Abstract
Indoor residual spraying (IRS) of insecticides is a major vector control strategy for malaria prevention. We evaluated the impact of a single round of IRS with the organophosphate, pirimiphos-methyl (Actellic 300CS), on entomological and parasitological parameters of malaria in Migori County, western Kenya in 2017, in an area where primary vectors are resistant to pyrethroids but susceptible to the IRS compound. Entomological monitoring was conducted by indoor CDC light trap, pyrethrum spray catches (PSC) and human landing collection (HLC) before and after IRS. The residual effect of the insecticide was assessed monthly by exposing susceptible An. gambiae s.s. Kisumu strain to sprayed surfaces in cone assays and measuring mortality at 24 hours. Malaria case burden data were extracted from laboratory records of four health facilities within the sprayed area and two adjacent unsprayed areas. IRS was associated with reductions in An. funestus numbers in the intervention areas compared to non-intervention areas by 88% with light traps (risk ratio [RR] 0.12, 95% CI 0.07–0.21, p < 0.001) and 93% with PSC collections (RR = 0.07, 0.03–0.17, p < 0.001). The corresponding reductions in the numbers of An. arabiensis collected by PSC were 69% in the intervention compared to the non-intervention areas (RR = 0.31, 0.14–0.68, p = 0.006), but there was no significant difference with light traps (RR = 0.45, 0.21–0.96, p = 0.05). Before IRS, An. funestus accounted for over 80% of Anopheles mosquitoes collected by light trap and PSC in all sites. After IRS, An. arabiensis accounted for 86% of Anopheles collected by PSC and 66% by CDC light trap in the sprayed sites while the proportion in non-intervention sites remained unchanged. No sporozoite infections were detected in intervention areas after IRS and biting rates by An. funestus were reduced to near zero. Anopheles funestus and An. arabiensis were fully susceptible to pirimiphos-methyl and resistant to pyrethroids. The residual effect of Actellic 300CS lasted ten months on mud and concrete walls. Malaria case counts among febrile patients within IRS areas was lower post- compared to pre-IRS by 44%, 65% and 47% in Rongo, Uriri and Nyatike health facilities respectively. A single application of IRS with Actellic 300CS in Migori County provided ten months protection and resulted in the near elimination of the primary malaria vector An. funestus and a corresponding reduction of malaria case count among out-patients. The impact was less on An. arabiensis, most likely due to their exophilic nature.
Similar content being viewed by others
Introduction
Over the last two decades, malaria control has been scaled up throughout sub-Saharan Africa with an emphasis on the distribution of long-lasting insecticidal nets (LLINs), targeted application of indoor residual spraying (IRS), and improved diagnostics and case management. As a result, the burden of malaria has declined substantially with a 40% reduction in incidence and a 50% reduction in prevalence between 2000 and 2015. While LLINs contributed an estimated 68% of the decline in malaria prevalence, IRS was responsible for 13%1.
The efficacy of insecticide-treated nets was demonstrated in a series of cluster randomized, controlled trials2. Formal randomized controlled trials of IRS have also demonstrated the efficacy of IRS3,4. Furthermore, there is a long history of programmatic implementation of IRS in many settings of the world which resulted in reduced malaria burden and even elimination in some settings2. The use of both LLINs and IRS for malaria control has a direct impact on mosquito bionomics. LLINs and IRS have multiple effects on mosquito populations which may result in reduced malaria transmission including: reduced indoor Anopheles densities5,6,7, shifts in vector species composition8, changes in the time and location of mosquito biting9,10,11, and changes in host selection12, and increases in early exophily13.
In western Kenya, vector control included universal coverage of LLINs through periodic mass campaigns and routine distribution to high-risk groups as well as IRS in specifically targeted areas. The first mass LLIN distribution occurred in 2006 and targeted children <5 years of age. Additional distributions aiming for universal coverage occurred in 2011 and 2014 leading to 54% of households in the lake endemic zone having one LLIN for every two residents14. The region also bears the highest malaria burden nationally14,15,16. Implementation challenges facing LLINs include incomplete coverage14,17,18,19, widespread pyrethroid resistance20,21,22,23 and possible changes in vector behaviour9.
IRS in western Kenya was based exclusively on pyrethroids until 201224,25,26. However, spraying was interrupted between 2013 and 2017 due to widespread pyrethroid resistance in local malaria vector populations and the lack of a registered, non-pyrethroid insecticide in the country. In response to widespread pyrethroid resistance, the Kenyan National Malaria Control Programme (NMCP) developed an insecticide resistance management strategy involving the rotation of different non-pyrethroid classes of insecticides used in IRS every two years in endemic and epidemic-prone areas where 80% or more households own one or more LLIN27. This is in accordance with global insecticide resistance management strategy aimed at delaying the rise and spread of insecticide resistance to new classes of insecticide while preserving pyrethroids for use in bednets28. In 2017, IRS was re-introduced using a microencapsulated formulation of pirimiphos-methyl (Actellic 300CS (capsule suspension)). The insecticide has been reported to be effective against pyrethroid-resistant Anopheles mosquitoes29,30,31 and has a relatively long residual effect on sprayed wall surfaces, of up to twelve months29,30,32.
Between 2008 and 2012, IRS campaigns in western Kenya were conducted using pyrethroid based insecticides. These campaigns resulted in significantly lower parasitaemia and cases of clinical malaria in IRS districts compared to non-IRS districts26. The protective efficacy of ITN plus IRS compared with ITN only in the same area was measured at 62%, whereas, 60% fewer anophelines were observed in the sprayed districts compared to unsprayed districts post-IRS25. Anopheles funestus accounted for 1.7% of total anophelines collected in the sprayed areas before IRS and 2.7% after IRS25, an indication that the species was not affected by spraying. Following reports of increasing insecticide resistance, the malaria vector control policy changed to the use of non-pyrethroid insecticides for IRS. IRS was reintroduced in 2017 with an organophosphate, Actellic 300CS. We sought to determine efficacy of the Actellic CS based IRS campaign on entomological and epidemiological indices against a background of moderate to high coverage of pyrethroid LLINs14 and extensive pyrethroid resistance.
