (UV-Vis) using EDTA and DDTC as complexing agents for high (50–1000 ppm) and low (0.1–50 ppm) concentration, respectively. The absorption wavelength for Cu2+-EDTA and Cu2+-DDTC is 730 nm and 452 nm, respectively. Handwashing test was conducted by washing 1 g of zeolite cotton or impregnated using 50 mL deionized water for three times. To optimize tube diameter, four tubes with different diameter (20 mm, 15 mm, 10 mm and 8 mm) were tested. In order to simulate the real contaminated waste water, 10 g zeolite-cotton was added into a 10 mm diameter tube, then heavy metal ions solution with a referenced concentration (Cu2+ = 4.29 ppm, Cd2+ = 3.12 ppm and Pb2+ = 14.19 ppm) was poured into the tube. Remained Cu2+, Cd2+ and Pb2+ concentration was characterized by inductively coupled plasma mass spectroscopy (ICP-MS).
Characterizations
The powder X-ray diffraction (XRD) patterns were recorded on a Rigaku Ultimate IV with Cu Kα radiation (10 °C min−1). The accelerating voltage and the applied current were 40 kV and 40 mA, respectively. The morphologies of prepared samples were observed via the field-emission scanning electron microscope (FE-SEM, Hitachi SU8010, Japan). The thermogravimetry analyses (METTLER, TGA/DSC 1/1100, Switzerland) were recorded under dynamic oxygen flow by heating the samples to 800 °C at a rate of 10 °C min−1.
Data availability
The final dataset and accompanying material are available on request.
References
Schwarzenbach, R. P., Egli, T., Hofstetter, T. B., von Gunten, U. & Wehrli, B. Global water pollution and human health. Annu. Rev. Env. Resour. 35, 109–136 (2010).
World Health Organization. Drinking water, https://www.who.int/en/news-room/fact-sheets/detail/drinking-water (2019).
World Health Organization. Household water treatment and safe storage, https://www.who.int/water_sanitation_health/water-quality/household/en/ (2019).
Sobsey, M. Managing water in the home: accelarate health gain from improved water supply, http://www.researchgate.net/publication/267362188 (2002).
Asiimwe, J. K., Quilty, B., Muyanja, C. K. & McGuigan, K. G. Field comparison of solar water disinfection (SODIS) efficacy between glass and polyethylene terephalate (PET) plastic bottles under sub-Saharan weather conditions. J. Water Health. 11, 729–737 (2013).
Joyce, T. M., McGuigan, K. G., ElmoreMeegan, M. & Conroy, R. M. Inactivation of fecal bacteria in drinking water by solar heating. Appl. Environ. Microb. 62, 399–402 (1996).
Polo-Lopez, M. I. et al. Microbiological evaluation of 5 L- and 20 L-transparent polypropylene buckets for solar water disinfection (SODIS). Molecules. 24, 1–14 (2019).
Conroy, R. M., Meegan, M. E., Joyce, T., McGuigan, K. & Barnes, J. Solar disinfection of water reduces diarrhoeal disease: an update. Arch. Dis. Child. 81, 337–338 (1999).
Wu, T. et al. Amidoxime-functionalized macroporous carbon self-refreshed electrode materials for rapid and high-capacity removal of heavy metal from water. ACS Cent. Sci. 5, 719–726 (2019).
Zeng, X. et al. Heavy metal exposure has adverse effects on the growth and development of preschool children. Environ. Geochem. Hlth. 41, 309–321 (2019).
Choi, Y., Park, K., Kim, I. & Kim, S. D. Combined toxic effect of airborne heavy metals on human lung cell line A549. Environ. Geochem. Hlth. 40, 271–282 (2018).
Dixit, D., Soppina, V. & Ghoroi, C. A non-electric and affordable surface engineered particle (SEP) based point-of-use (POU) water disinfection system. Sci. Rep-Uk. 9, 1–12 (2019).
Barakat, M. A. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 4, 361–377 (2011).
Kurniawan, T. A., Chan, G. Y. S., Lo, W. H. & Babel, S. Physico-chemical treatment techniques for wastewater laden with heavy metals. Chem. Eng. J. 118, 83–98 (2006).
Renu, Agarwal, M. & Singh, K. Heavy metal removal from wastewater using various adsorbents: a review. J. Water Reuse Desal. 7, 387–419 (2017).
