Abstract
Transition metal sulfide semiconductors have achieved significant attention in the field of photocatalysis and degradation of pollutants. MoS2 with a two dimensional (2D) layered structure, a narrow bandgap and the ability of getting excited while being exposed to visible light, has demonstrated great potential in visible-light-driven photocatalysts. However, it possesses fast-paced recombination of charges. In this study, the coupled MoS2 nanosheets were synthesized with ZnO nanorods to develop the heterojunctions photocatalyst in order to obtain superior photoactivity. The charge transfer in this composite is not adequate to achieve desirable activity. Therefore, heterojunction was modified by reduced graphene oxide (RGO) nanosheets and carbon nanotubes (CNTs) to develop the RGO/ZnO/MoS2 and CNTs/ZnO/MoS2 ternary nanocomposites. The structure, morphology, composition, optical and photocatalytic properties of the as-fabricated samples were characterized through X-ray diffraction (XRD), Fourier Transform Infrared (FTIR), Field Emission Scanning Electron Microscopy (FESEM), Transmission Electron Microscopy (TEM), Energy-Dispersive X-ray (EDX), elemental mapping, Photoluminescence (PL), Ultraviolet–Visible spectroscopy (UV-VIS), and Brunauer-Emmett-Teller (BET) techniques. The photo-catalytic performance of all samples was evaluated through photodegradation of aniline in aqueous solution. The combination of RGO or CNTs into the ZnO/MoS2 greatly promoted the catalytic activity. However, the resulting RGO/ZnO/MoS2 ternary nanocomposites showed appreciably increased catalytic performance, faster than that of CNTs/ZnO/MoS2. Charge carrier transfer studies, the BET surface area analysis, and the optical studies confirmed this superiority. The role of operational variables namely, solution pH, catalyst dosage amount, and initial concentration of aniline was then investigated for obtaining maximum degradation. Complete degradation was observed, in the case of pH = 4, catalyst dosage of 0.7 g/L and aniline concentration of 80 ppm, and light intensity of 100 W. According to the results of trapping experiments, hydroxyl radical was found to be the main active species in the photocatalytic reaction. Meanwhile, a plausible mechanism was proposed for describing the degradation of aniline upon ternary composite. Moreover, the catalyst showed excellent reusability and stability after five consecutive cycles due to the synergistic effect between its components. Total-Organic-Carbon concentration (TOC) results suggested that complete mineralization of aniline occurred after 210 min of irradiation. Finally, a real petrochemical wastewater sample was evaluated for testing the catalytic ability of the as-fabricated composites in real case studies and it was observed that the process successfully quenched 100% and 93% of Chemical Oxygen Demand (COD) and TOC in the wastewater, respectively.
Similar content being viewed by others
Introduction
Water scarcity and increasing amount of discharges of effluents from industries as well as agricultural runoff flowing into water resources, all as a consequence of civilization; make the recycle and treatment of water more and more vital. Aniline is a crucial raw and intermediate material that has various applications in the manufacture of polymer herbicides, pharmaceuticals, polyurethane, and dyes. Besides, it is widely produced in petrochemical plants. However, it is a toxic and cancer-causing substance, and its presence in the wastewater of these industries could result in accumulation in trace concentration in water bodies, creating a risk for the public health and wildlife. Therefore, it is urgent to develop a new practical method to minimize and eliminate this pollutant before being released into the environment. Up to now, many methods have been used for treatment of waste water, including membrane filtration, adsorption, ozonation, biological treatment, and electrolysis1,2,3,4,5,6. Efforts by researchers for finding the best methods or approaches to tackle water issues and find suitable alternative for conventional wastewater treatment have led to the discovery and development of the photocatalytic process7,8,9. In the photocatalytic systems, semiconductor-based materials including metal sulfides (ZnS, CdS), metal oxides (Fe2O3,WO3, TiO2), etc. are exploited under the illumination of light, and when a photon with sufficient energy reaches the semiconductor surface, it would be excited and consequently the electron-hole pairs would be generated. Then, these photo-induced charges are transferred to the photocatalyst surface where they undergo reduction and oxidation reactions with O2 and OH− to produce superoxide anions (.O2−) and hydroxyl radicals (.OH), which are capable of degrading various hazardous organic compounds10,11,12,13,14,15.
Recently, following the continuous progress in the photocatalysis field, there has been a notable focus on the development of the ideal semiconductor photocatalyst. For his purpose, finding a photocatalyst with a suitable bandgap for absorbing light in the visible region as well as being economically and practically favorable for large-scale deployment was of high request. Previous studies showed that, solar light is an intrinsic inexhaustible supply of environmental energy. It is noteworthy that only 4% of solar light is placed in the UV region, while nearly 46% of its spectrum is placed in the visible region which is far abundant. Hence, developing a robust solar-responsive photocatalyst is urgently required for the next-generation of photocatalysts16,17,18,19,20,21,22,23.
So far, transition metal sulfide semiconductors such as CdS, MoS2, and WS2 have achieved much attention because of their narrow bandgap and their ability to separate charges under the visible light24,25,26,27,28. MoS2 with a 2D layered structure and a narrow bandgap of 2 eV is capable to absorb noticeable amount of light, with excellent thermal and chemical stability. This semiconductor has demonstrated a great potential towards water splitting and degradation of pollutants. Nevertheless, pure MoS2 has a major demerit, which is poor quantum yield caused by the insufficient lifetime of photo-induced pairs of electron-hole, which is not considered to be desirable for the redox reaction. It also has a strong tendency towards aggregation and restacking which in turn leads to a significant decrease in the accessible surface area29,30,31,32.
ZnO, as one of the most studied semiconductor photocatalysts, has been extensively applied in degradation of pollutants because it is a metal-free, nontoxic, and low-cost photocatalyst and is effective for almost full mineralization of pollutants. ZnO with a bandgap of 3.37 eV has limited application in the presence of visible light. Furthermore, rapid recombination of photo-induced pairs of electron-hole in the photocatalytic reaction would cause a reduction in its efficiency. The previous studies showed that coupling this semiconductor with a narrow bandgap semiconductor has resulted in an improvement in its light absorption range and yielded better catalytic results33,34,35,36,37,38,39,40. For instance, Tan et al. reported that incorporation of ZnO into MoS2 could result in easy transfer of electrons to the ZnO surface. As a result, the charges could be separated effectively, and finally, the photocatalytic performance would be improved41.
A number of studies have been conducted on graphene, as a 2D carbon sheet; for the fabrication of graphene-based semiconductors due to its exceptional features and also graphene (GR) is considered to be one of the main materials to be used in environmental remediation technologies in future, particularly for wastewater treatment. The superior conductivity property of graphene causes the facilitation regarding the transfer of produced electrons from photocatalyst surface to its conjugated plane. Graphene is known to be a zero bandgap material with low Fermi level, therefore; it acts as an electron storage which receives electrons from the conduction band of the photocatalyst which in turn limits the recombination of electrons and holes. As a consequence, the photocatalytic performance could be boosted15,42,43,44,45,46. Yu et al. in their study showed that incorporation of graphene into MoS2 nanosheets caused a significant effect on the activity of MoS2, and the GR/MoS2 hetero-structures showed more than twice photocatalytic performance for H2 generation than bare MoS2 nanosheets47. Yuan et al. in a study improved the electrical conductivity and photocatalytic activity of MoS2 by coupling it with reduced graphene nanosheets. The RGO sheets acted as a charge transfer highway caused the easy transfer of charges resulting in the limitation in the recombination of electron and holes and improvement of H2 evolution48. Interestingly, many researchers have emphasized the extraordinary effect of graphene on improving the photocatalytic performance of semiconductor which is somehow similar to that of CNT-based photocatalysts. However, the effect of these carbon-based materials on semiconductor photocatalysts such as MoS2 has been investigated separately, and usually a thoughtful comparison between graphene and CNT-semiconductor photocatalysts and their ability for degradation of organic pollutants has been left untouched49,50,51,52.