Methods
Study sites
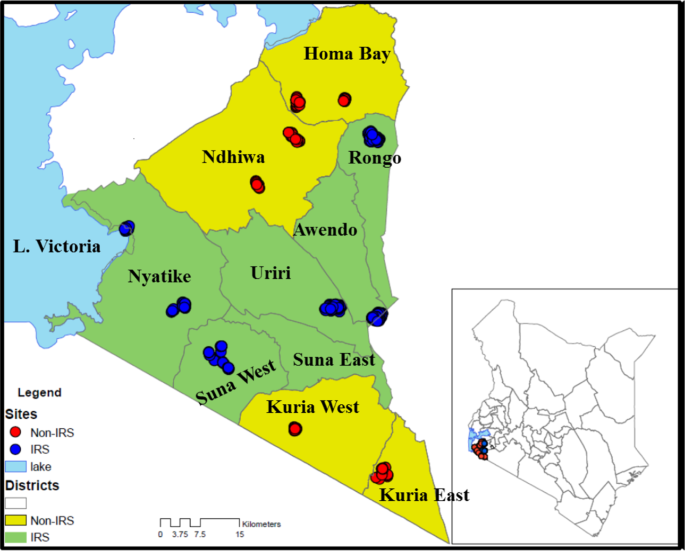
Entomological monitoring was conducted in 12 villages in Migori (−1.0667S; 34.4667E) and Homa Bay (−0.5396S; 34.4565E) counties from July 2016 to February 2018. Six IRS intervention sub-counties were in Migori County, and six control sub-counties were in neighbouring Homa Bay County (n = 4) and unsprayed areas of Migori County (n = 2) (Fig. 1). The residents in the study area are mainly of the Luo ethnic group and are subsistence farmers with a few growing cash crops such as sugar cane and tobacco. Residents mostly live in small houses, clustered into family social units called compounds. The region has bimodal peaks of rainfall with the long rains between April and June and short rains in October and November. The Lake Victoria region of western Kenya is malaria endemic; the most recent Malaria Indicator Survey in 2015 documented a malaria prevalence of 27% by microscopy. The last mass net campaign in the region was conducted in April 2017 with DawaPlus 2.0, a deltamethrin long-lasting (coated) insecticidal net distributed in this area. Though 87% of households own at least one LLIN and 60% own more than one LLIN, only 54% of households have an adequate number of nets, defined as one LLIN for every two residents. Among residents of western Kenya, 68.4% reported to have slept under any net the night before the survey while 66.9% had slept under LLINs14. Anopheles funestus, An. arabiensis and An. gambiae s.s. are the main malaria vectors in the region33,34. Insecticide resistance among major malaria vectors in Kenya has been reported for pyrethroids, carbamates, and organochlorines35. The reported resistance mechanisms include both target site mutations and increased activity of enzymes involved in metabolic detoxification21,22,23.
Map of Kenya, showing study sites in western Kenya with the names of sub-counties. Yellow shading represents non-intervention sites and red dots represent sampled houses. The green shading is the intervention site with the blue dots representing sampled houses (The map was created on ArcGIS 10.2.2).
IRS campaign
IRS was conducted in February-March 2017. A total of 212,029 houses in Migori County were sprayed, representing coverage of 97.7% of houses sprayed against houses found. The campaign covered a population of 906,388 people, including 16,932 pregnant women and 127,157 children below five years of age36.
Vector surveillance
Vector surveillance was conducted in the twelve villages from July 2016 to February 2018. Houses were randomly selected in each village every month for mosquito collections by PSC and indoor CDC light trap (CDC-LT). Household information including roof type, wall type, open or closed eaves, the presence of nets, number of people that slept under a net the previous night and those that did not, and the presence of cattle were collected on a tablet computer. The mosquito density for each method were expressed as the mean number of mosquitoes caught per house per collection visit.
Indoor-resting mosquitoes were collected between 07:00 and 11:00 by PSC in five houses per site per month. PSCs were done by laying white sheets on the floor and over the furniture within the house. Two collectors, one inside the house and another outside, sprayed around the eaves with 0.025% pyrethrum emulsifiable concentrate mixed with 0.1% piperonyl butoxide (supplied by the Kenya Pyrethrum Board) in kerosene. The collector inside the house then sprayed the roof space. The house was closed for 10–15 minutes after which knocked-down mosquitoes were collected from the sheets and transferred to the laboratory in scintillation vials containing 70% ethanol.
Indoor host-seeking mosquitoes were collected by CDC-LT in 10 houses per site once per month. A single 12-volt CDC-LT was hung in each house in the sleeping area, approximately 1.5 meters from the floor, adjacent to an occupied bed net owned by a member of the household. The traps were run from 18:00 to 07:00 the following morning. The trapped mosquitoes were transferred into paper cups and transported to the laboratory for further analysis.
Human landing catches (HLC) were used to assess biting time and location (indoor vs outdoor) of the local vector population before and after spraying. HLC was done during the short rains pre-IRS in November 2016, and after the long rains in June 2017. Collections were performed at six sites used for routine surveillance, two in non-IRS areas and four IRS areas. In each site, five houses were randomly selected, and collections were performed for five consecutive nights in each house once before and after IRS.
During HLC, one volunteer sat outside within 5 meters from the house, and another sat inside the house in the living room. Collectors kept their trousers folded to knee length and aspirated any mosquitoes landing on their lower legs. Each house had a team of six collectors, each working in pairs during one of three six-hour shifts running from 17:00 to 11:00 the next morning. Collections were performed for 45 minutes, and the collectors rested for 15 minutes in each collection hour. The collectors recorded the location of members of the household observed at the end of each hour as either outdoor, in the living room, or in the bedroom. Collected mosquitoes were separated by time and location of collection and sustained on 10% sugar solution before being transported to the laboratory for analysis. Estimation of exposure of individuals to bites by An. funestus was performed using models previously described by Seyoum et al.37.
Persistence of insecticidal activity on sprayed walls
To assess the persistence of insecticidal activity on sprayed walls following IRS, WHO cone bioassays38 were conducted each month using laboratory-reared, 2–5 day old, non-blood fed susceptible colony of An. gambiae s.s. Kisumu strain. Mosquitoes were exposed in 10 randomly selected sprayed houses, seven with mud walls and three with cement walls, in each of four sub-counties in Migori county. Exposures were performed with ten mosquitoes per cone each month in the same houses at three different heights (0.5 m, 1 m, and 1.5 m) from the floor for 30 minutes, on three different walls of the living room of each sprayed house. A control cone with ten mosquitoes was set on an unsprayed plywood board outside of each sprayed house in a shaded area close to the house. Temperature and relative humidity were recorded at every house where mosquitoes were exposed.
Insecticide resistance monitoring
WHO insecticide susceptibility tests were performed in Rongo, Nyatike, Awendo and Uriri sub-counties in Migori County (IRS sites) and Homa Bay and Ndhiwa sub-counties in Homa Bay County (no IRS). Larval stages of An. gambiae s.l. were collected from Homa Bay, Ndhiwa, Rongo, Nyatike and Awendo sub-counties before IRS and in Homa Bay, Ndhiwa and Rongo after IRS. Larval samples were collected mainly from borrow pits, tire tracks, farmlands and trenches. The collected larvae were raised to three-day-old adults before testing. Adult An. funestus were also collected by hand aspiration inside houses for insecticide resistance tests, as larvae were difficult to find. Collections were performed in Homa Bay and Ndhiwa, Rongo, Awendo and Uriri sub-counties before IRS. However, after IRS few adult mosquitoes were in the sprayed sites, so no An. funestus s.l. were available for testing from these areas. CDC bottle intensity assay was used to assess intensity of resistance for An. arabiensis from Awendo, Ndhiwa and Homa Bay counties. Three-day-old adults raised from field-collected larvae were exposed in bottle coated with doses of 1X, 2X, 5X and 10X of permethrin. Survival frequency of An. arabiensis to the different doses was determined at 30 minutes exposure period.
Insecticide resistance status was assessed using the WHO diagnostic concentrations of deltamethrin (0.05%), permethrin (0.75%), pirimiphos-methyl (0.25%) and alpha-cypermethrin (0.05%). All papers were prepared by the WHO collaborating centre, Universiti Sains Malaysia. The WHO bioassay was done using 2- to 5-day-old An. gambiae s.l. emerging from collected larvae or by direct exposure of field-collected adult An. funestus since these were difficult to collect as larvae. At least 100 mosquitoes (four replicates of 25) of each species were exposed to each insecticide per sub-county. The samples were then transferred to a holding tube, provided with cotton wool soaked in 10% sugar solution and held for 24 hours. Mortality was scored 24 hours after exposure.
