Abstract
Single substances within complex vertebrate chemical signals could be physiologically or behaviourally active. However, the vast diversity in chemical structure, physical properties and molecular size of semiochemicals makes identifying pheromonally active compounds no easy task. Here, we identified two volatile cyclic dipeptides, cyclo(L-Leu-L-Pro) and cyclo(L-Pro-L-Pro), from the complex mixture of a chemical signal in terrestrial vertebrates (lizard genus Sceloporus), synthesised one of them and investigated their biological activity in male intra-specific communication. In a series of behavioural trials, lizards performed more chemosensory behaviour (tongue flicks, lip smacks and substrate lickings) when presented with the synthesised cyclo(L-Pro-L-Pro) chemical blend, compared to the controls, the cyclo(L-Leu-L-Pro) blend, or a combined blend with both cyclic dipeptides. The results suggest a potential semiochemical role of cyclo(L-Pro-L-Pro) and a modulating effect of cyclo(L-Leu-L-Pro) that may depend on the relative concentration of both compounds in the chemical signal. In addition, our results stress how minor compounds in complex mixtures can produce a meaningful behavioural response, how small differences in structural design are crucial for biological activity, and highlight the need for more studies to determine the complete functional landscape of biologically relevant compounds.
Similar content being viewed by others
Introduction
Chemical signals of terrestrial vertebrates tend to be complex mixtures of compounds1. However, this does not necessarily mean that numerous compounds are always needed for recognition by a signal receiver (e.g.2,3,4). Single compounds, or even a selected profile from all mixture components, could be physiologically or behaviourally active in different contexts5,6,7,8. Intra-specific chemical signals, often liberally referred to as “pheromones” in the extensive literature, can vary considerably in their chemical structure, physical properties and molecular size9, and there is currently no simple way to rule out the biological roles of additional mixture components. For example, even in an extensively studied model system such as the house mouse, the biological roles of volatile ligands, compared to the lipocalin proteins that are involved in different chemosensory functions10,11,12,13, are relatively unknown. Using an interdisciplinary approach, here we characterise two volatile cyclic dipeptides from the complex mixture of a chemical signal in terrestrial vertebrates (lizard genus Sceloporus) and investigate their biological activity in intra-specific communication.
The structural diversity of compounds documented in terrestrial vertebrates is enormous14, and it has been difficult to associate specific structural designs or features with chemical signalling in general1. It has been somewhat useful to divide potential chemosignals according to their volatility: while volatile pheromones can act in longer distance signalling, protein-like molecules and other highly polar substances with very low vapour pressure (e.g. polypeptides) require direct contact between the receiver’s chemosensory structures and the signaller or their scent marks15. Similar considerations may apply to kairomones in predator-prey communication5. One common feature among some proven or putative volatile pheromonal ligands is the incorporation of nitrogen atoms into their structures4,16,17,18,19. However, other structurally diverse volatile chemosignals have been documented (for review, see1,11,14), all pertaining to terrestrial vertebrates and their thus far known semiochemistry, and there is still much to be learned about how chemical structures relate to biological function.
There are two entirely different strategies to identify the physiologically and behaviourally active components of highly complex mixtures sampled from vertebrates: (i) the response-guided strategy and (ii) the chemical image strategy20. In the first strategy, the stimulus mixture (e.g. glandular extract) is subjected to isolation and fractionation, each followed with a bioassay, until the isolated chemical compound is structurally identified and ultimately proven as biologically active. The chemical image strategy relies on the capability to cover an entire profile of substances, assuming that many (if not all) profile constituents are involved in the complete biological response. The first strategy has particularly been fruitful in relatively simple cases such as insects21, while the chemical image strategy implies that enormous complexity is associated with a complete behavioural or physiological response. The downside of the response-guided strategy is that repeated fractionation of a complex stimulus-containing mixture can lead to a loss of biological activity if more than one component is needed for a robust biological response. Additionally, this approach can be procedurally tedious. From a chemist’s perspective, looking for structurally unusual compounds that consistently appear in a complex profile of substances, rather than systematically testing each and every compound, can sometimes be profitable. As we demonstrate in this study, two structurally unique compounds in a chemical mixture were positively identified from the femoral gland secretions of Sceloporus virgatus lizards through their mass-spectral (MS) data and a capillary gas chromatography-mass spectrometry (GC-MS) profiling technique. These mixture constituents, tentatively identified as “heterocyclic compounds” when first discovered22, we now report are cyclic dipeptides (Fig. 1), whose relative hydrophobicity imparts sufficient volatility to act as longer-range chemosignals.
Cyclic dipeptides, which can be classified structurally as diketopiperazines or pyrazine derivatives, have received considerable attention in recent years due to their structural stability and significant pharmacological potential related to their reported bioactivity as antibacterial, antifungal and antiviral agents23, but are hardly known in semiochemical roles. In nature, they are predominantly synthesised by microorganisms24. In animals, enzymatic pathways for production of cyclic dipeptides have been reported for the annelid worm Platynereis dumerolii25 and for the starlet sea anemone Nematostella vectensis26. Pyrazines of low molecular weights such as alkylated or alkoxylated derivatives are ubiquitous in nature. They are highly odoriferous, and not surprisingly, involved in signalling as insect alarm pheromones27. Another pyrazine derivative, 2,5-dimethylpyrazine, has been identified as a key component of the puberty-delaying pheromone of female mice4,28 and as a behaviourally relevant compound of the scent signals of male tree-shrews29. Moreover, different pyrazines are speculated to act as “classical alerting signals functioning as deterrents or attractants”30.
Among vertebrates, reptiles possess a highly developed olfactory system, characterised by the presence of the vomeronasal organ (VNO), a specialised sensory organ for processing semiochemicals31. The chemosensory lives of reptiles are very rich, as they use chemical cues and signals for foraging, social and spatial organization, species and sex recognition, and reproductive behaviour32,33,34,35, and thus chemical communication can importantly affect their fitness. One of the main sources of chemical cues in lizards are their femoral glands (FG), whose secretions are deposited on substrates as lizards move, both passively and actively36,37. The chemical components of femoral gland secretions (FGS), a mix of lipids and proteins, potentially serve different biological roles, not only as chemical signalling compounds, but also as structural stabilisers, antioxidants or signal enhancers33,38,39,40,41, yet the functions of individual compounds identified in lizard glandular secretions remain largely unknown (but see42,43).