In the present study, we have focused on developing novel ternary nanocomposites by combining RGO or CNTs with ZnO to enhance the photocatalytic activity of MoS2 for degradation of aniline under exposure to visible light. To the best of our knowledge, there have been no studies which used these nanocomposites for photocatalytic degradation of organic pollutants, especially aniline. Besides, no detailed studies can be found which systematically compare the impact of RGO and CNTs in composite with ZnO/MoS2. In addition, the effects of various parameters including the concentration of aniline, the pH of the aniline solution, the catalyst dosage amount, and the presence of radical scavengers were also evaluated. Furthermore, the reaction was repeated several times in order to determine the reusability of the composites. Moreover, a probable mechanism was proposed for describing the process of photocatalysis. Finally, the efficiency of the fabricated samples was evaluated in terms of wastewater treatment using a real wastewater sample.
Materials and experimental procedure
Materials
Sodium molybdate dihydrate (Na2MoO4.2H2O) and thiourea (CH4N2S) as precursors of MoS2 and zinc acetate as a precursor of ZnO were purchased from Sigma-Aldrich, USA. Aniline was supplied from Merck, Germany. Graphene oxide (GO) and Multi-walled carbon nanotubes (MWCNTs, with high purity >97%, OD < 7 nm, ID = 2–5 nm, length = 10–30 µm) were obtained from US Research Nanomaterials Inc., USA. Other chemicals such as sodium hydroxide (NaOH), hydrochloric acid (HCl), ethanol, p-benzoquinone, isopropanol, and disodium ethylene diamine tetra acetic acid were purchased from Merck, Germany.
Wastewater sampling method
A real petrochemical wastewater sample was prepared from the effluent of a petrochemical company, in Iran. The sample was kept in a 10 L container at 4 °C53.
Synthesis of MoS2 nanosheets
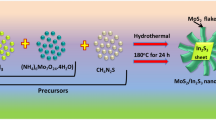
For preparation of MoS2 nanosheets, 1.9 g of thiourea, as a source of sulfur content was dissolved in 80 mL of DI water. Simultaneously, 1.03 g of sodium molybdate was added to this solution accompanied by constant stirring. Next, the mixture was poured into a 100 mL Teflon-sealed autoclave and was heated for 15 hour at 200 °C. The black product was filtered and was washed several times using ethanol and DI water. Finally, the sample was annealed for 2 hour at 700 °C to improve its crystallinity34.
Synthesis of ZnO nanorods
In order to develop ZnO nanorods, 2.195 g of Zn(CH3COOH)2 was dissolved in 80 mL of ethanol. Then, 0.8 g of NaOH was added to the solution and was stirred for 20 min. Next, the solution was poured into a 100 mL Teflon-sealed autoclave and was heated at 150 °C for 12 hour. After the reaction, ZnO nanorods were collected through centrifugation and were washed using ethanol and water. In order to improve the crystallinity and to obtain the Wurtzite phase, the sample was annealed at 700 °C for 2 hour53,54.
Synthesis of binary and ternary samples
CNT/ZnO/MoS2 nanocomposite was synthesized by a typical hydrothermal method. First, a certain amount of CNTs (0.07, 0.1 and 0.13 gr CNTs) was dispersed into a mixture of ethanol (40 mL) and water (40 mL) through ultrasonic treatment at 60 watts for 3 hour. Then, 0.8 g of MoS2 and 0.2 g of ZnO were added to the above-mentioned solution and were sonicated again for 1 hour. The resulting solution was put into a 100 mL hydrothermal autoclave and was kept at 250 °C for 15 h. Then, the prepared composite was centrifuged and was washed several times using distilled water and was dried at 60 °C overnight. Finally, the nanocomposite was annealed for 2 hour at 300 °C for obtaining intimate contact between the components. Similar procedure was carried out to prepare samples of RGO/ZnO/MoS2 nanocomposite. Also, the binary nanocomposite (ZnO/MoS2) was prepared without using carbonaceous materials55.
Characterization
X-ray diffraction (XRD) experiment was conducted using PHILIPS PW1730 (Netherland) Diffractometer with Cu Kα radiation in scanning range of 2θ = 5°–70°. A THERMO AVATAR (USA) Fourier Transform Infrared (FTIR) spectrometer was utilized to obtain the spectra with the wavelength range of 400–4000 cm−1. The surface morphology of the samples was evaluated using Field Emission Scanning Electron Microscopy (FESEM-FEI MOVA NANOSEM 450, USA). The Transmission Electron Microscopy (TEM) was obtained by Zeiss EM 900 apparatus. Energy-Dispersive X-ray (EDX) spectroscope and elemental mapping were applied in order to determine the composition of the samples. The Barrett-Joyner-Halenda (BJH) method was applied to determine the pore size and Brunauer-Emmett-Teller (BET) method was employed to assess the specific surface area based on the nitrogen adsorption/desorption isotherms using BELSORP MINI II (Japan) instrument. All photocatalysts samples were degassed at 120 °C for 2 hour before the analysis. Photoluminescence (PL) spectra were obtained using AvaSpec-2048 apparatus. UV-vis spectra of the samples were analyzed by an Avantes (AvaSpec-2048-TEC) spectrophotometer in the range of 200–1200 nm. The mineralization was studied through determination of Total-Organic-Carbon-concentration (TOC) using TOC-VCSH Shimadzu analyzer, Japan. The components of real wastewater sample prepared from the effluent of the petrochemical company were identified using Agilent 7890 GC-MS, USA. COD determination was done using open-reflux method, based on the available standard methods for water and wastewater examination.
Experimental procedure
Aniline was applied as a study model to measure the photocatalytic performance of samples at ambient temperature. First, all the as-fabricated samples were evaluated under the same condition in order to select the best photocatalyst in terms of photocatalytic performance. Initially, 1.2 g of the nanocomposite was added into 100 mL of 100 ppm aniline solution at pH = 7. Prior to the reaction, the solution was vigorously stirred in dark to establish the adsorption-desorption equilibrium time between the photocatalyst and aniline solution. Then, the experiment was initiated under the exposure to light for 120 min using two 50 W LED lamps, as a source of visible light and they were placed 10 cm away from the reaction media. During the reaction, 2 mL of aniline solution was taken at a regular interval of 15 min using a syringe and after centrifugation, the variation in the aniline concentration was monitored using UV-vis spectrophotometer (Unico, USA) at maximum absorbance of 230 nm (λmax = 230 nm). The results showed that RGO10%/ZnO/MoS2 had the highest photocatalytic performance among all samples, therefore; it was chosen for performing the optimization of the operational parameters. Finally, the effect of operational parameters such as pH, the catalyst dosage amount, and aniline concentration was evaluated during the course of study. The value for removal and total mineralization of aniline in the photocatalytic degradation process was calculated by the following equations56:
where C0 represents initial concentration of aniline, and Ct represents the concentration of aniline at time t. TOC0 represents the initial TOC value and TOCt represents the value of TOC solution at time t.
The pHZPC of the as-fabricated sample was calculated by adding 0.1 g of catalyst sample to 25 mL of NaCl solution. Then, the pH of the solution was adjusted by adding specific amounts of HCl or NaOH to reach to the pH in the range of 2–12. After that, the solutions with different pH values were shaken constantly for 48 hour. Finally, the pHZPC of the catalyst was obtained similar to the initial pH value.
