Abstract
Despite its popularity, the construct of biological motion (BM) and its putative anomalies in autism spectrum disorder (ASD) are not completely clarified. In this article, we present a meta-analysis investigating the putative anomalies of BM perception in ASD. Through a systematic literature search, we found 30 studies that investigated BM perception in both ASD and typical developing peers by using point-light display stimuli. A general meta-analysis including all these studies showed a moderate deficit of individuals with ASD in BM processing, but also a high heterogeneity. This heterogeneity was explored in different additional meta-analyses where studies were grouped according to levels of complexity of the BM task employed (first-order, direct and instrumental), and according to the manipulation of low-level perceptual features (spatial vs. temporal) of the control stimuli. Results suggest that the most severe deficit in ASD is evident when perception of BM is serving a secondary purpose (e.g., inferring intentionality/action/emotion) and, interestingly, that temporal dynamics of stimuli are an important factor in determining BM processing anomalies in ASD. Our results question the traditional understanding of BM anomalies in ASD as a monolithic deficit and suggest a paradigm shift that deconstructs BM into distinct levels of processing and specific spatio-temporal subcomponents.
Similar content being viewed by others
Introduction
Our brain is constantly facing a plethora of sensory stimuli that need to be properly sampled and organized to construct a meaningful perceptual experience. Since seminal studies on point-light displays (PLD) on the perception of distinct types of motion patterns (e.g., walking, running and dancing1), the robust tuning of the human visual system to biological motion (BM) has represented an intriguing challenge for scientists2,3,4,5. Compared to full body motion, stimuli created with PLD permit to profitably separate motion processing from other features such as color or shape, representing an essential tool to investigate the principles behind our perceptual tuning to BM. In light of its implications for cognitive science, developmental and clinical neuroscience, the study of BM processing raised considerable attention in recent years6,7. In particular, its putative implications in social cognition have been widely debated in the context of Autism Spectrum Disorder (ASD), raising the hypothesis that anomalies in BM processing could be considered a marker or an intermediate phenotype of ASD5. The present work has multiple aims. First, to test whether (and eventually to what extent) the processing of BM, in its most low-level and controlled form, namely PLD stimuli, is effectively anomalous in ASD. Second, to consider a different theoretical and conceptual model that deconstructs BM into distinct levels of processing and according to distinct perceptual manipulations. Third, to present a quantitative meta-analysis of putative BM processing anomalies in ASD that follows this alternative conceptualization.
Being characterized by early onset, lifelong and debilitating behavioral symptomatology, ASD represents a serious concern for families, clinicians and generally for societies8,9. To date, the clinical diagnosis of ASD is based on behavioral symptoms and according to the DSM 5, it is characterized by restricted and repetitive patterns of behaviors, interests or activities, and difficulties in the social communication/interaction domain10. Hyper- or hypo-reactivity to sensory stimuli or unusual interest for specific sensory aspects are also considered important features of the disorder11. Some of the major concerns in dealing with ASD are the heterogeneity in severity and behavioral manifestations as well as the challenges in its early detection, when behavioral signs are less evident12. These aspects significantly impact on the clinicians’ ability to provide early and specific interventions13. Although the growing body of studies targeting neural structures implicated in the pathophysiology of ASD has improved our insights on the condition14,15,16,17, the neurobiology of ASD is far from being clarified12,18,19,20,21. Similarly, neither brain functional and structural connectivity approaches22,23 nor genetics24,25 have definitely solved the puzzle. Efforts in understanding ASD are hampered by substantial disconnection between neurobiological findings aiming to characterize the neural/genetic components associated with the condition, and its core behavioral phenotype. Thus, research investigating endophenotypes of ASD, i.e. measurable and quantifiable links between neurobiological underpinnings and behavioral symptoms26,27, represents a critical challenge for the future28,29. In recent years, a growing body of studies on endophenotypes of ASD has been performed exploring complex functions such as visual attention30,31,32,33,34,35, visual perception36,37,38,39, motor cognition40,41,42,43,44,45 and sensory processing11,46,47,48,49,50.