Species of the large genus Sceloporus (90 + species44) are characterised by the presence of a row of femoral pores along each of their inner thighs that exude femoral gland secretions. As in many lizards34, both male and female Sceloporus use these secretions to signal individual and species identity, sex, and physiological state36,38, although males produce secretions more abundantly with peak production during the breeding season36,45. Earlier reports on FGS of Sceloporus list proteins, sterols and some other fairly common volatile organic compounds as part of their composition22,39,46. While studying evolutionary interactions between visual and chemical signals in males of four Sceloporus species, namely S. cozumelae, S. parvus, S. siniferus, and S. merriami22, we observed a number of carboxylic acids and steroids together with a series of structurally unidentified “heterocyclic compounds” with no known function. These heterocyclic compounds found in all four investigated Sceloporus species are the cyclic dipeptides cyclo(L-Leu-L-Pro) 1 and cyclo(L-Pro-L-Pro) 2 (Fig. 1), which can be chemically classified as diketopiperazines. We have now identified these compounds in an additional species, the lizard S. virgatus, and provided the synthetic analogues, one commercial and one in-house synthesized analogue, of the identified cyclic dipeptides to (i) authenticate the presumed cyclic dipeptide mixture components; and (ii) supply sufficient amounts for testing their potential biological role in intra-specific communication in a series of behavioural trials.
Results
Chemical composition of femoral gland secretions (FGS)
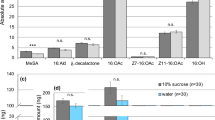
We identified compounds by comparing mass spectra and retention times against reference compound spectra and the National Institute of Standards and Technology (NIST) database. Samples and standard compounds were analysed by scanning the MS total ion chromatograms (TICs) in the mass range between 40–350 amu using the positive electron ionization (EI) mode as described in Pruett et al.22. After the MS recording, we extracted selective-ion currents from TICs using appropriate m/z ions as filters to obtain selected-ion chromatograms (SICs) where we measured the peak areas to compare compound abundances. The SIC peak areas were divided by the peak area of the internal standard peak area (SIC m/z 113) and by the sample weight (mg) in each sample to obtain normalised data values per weight. A total of 24 volatile compounds assigned to 8 different chemical classes were identified in the lipophilic fraction of FGS of adult male S. virgatus (Table 1). Short-chain fatty acids were the most abundant constituents of FGS (81.5%) and we confirmed the presence of the two volatile cyclic dipeptides in this species, cyclic dipeptide 1, cyclo(L-Leu-L-Pro), and cyclic dipeptide 2, cyclo(L-Pro-L-Pro), as shown in extracted m/z 70 ion currents (Fig. 2). Cyclic dipeptides 1 and 2 were not fully resolved in S. virgatus samples and we estimated peak areas using an integration approach (Fig. S1). These cyclic dipeptides were present at lower quantities than those found in congener lizard species, e.g. S. merriami22 (Figs. 2 and 3) and, overall, cyclic dipeptides were the least abundant class of compounds in FGS of S. virgatus (~0.1%). Generally, cyclic dipeptide 2 appeared in higher concentrations than cyclic dipeptide 1 in all FGS samples.
Post-run selected ion chromatogram (SIC), with m/z 70, from the lizard femoral gland extract of S. virgatus (A), S. merriami (B) and the reference standard compounds cyclic dipeptide 1 (C), and cyclic dipeptide 2 (D). Cyclic dipeptide 1, identified as cyclo(L-Leu-L-Pro), with retention time (Rt) 47.99 min and cyclic dipeptide 2, identified as cyclo(L-Pro-L-Pro), with Rt 48.12 min, are not fully resolved in S. virgatus but exhibit characteristic mass spectra as seen in other congeners, e.g. S. merriami22 (B)—shown here for comparison purposes—where they occur at higher concentrations. Peak areas for cyclic dipeptide 1 were 0.09 × 106 and 0.26 × 106 for S. virgatus and S. merriami, respectively. Peak areas for cyclic dipeptide 2 were 0.27 × 106 and 1.1 × 106 for S. virgatus and S. merriami, respectively.
Biological activity of cyclic dipeptides
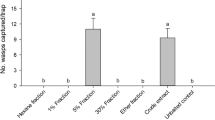
Chemosensory behaviour of S. virgatus differed among treatments during behavioural trials (Χ24 = 15.08, P = 0.045). Lizards performed more tongue flicks, lip smacks and substrate lickings when presented with the synthesised cyclic dipeptide 2 (CDP 2) compared to the blank control (BC; coefficient estimate ± S.E.: 0.51 ± 0.16, Z = −3.28, P = 0.001), the matrix control (MC: 0.35 ± 0.15, Z = −2.38, P = 0.017), the cyclic dipeptide 1 (CDP 1: 0.42 ± 0.15, Z = −2.79, P = 0.005), or the combined blend of CDP1 and CDP2 (CDP1 + CDP2: 0.48 ± 0.15, Z = −3.12, P = 0.002) (Fig. 4). However, we found no differences between spontaneous chemosensory behaviour in the presence of an unscented pebble and the chemosensory behaviour elicited by MC (Z = 0.94, P = 0.347), CDP1 (Z = 0.18, P = 0.859) or CDP1 + CDP2 (Z = 0.52, P = 0.601) (Fig. 4).
Chemosensory behaviour (number of tongue flicks, lip smacks, and substrate lickings of the pebble) of 20 male S. virgatus in response to a blank control (BC; an unscented pebble) and each of four different chemical blends: MC: matrix control; CDP 1: cyclic dipeptide 1, cyclo(L-Leu-L-Pro); CDP 2: synthesised cyclic dipeptide 2, cyclo(L-Pro-L-Pro); CDP1 + CDP2: a blend of CDP1 and CDP2 in equal amount. All blends included a matrix of the three most common saturated fatty acids in Sceloporus, in representative proportions, an acetone carrier and a non-volatile binding agent (PEG; see Methods). Shown are means ± 1 S.E. Different letters denote significantly different groups.
Discussion
In this study, we characterised and confirmed the presence of two cyclic dipeptides in the femoral gland secretions (FGS) of S. virgatus, of which at least one elicited a chemosensory response typical of social communication via olfaction and vomerolfaction32. Cyclic dipeptide 1, cyclo(L-Leu-L-Pro), and cyclic dipeptide 2, cyclo(L-Pro-L-Pro) are relatively hydrophobic (non-zwitterionic) dipeptides and, unlike most diketopiperazines, they are apparently detectable in the gas phase. Here, they accounted for ~0.1% of the total content of FGS. This makes S. virgatus the Sceloporus species in which these two cyclic dipeptides have been found in the lowest proportion to date22,39, presenting a great opportunity to test the biological activity of rare volatile constituents of complex signalling mixtures in a terrestrial vertebrate.