Results and discussion
Characterization of nanocomposites
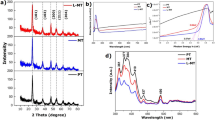
In order to characterize the crystallinity and phase purity of MoS2, ZnO20%/MoS2, RGO10%/ZnO/MoS2 and CNTs10%/ZnO/MoS2 nanocomposite samples, their XRD patterns were evaluated. Figure 1 shows the XRD patterns related to the as-fabricated samples. Figure 1(a), shows that bare MoS2 clearly showed the diffraction peaks at 2θ = 14.49°, 32.68°, 35.9°, 39.56°, 44.1°, 49.84°, 58.32°, and 60.14° which could be attributed to (002), (100), (102), (103), (006), (105), (110) and (112), respectively, and they represented the hexagonal structure of MoS2 (JCPDS No. 37–1492)32. The abovementioned characteristic peaks of MoS2 were also observed in the XRD pattern for binary and ternary nanocomposites (Fig. 1(b–d)). Figure 1(b–d) shows that multiple peaks of ZnO were also observed in these nanocomposite samples at 2θ = 31.82°, 34.5°, 36.3°, 47.62°, 56.68°, 63.08°, and 68° which are assigned to (100), (002), (101), (102), (110), (103), and (112) lattice planes of hexagonal Wurtzite structure of ZnO (JCPDS No. 36–1451)37. In the XRD pattern for RGO/ZnO/MoS2 nanocomposite, the sharp diffraction peak of GO at 2θ = 10° did not exist, however; a very weak diffraction peak emerged at 2θ = 26° which is attributed to (002) reflections of RGO. This implies that GO sheets were transformed into RGO after hydrothermal reduction (Fig. 1(c)). Figure 1(d) shows that there is not any obvious peak related to CNT. This could be due to the low content and amorphous property of CNT. A broad noticeable diffraction peak was observed at 2θ = 14.49° with interlayer space of about 0.61 nm indicating that the samples might compose of a few layers of MoS255. In addition, the crystallite sizes of the samples were measured by the Scherrer’s equation. The mean crystallite size of the MoS2, ZnO20%/MoS2, CNT10%/ZnO/MoS2, and RGO10%/ZnO/MoS2 samples were found to be equal to 66 nm, 64 nm, 54 nm, and 53 nm, respectively at the highest peak for (002) plane. It can be concluded that, carbonaceous materials caused prevention in the growth of the crystallinity for the MoS2 nanosheets.
FTIR was conducted to determine the functional groups in the samples (Fig. 2). The results revealed that MoS2 showed the following absorption peaks at 470 cm−1, 1100 cm−1, 1640 cm−1, and 3436 cm−1. The absorption peaks at 470 cm−1 and 1640 cm−1 are assigned to the presence of Mo-S and Mo-O bands in the sample, respectively. The peak at 1100 cm−1 and 3436 cm−1 were formed as a result of the hydroxyl stretching vibration due to the absorbed water molecules. All these characteristic peaks also existed in the binary and ternary samples. Moreover, in the case of ZnO20%/MoS2, the peak of Mo-S bond at 470 cm−1 had overlap with the Zn-O peak (at 500 cm−1) resulted in formation of a large peak at 470 cm−1. In the spectra of CNT10%/ZnO20%/MoS2, the peaks at 2700 cm−1 and 1720 cm−1 were formed due to the vibration of –COOH groups and C=O stretching, respectively. Also, in the spectra of RGO10%/ZnO20%/MoS2, the peaks appearing at 2854 cm−1 and 2922 cm−1 were formed due to the C-H stretching vibration, and the peak centered at 1726 cm−1 is corresponded to the C=O stretching frequency from the residual carboxyl groups of RGO34,37,57,58,59.
The FESEM images of the as-fabricated samples along with their corresponding EDX results are presented in Fig. 3(a–d). Figure 3(a) clearly shows the formation of large MoS2 nanosheets. As mentioned before, the strong peak at 2θ = 14.4 suggests that the fabricated MoS2 is few-layered according to the FESEM image of MoS2. The average thickness of each sheet is about 11 nm. Figure 3(b) displays the FESEM image of ZnO20%/MoS2 nanocomposite. ZnO particles mainly with a rod-like form and hexagonal shape were compactly deposited on the surface of MoS2 nanosheets while random dispersion was also observed. For better evaluation of the size and morphology of ZnO particles, at the Fig. 3(e,f) the TEM image is provided which confirmed the FESEM result and indicate that rod-like ZnO nanostructures are distributed on the surface of MoS2 nanosheets. It can be seen that the length and diameter of ZnO nanorods are about 100–200 nm and 50–75 nm, respectively. For the sample of CNTs10%/ZnO20%/MoS2, CNTs with an average diameter of 20 nm obviously adhered to the ZnO20%/MoS2 material which is considered to be beneficial for efficient charge transfer. Finally, in the RGO10%/ZnO20%/MoS2 structure, after incorporation of RGO nanosheets, the morphology of the MoS2 nanosheets and ZnO nanorods was not changed. Instead, they were embedded into the MoS2 nanosheets, leading to provision of enlarged surface area and improvement of electrical performance for the ternary sample. The results of EDX analysis only showed the peaks for Mo, S, Zn, O, and carbon elements, which confirmed the successful formation of all nanocomposite samples and high elemental purity of them. Additionally, elemental mapping was used for further identification of elemental content and distribution of CNTs/ZnO/MoS2 and RGO/ZnO/MoS2 nanocomposite samples (Fig. 4). Homogeneous distribution of Mo, Zn, C, and O was observed in the ternary composites, indicating well-organized structure with desirable interaction.
The adsorption-desorption isotherm of samples (Fig. 5A), surface area, BJH results (Fig. 5B) and pore sizes (Table 1) were calculated to investigate the effect of ZnO and carbonaceous materials on the enhancement of surface area for the MoS2. Figure 5A shows that pure MoS2 and ZnO20%/MoS2 both showed similar type V isotherms with H3 type hysteresis loop, while the ZnO/MoS2-carbon materials showed type IV isotherms. These findings indicated that the pore volumes are supplied by mesopores in all samples providing efficient channels for mass transport and enhancing the interface contacts between catalyst surface and pollutant. Pure MoS2 had a surface area of 59.94 m2g−1 and the addition of ZnO nanorods resulted in a slight rise in the surface area, yielding a value of 60.95 m2g−1 for ZnO20%/MoS2. After modification using carbonaceous materials, the BET surface area reached 344.97 m2g−1 and 420.75 m2g−1 for CNT10%/ZnO20%/MoS2 and RGO10%/ZNO20%/MoS2, respectively, indicating that carbonaceous materials especially RGO are considered to be excellent support material for ZnO/MoS2. The pore size studies revealed that the average pore size of ternary composites was lower than that of pure MoS2 and binary composite. However, the extended surface area of the ternary photocatalysts is still capable to supply more active sites for the adsorption of aniline and enhancing the photocatalytic performance52.
The UV-vis analysis of as-fabricated photocatalysts was performed to investigate the optical properties. Figure 6(A) shows the UV-vis adsorption spectra for MoS2, ZnO20%/MoS2, CNT10%/ZnO20%/MoS2 and RGO10%/ZNO20%/MoS2 in the range of 190–800 nm. These results are attributed to the color of composite influencing the adsorption capability of the composite powders. Incorporation of ZnO into MoS2 catalyst caused a decrease in the cut-off wavelength which has led to higher bandgap value due to color conversion from black to gray for MoS2 and ZnO20%/MoS2 catalysts, respectively. However, adding graphene and CNT to the final composite caused an increase in the cut-off wavelength. Moreover, the adsorption edge of RGO10%/ZNO20%/MoS2 and CNT10%/ZnO20%/MoS2 composites showed a red-shift near the wavenumbers of bare MoS2, which is attributed to successful interaction between graphene nanosheets and CNT layers. Hence, the presence of RGO or CNT cause the enhancement in the optical response and light adsorption intensity of ZnO20%/MoS2 composite which are considered to be influential factors for enhancement of photocatalytic degradation in the industrial plants. The bandgap value for each photocatalyst was calculated using Planck equation:
where, h and c represent the planck constant (4.13566 × 10−15) and the light-speed (2.99 × 108 m/s), respectively, and λ represents the cut-off wavelength. The bandgap value and cut-off wavelength for each catalyst is presented in Table 2. The results showed that the bandgap value for photocatalyst containing graphene was higher than that of photocatalyst containing CNT. The obtained results can be attributed to the high charge migration rate between graphene layers and semiconductors and the potential charactristic of graphene in light adsorption60.