Mosquito species identification, sporozoite infection and blood meal identification
All Anopheles collected were identified morphologically to species using the keys of Gillies and DeMeillon or Gillies and Coetzee39,40. The physiological status was determined by observation of the abdomen to classify female mosquitoes as either blood-fed, gravid, half gravid or unfed. Female mosquitoes were dissected into three parts for various procedures: heads and thoraces were used for determination of Plasmodium falciparum sporozoite infection by enzyme-linked immunosorbent assay (ELISA) using the MR4 Methods in Anopheles Research adapted from Wirtz et al.41,42; the abdomens of blood-fed females were used to determine the source of mosquito blood meals by targeting cytochrome b protein using a multiplexed PCR protocol43, with slight modifications. The legs and wings were used in PCR analyses to identify to species level members of the An. gambiae species complex and Anopheles funestus group44. All mosquitoes morphologically identified as An. gambiae s.l. and 20% of randomly selected An. funestus s.l., were analyzed by PCR each month. This approach was done due to the greater number of An. funestus collected and based on previous studies in the area showing that An. gambiae and An. arabiensis are found in sympatry, while An. funestus s.s. was the only member of the species group routinely collected33,34.
Health facility surveillance
Health facility laboratory data were collected from Rongo, Uriri, and Macalder sub-county hospitals within Migori County (IRS) and Marindi health centre and Ndhiwa sub-County hospital in Homa Bay County (No IRS). The facilities were chosen based on proximity to entomological surveillance sites, availability of health records and catchment area as falling within either IRS or non-IRS area. Febrile cases were tested by health facility staff using light microscopy as part of routine health care and data were recorded in registers provided by the Kenya Ministry of Health. Data were abstracted from laboratory registers of the selected health facilities for the period from January 2015 until June 2018. Each page of the register was photographed using a smartphone camera, and the photographs converted to PDF files using CamScanner-Phone PDF creator, (INTSIG Information Co., Ltd). To ensure confidentiality, the column containing the patient’s name was covered when taking the photograph. The PDF copies were then printed and filed.
Data management and analysis
Field entomological data collection used Open Data Kit software (ODK) run on tablets with an interface designed to limit data entry errors. Data entry screens used drop-down menus and automatic data checks to reduce errors. Each house sampled received a unique code and a study number. Individual mosquitoes were placed in Eppendorf tubes labeled with pre-printed barcodes and linked to the field data by house code and a unique study number. Results of additional testing, including sporozoite ELISA, species identification by PCR and blood meal analysis, were linked to an individual mosquito by the unique barcode label. Individual patient records included date of testing, age, gender, village, clinical diagnosis, test performed, and test results from scanned copies of health facility registers. Data were entered into a Microsoft Access database.
Data analysis was performed using R statistical software version 3.4.1 or SAS version 9.4. The risk ratio (RR) was used to assess the statistical significance of differences in mosquito densities pre and post IRS, between intervention and non-intervention sites. Data were fitted using Generalized Linear Mixed Effects Statistical Models (GLMMs). Since the data were over-dispersed, we used the package Generalized Linear Mixed Models using Template Model Builder (glmmTMB) or PROC GLIMMIX, to fit negative binomial distribution models for the analysis of mosquito numbers. The numbers of female Anopheles mosquitoes were assessed as a function of the period of collection (before or after IRS) and intervention status (sprayed or non-sprayed) as a fixed effect, while village was treated as a random effect. To obtain the risk ratios (RR) and confidence intervals, we exponentiated the model coefficients. Models were adjusted for reported net use, the presence of open eaves, and the presence of cattle on the compound. A test of interaction was performed to compare differences in estimates of mosquito numbers between the period of mosquito collection and intervention status45. Conditional estimates of the change in mosquito densities pre- and post-IRS conditional on the IRS or non-IRS County were generated. To analyse Anopheles species proportions pre-and post-IRS in intervention and non-intervention areas for each trapping method, a binomial GLM model was used. The model was also used to analyse sporozoite rates (proportion of sporozoite ELISA tests that are positive) and human biting rates between intervention and non-intervention sites, before and after IRS and proportions of the types of mosquito host blood meals.
To detect changes in numbers of malaria cases before and after IRS within each health facility Auto-Regressive Integrated Moving Average (ARIMA) analysis was performed. Data from each facility was analysed using the “Time Series Analysis” (TSA)46 and “Alternative Time Series Analyses” (aTSA)47,48 packages in R. The ARIMA model was derived by observation of the autocorrelation and partial-autocorrelation functions to determine the most parsimonious solution of the “order” (p), “differencing” (d), and “moving-average” (q) parameter values. The model was then regressed on the absence (prior to) or presence of IRS in the village to estimate the value of the number of positive malaria cases prior to, and during the period of IRS.
Ethical considerations
The study was approved by the Kenya Medical Research Institute/ Scientific and Ethics Review Unit (KEMRI/SERU), number 2776 and by CDC through a reliance agreement with KEMRI/SERU (CDC IRB 6728). Individuals participating in HLC gave informed consent. They were screened for malaria before the start of the study and treated if positive. Collectors were placed on mefloquine malaria prophylaxis, (Mephaquin, Acino Pharma AG, Switzerland) one week before collections began, with repeat doses once every week through the collection period, until four weeks after collections ended. During routine mosquito collections, verbal consent was sought from the household head to use CDC-LT and PSC in their compound. All methods were performed in accordance with relevant guidelines and regulations.
Results
Vector species composition and seasonality
The mean number of An. funestus and An. gambiae. s.l. found in indoor CDC-LT and PSCs are presented by IRS status and period (pre- or post-IRS) in Table 1. The number of each species of mosquito collected by the two different methods was compared using negative binomial regression models incorporating IRS status, period, an interaction between IRS status and period, net use, the presence of open eaves and the presence of cattle on the compound (Supplemental Tables 1–4). For all models except for the An. gambiae s.l. collected by PSC, the interaction term was statistically significant indicating a differential effect of period based upon the IRS status. Conditional estimates of the effect of period controlling for IRS status are provided in Table 1.
The number of An. funestus collected in light traps in intervention sites were significantly lower in the post-IRS compared to pre-IRS period (RR = 0.12, 95% CI: 0.07–0.19, P < 0.001). No significant difference in the mean number of An. funestus was observed in the non-intervention sites between pre- and post-IRS (IRR = 0.98, 95% CI: 0.69–1.38, p = 0.899). A statistically significant difference-of-differences between the period of mosquito collection and intervention status was observed based on the statistically significant interaction term (RR = 0.12, 95% CI: 0.07–0.21) (Supplemental Table 1). From PSC collections, significantly fewer numbers of An. funestus were observed in both IRS and non-IRS sites in the post-IRS period compared to pre-IRS period (RR = 0.04, 95% CI: 0.02–0.07, p < 0.001). The number of An. funestus in the non-IRS area also declined but the conditional difference between pre-IRS and post-IRS was not statistically significant (RR = 0.64, 95% CI: 0.41–1.00, p = 0.052). A statistically significant difference-of-differences was observed between period of mosquito collection and intervention status post-IRS indicating a stronger decline in the IRS sites compared to the non-IRS sites (RR = 0.06, 95% CI: 0.03–0.13, p < 0.001) (Supplemental Table 2).