Even in the most studied of taxa (terrestrial mammals), it has been difficult to ascribe function to specific chemical structures1,11,14. For example, here, cyclic dipeptide 1 and cyclic dipeptide 2 have, relatively, very similar chemical structures (Fig. 1), including a diketopiperazine ring with nitrogen atoms, yet the biological response to each of their chemical blends was significantly different (Fig. 4); only CDP 2, when presented alone, elicited a significant chemosensory response. This disparity in the behavioural responses toward CDP1 and CDP2, together with the fact that the matrix control elicited an equivalent response to spontaneous lizard behaviour, demonstrate that the effect of CDP2 was not the result of compound class (diketopiperazine) nor compound novelty per se. Because the here tested compound quantities were within the naturally occurring range found in natural secretions of Sceloporus lizards22,39, it is unlikely that CDP2 acted through trigeminal chemoreception (pungency). In fact, we know that whole FGS elicit a comparable chemosensory behavioural response to CDP2, if not higher, from conspecific S. virgatus39,47, whose FGS samples contain approximately between below detection limit-282 ng of cyclic dipeptide 1 and 19–295 ng of cyclic dipeptide 2 (with m/z 70). Unexpectedly, the combined blend CDP1 + CDP2 evoked the same response as either of the controls, suggesting that CDP1 interferes with the effects of CDP2 and could mask the presence of the latter in the complete scent. However, cyclic dipeptide 2 consistently appears in higher concentrations than cyclic dipeptide 1 in the FGS of these lizards39 (Table 1); instead, our combined blend used an equal amount of both compounds. This allows for the possibility of CDP2 conserving its biological activity amid compounds in natural FGS. Overall, these results support the idea that biological activity resides in the nuances of structural design (i.e. it has a high specificity), relative compound proportion and/or chemical context (e.g.48).
To date, both cyclic dipeptide 1, cyclo(L-Leu-L-Pro), and cyclic dipeptide 2, cyclo(L-Pro-L-Pro), have been found in at least other four Sceloporus lizards22,49, the only vertebrates on the list. Cyclic dipeptide 1 has also been identified in benthic marine diatoms50 and different Bacteria phyla, including the mangrove rhizosphere bacterium Bacillus amyloliquefaciens51,52 and chili pepper rhizosphere bacterium B. vallismortis53 (Firmicutes), Streptomyces spp.48 (Actinobacteria), the marine bacteria Rheinheimera japonica54 and Pseudomonas fluorescens55, and Achromobacter xylosoxidans56 (Proteobacteria). Likewise, it is present in fungal cultures of Aspergillus flavipes57 and in ants58. Cyclic dipeptide 2 has been identified in the Antarctic psychrophilic bacterium Pseudoalteromonas haloplanktis59, the fungus Aspergillus fungi60, blowflies61 and bumblebees62. The taxonomical breadth in which these two compounds are naturally found thus seems to be quite extensive, and as diverse as the environments in which they occur. More generally, cyclic dipeptides are common by-products of anabolic and catabolic biochemical pathways, endogenous to many protists, fungi, plants and animals63, suggesting that these compounds may be far more frequent in animal skins64 and gland secretions62,65. A possible microbial source of cyclic dipeptides 1 and 2 within the femoral pore opening could also be considered39,66.
The fact that CDP 2 elicited increased chemosensory behaviour from male S. virgatus conspecifics suggests that cyclo(L-Pro-L-Pro) may potentially play a role in intra-specific communication in this species without the need of actual physical contact between individuals22,47. Furthermore, because S. virgatus is not the only Sceloporus species that excretes this compound, cyclic dipeptide 2 might also potentially operate in an inter-specific signalling context between sympatric congeners, but these hypotheses require further experimental testing. In other taxa, cyclo(L-Leu-L-Pro) has demonstrated anti-microbial and anti-mutagenic properties in vitro48,52 while cyclo(L-Pro-L-Pro) functions as a mate attractant in diatoms50 and has demonstrated anti-bacterial activity in vitro60,61. Thus, the fact that cyclo(L-Pro-L-Pro) could act as a pheromone in male-male communication of S. virgatus is congruent with previous reports of biological activity.
CDP 1 showed no apparent biological activity in intra-specific communication. There are several reasons why we may have not observed a significant behavioural response. First, behavioural responses to some pheromones sometimes require co-presentation with other constituents (e.g.67). Second, CDP 1 may be meaningful in other Sceloporus species, where increased concentrations of cyclic dipeptides in FGS occur, and its presence in S. virgatus is the result of phylogenetic conservatism. Third, CDP 1 may not be relevant to male conspecifics, although it may to females (e.g.68) or to allospecifics. Alternatively, CDP1 could modulate the effects of CDP2, as suggested by the lack of response to the combined blend CDP1 + CDP2, or it may have a structural function in FGS. For example, it may increase signal effectiveness by protecting the integrity and/or enhancing the durability of chemical scents deposited on the substrate, perhaps by slowing bacterial degradation owing to its anti-microbial effects. In ants, cyclic dipeptide 1 is putatively responsible for the bitter taste of ant venom gland secretions58,65. Even humans can taste relatively low levels of CDP1 (25 ppm) as metallic taste in cocoa nibs69. Thus, we cannot completely discard a biological role of CDP 1 and further studies are needed to discern among these and other possibilities. Follow-up studies should investigate, for example, how differences in absolute concentration, relative concentration, or the combination with additional compounds within the FGS affect behavioural responses to CDP1 and CDP2, and whether these responses differ between male and female conspecifics. To determine whether the molecular context might be important to elicit behavioural responses, it should also be instructive to present these two compounds in a different solvent, absent from FGS.