Obaid, S. S., Gaikwad, D. K., Sayyed, M. I., AL-Rashdi, K. & Pawar, P. P. Heavy metal ions removal from waste water bythe natural zeolites. Mater. Today-Proc. 5, 17930–17934 (2018).
Charerntanyarak, L. Heavy metals removal by chemical coagulation and precipitation. Water Sci. Technol. 39, 135–138 (1999).
Matlock, M. M., Howerton, B. S. & Atwood, D. A. Chemical precipitation of heavy metals from acid mine drainage. Water Res. 36, 4757–4764 (2002).
Caprarescu, S., Vaireanu, D. I., Cojocaru, A., Maior, I. & Purcar, V. A 3-cell electrodialysis system for the removal of copper ions from electroplating wastewater. Optoelectron. Adv. Mat. 5, 1346–1351 (2011).
Shafaei, A., Rezayee, M., Arami, M. & Nikazar, M. Removal of Mn2+ ions from synthetic wastewater by electrocoagulation process. Desalination. 260, 23–28 (2010).
Wang, Y. J. et al. Tunable surface charge of ZnS:Cu nano-adsorbent induced the selective preconcentration of cationic dyes from wastewater. Nanoscale. 4, 3665–3668 (2012).
Jiang, L., Tu, Y., Li, X. M. & Li, H. X. Application of reverse osmosis in purifying drinking water. E3s. Web. Conf. 38, 1–6 (2018).
Albergamo, V. et al. Removal of polar organic micropollutants by pilot-scale reverse osmosis drinking water treatment. Water Res. 148, 535–545 (2019).
Bolisetty, S. & Mezzenga, R. Amyloid-carbon hybrid membranes for universal water purification. Nat. Nanotechnol. 11, 365–371 (2016).
Guo, T. J., Englehardt, J. & Wu, T. T. Review of cost versus scale: water and wastewater treatment and reuse processes. Water. Sci. Technol. 69, 223–234 (2014).
Inglezakis, V. J., Stylianou, M. A., Gkantzou, D. & Loizidou, M. D. Removal of Pb(II) from aqueous solutions by using clinoptilolite and bentonite as adsorbents. Desalination. 210, 248–256 (2007).
Argun, M. E. Use of clinoptilolite for the removal of nickel ions from water: Kinetics and thermodynamics. J. Hazard Mater. 150, 587–595 (2008).
Jarup, L. Hazards of heavy metal contamination. Br. Med. Bull. 68, 167–182 (2003).
Yu, L. S. et al. A tightly-bonded and flexible mesoporous zeolite-cotton hybrid hemostat. Nature Communications. 10, 1–9 (2019).
Bora, A. J. & Dutta, R. K. Removal of metals (Pb, Cd, Cu, Cr, Ni, and Co) from drinking water by oxidation-coagulation-absorption at optimized pH. J. Water Process Eng. 31, 1–6 (2019).
Barkouch, Y., Flata, K., Melloul, A. A., Khadiri, M. E. & Pineau, A. Study of filter height effect on removal efficiency of Cd, Cu, Pb and Zn from water by slow sand filtration. Desalin. Water Treat. 161, 337–342 (2019).
Shukla, A. K. et al. Removal of heavy metal ions using a carboxylated graphene oxide-incorporated polyphenylsulfone nanofiltration membrane. Environ. Sci-Wat. Res. 4, 438–448 (2018).
Kirincic, S. et al. Lead and cadmium in foods/drinking water from Slovenian market/taps: Estimation of overall chronic dietary exposure and health risks. Food Addit. Contam. A 36, 1584–1588 (2019).
Trueman, B. F. et al. Manganese increases lead release to drinking water. Environ. Sci. Technol. 53, 4803–4812 (2019).
Yurekli, Y. Removal of heavy metals in wastewater by using zeolite nano-particles impregnated polysulfone membranes. J. Hazard Mater. 309, 53–64 (2016).
Mengistu, H., Yibas, B., Diop, S., Alemayehu, T. & Demile, M. Investigation of phosphatic-vermiculite-heavy metal interaction and the implication to pollution of Solati River in Limpopo Province, South Africa. International Mine Water Association Symposium. (2012: Bunbury, Western Australia) Proceedings, 713–721 (2012).