The mean numbers of An. arabiensis collected in indoor CDC-LTs in both intervention and non-intervention sites increased in the post-IRS compared to pre-IRS period with a statistically significant increase in the non-IRS sites (IRS sites: RR = 1.39, 95% CI: 0.78–2.47, p = 0.266; non-IRS sites: RR = 3.06, 95% CI: 1.59–5.92, p = 0.001). The conditional estimates are provided in Table 1 although the interaction term was not significant (RR = 0.45, 95% CI: 0.2–1.01, p = 0.052) (Table 1) indicating the increase was not statistically greater in the non-IRS sites than the IRS sites (Supplemental Table 3).
The mean numbers of An. arabiensis collected by PSC in the intervention sites were not significantly different in the post-IRS compared to pre-IRS period (RR = 0.60, 95% CI: 0.33–1.09, p = 0.093). For the non-IRS areas, the number of An. arabiensis collected by PSC increased although not significantly (RR = 1.64, 95% CI: 0.87–3.09, p = 0.123). Although no significant difference in the mean numbers of An. arabiensis was observed pre- and post-IRS in either the IRS or the non-IRS sites, a statistically significant difference-of-differences was observed between the period of mosquito collection and intervention status indicating a significant difference between the IRS and non-IRS areas after IRS implementation (RR = 0.36, 95% CI: 0.16–0.82, p = 0.015) (Supplemental Table 4).
The mean number of An. funestus collected by CDC-LT and PSC in both intervention and non-intervention areas varied by month, with the highest numbers collected during the short rainy season before IRS (Nov–Dec 2016) and during the long rainy season in the unsprayed area (March–June 2017) (Fig. 2). After IRS, the mean numbers collected by both CDC-LT and PSC in the intervention areas remained low, with no seasonal variation throughout the study period. The mean number of An. arabiensis collected by either method was lower compared to An. funestus with little monthly variation before and after IRS. No clear difference was observed in the seasonality of An. arabiensis before and after IRS (Fig. 2).
Mean number of observed female Anopheles (means ± std error) per trap-night per month in indoor CDC light trap and PSC before and after IRS in sprayed and unsprayed areas. The grey shaded area is the period post-spray with residual efficacy above 80%. The primary scale shows Anopheles density while the secondary scale shows rainfall in milliliters. Sixty trap-nights for CDC-LT and 30 for PSC per study arm per month. Standard errors were calculated by dividing the standard deviation by the square root of the sample size, a function was developed in R that generated the standard error around each mean.
Differences in Anopheles species composition were observed in intervention areas before and after IRS. In both CDC-LT and PSC collections, An. funestus comprised over 80% of the total Anopheles collected in both intervention and non-intervention sites before IRS. While An. funestus remained predominant in non-intervention sites after IRS, An. arabiensis formed the bulk of all Anopheles collected in the intervention sites after IRS (Fig. 3).
Insecticide decay rate and insecticide resistance monitoring
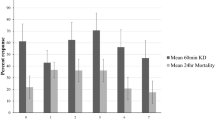
Mortality rates of susceptible An. gambiae s.s females were over 80% up to 10 months post-IRS across all study sites on both mud and concrete walls as measured by WHO cone assay (Supplemental Fig. 1). Using WHO tube bioassays both An. funestus (Supplemental Fig. 2A) and An. arabiensis (Supplemental Fig. 2B) before IRS and An. arabiensis after IRS (Supplemental Fig. 2C) were fully susceptible to pirimiphos-methyl and bendiocarb but resistant to the pyrethroids, deltamethrin, permethrin, and alpha-cypermethrin. Survival frequencies of An. arabiensis to different doses of permethrin varied between sites (Supplemental Fig. 3).
Factors affecting Anopheles numbers
Table 2 presents data showing modelled estimates of the effect of net use, open eaves, and presence of cattle in the compound on the indoor occurrence of An. funestus and An. arabiensis in sprayed and unsprayed houses, measured by CDC-LT and PSC collections. For An. funestus, significantly fewer were collected by light traps in houses with completely closed eaves (RR = 0.68, 95% CI: 0.48–0.96, p = 0.030) while significantly more were collected from houses where cattle were kept on the compound (RR = 1.62, 95%CI: 1.22–2.13, p = 0.001). No other comparisons were statistically significant. By PSC, there were again significantly more An. funestus collected in households where cattle were kept on the compound (RR = 1.63, 95% CI: 1.12–2.35, p = 0.010). There were significantly more An. funestus in houses where some but not all residents used a net the previous night compared to houses where no one used a net (RR = 2.02, 95% CI: 1.13–3.59, p = 0.017). No other comparisons were statistically significant.
From light trap collections, closed eaves were associated with significantly lower numbers of An. arabiensis (RR = 0.57, 95% CI: 0.33–0.96, p = 0.033) while significantly more An. arabiensis were collected in houses where some but not all residents of the household used a net the night before compared to houses where no one used a net (RR = 2.17, 95% CI: 1.02–4.62, p = 0.045). The number of An. arabiensis collected by PSC also was significantly lower in houses with closed eaves compared to those with open eaves (RR = 0.34, 95% CI: 0.18–0.67, p = 0.002). No other comparisons were statistically significant.
Sporozoite infection rates
Sporozoite infection rates in Anopheles mosquitoes were determined in intervention and non-intervention sites before and after IRS. Before IRS, 4.8% (48/1,000) of An. funestus were sporozoite positive in non-intervention sites compared to 2.2% (10/447) in the intervention sites whereas for An. arabiensis, sporozoite positivity rate was 2.8% (10/357) in non-intervention sites and 1.5% (3/192) in the intervention sites before IRS. Sporozoite infection rates for both species combined were not significantly different between intervention and non-intervention sites before IRS (RR = 0.27, 95% CI: 0.06–1.26, P = 0.09). After IRS, sporozoite infections were detected only in the non-intervention sites, where 3.5% (40/1,132) of An. funestus and 3.3% (22/643) of An. arabiensis were positive. (Fig. 4).
Vector biting behaviour
We estimated the exposure of humans to the risk of mosquito bites based on their observed behaviour and time and location of An. funestus biting. The numbers of An. arabiensis were insufficient to be included in the analysis. Over 70% of people within the study area were observed to be outdoors at 17:00, the beginning of mosquito collection. The number of people outdoors declined steadily over time with an increase in the number of individuals observed indoors, either in the living room (indoors not asleep) or in the bedroom (indoors and in bed). Over 90% of the people were observed to be indoors and in bed between 23:00 and 05:00 (Supplemental Fig. 4). In both intervention and non-intervention sites, before IRS, exposure to An. funestus was estimated to occur mostly, although not exclusively, indoors, late at night when people were asleep (Fig. 5a,b). In the post-IRS period, no change in the estimated exposure to bites by An. funestus was observed in the non-intervention sites (Fig. 5c). However, in the intervention sites, the risk of exposure to mosquito bites was nearly zero post-IRS (Fig. 5d). The relative proportion of bites by An. funestus increased both indoors and outdoors at dawn (05:00 am–08:00 am), corresponding to the time when most individuals woke up. Low levels of biting continued until 11:00 am when collection ceased.