Many volatile constituents are likely by-products of general metabolism without any signalling function. In vertebrates, cyclic dipeptides (diketopyrazines) are not known in semiochemical roles and it is possible that other compounds within the FGS of S. virgatus, either lipids or proteins, have semiochemical activity. None of the known putative lizard pheromones, including cholesterol, cholesta-5,7-dien-3-ol and ergosterol (steroids), linoleic acid (polyunsaturated fatty acid), hexadecanol and octadecanol (alcohols), squalene (triterpene) and tocopherol (vitamin E)33,66 were detected in FGS of S. virgatus (Table 1), and thus they are unlikely to be semiochemicals in this species. In addition, we have experimentally tested other two likely candidates, namely the only steroid and the odorous ester methyl dihydrojasmonate, and found no apparent effect (C.R.D. unpub. data). In snakes, squalene and several long-chain methyl ketones (ketone) are well-characterized sex pheromones37,66, and the ratio of unsaturated-to-saturated ketones of pheromone blends (ranging from 10 to 18 unique methyl ketones) determines the attractiveness70. However, we found only two medium-chain saturated ketones in S. virgatus, suggesting that a similar mechanism is unlikely to operate here. Thus, any other potential semiochemicals within the FGS of S. virgatus remain to be identified.
In sum, we were able to characterize two cyclic dipeptides in the chemical signal of a terrestrial vertebrate, and demonstrate biological activity of cyclo(L-Pro-L-Pro), which may potentially be involved in intra-specific (male-male) communication of S. virgatus. This finding supports the idea that even minor components in complex mixtures can be meaningful and perhaps enough to produce a complete behavioural response2,3,7,13. Importantly, our results highlight the need for more detailed studies to determine the functional landscape of biologically relevant compounds in the complex mixtures of Sceloporus lizards, and more generally, of terrestrial vertebrates.
Methods
Study species
Sceloporus virgatus is a small (up to 70 mm, adult snout-to-vent length [SVL]) Phrynosomatid lizard that commonly occurs in Madrean pine-oak woodlands and Petran conifer forests of the Chiricahua Mountains in Arizona, USA. Like its congeners, S. virgatus uses multimodal communication, namely visual (motion and colour) and chemical signals in intra- and inter-specific interactions38,47,71. Males defend territories mainly for breeding purposes72,73, which they patrol, performing broadcast displays and depositing scent marks74, and engage in male-male competition for access to females75. In comparison with other Sceloporus species, S. virgatus has a higher rate of basal chemosensory behaviour and previous studies suggest that they rely more on chemical cues47,71,76.
Sample collection
We collected femoral gland secretions (FGS; waxy plugs <1.0 mm diameter) from 17 adult male S. virgatus in the field in May 2012. We used nitrile gloves to handle lizards, and pulled waxy plugs from femoral pores on both legs using clean forceps, storing secretions in 2 mL glass vials with Teflon®-lined screw caps at −20 °C until analysis at Indiana University’s Institute for Pheromone Research. Because individual lizard samples were too small for separate chemical analyses (<1 mg22), we pooled secretions from various individuals to create six samples weighing 1.6 mg each and used stir bar sorptive extraction to analyse them77.
Gas chromatography-mass spectrometry (GC-MS)
We characterised the volatile lipidic fraction of FGS of male S. virgatus using gas chromatography-mass spectrometry. The samples were weighed and placed in 20 mL glass scintillation vials, 8 ng of the internal standard 7-tridecanone (Sigma-Aldrich, Saint Louis, MO) dissolved in 5 μL methanol (Baker Analyzed®, Mallinckrodt Baker Inc., Phillipsburg, NJ), 2 mL of OmniSolv™ water (EMD Millipore Corporation, Billerica, MA) and 50 mg of ammonium sulfate (99.99%, Sigma-Aldrich, St.Louis, MO) were added to each vial. Cyclo(L-Leu-L-Pro) (99.9 + %), henceforth “cyclic dipeptide 1”, was obtained from BOC Sciences, Shirley, NY. Cyclo(L-Pro-L-Pro), henceforth “cyclic dipeptide 2”, was synthesised at Indiana University, Department of Chemistry (see details below) since pure chiral forms were not commercially available. All other reference compounds were purchased from Sigma-Aldrich (St. Louis, MO).
Synthesis of the cyclic dipeptide 2
(5aS,10aS)-Octahydrodipyrrolo[1,2-a:1′,2′-d]pyrazine-5,10-dione (2)
The piperazine-2,5-dione 2 was prepared following the literature report of78. In our study, L-proline (23.0 g; 200 mmol) was dissolved in tetrahydrofuran (THF; 200 mL). Phosphorous trichloride (8.7 mL; 100 mmol) was dissolved in 30 mL of THF, and this solution was added into the reaction flask in approximately 10 mL quantities at 22 °C with stirring. After the addition was completed, the mixture was stirred at 22 °C for 1 h and subsequently heated to reflux for an additional 2 h. Upon cooling, the reaction mixture was concentrated under reduced pressure and water (30 mL) and then saturated aqueous sodium bicarbonate were added to adjust the pH 7–8. The precipitate was collected by filtration and washed with water (3 × 50 mL). Following silica gel column chromatography of this precipitate (methanol/ethyl acetate 1:5 by volume), the desired cyclic dipeptide 2 was obtained in 52% yield. Our bulk sample of the 2,5-diketopiperazine 2 was recrystallised three times from ethyl acetate to give fine white crystals of the pure product for biological studies.
The pure product 2 was fully characterised after drying in vacuo. Spectroscopic data were in agreement with the reported values78,79. Lit. 1H NMR (CDCl3) δ 4.16 (t, 2H), 3.49–3.54 (m, 4H), 1.88–2.33 (m, 8H)78,79. Mp 146–148 °C; IR (solid) 2975, 2958, 1655, 1430, 1336, 1280, 1258, 1160 cm−1; 1H NMR (CDCl3) δ = 4.18 (m, 2H), 3.52 (m, 4H), 2.29–2.17 (m, 4H), 2.0–1.92 (m, 4H); 13C NMR (CDCl3) δ 166.4 (C = O), 60.4 (CH), 45.1 (NCH2), 27.7 (CH2), 23.4 (CH2), HRMS [M + 1] calcd 195.1128; found 195.1126; [α] 22D –145 (c 1, CH3OH).
Testing of cyclic dipeptides
In May 2018, we captured 20 adult (mean SVL: 56.6 ± 0.3 mm), male S. virgatus by noose from a population surrounding the Southwestern Research Station (SWRS) in Cochise County (AZ, USA). We housed them individually in glass terraria (50.8 × 27.9 × 33.0 cm) containing a paper substrate and a wooden perch in the Live Animal Holding Facilities at SWRS. Terraria were placed on shelves in a screened concrete porch and hence received indirect sunlight and were subjected to natural daily variation in ambient air temperatures. Terraria were misted with water every two days, and a 60 W lamp located towards one end of the terrarium provided heat on a 12:12 h light:dark photoperiod. Lizards were visually isolated from one another, fed two crickets every other day and allowed 48 hours of acclimation to captivity before the beginning of behavioural trials, which occurred in their home terraria.