A significant interest in the literature has been directed to the idea that individuals with ASD have a different perceptual experience of the world and, even more critical for our aims, that sensory/perceptual anomalies in ASD are not just a secondary effect of reduced social interactions. Accordingly, sensory/perceptual anomalies would represent a key and primary component both in terms of symptom development and pathophysiology11. A specific focus has been oriented to BM processing in ASD. Following the first report of BM impairment in ASD51, the number of studies investigating this question has constantly increased over the years (see Fig. S1 in Supplementary Information). However, factors such as heterogeneous experimental designs, weak understanding of potential confounds and inconsistency among the classes of information conveyed by BM may impact on the results reliability. Despite a general agreement on the supposed BM anomalies in ASD, awareness for both the epistemological nature of these anomalies and their quantification is to date lacking5,52,53.
Biological motion processing: an early emerging ability
We may expect that BM processing, being a phylogenetically highly preserved mechanism, would show an early developmental progression during ontogenesis54. However, it still remains to be clarified whether, and eventually to what extent, being tuned to BM is dependent on experience. A seminal study indicates that at 3 months of age infants are able to discriminate BM stimuli vs. their scrambled counterparts55. This trend is observed also at 4–5 months of age55,56. More recently, a convincing argument in favor of the hypothesis that BM processing is largely independent of experience was provided by a study testing the ability of newborns to discriminate BM stimuli4. The sensitivity to BM was tested in 2-day-old babies using hen-walking animations as compared to non-BM stimuli (i.e., the same pattern of elements moving in a random manner) and inverted (upside -down) hen-walking stimuli. The use of hen stimuli allowed to exclude any possible (although remote) learning mechanisms taking place in the first 2 days of life. The results indicate that newborns manifest a spontaneous preference for BM stimuli, supporting the idea that this processing is largely (or even completely) independent from experience4. Although the authors could not rule out the possible role of fetal experience in shaping BM predispositions (see for example5), these findings support a certain degree of innate or nearly innate tuning of the human visual system to BM.
The developmental trajectory of the sensitivity to BM stimuli has also been extensively explored in comparison to global motion57. Testing different groups of children (aged 6–8, 9–11 and 12–14 years, respectively) and adults, Hadad and colleagues57 claimed that developmental trajectories for coherent motion and BM are similar. In both cases, the authors found that thresholds achieved adult-like profiles around the age of 14, following a sort of monotonic developmental trajectory of improvement from childhood. In contrast, testing after surgery the BM and global motion processing in dense bilateral congenital cataract patients that suffered from early visual deprivation, showed that marked deficits in global motion are not accompanied by similar difficulties in BM processing58. Importantly, a recent EEG study showed that the BM perception is not only preserved in these patients at the behavioral level, but also that the same neural processing is involved. Indeed, in patients with congenital cataracts, the N1 component of event-related potentials was modulated post-surgery by the processing of BM as in the control group59. Spared abilities in the processing of BM after early visual deprivation are surprising in light of the data reporting impairments in similar cohorts of patients both in global motion60 and in holistic face processing61,62.
Taken together, these data indicate a composite picture in which clear similarities57 and marked differences58 between basic and more complex mechanisms (e.g., global motion and BM processing) coexist. Such coexistence should stimulate further studies aiming at characterizing shared developmental patterns vs. neuroplastic mechanisms. For shared patterns, a classical approach refers to the well-established neuroconstructivist view supporting the idea that very early not-detailed, domain-general functions impact on the development of more complex, domain-specific, and even “social” functions63,64. For compensatory mechanisms, examples from heterogeneous clinical pictures may provide intriguing insights58 (for example, related to other functions, see also65,66). This overview of BM ontogenesis suggests that oversimplifying this construct, as if it were a monolithic process, can be misleading. For this reason, we believe that a paradigm shift in approaching the understanding of BM is needed.