The efficient separation of photo-induced electron-hole pairs is considered as a prominent factor for an ideal photocatalyst. Therefore, the photoluminescence (PL) emission spectra formed as a result of the recombination of photo-excited charge carriers on the surface of the semiconductor was investigated for further study of the migration, separation, and recombination of carriers. The PL spectrum of the bare MoS2, binary, and ternary composites are illustrated in Fig. 6(B). Bare MoS2 showed an intense PL emission centered at around 388 nm with an excitation wavelength of 340 nm. The PL spectra for other samples were similar to that of bare MoS2, but the presence of ZnO, CNT, and RGO caused to the reduction in their PL intensity. This reduction was found to be significant when the carbonaceous materials were incorporated. This could happen since, carbonaceous materials possess excellent electro-conductivity and high electron storage capacity, therefore; the migration of photo-excited electrons could be accelerated from the conduction band of MoS2 to the surface of these materials, and therefore, the recombination of charges would be prevented. RGO10%/ZnO20%/MoS2 had lower recombination rate compared to CNT10%/ZnO20%/MoS2 which is accounted to be better for photocatalytic reaction60,61.
Photocatalytic degradation of aniline
Study on the role of ZnO substrate
For evaluating the catalytic performance of MoS2-based composites synthesized with different catalyst materials, aniline solution was selected as a model of organic pollutant to be degraded under the exposure to visible light.
In order to investigate the effect of ZnO substrate, different amounts of ZnO loading (0, 10, 20, and 40 wt.%) were used (Fig. 7A). Furthermore, to investigate the structural stability and photosensitization of aniline under the visible light irradiation, a blank test was carried out in a case without catalyst involvement. Figure 7(A) shows that no obvious self-degradation was observed in the absence of a catalyst, suggesting that aniline is persistent and cannot be degraded under visible light. As expected, in the presence of the catalyst, the single-component had an inadequate degradation rate by 35% which is due to the insufficient absorption sites and fast recombination of charges. The degradation was further improved by loading ZnO content. Increasing the amount of ZnO from 10% to 20% caused an increase in the degradation efficiency from 44% to 58%. However, when the content of ZnO increased to 40%, the catalytic performance decreased to 52%. The reduction in the degradation efficiency of the sample containing the excessive amount of ZnO content might be attributed to the shading effect and increasing opacity of sample blocking the adsorption light of MoS2, and high amount of ZnO content could cause severe agglomeration which results in the reduction of charge transfer capability. It can be concluded that, the photo-activity of the samples depends on the mass ratio of MoS2 and ZnO, indicating that the proper amount of ZnO is of great importance for the synergistic effect between MoS2 and ZnO. Therefore, the selected amount of ZnO content in the ZnO/MoS2 is equal to 20 wt.%, and it is considered to be proper for further investigation62,63,64,65.
The effects of operational parameters on photocatalytic degradation of aniline after being exposed to visible light irradiation for 120 min, (A) Effect of ZnO amount at pH value of 7, catalyst dosage of 1 g/L, aniline concentration of 100 ppm; (B) Effect of CNT amount at ZnO content of 20%, pH value of 7, catalyst dosage of 1 g/L, aniline concentration of 100 ppm; (C) Effect of RGO amount at ZnO content of 20%, pH value of 7, catalyst dosage of 1 g/L, aniline concentration of 100 ppm; (D) Effect of pH value on RGO/ZnO/MoS2 at catalyst dosage of 1 g/L, aniline concentration of 100 ppm; (E) Effect of RGO10%/ZnO20%/MoS2 dosage at pH value of 4, aniline concentration of 100 ppm; and (F) Effect of aniline concentration at pH value of 4 and catalyst dosage of 0.7 g/L.
Study on the role of carbonaceous materials substrate
In order to investigate the effect of carbonaceous materials as support material for catalytic behavior of ZnO20%/MoS2, the degradation of aniline was evaluated. Figure 7(B) clearly shows that the presence of RGO had a substantial effect on the photocatalytic ability of ZnO20%/MoS2. This result is attributed to the excellent properties of RGO nanosheets incorporated into ZnO20%/MoS2 which are explained as follows: (1) incorporation of RGO caused an increase in the surface area and enhancement in the adsorption capacity through providing a 2D support material for ZnO20%/MoS2. As a result, more pollutant molecules can be absorbed through absorption sites. (2) More importantly, incorporation of RGO would result in a delay in the recombination of charges by separating them effectively. This delay could be attributed to the characteristics of carbonaceous materials including having remarkable electron storage capacity and the capability to act as the electron sink, such that photo-induced electrons can be accumulated on their structures. Subsequently, these electrons can be trapped by the dissolved oxygen to form superoxide and hydroxide radicals. Concurrently, the holes in the VB could be reacted with the pollutants to degrade them or react with water molecules and produce more.OH radical. With the increase in RGO content from 7% to 10%, the degradation increased gradually from 68.5% to 84%, while by increasing RGO substrate to 13%, the degradation rate was observed to have a decreasing trend (Fig. 7B).
The major reasons for a decreased catalytic ability caused by excessive incorporation of RGO included the role of RGO as a center of recombination of charges and also the shielding effect of the overloaded RGO causing less light to reach to the surface of the catalyst. Same performance was observed after modification of ZnO20%/MoS2 using CNTs (Fig. 7C). The comparison made between photo-activity performances of ternary nanocomposites showed that RGO is a better alternative for promoting the efficiency of ZnO20%/MoS2 nanocomposite. Although graphene and CNT have similar superior properties in common, such as great adsorption capacity, high electron transition rate, and large specific surface area, they were not similar in enhancing the photocatalytic performance, when used as a composite with ZnO/MoS2. After being exposed to 120 min of irradiation, degradation rate of aniline over the optimized RGO10%/ZnO20%/MoS2 reached to 84% while for CNT10%/ZnO20%/MoS2, this value was equal to 76%. Adsorption capacity of the composite sample is one of the major factors which influence its behavior. Obviously, the adsorption capacity of RGO modified ZnO/MoS2 is higher than that of CNTs modified ZnO/MoS2 catalyst, which should be due to its larger SBET. Based on the optical studies, slight narrowed band gap of RGO10%/ZnO20%/MoS2 compared with CNT10%/ZnO20%/MoS2 suggests that interaction between RGO and ZnO/MoS2 is stronger than that of CNT modified ZnO/MoS251,66.
In addition, the PL studies confirmed the improved light-harvesting properties in RGO10%/ZnO20%/MoS2. Charge carrier transfer studies provided more evidence regarding the effective migration and separation of charges as well as a reduction in the recombination of charges in RGO10%/ZnO20%/MoS2 compared to CNT10%/ZnO20%/MoS2. All these findings strongly suggest that RGO has more effect on the photo-degradation of aniline under visible light than CNTs.
In order to make a quantitative comparison between the photo-activity of all nanocomposite samples, the kinetics study was carried out. The photocatalytic degradation of aniline was found to follow the pseudo-first order equation (Fig. S1(A)).
where C0 represents the initial concentration of aniline, C represents the concentration of aniline at time t, and k represents the apparent reaction rate constant.
The rate constants values was obtained from Fig. S1(B). According to the k value, RGO10%/ZnO20%/MoS2 sample showed a rapid increase in photo-degradation rate suggesting that RGO is beneficial for promoting the photo-activity. As a result, this nanocomposite was chosen as the desired ternary sample for the following investigation.
Study on the role of pH
Solution pH is considered as one of the main parameters that can dramatically influence the photocatalytic reaction since it influences the formation of active radicals and the surface charge of the adsorbate. Due to the formation of intermediate species which may alter the pH of the solution, the pH was monitored during the process. Figure 7(D) depicts the degradation profile of aniline as a function of time at different pH values. Clearly, the degradation rate reached to its maximum level in acidic medium, and it decreased along with increasing pH value. This can be explained according to the surface charge of photocatalyst and the state of aniline at different pH values. Typically, the zero point of charge (pHzpc) for the RGO10%/ZnO20%/MoS2 influenced its surface charge at different pH values. For the RGO10%/ZnO20%/MoS2, the pHzpc was reported to be equal to 2.8. Thus, at pH value < 2.8, the surface of the composite is positively charged, while at pH value > 2.8, it is negatively charged. Besides, the acid dissociation constant (pKa) of aniline was reported to be equal to 4.6 meaning that aniline at values below than this value is positively charged and at values above than this value, it is negatively charged. As a consequence, at high value of pH, both aniline and catalyst are negatively charged. Thus, the interaction between them becomes repulsive, resulting in a decrease in the degradation rate. Conversely, at pH = 4, the electrostatic attraction forces between the positively charged aniline and negatively charged RGO10%/ZnO20%/MoS2 leads to an increase in the degradation rate. Moreover, the recombination of e-h pairs is less likely to occur in the acidic medium which is considered to be favorable for photocatalytic reaction. It should be noted that due to the excess amount of H+ ions at the low value of pH, more electrons migrate to the surface of the photocatalyst to react with O2 to generate active species (superoxide and hydroxyl radicals). Prior studies reported a similar effect of pH value on photo-degradation of aniline67,68,69.