We presented each lizard with four different chemical blends and a blank control, in random order. One of the chemical blends, the matrix control, was composed of 2 mL of acetone, a fatty acid matrix with the three most common saturated fatty acids found across Sceloporus secretions in representative relative proportions22 (i.e. 25 µL tetradecanoic acid, 150 µL hexadecanoic acid and 50 µL octadecanoic acid, corresponding to 250 ng, 1500 ng, and 500 ng in the applied 20 μL of test solution, respectively), and 60 mg of polyethylene glycol (PEG). The other three treatment blends, additionally included 50 µL of one or each of the two cyclic dipeptides of interest diluted in acetone, with each corresponding to 500 ng in the applied 20 μL of test solution. These tested compound quantities are within the naturally occurring range found in FGS samples of Sceloporus lizards (i.e. cyclic dipeptide 1: 12–529 ng; cyclic dipeptide 2: 19–791 ng)22,39. The saturated fatty acids on the blend’s matrix are also very common in FGS of other lizard taxa and are associated with a structural, non-informative function33,80. PEG is a non-volatile, odourless, and colourless polymeric binding agent that entraps temporarily the volatile compounds in the blend. Due to their hydrophobic and volatile nature, cyclic dipeptides were not presented alone. By embedding the cyclic dipeptides in the matrix control we were able to test the effects of these compounds in analogous conditions to those in which they appear in nature while avoiding their premature evaporation during transfer onto the cue surface.
Thus, to one of the treatment blends, hereafter “CDP 1”, we added the commercially available cyclic dipeptide 1, cyclo(L-Leu-L-Pro); to a second blend, hereafter known as “CDP 2”, we added the laboratory synthesised cyclic dipeptide 2, cyclo(L-Pro-L-Pro) (see above). We made a third blend by adding an equal quantity of each of the two cyclic dipeptides (“CDP1 + CDP2”). The fourth blend acted as a matrix control (“MC”) and had no added cyclic dipeptides, but contained acetone, the fatty acid matrix and PEG (see above). Blends were mixed in capped glass vials, stirred homogeneously using a vortex and stored at 4–6 °C until use. Wearing nitrile gloves, we used a 50 µL Hamilton syringe (Hamilton Company, Reno, NE) to apply 20 µL of treatment solution onto a pebble and deposited it inside the lizard’s terrarium on top of a 15 × 15 cm glazed tile. We cleaned the syringe and pebbles with acetone between applications. In the blank control treatment (hereafter “BC”), we replicated this presentation procedure but deposited an unscented pebble with no added test solution. Upon presentation, we video-recorded lizard behaviour during 15 min and later scored chemosensory behaviour, namely, the number of tongue flicks, lip smacks, and substrate licking (directed at the pebble; Table S1). Chemosensory behavioural acts involve gustation, olfaction, and vomerolfaction in lizards and their frequency reflects the strength of the response to a particular chemical stimulus34,81.
All procedures described adhere to national and international guidelines for the ethical use of animals in research and were approved by Arizona State University Institutional Animal Care and Use Committee (protocol 17-1597 R to E.P.M.). Animal collection was permitted by Arizona Game and Fish Department (LIC #SP621793) and the US Forest Service.
Statistical analyses
To test for differences in the response to different chemical blends, we analysed the scored chemosensory behaviour in R statistical software82, using generalised linear mixed models (GLMM). To account for repeated measures of the same individual we used individual ID as a random factor. We used package lme483 and models with a Poisson distribution and a log link. We used pairwise post-hoc comparisons with a Holm-Bonferroni correction84 and verified model assumptions on the residuals.
References
Apps, P. J., Weldon, P. J. & Kramer, M. Chemical signals in terrestrial vertebrates: search for design features. Nat. Prod. Rep. 32, 1131–1153, https://doi.org/10.1039/c5np00029g (2015).
Schaal, B. et al. Chemical and behavioural characterization of the rabbit mammary pheromone. Nature 424, 68, https://doi.org/10.1038/nature01739 (2003).
Rasmussen, L. E., Lee, T. D., Zhang, A., Roelofs, W. L. & Daves, G. D. Jr. Purification, identification, concentration and bioactivity of (Z)-7-dodecen-1-yl acetate: sex pheromone of the female Asian elephant, Elephas maximus. Chem. Senses 22, 417–437, https://doi.org/10.1093/chemse/22.4.417 (1997).
Novotny, M. V., Jemiolo, B., Harvey, S., Wiesler, D. & Marchlewska-Koj, A. Adrenal-mediated endogenous metabolites inhibit puberty in female mice. Science 231, 722–725, https://doi.org/10.1126/science.3945805 (1986).
Apfelbach, R., Parsons, M., Soini, H. A. & Novotny, M. V. Are single odorous components of a predator sufficient to elicit defensive behaviors in prey species? Front. Neurosci. 9, https://doi.org/10.3389/fnins.2015.00263 (2015).
Harvey, S., Jemiolo, B. & Novotny, M. Pattern of volatile compounds in dominant and subordinate male mouse urine. J. Chem. Ecol. 15, 2061–2072, https://doi.org/10.1007/bf01207438 (1989).
Novotny, M. V., Harvey, S. & Jemiolo, B. Chemistry of male dominance in the house mouse, Mus domesticus. Experientia 46, 109–113, https://doi.org/10.1007/bf01955433 (1990).
Ma, W., Miao, Z. & Novotny, M. V. Induction of estrus in grouped female mice (Mus domesticus) by synthetic analogues of preputial gland constituents. Chem. Senses 24, 289–293, https://doi.org/10.1093/chemse/24.3.289 (1999).
Brennan, P. A. & Zufall, F. Pheromonal communication in vertebrates. Nature 444, 308–315, https://doi.org/10.1038/nature05404 (2006).
Zidek, L. et al. NMR mapping of the recombinant mouse major urinary protein I binding site occupied by the pheromone 2-sec-butyl-4,5-dihydrothiazole. Biochemistry 38, 9850–9861, https://doi.org/10.1021/bi990497t (1999).
Liberles, S. D. Mammalian Pheromones. Annu. Rev. Physiol. 76, 151–175, https://doi.org/10.1146/annurev-physiol-021113-170334 (2014).