Motor, spatio-temporal and social aspects of biological motion processing
It has been hypothesized that the ability to detect, recognize and interpret BM is related to the expertise in performing similar movements or actions and, to some extent, also to one’s social abilities5. Beyond the attractiveness of this view, whether and how the ability to process BM is linked tout court to the expertise in producing similar movements or actions requires a cautious interpretation. Recent experimental and theoretical investigations on mirror mechanisms clearly indicate that humans benefit from their own motor representations to understand the actions of others directly (“motorically”, “from the inside”67). Notably, mirroring phenomena may occur at distinct levels of abstractness, as if we are able to represent distinct components of an action in a motor way67,68. Such mechanisms have been hypothesized to be anomalous in ASD43,69.
Two studies reporting seemingly diverging results may help in furnishing further insights on this point. First, adolescents born preterm and suffering from periventricular leukomalacia, a clinical condition characterized by white matter lesions near the lateral ventricles, were tested in their ability to process BM5,70,71. These participants varied in their motor ability, ranging from complete walking disability to typical or near-typical motor patterns70. Impairment in the execution of actions with the lower and upper limbs correlated with the lateral extent of periventricular lesions but did not correlate with the performance at the BM task. Interestingly, sensitivity to the processing of BM negatively correlated with the volume of the lesions over parieto-occipital areas, whereas no correlation over the frontal and temporal regions was found70. Second, the link between action perception and action execution was explored by Casile and Giese72, who tested whether the acquisition of novel motor behaviors could improve the processing of BM stimuli. The authors demonstrated that nonvisual motor training selectively improves the visual recognition of novel BM patterns, thus suggesting that motor learning, independently of visual feedback (i.e., the participants were blindfolded), impacts on BM recognition73. Taken together, these two studies outline a more articulated picture. The hypothesis that BM recognition is linked to some extent to the ability in producing specific motor patterns is fascinating, yet simplistic73. At the same time, BM processing seems to benefit from more efficient and unexpected compensatory mechanisms, in turn suggesting that functional networks supporting BM processing are more complex70,74.
Another source of debate in the literature is how spatio-temporal features of BM stimuli impact on the BM processing itself. BM processing entails both spatial and temporal dynamics, but the exact contribution of these components and individual tolerance to their perturbation have not yet been clarified in ASD57,75. This may offer insights toward the understanding of apparently controversial findings reported in the ASD literature, given that being tolerant to spatial and/or temporal perturbations is supported by potentially distinct neural mechanisms. Recent evidence refined traditional views on timing by attributing a key role to the cerebellum76. Nowadays, cerebellar contributions to non-motor functions, such as sensation and perception, are well-established77,78. Interestingly, the cerebellum also contributes to the pathophysiology of ASD15 and – more specifically – to the sensory/perceptual anomalies recently explored in ASD14,79.
The complexity of networks supporting BM processing and the neuroplasticity of the compensatory mechanisms may be considered cues of the functional relevance of BM. A classical view suggests that the perception of BM is tightly linked with individual abilities in the social domain, leading back this hypothesis to the evolutionary benefit in distinguishing efficiently biological from non-BM motion. This “social” interpretation of BM posits that performance in BM tasks may serve as benchmark for unveiling difficulties in social functioning, for example in neurodevelopmental conditions, such as ASD5,7. Beyond the considerable success of this view80, refined theoretical models and robust quantitative approaches (i.e., meta-analysis) are limited and whether and how BM processing is linked to social functioning is not clear. One possibility is to interpret BM processing as a building block of more complex social abilities in the context of the so-called “social brain”. Poor performance in BM discrimination in ASD would then be a marker of their social functioning difficulties5,80. The exact nature of the “social brain” construct30,81,82 and of other related constructs such as “theory of mind” or “mindreading”83,84,85, which gained considerable attention in the last decades in a wide range of disciplines, remains however often vague and inconsistent among studies. The methodologies employed for exploring these constructs are often heterogeneous and the associated neurobiological underpinnings are unclear86, suggesting the need for a perspective shift.