Study regarding the role of nanocomposite dosage
Catalyst dosage is another key parameter influencing the efficiency of the photocatalytic reaction. To determine the role of catalyst dosage, different amounts of RGO10%/ZnO20%/MoS2 were used varying from 0.4 to 1 g/L. The results are shown in Fig. 7(E). It was found that by increasing the amount of catalyst dosage from 0.4 g/L to 0.7 g/L, the degradation rate increased from 72% to 95%. However, beyond this value, a slight decrease was observed in the rate of reaction. The increase in the degradation rate caused by increasing the catalyst dosage was accompanied by the abundance of active sites, and the generation of active radicals. In higher amounts of catalyst dosage, the decrease in the activity was observed, which is due to the fact that the number of aniline molecules are not enough in the reaction medium to be absorbed on the semiconductor sites. In other words, the additional amount of catalyst substrates does not have any effect in the reaction. Moreover, when high amounts of catalyst is used, the reaction mixture becomes opaque, and the penetration of light to the surface of substrates would be hindered70,71.
Study on the role of aniline concentration
After adjusting the catalyst dosage and pH of the solution, in order to determine the proper amount of aniline concentration, the photo-degradation process was carried out using three different concentration of aniline and the results are presented in Fig. 7(F). It was observed that the increase the aniline concentration resulted in the decrease in the removal rate. The trends for degradation of aniline were similar to the trends for degradation of other organic pollutants. A possible explanation for this observation is that, as the concentration of aniline increases, a significant amount of light might be absorbed by aniline molecules rather than by catalyst, which in turn less active species would be produced. Besides, the catalyst sample has a restricted number of active sites which are saturated with aniline molecules. Therefore, the adsorption of OH− on the catalyst surface would be decreased which leads to the reduction in the formation of .OH and .O2 radicals. Furthermore, the formation of intermediates during the photocatalytic process influences the overall aniline degradation rate. Since by increasing the initial concentration, the amount of formation of intermediates increases which competitively attach to the surface of the catalyst and also competitively react with oxidant species70,72.
Herein, for a better evaluation, the catalytic activity of the as-fabricated nanocomposite in the degradation of aniline was compared with those reported in previous studies (see Table 3). The RGO/ZnO/MoS2 showed performance than did the nanomaterials used in previous works, since it decompose higher amount of aniline in less time under visible light. To sum up, RGO/ZnO/MoS2 is an efficient photocatalyst for decontamination of aqueous medium containing organic pollutants.
Mineralization
The TOC analysis was performed to identify the remained intermediates in the reaction medium73. Therefore, an experiment was carried out in the optimal aniline degradation condition which is as follows: pH value of 4, RGO10%/ZnO20%/MoS2 dosage of 0.7 g/L, and initial aniline concentration of 80 ppm. The complete degradation of aniline was achieved over the time period of 120 min, however, the TOC removal was found to be by 40%, and the complete removal of TOC was obtained during the time period of 210 min because aniline molecules were degraded into other organic intermediates. Figure S2 shows the results obtained during mineralization process of aniline.
Study on the effect of radical scavenger
Trapping experiments were carried out with the purpose of identifying the active species in degradation of aniline and determining the mechanism of the photocatalytic reaction. Holes (h+) and radicals such as hydroxyl radicals and superoxide radicals are the common oxidative species in the photocatalytic process. To identify the predominant species for aniline degradation over the RGO10%/ZnO20%/MoS2 nanocomposite in the reaction, three active species of scavengers, namely, disodium ethylene diamine tetra acetic acid (Na-EDTA, 10 mL), benzoquinone (BQ, 10 mL), and 10 mL of isopropanol (IPA, 10 mL) were added to the reaction media for evaluating the effect of radical scavengers h+, .O2, and .OH, respectively. Figure 8(a) shows that after addition of radical scavengers, the degradation percentage achieved in the order of EDTA > BQ > IPA. EDTA had less effect on the degradation percentage which suggests that h+ contributed to the minor extent in the removal of aniline and only a few number of h+ species were produced in the degradation process. On the other hand, the reaction efficiency was greatly impeded following the addition of IPA and BQ, suggesting that .OH and .O2 are the main active species in the photocatalytic reaction74.
According to the abovementioned information, a probable mechanism for degradation of aniline over the RGO/ZnO/MoS2 photocatalyst was proposed using the information presented in Fig. S3. Due to the narrow bandgap of MoS2, firstly, the electrons are excited from VB of MoS2 to the CB. Similarly, ZnO is excited under visible light after modification. Then, the excited electrons in the CB of MoS2 migrate to the CB of ZnO. Subsequently, the generated holes in the VB of ZnO migrate to the VB of MoS2. The positively charged holes react with water and then translate into hydroxyl radicals. In this case, the charges are successfully separated to avoid being recombined again. Besides, given that RGO has superior electron mobility and storage capacity, it can act as an electron reservoir. It can advance the suppression of electron, hole recombination and extend the lifetime of charge carriers, as confirmed by the PL spectra discussed earlier. The accumulated electrons on the conduction band of ZnO can move to RGO sheets. These electrons can reduce the oxygen into superoxide radicals. These radicals can directly degrade aniline or can be translated to hydroxyl radicals for further degradation of aniline. In conclusion, the synergistic effects of MoS2, ZnO, and RGO facilitates the separation of charges and consequently, the degradation of aniline is improved by the use of RGO10%/ZnO20%/MoS2.
Reusability and stability
In addition to catalytic efficiency, the reproducibility and stability is another essential feature of the photocatalyst, which is referred to its cost-effective practical usage. For investigation of the reusability of the RGO10%/ZnO20%/MoS2 sample, the experiment of photocatalytic degradation of aniline at optimal condition (pH value of 4, photocatalytic dosage of 0.7 g/L and aniline concentration of 80 ppm) was repeated for five times. After each run, RGO10%/ZnO20%/MoS2 sample was collected using centrifuge, and was washed using distilled water. Then, it was dried, and reused for the next run. Figure 8(B) shows that there is no significant loss of catalytic activity after five cycles of experiment and the negligible loss is maybe due to the loss in the amount of RGO10%/ZnO20%/MoS2 during its preparation for each cycle, or reduction of surface area caused by absorbing the untreated by-products to the surface of the photocatalyst. Furthermore, after the five cycles, the sample was subjected to XRD, FTIR, BET, and FESEM analysis to investigate the structural, textural, and morphological stability of RGO10%/ZnO20%/MoS2 (Fig. S4(A–D)). The XRD and FTIR patterns of the recovered sample (Fig. S4(A,B)) shows that, no additional peak was appeared and the crystalline nature of photocatalyst has been preserved after five consecutive cycles. Figure S4(C) shows the FESEM image of the nanocomposite after five cycles, and it reveals that the morphological features remained unchanged. Figure S4(D) exhibits the texture properties of the samples after five runs, and it indicates that there was not any obvious change in the type of nitrogen adsorption-desorption isotherms. However, BET measurement data shows slight decrease in the SBET which might be attributed to the fact that during the cycles, a number of untreated intermediates attached on the nanocomposite surface and block the pores, resulting in the reduction of surface area of the nanocomposite.
Consequently, RGO10%/ZnO20%/MoS2 showed excellent reusability and stability without any photo-corrosion in the photocatalytic reaction, which makes it to be considered for long-run utilization in the tests for the elimination of organic pollutants.