Profiles of biting by An. funestus experienced by the human population in intervention and non-intervention sites before and after IRS. The black area represents biting that occurs outdoors, the dark red represents biting that occurs indoors when people are away from their bed nets and the blue represents biting that occurs while people are asleep.
Blood meal type
Blood meal analysis using mosquitoes collected by PSC was conducted on 236 fed Anopheles mosquitoes, 151 An. funestus and 85 An. arabiensis. An. funestus fed mostly on humans 52.3% (79/151), followed by cattle 40.4% (61/151), goat 3.3% (5/151), pig 0.7% (1/151) and mixed-blood meals 3.3% (5/151, 2 human/cow, 2 human/goat and 1 human/pig). An. arabiensis had fed mostly on cattle blood 70.6% (60/85), followed by pig 12.9% (11/85), human 9.4% (8/85), goat 4.7% (4/85) and mixed-blood meal, human/goat 1.9% (1/85) (Fig. 6).
Malaria case count
A total of 137,972 laboratory test records from patients attending the out-patient departments were extracted from the five health facilities. For the two-year period before IRS (January 2015–February 2017), malaria test positive proportions were similar at 33.2% (18,036/54,404) in intervention and 33.3% (12,920/38,835) in non-intervention sites respectively. For the post-IRS period (March 2017–May 2018), the test positivity rates were 30.4% (6,347/20,882) in the non-intervention sites and 20.6% (4905/23,851) in the intervention sites.
ARIMA analysis of malaria case counts for health facilities within IRS areas showed a reduction in malaria cases at the facilities post-IRS. Estimated mean monthly malaria cases in Rongo sub-county hospital dropped by 44% from 323 cases per month before IRS to 178 after IRS [mean difference = −142; 95% CI: −236 to −48; P = 0.003]. A similar reduction in mean monthly malaria cases with a 65.0% drop from 301 before IRS to 78 cases after IRS [mean difference = −196; 95% CI: −345 to −47; P = 0.01] was observed in Uriri sub-county hospital. In Nyatike sub-county hospital, the mean monthly malaria cases dropped by 47.4% from 118 cases before IRS to 72 after IRS [mean difference = −56; 95% CI: −123 to 11; P = 0.1]. For the two health facilities within non-IRS sites, no significant changes in malaria case counts were observed post-IRS, [Ndhiwa hospital: mean difference = −82; 95%CI: −230 to 65; P = 0.3; Marindi hospital: mean difference = 9.3; 95%CI: −132 to 151; = 0.9]. A plot of positive malaria cases over time, before and after IRS, showed a decline in the number of cases detected at facilities within sprayed areas compared to those in unsprayed regions (Fig. 7).
Discussion
Our results demonstrate a significant reduction in An. funestus indoor resting densities, biting rates and sporozoite infections, as well as a decline in malaria test positivity rates and case-count at health facilities after one round of Actellic 300CS IRS in Migori County, western Kenya. Human biting rates and sporozoite infections in Anopheles mosquitoes are the most direct entomological measures of malaria infection risk. We observed moderate biting and sporozoite rates in both intervention and non-intervention sites before IRS and the unsprayed sites after IRS. However, after IRS, An. funestus biting rates were nearly zero, and no sporozoite infections were detected post-IRS. Susceptibility tests confirmed that the major vector species, An. arabiensis and An. funestus, were both resistant to pyrethroid insecticides but were susceptible to pirimiphos-methyl, the active ingredient in Actellic 300CS.
Similar reductions in An. funestus populations to near elimination were observed in the Asembo Bay area of western Kenya following the scale-up of pyrethroid-treated nets5 although An. funestus later returned as the primary malaria vector in the region presumably due to the development of pyrethroid resistance34. The complete elimination of An. funestus following effective IRS campaigns have been reported in South Africa, Mauritius and the Pare/Taveta area of Tanzania and Kenya49,50. An. funestus is particularly sensitive to effective insecticides used in IRS, probably due to the highly endophilic and anthropophilic nature of the species51,52,53, leading to high levels of exposure to treated surfaces.
In contrast, An. arabiensis indoor resting densities, human-biting rates, and sporozoite infection rates all reduced only marginally in sprayed areas post-IRS. With the decline in An. funestus, An. arabiensis became the predominant vector species in the sprayed areas (although the densities of An. arabiensis did not increase). IRS has a limited impact on the population of An. arabiensis, despite full susceptibility to pirimiphos-methyl in WHO susceptibility tests. This lesser impact of IRS on An. arabiensis is therefore unlikely to be due to insecticide resistance but may be attributable to the behaviour of this species. Blood-meal host analysis showed that An. arabiensis fed more frequently on cattle than humans, unlike An. funestus that fed more frequently on humans. This finding is supported by a previous study in western Kenya that reported An. arabiensis fed predominantly on cattle (65% of blood meals on cattle; 22% mixed bovine/human; 13% human)8. Furthermore, results from a deterministic model, developed using data from Kilombero, Tanzania, suggested that An. arabiensis fed outdoors on both humans and cattle and rapidly exited houses without fatal exposure to insecticidal nets or IRS54. A recent trap evaluation in western Kenya observed over sevenfold more An. arabiensis collected by cow odour compared to human odour outdoor55. Therefore, it is likely that a significant population of An. arabiensis rests predominantly outdoors and feeds primarily on cattle, but occasionally bites humans and transmits malaria, albeit less efficiently than the more anthropophilic vector An. funestus. Therefore, it is possible that the population of An. arabiensis collected indoors by light traps and PSC represents only a proportion of a larger outdoor population. These factors may explain the lesser impact of IRS on An. arabiensis.
IRS with Actellic 300CS had a prolonged residual activity of at least ten months post-IRS, as measured by wall bioassays. As spraying was conducted in February, the insecticide provided protection throughout the periods of highest malaria transmission during the long (April–June) and short (October–November) rainy seasons. This long residual efficacy of Actellic 300CS makes the insecticide particularly useful in providing all-year-round protection with just one spray round each year. Similar prolonged residual activity of Actellic 300CS and control of pyrethroid-resistant mosquitoes have been reported in other countries32. As this is the first report of the efficacy of IRS with a non-pyrethroid insecticide in Kenya in the wake of pyrethroid resistance, strict adherence to the insecticide resistance management strategy27,28 is needed. The Kenya National Malaria Control Program (NMCP) has a responsibility to fast track registration of additional non-pyrethroid classes of insecticides to enable rotation of insecticide in IRS for effective resistance management.
This study presents three novel findings that are crucial for malaria control and elimination in Kenya. Our study demonstrated that a single spray-round with Actellic 300CS is effective for year-round control of malaria in the region. Well-timed IRS, just before the peak malaria transmission, suppressed the local malaria vector populations through both the long and short rain periods, hence reducing disease transmission even during the peak transmission seasons that coincide with the long and short rain seasons. Second, the impact of IRS on An. arabiensis was less than that on other vectors, making it the dominant malaria vector sustaining residual transmission. While IRS was effective in controlling An. funestus, An. arabiensis would require alternative control approaches for malaria elimination. Finally, the study demonstrates late morning biting by An. funestus, a phenomenon not before investigated in the region. This presents a new challenge facing vector control given that the tools currently deployed for vector control are limited to the household and mostly only to the times when people are asleep (LLINs).