Aim
As outlined in the previous sections, BM perception (i) is phylo- and onto-genetically robust, (ii) has been hypothesized to be a putative marker for ASD5,28,87, and (iii) seems to be preserved, at least to a certain degree, when the BM stimuli are perturbed (or scrambled), spatially and/or temporally75.
Despite the fact that a rigorous selection of comparable studies for conducting a quantitative meta-analysis has the disadvantage of losing some information on the characterization of the BM phenomenon at large, such an approach can help systematizing the heterogeneity of the findings reported so far. In our meta-analysis, we selected only studies that assessed the BM processing in ASD using PLD stimuli. As stated above, this kind of stimuli allows to investigate the essence of BM perception without the interference of other higher-level visual features that cannot be easily controlled for. We believe that focusing on low-level features is critical in our attempt to shed light on possible anomalies of BM processing in ASD. Moreover, this choice is consistent with other theoretical proposals which focused on findings from PLD stimuli to disentangle BM processing in the neurotypical population72,88. To better understand BM processing anomalies in ASD, it is critical to investigate the impact of both the type of processing involved in different BM tasks, and the type of stimuli that are used to assess this ability. These critical points will be addressed in the following subsections.
Deconstructing biological motion processing: a multi-level conceptual model
Given the high variability of the BM tasks employed with ASD individuals, we propose a conceptual classification of these tasks that aims at deconstructing the complex notion of BM in distinct (sub)components. The need for such deconstruction has been previously proposed also for other complex constructs such as “theory of mind”89,90,91, “social brain”82,92 or “imitation”65,93,94,95. Indeed, when these constructs are treated as monolithic ones, we may run into misleading consequences. From a conceptual and epistemological perspective, they may convey different meanings and could translate into highly heterogeneous experimental protocols, which in turn can threat the reproducibility of findings and the reliability of results86.
In the literature, others have attempted to identify distinct (sub)components that play a role in BM processing. Troje88, for example, proposed a hierarchical model with four levels of processing with different complexity: (1) life detector, a cue of an animate object moving on the ground driven by the local motion, (2) structure-from-motion driven by the global motion, (3) action recognition and (4) style recognition, such as the recognition of emotional states. The author pointed out that the BM is a complex phenomenon characterized by a local spatio-temporal, ballistic-velocity profile due to the gravity force, which can convey high-level information. Also, Casile and Giese72 proposed an interesting model that tries to explain the phylo- and onto-genetically highly preserved inclinations in processing BM. The authors hypothesized a hierarchy of neural detectors that extract motion features with different BM complexities: (1) local motion energy detectors, (2) detectors for horizontal and vertical opponent motion, (3) detectors for complex global optic flow patterns and (4) detectors for complete biological motion patterns. Both these models highlighted a clear hierarchical organization in BM processing and have the merit of focusing on the need of deconstructing BM. However, such models use categories that are less informative to our specific aim, namely better understanding BM anomalies in ASD. For that reason, we hereby propose a new clusterization across different levels of BM processing according to task complexity. We hypothesize that these distinct levels of BM processing could be differently affected in participants with ASD. Specifically, we propose a three-level model that distinguishes between: (I) first-order processing of BM, (II) direct processing of BM, and (III) instrumental processing of BM.
-
(I)
First-order processing of BM refers to the implicit detection of BM that implicates a behavioral response, which does not entail any explicit recognition or categorization of BM. Experimenters infer that participants have detected a BM stimulus by simply evaluating their spontaneous behavioral response (e.g., looking time in preferential looking paradigms).
-
(II)
Direct processing of BM involves an explicit discrimination between distinct features of the stimuli, using tasks with different types of BM stimuli (e.g. rightward vs. leftward walker), or an explicit detection and/or recognition of BM when both BM and non-BM stimuli are employed (e.g., human vs. vehicle; biological vs. its scrambled version).