Real wastewater treatment
In order to determine whether the catalyst sample fabricated here was proper for practical purposes, a real petrochemical wastewater sample was prepared to be treated using the RGO10%/ZnO20%/MoS2. The properties of petrochemical wastewater sample are presented in Table S1. In order to find the untreated wastewater components, GC-MASS analysis was carried out. The main components of untreated wastewater such as benzene (14.486); 1-Methyl-2-nitro-benzene (15.985); 4-Nitrotoluene (15.985); Aniline (17.773); Phenol (22.781); 3-Methyl-benenamineo (23.845); 4-Chlorophenol (30.510); 2-(5H)-Furanone (31.451); o-Terphenyl (32.092); Naphthalene (32.211); Hexane (34.001); 3-Phenyl-thioethane (34.255); 1,4-Dicyclohexane (34.862); 2-Methyl-benzaldehyde (35.611) and 9,10-Anthracenedione (35.355) (Fig. S5).
The efficiency of the as-fabricated photocatalyst in terms of TOC and COD removal for the petrochemical wastewater sample was found to be equal to 93% and 100%, respectively, in the presence of RGO10%/ZnO20%/MoS2 photocatalyst under the optimum degradation condition (pH value of 4, catalyst dosage of 0.7 g/L) over the time period of 440 min (Figs. S6 and S7). Photocatalyst is identified as a reliable material for efficient long term mineralization and partial oxidation of organic compounds75.
Conclusions
In summary, ternary RGO/ZnO/MoS2 and CNTs/ZnO/MoS2 nanocomposites were synthesized with different amount of RGO and CNTS contents by a typical hydrothermal method and their catalytic ability was evaluated in terms of photo-degradation of aniline. The RGO/ZnO/MoS2 showed the best efficiency compared to that of CNTs/ZnO/MoS2, ZnO/MoS2, and MoS2. The outcoming of the present study suggested that RGO has a substantial effect on the catalytic behavior of ZnO/MoS2. The results of characterization also confirmed the significant effect regarding the incorporation of RGO. The UV-vis analysis and PL spectra showed that RGO/ZnO/MoS2 possess extended visible absorption, and as a result the separation of photo-induced charges was promoted. The BET analysis showed that the surface area also increased greatly in the presence of RGO. In addition, the degradation process was conducted under various operational parameters, and complete degradation of aniline was achieved at pH value of 4, catalyst dosage of 0.7 g/L, and aniline concentration of 80 ppm after being exposed to light irradiation for 120 min. Trapping experiments were performed with the purpose of recognizing active radicals in the reaction and the results suggested that .OH played a paramount role in degradation progress. In the real wastewater sample, the COD and TOC ratios decreased to zero and 7%, respectively after 440 min under the same operational conditions. The obtained results revealed that RGO/ZnO/MoS2 has great potential for the remediation of wastewater containing different kinds of organic pollutants due to its tremendous catalytic ability, stability and reusability.
References
Albadarin, A. B. et al. Activated lignin-chitosan extruded blends for efficient adsorption of methylene blue. Chem. Eng. J. 307, 264–272 (2017).
Alshehri, S. M., Naushad, M., Ahamad, T., Alothman, Z. A. & Aldalbahi, A. Synthesis, characterization of curcumin based ecofriendly antimicrobial bio-adsorbent for the removal of phenol from aqueous medium. Chem. Eng. J. 254, 181–189 (2014).
Naushad, M., Ahamad, T., Al-Maswari, B. M., Abdullah Alqadami, A. & Alshehri, S. M. Nickel ferrite bearing nitrogen-doped mesoporous carbon as efficient adsorbent for the removal of highly toxic metal ion from aqueous medium. Chem. Eng. J. 330, 1351–1360 (2017).
Piotrowska, G. & Pierozynski, B. Electrodegradation of phenol through continuous electrolysis of synthetic wastewater on platinized titanium and stainless steel anodes. Int. J. Electrochem. Sci. 12, 4444–4455 (2017).
Bódalo, A. et al. Nanofiltration membranes to reduce phenol concentration in wastewater. Desalination 245, 680–686 (2009).
Kumar, A. et al. Wide spectral degradation of Norfloxacin by Ag@BiPO4/BiOBr/BiFeO3 nano-assembly: Elucidating the photocatalytic mechanism under different light sources. J. Hazard. Mater. 364, 429–440 (2019).
Zhao, D., Zhou, J. & Liu, N. Characterization of the structure and catalytic activity of copper modified palygorskite/TiO2(Cu2+-PG/TiO2) catalysts. Mater. Sci. Eng. A 431, 256–262 (2006).
Zeng, X. et al. Highly dispersed TiO2 nanocrystals and WO3 nanorods on reduced graphene oxide: Z-scheme photocatalysis system for accelerated photocatalytic water disinfection. Appl. Catal. B Environ. 218, 163–173 (2017).
Xu, Y. et al. Simple synthesis of ZnO nanoflowers and its photocatalytic performances toward the photodegradation of metamitron. Mater. Res. Bull. 76, 235–239 (2016).
Gnanasekaran, L., Hemamalini, R. & Naushad, M. Efficient photocatalytic degradation of toxic dyes using nanostructured TiO2/polyaniline nanocomposite. Desalin. Water Treat. 108, 322–328 (2018).
Jia, Y., Ma, H. & Liu, C. Au nanoparticles enhanced Z-scheme Au-CoFe2O4/MoS2 visible light photocatalyst with magnetic retrievability. Appl. Surf. Sci. 463, 854–862 (2019).
Saravanan, R. et al. Mechanothermal synthesis of Ag/TiO2 for photocatalytic methyl orange degradation and hydrogen production. Process Saf. Environ. Prot. 120, 339–347 (2018).
Vaishnav, J. K., Arbuj, S. S., Rane, S. B. & Amalnerkar, D. P. One dimensional CdS/ZnO nanocomposites: An efficient photocatalyst for hydrogen generation. RSC Adv. 4, 47637–47642 (2014).
Ma, G. et al. Synergistic effect of Cu-ion and WO3 nanofibers on the enhanced photocatalytic degradation of Rhodamine B and aniline solution. Appl. Surf. Sci. 451, 306–314 (2018).
Labhane, P. K., Patle, L. B., Sonawane, G. H. & Sonawane, S. H. Fabrication of ternary Mn doped ZnO nanoparticles grafted on reduced graphene oxide (RGO) sheet as an efficient solar light driven photocatalyst. Chem. Phys. Lett. 710, 70–77 (2018).
Han, W. et al. Enhanced photocatalytic activities of three-dimensional graphene-based aerogel embedding TiO2 nanoparticles and loading MoS2 nanosheets as Co-catalyst. Int. J. Hydrogen Energy 39, 19502–19512 (2014).
Hu, X. et al. Synthesis of novel ternary heterogeneous BiOCl/TiO2/sepiolite composite with enhanced visible-light-induced photocatalytic activity towards tetracycline. J. Colloid Interface Sci. 533, 238–250 (2019).
Kumar, A. et al. Quaternary magnetic BiOCl/g-C3N4/Cu2O/Fe3O4 nano-junction for visible light and solar powered degradation of sulfamethoxazole from aqueous environment. Chem. Eng. J. 334, 462–478 (2018).
Rathi, A. K. et al. Significant Enhancement of Photoactivity in Hybrid TiO2/g-C3N4 Nanorod Catalysts Modified with Cu–Ni-Based Nanostructures. ACS Appl. Nano Mater. 1, 2526–2535 (2018).
Zhao, H. et al. Light-Assisted Preparation of a ZnO/CdS Nanocomposite for Enhanced Photocatalytic H2 Evolution: An Insight into Importance of in Situ Generated ZnS. ACS Sustain. Chem. Eng. 3, 969–977 (2015).
Barik, A. J. & Gogate, P. R. Degradation of 4-chloro 2-aminophenol using combined strategies based on ultrasound, photolysis and ozone. Ultrason. Sonochem. 28, 90–99 (2016).
Liu, L., Bai, H., Liu, J. & Sun, D. D. Multifunctional graphene oxide-TiO2-Ag nanocomposites for high performance water disinfection and decontamination under solar irradiation, J. Hazard. Mater. 261(2013).