Biting by An. funestus in the intervention and non-intervention areas before IRS and non-intervention areas after IRS occurred mostly indoors late at night corresponding to the period when most people were indoors and in bed. Late night, indoor biting by An. funestus has been previously reported, dating back to the pre-bed net era. For instance, in 1975, 94% of An. funestus were observed to bite after midnight, with another a peak in the hours before dawn in Kano plain of western Kenya56. Similar late night, indoor biting was observed in the same study area in 199653 and more recently the vector species have been reported to persistently bite indoors, late in the night despite high coverage in insecticide-treated nets52. While all these studies observed a biting peak at dawn, the collections ceased at 07:00 hours. With extended collections to monitor An. funestus biting in the morning, we observed biting until 11:00 hours. Similar findings of day-biting An. funestus in the presence of LLINs have been recently reported in Senegal9 and Benin57. This observed extended biting behaviour in An. funestus not previously investigated may potentially undermine the effectiveness of LLINs as people may be exposed to mosquito bites while away from the protection of their bed nets. However, one round of IRS with Actellic 300CS substantially reduced the number of An. funestus collected and it was not possible to detect biting either indoors or outdoors in the sprayed areas post-IRS.
In an analysis of risk factors associated with the indoor occurrence of mosquitoes, fewer An. funestus and An. arabiensis were collected by light traps in houses with closed eaves. Similar results were observed for An. arabiensis collected by PSC. Open eaves are known to be the main route for indoor entry of Anopheles mosquitoes11,58 and blocking them has been demonstrated to be effective in preventing Anopheles house entry59. Closing eave spaces or deploying vector control tools in these spaces may present an additional intervention to the current vector control tool kit for reducing the indoor occurrence of mosquitoes in addition to IRS and LLINs. However, new tools targeting outdoor Anopheles populations, in particular, An. arabiensis, are needed to control vector species that are less well controlled by interventions that target the indoor environment such as IRS and LLINs.
Reductions in malaria cases at the health facilities within sprayed areas post-IRS provided further evidence of the impact of a single round of IRS on malaria transmission. Health facility-based surveys of malaria cases in febrile patients have been useful as part of rapid analysis of changes in local malaria epidemiology60,61,62,63. Malaria infection is highly correlated with febrile cases reported at the health facilities62. Furthermore, a systematic review of febrile illness over 20 years in sub-Saharan Africa reported a dramatic reduction in the proportion of fevers associated with Plasmodium falciparum malaria64. Consequently, reductions in malaria cases likely contribute considerably to the reductions in febrile illnesses presenting at health facilities. The use of routine Health Management Information System (HMIS) data to evaluate malaria control interventions63, however, suffers from incompleteness in reporting and variation in the utilization of the health system65. We extracted data from the primary records, health facility laboratory registers and observed a reduction in confirmed malaria cases in health facilities within sprayed areas post-IRS, with no change in the number of cases detected at the facilities in control regions. Reduction in An. funestus densities, sporozoite rates and man-biting rates coupled with reduced malaria cases following one round IRS with pirimiphos-methyl provide compelling evidence of the effectiveness of IRS in malaria transmission reduction when implemented with an effective insecticide to which mosquito populations are susceptible.
This study compared entomological indices and malaria test positivity rates between one district receiving IRS in addition to LLINs and the neighboring districts receiving only LLINs. This approach was driven by the fact that it was an effectiveness study rather than a highly controlled study to assess efficacy. However, the study does raise questions about the optimal approach to evaluate vector control interventions such as IRS or larval source management which target population-wide suppression of malaria vectors and where the scale of the study may affect the magnitude with which the results can be generalized. While cluster randomized trials might be ideal for the evaluation of these tools, the flight range of mosquitoes should be accounted for as migration from outside clusters and/or spill-over effects from intervention clusters into control clusters may bias results towards the null66. However, larger clusters, in addition to being logistically unfeasible, may result in clusters that are spatially distant and less similar which may also bias the results. In this study, the non-random selection of areas for IRS, the low number of clusters and their spatial distance are a potential limitation of the study. However, the before and after design coupled with multiple endpoints indicating similar trends in mosquito densities and malaria-related outcomes strongly suggest that the results are robust.
Conclusion
IRS with pirimiphos-methyl was highly effective for the control of indoor biting and indoor resting, pyrethroid-resistant An. funestus and resulted in substantially reduced numbers of this primary vector species coupled with reduced malaria cases. Due to the long residual effect of pirimiphos-methyl, it was possible to achieve year-round protection with a single round of IRS. Sustaining these gains is a priority for the Kenya NMCP and development partners and IRS should continue to be implemented to sustain the impact on An. funestus. However, there was less of an impact of spraying on An. arabiensis populations, likely due to their exophilic nature. Additional control measures are needed to control outdoor biting and resting An. arabiensis.
Data availability
All data generated or analyzed during this study are included in this published article and its Supplementary Information files.
References
Bhatt, S. et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 526, 207–211, https://doi.org/10.1038/nature15535 (2015).
Pluess, B., Tanser, F. C., Lengeler, C. & Sharp, B. L. Indoor residual spraying for preventing malaria. Cochrane Database Syst Rev, CD006657, https://doi.org/10.1002/14651858.CD006657.pub2 (2010).
Protopopoff, N. et al. Combination of Insecticide Treated Nets and Indoor Residual Spraying in Northern Tanzania Provides Additional Reduction in Vector Population Density and Malaria Transmission Rates Compared to Insecticide Treated Nets Alone: A Randomised Control Trial. PloS one 10, e0142671, https://doi.org/10.1371/journal.pone.0142671 (2015).
Protopopoff, N. et al. Effectiveness of a long-lasting piperonyl butoxide-treated insecticidal net and indoor residual spray interventions, separately and together, against malaria transmitted by pyrethroid-resistant mosquitoes: a cluster, randomised controlled, two-by-two factorial design trial. The Lancet 391, 1577–1588, https://doi.org/10.1016/s0140-6736(18)30427-6 (2018).
Gimnig, J. E. et al. Effect of permethrin-treated bed nets on the spatial distribution of malaria vectors in western Kenya. American Journal of Tropical Medicine and Hygiene 68, 115–120 (2003).
Gimnig, J. E. et al. Impact of permethrin-treated bed nets on entomologic indices in an area of intense year-round malaria transmission. American Journal of Tropical Medicine and Hygiene 68, 16–22 (2003).
Mbogo, C. N., Baya, N. M., Ofulla, A. V., Githure, J. I. & Snow, R. W. The impact of permethrin-impregnated bednets on malaria vectors of the Kenyan coast. Medical Veterinary Entomology 10, 251–259 (1996).
Bayoh, M. N. et al. Anopheles gambiae: historical population decline associated with regional distribution of insecticide-treated bed nets in western Nyanza Province, Kenya. Malaria journal 9, 62, https://doi.org/10.1186/1475-2875-9-62 (2010).
Sougoufara, S. et al. Biting by Anopheles funestus in broad daylight after use of long-lasting insecticidal nets: a new challenge to malaria elimination. Malaria journal 13, 125, https://doi.org/10.1186/1475-2875-13-125 (2014).