Kuntova, B., Stopkova, R. & Stopka, P. Transcriptomic and proteomic profiling revealed high proportions of odorant binding and antimicrobial defense proteins in olfactory tissues of the house mouse. Front. Genet. 9, 26, https://doi.org/10.3389/fgene.2018.00026 (2018).
Novotny, M. V., Ma, W., Wiesler, D. & Zidek, L. Positive identification of the puberty-accelerating pheromone of the house mouse: the volatile ligands associating with the major urinary protein. Proc. R. Soc. Lond. B Biol. Sci. 266, 2017–2022, https://doi.org/10.1098/rspb.1999.0880 (1999).
Burger, B. V. In The Chemistry of Pheromones and Other Semiochemicals II Topics in Current Chemistry (ed. Stefan Schulz) 231-278 (Springer Berlin Heidelberg, 2005).
Hurst, J. L. et al. Individual recognition in mice mediated by major urinary proteins. Nature 414, 631, https://doi.org/10.1038/414631a (2001).
Vernet-Maury, E. In Olfaction and Taste Vol. VII (ed. van der Starre, H.) 407 (IRL Press, 1980).
Jemiolo, B., Gubernick, D. J., Catherine Yoder, M. & Novotny, M. Chemical characterization of urinary volatile compounds of Peromyscus californicus, a monogamous biparental rodent. J. Chem. Ecol. 20, 2489–2500, https://doi.org/10.1007/bf02036186 (1994).
Jemiolo, B. & Novotny, M. Inhibition of sexual maturation in juvenile female and male mice by a chemosignal of female origin. Physiol. Behav. 55, 519–522, https://doi.org/10.1016/0031-9384(94)90110-4 (1994).
Soini, H. A. et al. Investigation of scents on cheeks and foreheads of large felines in connection to the facial marking behavior. J. Chem. Ecol. 38, 145–156, https://doi.org/10.1007/s10886-012-0075-0 (2012).
Albone, E. S. Mammalian semiochemistry: The investigation of chemical signals between mammals. xii + 360 (John Wiley & Sons. Ltd, 1984).
Renou, M. In Neurobiology of Chemical Communication (ed. Mucignat-Caretta, C.) Ch. 2, (CRC Press/Taylor & Francis, 2014).
Pruett, J. A. et al. Evolutionary interactions between visual and chemical signals: Chemosignals compensate for the loss of a visual signal in male Sceloporus lizards. J. Chem. Ecol. 42, 1164–1174, https://doi.org/10.1007/s10886-016-0778-8 (2016).
Borthwick, A. D. 2,5-Diketopiperazines: Synthesis, Reactions, Medicinal Chemistry, and Bioactive Natural Products. Chem. Rev. 112, 3641–3716, https://doi.org/10.1021/cr200398y (2012).
Belin, P. et al. The nonribosomal synthesis of diketopiperazines in tRNA-dependent cyclodipeptide synthase pathways. Nat. Prod. Rep. 29, 961–979, https://doi.org/10.1039/c2np20010d (2012).
Aravind, L., de Souza, R. F. & Iyer, L. M. Predicted class-I aminoacyl tRNA synthetase-like proteins in non-ribosomal peptide synthesis. Biol. Direct 5, 48, https://doi.org/10.1186/1745-6150-5-48 (2010).
Seguin, J. et al. Nonribosomal peptide synthesis in animals: the cyclodipeptide synthase of Nematostella. Chem. Biol. 18, 1362–1368, https://doi.org/10.1016/j.chembiol.2011.09.010 (2011).
Wheeler, J. W. & Blum, M. S. Alkylpyrazine Alarm Pheromones in Ponerine Ants. Science 182, 501–503, https://doi.org/10.1126/science.182.4111.501 (1973).
Ma, W., Miao, Z. & Novotny, M. V. Role of the adrenal gland and adrenal-mediated chemosignals in suppression of estrus in the house mouse: the lee-boot effect revisited. Biol. Reprod. 59, 1317–1320, https://doi.org/10.1095/biolreprod59.6.1317 (1998).
von Stralendorff, F. A behaviorally relevant component of the scent signals of male Tupaia belangeri: 2,5-dimethylpyrazine. Behav. Ecol. Sociobiol. 11, 101–107, https://doi.org/10.1007/bf00300098 (1982).
Woolfson, A. & Rothschild, M. Speculating about pyrazines. Proc. R. Soc. Lond. B Biol. Sci. 242, 113–119, https://doi.org/10.1098/rspb.1990.0113 (1990).
Schwenk, K. Of tongues and noses: chemoreception in lizards and snakes. Trends Ecol. Evol. 10, 7–12, https://doi.org/10.1016/S0169-5347(00)88953-3 (1995).
Cooper, W. E. Chemical discrimination by tongue-flicking in lizards: A review with hypotheses on its origin and its ecological and phylogenetic relationships. J. Chem. Ecol. 20, 439–487, https://doi.org/10.1007/bf02064449 (1994).
Martín, J. & López, P. In The Reproductive Biology and Phylogeny of Lizards and Tuatara (eds. Rheubert, Siegel, & Trauth) Ch. 3, 43–77 (CRC Press, 2014).
Mason, R. T. In Hormones, Brain, and Behavior Vol. 18, Physiology E Biology of the Reptilia (eds. Carl Gans & David Crews) Ch. 4, 114–206 (University of Chicago Press, 1992).
Halpern, M. In Hormones, Brain and Behaviour. Biology of the Reptilia Vol. 18, Physiology E (eds. Carl Gans & David Crews) 423–523 (The University of Chicago Press, 1992).
Martins, E. P., Ord, T. J., Slaven, J., Wright, J. L. & Housworth, E. A. Individual, sexual, seasonal, and temporal variation in the amount of sagebrush lizard scent marks. J. Chem. Ecol. 32, 881–893, https://doi.org/10.1007/s10886-006-9029-8 (2006).
Mason, R. T. & Parker, M. R. Social behavior and pheromonal communication in Reptiles. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 196, 729–749, https://doi.org/10.1007/s00359-010-0551-3 (2010).
Hews, D. K. & Martins, E. P. In Reptiles in Research: Investigations of Ecology, Physiology and Behavior from Desert to Sea (ed. Lutterschmidt, W. L.) Ch. 7, 111–141 (Nova Publishers, 2013).