Dhiman, P. et al. Nano FexZn1− xO as a tuneable and efficient photocatalyst for solar powered degradation of bisphenol A from aqueous environment. J. Cleaner Prod. 165, 1542–1556 (2017).
Ma, X., Zhao, F., Qiang, Q., Liu, T. & Wang, Y. Fabrication of selective interface of ZnO/CdS heterostructures for more efficient photocatalytic hydrogen evolution. Dalt. Trans. 47, 12162–12171 (2018).
Huang, T., Luo, Y., Chen, W., Yao, J. & Liu, X. Self-assembled MoS2-GO Framework as an Efficient Cocatalyst of CuInZnS for Visible-Light Driven Hydrogen Evolution. ACS Sustain. Chem. Eng. 6, 4671–4679 (2018).
Tian, L., Yang, X., Cui, X., Liu, Q. & Tang, H. Fabrication of dual direct Z-scheme g-C3N4/MoS2/Ag3PO4 photocatalyst and its oxygen evolution performance. Appl. Surf. Sci. 463, 9–17 (2019).
Yuan, Y. et al. Construction of g-C3N4/CeO2/ZnO ternary photocatalysts with enhanced photocatalytic performance. J. Phys. Chem. Solids 106, 1–9 (2017).
Zhou, C., Qian, J., Yan, J., Dong, X. & Zhou, B. A ternary photocatalyst of graphitic carbon nitride/cadmium sulfide/titania based on the electrostatic assembly using two-dimensional semiconductor nanosheets. J. Colloid Interface Sci. 491, 367–374 (2017).
Wu, M. et al. Molybdenum disulfide (MoS2) as a co-catalyst for photocatalytic degradation of organic contaminants: A review. Process Saf. Environ. Prot. 118, 40–58 (2018).
Lu, D. et al. Highly Efficient Visible-Light-Induced Photoactivity of Z-Scheme g-C3N4/Ag/MoS2 Ternary Photocatalysts for Organic Pollutant Degradation and Production of Hydrogen. ACS Sustain. Chem. Eng. 5.2, 1436–1445 (2017).
Chakrabarty, S., Mukherjee, A. & Basu, S. RGO-MoS2 Supported NiCo2O4 Catalyst toward Solar Water Splitting and Dye Degradation. ACS Sustain. Chem. Eng. 6, 5238–5247 (2018).
Ren, B., Shen, W., Li, L., Wu, S. & Wang, W. 3D CoFe2O4 nanorod/flower-like MoS2 nanosheet heterojunctions as recyclable visible light-driven photocatalysts for the degradation of organic dyes. Appl. Surf. Sci. 447, 711–723 (2018).
Saud, P. S. et al. One-pot synthesis of Ag3PO4/MoS2 nanocomposite with highly efficient photocatalytic activity. J. Environ. Chem. Eng. 5, 5521–5527 (2017).
Behtaj Lejbini, M. & Sangpour, P. Hydrothermal synthesis of α-Fe2O3-decorated MoS2 nanosheets with enhanced photocatalytic activity. Optik (Stuttg). 177, 112–117 (2019).
Wang, S., Ren, C., Tian, H., Yu, J. & Sun, M. MoS2/ZnO van der Waals heterostructure as a high-efficiency water splitting photocatalyst: A first-principles study. Phys. Chem. Chem. Phys. 20, 13394–13399 (2018).
Tian, N. et al. Utilization of MoS2 Nanosheets to Enhance the Photocatalytic Activity of ZnO for the Aerobic Oxidation of Benzyl Halides under Visible Light. Ind. Eng. Chem. Res. 55, 8726–8732 (2016).
Awasthi, G. P. et al. Facile synthesis of ZnO flowers modified graphene like MoS2 sheets for enhanced visible-light-driven photocatalytic activity and antibacterial properties. J. Alloys Compd. 682, 208–215 (2016).
Benavente, E., Durán, F., Sotomayor-Torres, C. & González, G. Heterostructured layered hybrid ZnO/MoS2 nanosheets with enhanced visible light photocatalytic activity. J. Phys. Chem. Solids 113, 119–124 (2018).
Zhang, S. et al. MoS2-coated ZnO nanocomposite as an active heterostructure photocatalyst for hydrogen evolution. Radiat. Phys. Chem. 137, 104–107 (2017).
Thakur, M. et al. Efficient photocatalytic degradation of toxic dyes from aqueous environment using gelatin-Zr(IV) phosphate nanocomposite and its antimicrobial activity. Colloids Surf. B Biointer. 157, 456–463 (2017).
Tan, Y. H., Yu, K., Li, J. Z., Fu, H. & Zhu, Z. Q. MoS2@ZnO nano-heterojunctions with enhanced photocatalysis and field emission properties. J. Appl. Phys. 116, 064305 (2014).
Yan, H., Song, P., Zhang, S., Yang, Z. & Wang, Q. Facile synthesis, characterization and gas sensing performance of ZnO nanoparticles-coated MoS2 nanosheets. J. Alloys Compd. 662, 118–125 (2016).
Zhang, C. et al. In situ fabrication of Bi2WO6/MoS2/RGO heterojunction with nanosized interfacial contact via confined space effect toward enhanced photocatalytic properties. ACS Sustain. Chem. Eng. 4, 5936–5942 (2016).
Kumar, S. et al. Efficient Electron Transfer across a ZnO–MoS2–Reduced Graphene Oxide Heterojunction for Enhanced Sunlight-Driven Photocatalytic Hydrogen Evolution. ChemSusChem 10, 3588–3603 (2017).
Yuan, Y.-J. et al. Promoting Charge Separation in g-C3N4/Graphene/MoS2 Photocatalysts by Two-Dimensional Nanojunction for Enhanced Photocatalytic H2 Production. ACS Appl. Energy Mater. 1.4, 1400–1407 (2018).
Sher Shah, M. S. A., Park, A. R., Zhang, K., Park, J. H. & Yoo, P. J. Green synthesis of biphasic TiO2-reduced graphene oxide nanocomposites with highly enhanced photocatalytic activity. ACS Appl. Mater. Interfaces 4, 3893–3901 (2012).
Yu, H. & Ching, C. B. Theoretical analysis of the adsorption effect on kinetic resolution of racemates catalyzed by immobilized enzymes in a batch reactor. Ind. Eng. Chem. Res. 47, 4251–4255 (2008).
Yuan, Y. et al. Excellent photocatalytic performance of few-layer MoS2/graphene hybrids. J. Alloys Compd. 700, 12–17 (2017).
Zhang, Z. et al. Noncovalent Functionalization of Monolayer MoS2 with Carbon Nanotubes: Tuning Electronic Structure and Photocatalytic Activity. J. Phys. Chem. C 121, 21921–21929 (2017).
Park, C. H., Lee, C. M., Choi, J. W., Park, G. C. & Joo, J. Enhanced photocatalytic activity of porous single crystal TiO2/CNT composites by annealing process. Ceram. Int. 44, 1641–1645 (2018).
Ye, A., Fan, W., Zhang, Q., Deng, W. & Wang, Y. CdS-graphene and CdS-CNT nanocomposites as visible-light photocatalysts for hydrogen evolution and organic dye degradation. Catal. Sci. Technol. 2, 969–978 (2012).
Yang, M.-Q., Zhang, N. & Xu, Y.-J. Synthesis of fullerene–, carbon nanotube–, and graphene–TiO2 nanocomposite photocatalysts for selective oxidation: a comparative study. ACS Appl. Mat. Interfaces 5.3, 1156–1164 (2013).
Hayati, F., Isari, A. A., Fattahi, M., Anvaripour, B. & Jorfi, S. Petrochemical wastewater through ZnO/TiO2 nanocomposite: influential operating factors. RSC Adv. 8.70, 40035–40053 (2018).
Moussa, H. et al. ZnO rods/reduced graphene oxide composites prepared via a solvothermal reaction for efficient sunlight-driven photocatalysis. Appl. Catal., B: Env. 185, 11–21 (2016).