Ototo, E. N. et al. Surveillance of malaria vector population density and biting behaviour in western Kenya. Malaria journal 14, 244, https://doi.org/10.1186/s12936-015-0763-7 (2015).
Takken, W. Do insecticide-treated bednets have an effect on malaria vectors? Tropical Medicine & International Health 7, 1022–1030 (2002).
Ndenga, B. A. et al. Malaria vectors and their blood-meal sources in an area of high bed net ownership in the western Kenya highlands. Malaria journal 15, 76, https://doi.org/10.1186/s12936-016-1115-y (2016).
Mathenge, E. M. et al. Effect of permethrin-impregnated nets on exiting behavior, blood feeding success, and time of feeding of malaria mosquitoes (Diptera: Culicidae) in western Kenya. J Med Entomol 38, 531–536 (2001).
KMIS. Kenya Malaria Indicator Survey (National Malaria Control Programe, Kenya Ministry of Heath, Nairobi, Kenya, 2015).
Noor, A. M. et al. The risks of malaria infection in Kenya in 2009. BMC Infectious Diseases 9, 180, https://doi.org/10.1186/1471-2334-9-180 (2009).
Wesolowski, A. et al. Quantifying the impact of human mobility on malaria. Science 338, 267–270, https://doi.org/10.1126/science.1223467 (2012).
Nyunt, M. H. et al. Challenges in universal coverage and utilization of insecticide-treated bed nets in migrant plantation workers in Myanmar. Malaria journal 13 (2014).
Atieli, H. E. et al. Insecticide-treated net (ITN) ownership, usage, and malaria transmission in the highlands of western Kenya. Parasites & vectors 4 (2011).
WHO. World Malaria Report. (WHO, Geneva, 2018).
Ochomo, E. O. et al. The efficacy of long-lasting nets with declining physical integrity may be compromised in areas with high levels of pyrethroid resistance. Malaria journal 12, 368, https://doi.org/10.1186/1475-2875-12-368 (2013).
Ochomo, E. et al. Presence of the knockdown resistance mutation, Vgsc-1014F in Anopheles gambiae and An. arabiensis in western Kenya. Parasites & vectors 8, 616, https://doi.org/10.1186/s13071-015-1223-5 (2015).
Ochomo, E. et al. Pyrethroid resistance in Anopheles gambiae s.s. and Anopheles arabiensis in western Kenya: phenotypic, metabolic and target site characterizations of three populations. Medical and veterinary entomology 27, 156–164, https://doi.org/10.1111/j.1365-2915.2012.01039.x (2013).
Ochomo, E. et al. Pyrethroid susceptibility of malaria vectors in four Districts of western Kenya. Parasit Vectors. 4, 310 (2014).
KMIS. Kenya Malaria Indicator Survey. (Division of Malaria Control, Ministry of Public Health and Sanitation, Nairobi, Kenya, 2010).
Hamel, M. J. et al. The combination of indoor residual spraying and insecticide-treated nets provides added protection against malaria compared with insecticide-treated nets alone. American Journal of Tropical Medicine and Hygiene 85, 1080–1086, https://doi.org/10.4269/ajtmh.2011.10-0684 (2011).
Gimnig, J. E. et al. The Effect of Indoor Residual Spraying on the Prevalence of Malaria Parasite Infection, Clinical Malaria and Anemia in an Area of Perennial Transmission and Moderate Coverage of Insecticide Treated Nets in Western Kenya. PloS one 11, e0145282, https://doi.org/10.1371/journal.pone.0145282 (2016).
NMCP. (ed Ministry of Health) (Nairobi, Kenya, 2015).
WHO. Global Plan For Insecticide Resistance Management In Malaria Vectors. (World Health Organization, Geneva, Switzerland, 2012).
Rowland, M. et al. A New Long-Lasting Indoor Residual Formulation of the Organophosphate Insecticide Pirimiphos Methyl for Prolonged Control of Pyrethroid-Resistant Mosquitoes: An Experimental Hut Trial in Benin. PloS one 8, 10.1371/ (2013).
Mashauri, F. M. et al. Indoor residual spraying with micro-encapsulated pirimiphos-methyl (Actellic(R) 300CS) against malaria vectors in the Lake Victoria basin, Tanzania. PloS one 12, e0176982, https://doi.org/10.1371/journal.pone.0176982 (2017).
Chanda, E. et al. Preventing malaria transmission by indoor residual spraying in Malawi: grappling with the challenge of uncertain sustainability. Malaria journal 14, 254, https://doi.org/10.1186/s12936-015-0759-3 (2015).
Haji, K. A. et al. Efficacy, persistence and vector susceptibility to pirimiphos-methyl (Actellic 300CS) insecticide for indoor residual spraying in Zanzibar. Parasites & vectors 8, 628, https://doi.org/10.1186/s13071-015-1239-x (2015).
Okara, R. M. et al. Distribution of the main malaria vectors in Kenya. Malaria journal 9, 69, https://doi.org/10.1186/1475-2875-9-69 (2010).
McCann, R. S. et al. Reemergence of Anopheles funestus as a vector of Plasmodium falciparum in western Kenya after long-term implementation of insecticide-treated bed nets. American Journal of Tropical Medicine and Hygiene 90, 597–604, https://doi.org/10.4269/ajtmh.13-0614 (2014).
Ondeto, B. M. et al. Current status of insecticide resistance among malaria vectors in Kenya. Parasites & vectors 10, 429, https://doi.org/10.1186/s13071-017-2361-8 (2017).
AIRS. Kenya End of Spray Report. (Abt Associates Inc, Bethesda, MD, May 2017).
Seyoum, A. et al. Human exposure to anopheline mosquitoes occurs primarily indoors, even for users of insecticide-treated nets in Luangwa Valley, South-east Zambia. Parasites & vectors 5 (2012).
WHO. Guidelines for testing mosquito adulticides for indoor residual spraying and treatment of mosquito nets (Worl Health Organization, Geneva, Switzerland, 2006).
Gillies, M. T. & Coetzee, M. A supplement to the Anophelinae of Africa South of the Sahara (Afrotropical region). (Johannesburg: South Africa Medical Resaerch Institute, 1987).
Gillies, M. T. & De Meillon, B. The Anophelinae of Africa, South of the Sahara (Ethopian zoogeographical region). 1968:1954 (Johannesburg: South African Institute for Madical Research; 1968).
Wirtz, R. A., Burkot, T. R., Graves, P. M. & Andre, R. G. Field evaluation of enzyme-linked immunosorbent assays for Plasmodiumfalciparum and Plasmodium vivax sporozoites in mosquitoes (Diptera: Culicidae) from Papua New Guinea. Journal of Medical Entomology 24, 433–437 (1987).
MR4. Methods in Anopheles Research. Vol. Chapter 5 267–269 (Mark Q. Benedict CDC, 2015).
Kent, R. J. & Norris, D. E. Identification of mammalian blood meals in mosquitoes by a multiplexed polymerase chain reaction targeting cytochrome B. The American journal of tropical medicine and hygiene 73, 336–342 (2005).
Scott, J. A., Brogdon, W. G. & Collins, F. H. Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. American Journal of Tropical Medicine and Hygiene 49, 520–529 (1993).