Campos, S. M. Communication breakdown: Evolution of territorial chemical signaling in a diverse lizard genus Ph.D. thesis, Indiana University, Bloomington, (2018).
Mangiacotti, M. et al. First experimental evidence that proteins from femoral glands convey identity related information in a lizard. Acta Ethol. 22, 57–65, https://doi.org/10.1007/s10211-018-00307-1 (2019).
Baeckens, S. et al. Environmental conditions shape the chemical signal design of lizards. Funct. Ecol. 32, 566–580, https://doi.org/10.1111/1365-2435.12984 (2018).
Martín, J. & López, P. Condition-dependent pheromone signaling by male rock lizards: More oily scents are more attractive. Chem. Senses 35, 253–262, https://doi.org/10.1093/chemse/bjq009 (2010).
Martín, J. & López, P. Links between male quality, male chemical signals, and female mate choice in Iberian rock lizards. Funct. Ecol. 20, 1087–1096 (2006).
Leaché, A. D. Species trees for spiny lizards (genus Sceloporus): identifying points of concordance and conflict between nuclear and mitochondrial data. Mol. Phylogen. Evol. 54, 162–171, https://doi.org/10.1016/j.ympev.2009.09.006 (2010).
Alberts, A. C., Pratt, N. C. & Phillips, J. A. Seasonal productivity of lizard femoral glands: relationship to social dominance and androgen levels. Physiol. Behav. 51, 729–733, https://doi.org/10.1016/0031-9384(92)90109-F (1992).
Alberts, A. C. Phylogenetic and adaptive variation in lizard femoral gland secretions. Copeia 1991, 69–79, https://doi.org/10.2307/1446249 (1991).
Hews, D. K., Date, P., Hara, E. & Castellano, M. Field presentation of male secretions alters social display in Sceloporus virgatus but not S. undulatus lizards. Behav. Ecol. Sociobiol. 65, 1403–1410, https://doi.org/10.1007/s00265-011-1150-1 (2011).
Rhee, K. H. Cyclic dipeptides exhibit synergistic, broad spectrum antimicrobial effects and have anti-mutagenic properties. Int. J. Antimicrob. Agents 24, 423–427, https://doi.org/10.1016/j.ijantimicag.2004.05.005 (2004).
Pruett, J. A. Chemical ecology of male Sceloporus lizards: an integrative approach to the study of multimodal signals, hormones, and behavior, Indiana State University, (2017).
Bondoc, K. G. V., Lembke, C., Vyverman, W. & Pohnert, G. Searching for a Mate: Pheromone-directed movement of the benthic diatom Seminavis robusta. Microb. Ecol. 72, 287–294, https://doi.org/10.1007/s00248-016-0796-7 (2016).
Gowrishankar, S., Poornima, B. & Pandian, S. K. Inhibitory efficacy of cyclo(l-leucyl-l-prolyl) from mangrove rhizosphere bacterium–Bacillus amyloliquefaciens (MMS-50) toward cariogenic properties of Streptococcus mutans. Res. Microbiol. 165, 278–289, https://doi.org/10.1016/j.resmic.2014.03.004 (2014).
Gowrishankar, S. et al. Cyclic dipeptide cyclo(l-leucyl-l-prolyl) from marine Bacillus amyloliquefaciens mitigates biofilm formation and virulence in Listeria monocytogenes. Pathog. Dis. 74, https://doi.org/10.1093/femspd/ftw017 (2016).
Noh, S. W. et al. Cyclic dipeptides from Bacillus vallismortis BS07 require key components of plant immunity to induce disease resistance in Arabidopsis against Pseudomonas infection. Plant. Pathol. J. 33, 402–409, https://doi.org/10.5423/ppj.oa.11.2016.0255 (2017).
Kalinovskaya, N. I., Romanenko, L. A. & Kalinovsky, A. I. Antibacterial low-molecular-weight compounds produced by the marine bacterium Rheinheimera japonica KMM 9513T. Antonie Van Leeuwenhoek 110, 719–726, https://doi.org/10.1007/s10482-017-0839-1 (2017).
Santos, O. C. S. et al. Investigation of biotechnological potential of sponge-associated bacteria collected in Brazilian coast. Lett. Appl. Microbiol. 60, 140–147, https://doi.org/10.1111/lam.12347 (2015).
Yan, P. S. et al. Cyclo(L-leucyl-L-prolyl) produced by Achromobacter xylosoxidans inhibits aflatoxin production by Aspergillus parasiticus. Appl. Environ. Microbiol. 70, 7466–7473, https://doi.org/10.1128/aem.70.12.7466-7473.2004 (2004).
Barrow, C. J. & Sun, H. H. Spiroquinazoline, a novel substance P inhibitor with a new carbon skeleton, isolated from Aspergillus flavipes. J. Nat. Prod. 57, 471–476, https://doi.org/10.1021/np50106a005 (1994).
Morgan, E. D. et al. Comparative survey of abdominal gland secretions of the ant subfamily Ponerinae. J. Chem. Ecol. 29, 95–114, https://doi.org/10.1023/a:1021928630441 (2003).
Mitova, M., Tutino, M. L., Infusini, G., Marino, G. & De Rosa, S. Exocellular peptides from Antarctic psychrophile Pseudoalteromonas haloplanktis. Mar. Biotechnol. 7, 523–531, https://doi.org/10.1007/s10126-004-5098-2 (2005).
Furtado, N. A. J. C. et al. Diketopiperazines produced by an Aspergillus fumigatus Brazilian strain. J. Braz. Chem. Soc. 16, 1448–1453, https://doi.org/10.1590/S0103-50532005000800026 (2005).
Huberman, L. et al. Antibacterial substances of low molecular weight isolated from the blowfly, Lucilia sericata. Med. Vet. Entomol. 21, 127–131, https://doi.org/10.1111/j.1365-2915.2007.00668.x (2007).
Baer, B., Maile, R., Schmid-Hempel, P., Morgan, E. D. & Jones, G. R. Chemistry of a mating plug in bumblebees. J. Chem. Ecol. 26, 1869–1875, https://doi.org/10.1023/a:1005596707591 (2000).
Milne, P. J. & Kilian, G. In Comprehensive Natural Products II: Chemistry and Biology Vol. 5 (eds. Lewis Mander & Hung-Wen Liu) Ch. 20, 657–698 (Elsevier, 2010).