Guan, Z. et al. Remarkable enhancement in solar hydrogen generation from MoS2-RGO/ZnO composite photocatalyst by constructing a robust electron transport pathway. Chem. Eng. J. 327, 397–405 (2017).
Pathania, D. et al. Photocatalytic degradation of highly toxic dyes using chitosan-g-poly(acrylamide)/ZnS in presence of solar irradiation. J. Photochem. Photobiol. A Chem. 329, 61–68 (2016).
Yang, Y., Asiri, A. M., Tang, Z., Du, D. & Lin, Y. Graphene based materials for biomedical applications. Biochem. Pharmacol. 16, 365–373 (2013).
Payan, A., Fattahi, M., Jorfi, S., Roozbehani, B. & Payan, S. Synthesis and characterization of titanate nanotube/single-walled carbon nanotube (TNT/SWCNT) porous nanocomposite and its photocatalytic activity on 4-Chlorophenol degradation under UV and solar irradiation. Appl. Surf. Sci. 434, 336–350 (2018).
Sasikala, R. et al. Nanohybrid MoS2-PANI-CdS photocatalyst for hydrogen evolution from water. Colloids Surfaces A Physicochem. Eng. Asp. 481, 485–492 (2015).
Nuengmatcha, P., Chanthai, S., Mahachai, R. & Oh, W. Journal of Environmental Chemical Engineering Visible light-driven photocatalytic degradation of rhodamine B and industrial dyes (texbrite BAC-L and texbrite NFW-L) by ZnO-graphene. Biochem. Pharmacol. 4, 2170–2177 (2016).
Wang, K., Link, B. G., Corrigan, P. W., Davidson, L. & Flanagan, E. Perceived provider stigma as a predictor of mental health service users’ internalized stigma and disempowerment. Psychiatry Res. 259, 526–531 (2018).
Xiao, M., Luo, B., Thaweesak, S. & Wang, L. Noble-metal-free MoS2/Ta3N5 heterostructure photocatalyst for hydrogen generation. Prog. Nat. Sci. Mater. Int. 28.2, 189–193 (2018).
Huang, S., Chen, C., Tsai, H., Shaya, J. & Lu, C. Photocatalytic degradation of thiobencarb by a visible light-driven MoS2 photocatalyst. Sep. Purif. Technol. 197, 147–155 (2018).
Sun, J.-X. et al. Fabrication of composite photocatalyst gC3N4–ZnO and enhancement of photocatalytic activity under visible light. Dalton Transactions. 41.22, 6756–6763 (2012).
Wang, F. et al. Fabrication of FeWO4@ ZnWO4/ZnO heterojunction photocatalyst: synergistic effect of ZnWO4/ZnO and FeWO4@ ZnWO4/ZnO heterojunction structure on the enhancement of visible-light photocatalytic activity. ACS Sustainable Chem. Eng. 4.12, 6288–6298 (2016).
Hu, S., Li, F. & Fan, Z. The property and photocatalytic performance comparison of graphene, carbon nanotube, and C60 modified TiO2 nanocomposite photocatalysts. Bull. Korean Chem. Soc. 34, 3671–3676 (2013).
Kumar, A. et al. Biochar-templated g-C3N4/Bi2O2CO3/CoFe2O4 nano-assembly for visible and solar assisted photo-degradation of paraquat, nitrophenol reduction and CO2 conversion. Chem. Eng. J. 339, 393–410 (2018).
Fakhri, A. Adsorption characteristics of graphene oxide as a solid adsorbent for aniline removal from aqueous solutions: Kinetics, thermodynamics and mechanism studies. J. Saudi Chem. Soc. 21, S52–S57 (2017).
Jourshabani, M., Shariatinia, Z. & Badiei, A. Controllable Synthesis of Mesoporous Sulfur-Doped Carbon Nitride Materials for Enhanced Visible Light Photocatalytic Degradation. Langmuir 33.28, 7062–7078 (2017).
Pirsaheb, M. et al. Photocatalytic degradation of Aniline from aqueous solutions under sunlight illumination using immobilized Cr:ZnO nanoparticles. Sci. Rep. 7, 1–12 (2017).
Al-Kahtani, A. A. Photocatalytic Degradation of Rhodamine B Dye in Wastewater Using Gelatin/CuS/PVA Nanocomposites under Solar Light Irradiation. J. Biomater. Nanobiotechnol. 08, 66–82 (2017).
Borji, S. H., Nasseri, S., Mahvi, A. H., Nabizadeh, R. & Javadi, A. H. Investigation of photocatalytic degradation of phenol by Fe(III)-doped TiO2 and TiO2 nanoparticles. J. Environ. Heal. Sci. Eng. 12, 1–10 (2014).
Hussain, I. et al. Heterogeneously degradation of aniline in aqueous solution using persulfate catalyzed by magnetic BiFeO3 nanoparticles. Catal. Today 310, 130–140 (2018).
Li, L. et al. Synthesis of barbituric acid doped carbon nitride for efficient solar-driven photocatalytic degradation of aniline. Appl. Surf. Sci. 428, 739–747 (2018).
Ahmadi, M. et al. Enhanced photocatalytic degradation of tetracycline and real pharmaceutical wastewater using MWCNT/TiO2 nano-composite. J. Environ. Manage. 186, 55–63 (2017).
Taghavi, M. et al. Feasibility of applying the LED-UV-induced TiO2/ZnO-supported H3PMo12O40 nanoparticles in photocatalytic degradation of aniline. Env. Mon. Assess. 190.4, 188 (2018).
Shahrezaei, F., Mansouri, Y., Zinatizadeh, A. A. L. & Akhbari, A. Photocatalytic degradation of aniline using TiO2 nanoparticles in a vertical circulating photocatalytic reactor. Int. J. Photoenergy 2012 (2012).
Norzaee, S., Djahed, B., Khaksefidi, R. & Mostafapour, F. K. Photocatalytic degradation of aniline in water using CuO nanoparticles. J. Water Supply Res. Technol. - AQUA 66, 178–185 (2017).
Ma, G. et al. Synergistic effect of Cu-ion and WO3 nanofibers on the enhanced photocatalytic degradation of Rhodamine B and aniline solution. Appl. Surf. Sci. 451, 306–314 (2018).
Guan, Y. et al. Constructing BiO1.1Br0.8 sheet-sphere junction structure for efficient photocatalytic degradation of aniline. Catal. Commun. 88, 22–25 (2017).
Author information
Authors and Affiliations
Contributions
Parisa Ghasemipour: Performed the experiments, analyzed data and wrote the draft of paper. Moslem Fattahi: Analyzed data, wrote the paper discussion and supervised the research. Behnam Rasekh: Analyzed data, co-wrote the paper discussion and supervised the research. Fatemeh Yazdian: Analyzed data, co-wrote the paper discussion and supervised the research.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ghasemipour, P., Fattahi, M., Rasekh, B. et al. Developing the Ternary ZnO Doped MoS2 Nanostructures Grafted on CNT and Reduced Graphene Oxide (RGO) for Photocatalytic Degradation of Aniline. Sci Rep 10, 4414 (2020). https://doi.org/10.1038/s41598-020-61367-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-61367-7
This article is cited by
-
Unleashing the power of solar light: WO3 nanorods decorated onto BiVO4 dendrites for tetracycline detoxification and water splitting
Environmental Science and Pollution Research (2023)
-
Efficiency of zero-dimensional and two-dimensional graphene architectural nanocomposites for organic transformations in the contemporary environment: a review
Journal of the Iranian Chemical Society (2023)
-
Facile green synthesis of ZnO/AC nanocomposites using Pontederia crassipes leaf extract and their photocatalytic properties based on visible light activation
Journal of Materials Science: Materials in Electronics (2023)
-
Photocatalytic degradation of chloroform using rGO-CuS nanocomposite under UV/VIS irradiation from gaseous phase
Environmental Science and Pollution Research (2023)
-
Adsorption and photocatalytic degradation of auramine–O dye using carbon nanoparticles and Carbon–CaO nanocomposites
Nanotechnology for Environmental Engineering (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.