Altman, D. G. & Bland, J. M. Interaction revisited: the difference between two estimates. BMJ 326, 219, https://doi.org/10.1136/bmj.326.7382.219 (2003).
Team, R. C. (ed. R Foundation for Statistical Computing) (Vienna, Austria, 2018).
Chan, K.-S. & Ripley, B. TSA: Time Series Analysis. R package version 1.2 (2018).
Qiu, D. aTSA: Alternative Time Series Analysis. R package version 3.1.2 (2015).
Smith, A. & Draper, C. C. Malaria in the Taveta area of Kenya and Tanganyika. Part I. Epidemiology. East Africa Medical Journal 36, 99 (1959).
Bruce-Chwatt, L. J. & Gockel, C. W. A study of blood feeding patterns of Anopheles mosquitoes through precipitin test. Bull. Wld. Hlth. Org 22, 685 (1960).
Githeko, A. K., Service, M. W., Mbogo, C. M., Atieli, F. K. & Juma, F. O. Origin of blood meals in indoor and outdoor resting malaria vectors in western Kenya. Acta tropica 58, 307–316 (1994).
Bayoh, M. N. et al. Persistently high estimates of late night, indoor exposure to malaria vectors despite high coverage of insecticide treated nets. Parasites & vectors 7, 380, https://doi.org/10.1186/1756-3305-7-380 (2014).
Githeko, A. K. et al. Some observations on the biting behavior of Anopheles gambiae s.s., Anopheles arabiensis, and Anopheles funestus and their implications for malaria control. Exp Parasitol 82, 306–315, https://doi.org/10.1006/expr.1996.0038 (1996).
Killeen, G. F., Govella, N. J., Lwetoijera, D. W. & Okumu, F. O. Most outdoor malaria transmission by behaviourally-resistant Anopheles arabiensis is mediated by mosquitoes that have previously been inside houses. Malaria journal 15, 225, https://doi.org/10.1186/s12936-016-1280-z (2016).
Abong’o, B. et al. Host Decoy Trap (HDT) with cattle odour is highly effective for collection of exophagic malaria vectors. Parasites & vectors 11, https://doi.org/10.1186/s13071-018-3099-7 (2018).
Chandler, J. A., Highton, R. B. & Hill, M. N. Mosquitoes of the Kano Plain, Kenya. I. Results of indoor collections in irrigated and nonirrigated areas using human bait and light traps. J Med Entomol 12, 504–510 (1975).
Moiroux, N. et al. Human exposure to early morning Anopheles funestus biting behavior and personal protection provided by long-lasting insecticidal nets. PloS one 9, e104967, https://doi.org/10.1371/journal.pone.0104967 (2014).
Lindsay, S. W. & Snow, R. W. The trouble with eaves; house entry by vectors of malaria. Trans R Soc Trop Med Hyg 82, 645–646 (1988).
Lindsay, S. W., Emerson, M. P. & D., C. J. Reducing malari a by mosquito-proofing houses. Trends in Parasitology 18, 510–514 (2002).
Oduro, A. R. et al. Monitoring malaria using health facility based surveys: challenges and limitations. BMC Public Health 16, 354, https://doi.org/10.1186/s12889-016-2858-7 (2016).
Githinji, S. et al. A national health facility survey of malaria infection among febrile patients in Kenya, 2014. Malaria journal 15, 591, https://doi.org/10.1186/s12936-016-1638-2 (2016).
Macedo de Oliveira, A. et al. Prevalence of malaria among patients attending public health facilities in Maputo City, Mozambique. The American journal of tropical medicine and hygiene 85, 1002–1007, https://doi.org/10.4269/ajtmh.2011.11-0365 (2011).
Ashton, R. A. et al. Use of Routine Health Information System Data to Evaluate Impact of Malaria Control Interventions in Zanzibar, Tanzania from 2000 to 2015. EClinicalMedicine, https://doi.org/10.1016/j.eclinm.2019.05.011 (2019).
D’Acremont, V., Lengeler, C. & Genton, B. Reduction in the proportion of fevers associated with Plasmodium falciparum parasitaemia in Africa: a systematic review. Malaria journal 9, 240, https://doi.org/10.1186/1475-2875-9-240 (2010).
Cibulskis, R. E. et al. Estimating trends in the burden of malaria at country level. The American journal of tropical medicine and hygiene 77, 133–137 (2007).
Hawley, W. A. et al. Community-wide effects of permethrin-treated bed nets on child mortality and malaria morbidity in western Kenya. The American journal of tropical medicine and hygiene 68, 121–127 (2003).
Acknowledgements
We would like to thank the residents of Migori and Homa Bay Counties from whose houses we conducted mosquito sampling. We also thank the County Health Management Teams in Migori and Homa Bay counties and health facility management for Uriri, Rongo, Sori Karungu and Nytike, Ndhiwa and Marindi health facilities for access to the hospital laboratory health records. Kate Zinszer provided invaluable help with the ARIMA analyses. Lastly, we thank the PMI-VectorLink Kenya project staff, Alexia Mshambala for participation in health facility surveillance and entomology staff who conducted mosquito collection and sample analysis, especially, Benjamin Oloo, Tobias Odongo, Amos Wabwile, Celestine Wekesa, and Mercy Nduta. The study was supported by the United States President’s Malaria Initiative through the United States Agency for International Development Africa Indoor Residual Spraying Project. The findings and conclusions in this report do not necessarily reflect the official position of the U.S. Department of Health and Human Services or the U.S. Centers for Disease Control and Prevention. The use of trade names is for identification only and does not imply endorsement by the U.S. Department of Health and Human Services or the U.S. Centers for Disease Control and Prevention.
Author information
Authors and Affiliations
Contributions
B.A., J.E.G., B.L. and R.M.O. wrote the protocol and designed the study. B.A., D.O., M.M. and R.M.O., participated in data collection and sample analysis. J.E.G., S.J.T., F.K., A.M.S., M.J.D., R.T.P., S.M., K.N. and R.M.O., provided technical oversight during the study. B.A., J.E.G., M.M., F.K., J.M. and M.J.D., analyzed the data and prepared manuscript figures. B.A., J.E.G. M.J.D. and R.M.O. wrote the manuscript. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abong’o, B., Gimnig, J.E., Torr, S.J. et al. Impact of indoor residual spraying with pirimiphos-methyl (Actellic 300CS) on entomological indicators of transmission and malaria case burden in Migori County, western Kenya. Sci Rep 10, 4518 (2020). https://doi.org/10.1038/s41598-020-61350-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-61350-2
This article is cited by
-
Early morning anopheline mosquito biting, a potential driver of malaria transmission in Busia County, western Kenya
Malaria Journal (2024)
-
Mosquito odour-baited mass trapping reduced malaria transmission intensity: a result from a controlled before-and-after intervention study
BMC Medicine (2024)
-
Malaria transmission heterogeneity in different eco-epidemiological areas of western Kenya: a region-wide observational and risk classification study for adaptive intervention planning
Malaria Journal (2024)
-
Comparison of different trapping methods to collect malaria vectors indoors and outdoors in western Kenya
Malaria Journal (2024)
-
Advances in the genetic characterization of the malaria vector, Anopheles funestus, and implications for improved surveillance and control
Malaria Journal (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.