Erspamer, V., Erspamer, G. F. & Cei, J. M. Active peptides in the skins of two hundred and thirty American amphibian species. Comparative Biochemistry and Physiology Part C: Comparative Pharmacology 85, 125–137, https://doi.org/10.1016/0742-8413(86)90063-0 (1986).
López, L. C. & Morgan, E. D. Explanation of bitter taste of venom of ponerine ant, Pachycondyla apicalis. J. Chem. Ecol. 23, 705–712, https://doi.org/10.1023/B:JOEC.0000006405.26872.ef (1997).
Weldon, P. J., Flachsbarth, B. & Schulz, S. Natural products from the integument of nonavian reptiles. Nat. Prod. Rep. 25, 738–756, https://doi.org/10.1039/b509854h (2008).
Novotny, M. V., Harvey, S., Jemiolo, B. & Alberts, J. Synthetic pheromones that promote inter-male aggression in mice. Proc. Natl. Acad. Sci. USA 82, 2059–2061, https://doi.org/10.1073/pnas.82.7.2059 (1985).
Martín, J. & López, P. Intersexual differences in chemosensory responses to selected lipids reveal different messages conveyed by femoral secretions of male Iberian rock lizards. Amphib-reptil. 29, 572–578, https://doi.org/10.1163/156853808786230479 (2008).
Stark, T. & Hofmann, T. Structures, sensory activity, and dose/response functions of 2,5-Diketopiperazines in roasted cocoa nibs (Theobroma cacao). J. Agric. Food Chem. 53, 7222–7231, https://doi.org/10.1021/jf051313m (2005).
LeMaster, M. P. & Mason, R. T. Variation in a female sexual attractiveness pheromone controls male mate choice in garter snakes. J. Chem. Ecol. 28, 1269–1285, https://doi.org/10.1023/a:1016294003641 (2002).
Hews, D. K. & Benard, M. F. Negative association between conspicuous visual display and chemosensory behavior in two phrynosomatid lizards. Ethology 107, 839–850, https://doi.org/10.1046/j.1439-0310.2001.00712.x (2001).
Abell, A. J. Estimating paternity with spatial behaviour and DNA fingerprinting in the striped plateau lizard, Sceloporus virgatus (Phrynosomatidae). Behav. Ecol. Sociobiol. 41, 217–226, https://doi.org/10.1007/s002650050382 (1997).
Rose, B. Factors affecting activity in Sceloporus virgatus. Ecology 62, 706–716, https://doi.org/10.2307/1937739 (1981).
Martins, E. P. In Lizard Ecology: Historical and Experimental Perspectives (eds. Vitt, L. J. & Pianka, E. R.) Ch. 6, 117–144 (Princeton University Press, 1994).
Smith, D. C. Home range and territory in the striped plateau lizard (Sceloporus virgatus). Anim. Behav. 33, 417–427, https://doi.org/10.1016/S0003-3472(85)80066-X (1985).
Herrmann, M. A. et al. The effects of chemical signal content in social communication of lizards. Integr. Comp. Biol. 59, E334, https://doi.org/10.1093/icb/icz004 (2019).
Soini, H. A. et al. Stir bar sorptive extraction: a new quantitative and comprehensive sampling technique for determination of chemical signal profiles from biological media. J. Chem. Ecol. 31, 377–392, https://doi.org/10.1007/s10886-005-1347-8 (2005).
Guo, Y.-C., Cao, S.-X., Zong, X.-K., Liao, X.-C. & Zhao, Y.-F. ESI-MSn study on the fragmentation of protonated cyclic-dipeptides. Spectroscopy 23, 131–139, https://doi.org/10.3233/SPE-2009-0388 (2009).
Gnanaprakasam, B., Balaraman, E., Ben-David, Y. & Milstein, D. Synthesis of peptides and pyrazines from beta-amino alcohols through extrusion of H2 catalyzed by ruthenium pincer complexes: ligand-controlled selectivity. Angew. Chem. Int. Ed. 50, 12240–12244, https://doi.org/10.1002/anie.201105876 (2011).
Martín, J. & López, P. Scent may signal fighting ability in male Iberian rock lizards. Biol. Lett. 3, 125–127, https://doi.org/10.1098/rsbl.2006.0589 (2007).
Cooper, W. E. Jr. & Burghardt, G. M. A comparative analysis of scoring methods for chemical discrimination of prey by squamate reptiles. J. Chem. Ecol. 16, 45–65, https://doi.org/10.1007/BF01021267 (1990).
R: A language and environment for statistical computing (R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL, http://www.R-project.org, 2018).
Pinheiro, J., Bates, D., DebRoy, S., Sarkar, D. & Team, t. R. D. C. nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1-108, http://CRAN.R-project.org/package=nlme (2013).
Holm, S. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 6, 65–70, https://doi.org/10.2307/4615733 (1979).
Acknowledgements
We thank Piyumika Suriyampola, Julio Rivera, Tamal Roy, Jennifer Flores and Melissa López for helpful comments on the manuscript. We are grateful to the staff at the Southwestern Research Station (of the American Museum of Natural History) for logistical support in the field. We appreciate permission from the Arizona Game and Fish Department (LIC #SP621793), US Forest Service, and Arizona State University Animal Care and Use Committee (protocol 17‐1597R to E.P.M.) to conduct this work. This material is based upon work supported by the National Science Foundation [grant numbers IOS-1050274 to E.P.M., IOS-1052247 to D.K.H. and CHE-1665356 to D.R.W.].
Author information
Authors and Affiliations
Contributions
E.P.M., D.K.H., M.V.N. and H.A.S. conceived the idea for the study; E.P.M. and D.K.H. obtained the necessary funding; D.R.W. and K.N.L. synthesised CDP 2; C.R.-D. designed the behavioural trials; C.R.-D., S.M.C. and M.A.H. collected behavioural data; S.M.C. and H.A.S. performed GC-MS analyses; H.A.S., M.V.N. and D.R.W. contributed to the cyclic peptide structural identification; C.R.-D. analysed the behavioural data and wrote the first manuscript draft; All authors read, contributed and approved the final draft.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Romero-Diaz, C., Campos, S.M., Herrmann, M.A. et al. Structural Identification, Synthesis and Biological Activity of Two Volatile Cyclic Dipeptides in a Terrestrial Vertebrate. Sci Rep 10, 4303 (2020). https://doi.org/10.1038/s41598-020-61312-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-61312-8
This article is cited by
-
Composition and compound proportions affect the response to complex chemical signals in a spiny lizard
Behavioral Ecology and Sociobiology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.