Anglewicz, P., VanLandingham, M., Manda-Taylor, L. & Kohler, H. P. Cohort profile: internal migration in sub-Saharan Africa-the migration and health in Malawi (MHM) study. Bmj. Open. 7, 1–12 (2017).
Yabe, J., Ishizuka, M. & Umemura, T. Current levels of heavy metal pollution in Africa. J. Vet. Med. Sci. 72, 1257–1263 (2010).
Okoro, H. K., Fatoki, O. S., Adekola, F. A., Ximba, B. J. & Snyman, R. G. Physico-chemical characteristics and 1-year monitoring of heavy metal pollution and its seasonal variation in seawater of cape town harbour, south africa. Fresen. Environ. Bull. 22, 2855–2866 (2013).
Elumalai, V., Brindha, K. & Lakshmanan, E. Human exposure risk assessment due to heavy metals in groundwater by pollution index and multivariate statistical methods: a case study from south africa. Water-Sui. 9, 1–16 (2017).
Hui, K. S., Chao, C. Y. H. & Kot, S. C. Removal of mixed heavy metal ions in wastewater by zeolite 4A and residual products from recycled coal fly ash. J. Hazard. Mater. 127, 89–101 (2005).
Curkovic, L., Cerjan-Stefanovic, S. & Filipan, T. Metal ion exchange by natural and modified zeolites. Water Res. 31, 1379–1382 (1997).
Yurekli, Y. Determination of adsorption characteristics of synthetic NaX nanoparticles. J. Hazard Mater. 378, 1–10 (2019).
Zamzow, M. J. & Murphy, J. E. Removal of metal cations from water using zeolites. Sep. Sci. Technol. 27, 1969–1984 (1992).
Panayotova, M. & Velikov, B. Kinetics of heavy metal ions removal by use of natural zeolite. J. Environ. Sci. Heal. A. 37, 139–147 (2002).
Dankovich, T. A., Levine, J. S., Potgieter, N., Dillingham, R. & Smith, J. A. Inactivation of bacteria from contaminated streams in Limpopo, South Africa by silver- or copper-nanoparticle paper filters. Environ. Sci-Wat. Res. 2, 85–96 (2016).
Jung, W. K. et al. Antibacterial activity and mechanism of action of the silver ion in Staphylococcus aureus and Escherichia coli. Appl. Environ. Microbiol. 74, 2171–2178 (2008).
Kwakye-Awuah, B., Williams, C., Kenward, M. A. & Radecka, I. Antimicrobial action and efficiency of silver-loaded zeolite X. J. Appl. Microbiol. 104, 1516–1524 (2008).
Li, W. R. et al. Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli. Appl. Microbiol. Biotechnol. 85, 1115–1122 (2010).
Nagy, A. et al. Silver nanoparticles embedded in zeolite membranes: release of silver ions and mechanism of antibacterial action. Int. J. Nanomedicine 6, 1833–1852 (2011).
Pichel, N., Vivar, M. & Fuentes, M. The problem of drinking water access: A review of disinfection technologies with an emphasis on solar treatment methods. Chemosphere. 218, 1014–1030 (2019).
Acknowledgements
This work was supported by National Natural Science Foundation of China (91545113, 91845203, 21802122, 21703050) and Shell Global Solutions International B.V. (PT71423, PT74557).
Author information
Authors and Affiliations
Contributions
J.F. and S.Z. conceived the project and designed the experiments. X.C. carried out the synthesis and characterizations. J.F. and X.C. wrote the manuscript. L.Y. and L.X. helped with the synthesis. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, X., Yu, L., Zou, S. et al. Zeolite Cotton in Tube: A Simple Robust Household Water Treatment Filter for Heavy Metal Removal. Sci Rep 10, 4719 (2020). https://doi.org/10.1038/s41598-020-61776-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-61776-8
This article is cited by
-
Chitosan-boehmite reinforced AgNPs prepared by the sol-gel method for cost-effective water disinfection
Journal of Sol-Gel Science and Technology (2024)
-
Experimental and Numerical Investigations of Using Nanoparticles in Groundwater Remediation
Arabian Journal for Science and Engineering (2023)
-
Novel metal based nanocomposite for rapid and efficient removal of lead from contaminated wastewater sorption kinetics, thermodynamics and mechanisms
Scientific Reports (2022)
-
Thiol-methyl-modified magnetic microspheres for effective cadmium (II) removal from polluted water
Environmental Science and Pollution Research (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.