-
(III)
Instrumental processing of BM implicates that the observer takes advantage of the processing of a BM stimulus for a secondary purpose, i.e. to disclose distinct and potentially more complex information, such as inferring about other’s intentionality, emotional states or actions.
A meta-analysis based on this three-level model allows to disentangle whether the richness of information conveyed by PLD-based BM could be, at least partially, the source of the variability of the findings of BM processing reported in ASD.
Do different features of BM stimuli imply different performance
Another possible source of variability in BM processing in the ASD population may stem from the fact that BM is characterized by a moving structure that requires the accurate decoding of both spatial and temporal information. Interestingly, Casile and Giese72 found that local motion seems to be an important feature supporting BM processing. Indeed, they showed that even if the spatial arrangement of the PLD dots is inconsistent with the human skeleton, a plausible local optic flow is enough to suggest the presence of a BM stimulus. This suggests that low-level features, such as spatio-temporal patterns, are extremely relevant in the processing of BM. Moreover, given the evidence of anomalies in low-level features processing in individuals with ASD11, we think that it could be extremely useful to test if the heterogeneity showed by ASD individuals in BM processing can be driven by the manipulation of different stimulus features. In other words, perceptual anomalies that are typically associated with ASD could potentially impact on BM processing more strongly when certain types of stimuli are employed. Therefore, the present work aims to preliminarily assess whether the difference between the performance of ASD and typical developing (TD) individuals is modulated by the type of low-level manipulation (spatial vs. temporal) of scrambled PLD stimuli used as comparison. Moreover, to assess whether spatio-temporal processing anomalies are specific to BM stimuli or whether they are generalized to other type of stimuli, we tested if there is a difference between the performance of ASD and TD individuals with non-BM stimuli.
Altogether, the present meta-analysis will compare the performance of ASD and TD groups according to the following four steps:
-
1.
A general analysis exploring all studies using PLD to investigate BM in ASD.
Biological motion in ASD vs. TD [1 = All BM studies];
-
2.
An analysis testing BM processing in ASD according to our three-levels model (i.e., first-order, direct and instrumental BM processing).
Level of processing of BM in ASD vs. TD [2 = Level of processing];
-
3.
An analysis investigating the contribution of spatial and temporal features in how individuals with ASD process BM.
Impact of low-level features of BM scrambled stimuli in ASD vs. TD [3 = Low-level features];
-
4.
An analysis testing ASD performance in non-BM stimuli.
Non-biological motion in ASD vs. TD [4 = Non-BM].
Methods
Literature search
Following the PRISMA-P guidelines96, we have established a protocol detailing the a priori rationale, the methodological and analytical approaches to which we adhered during the preparation of the present meta-analysis. This protocol does not represent an update of any other reports and it is not part of a systematic review on this topic.
An automatic literature search began on September 2017 and the last search was performed in 14th January 2020. We searched for all studies published in English in both PubMed and PsycInfo, by performing a search on titles, abstracts and keywords. For direct replication, we include the three Boolean queries used to the search the PubMed database: (1) ((“biology”[MeSH Terms] OR “biology”[All Fields] OR “biological”[All Fields]) AND (“motion”[MeSH Terms] OR “motion”[All Fields])) AND (“autistic disorder”[MeSH Terms] OR (“autistic”[All Fields] AND “disorder”[All Fields]) OR “autistic disorder”[All Fields] OR “autism”[All Fields] OR “asd”[All Fields] OR “autism spectrum disorder”[All Fields]); (2) “Human motion”[All Fields] AND (“autistic disorder”[MeSH Terms] OR (“autistic”[All Fields] AND “disorder”[All Fields]) OR “autistic disorder”[All Fields] OR “autism”[All Fields OR “asd”[All Fields] OR “autism spectrum disorder”[All Fields]); (3) point-light[All Fields] AND (“autistic disorder”[MeSH Terms] OR (“autistic”[All Fields] AND “disorder”[All Fields]) OR “autistic disorder”[All Fields] OR “autism”[All Fields] OR “asd”[All Fields] OR “autism spectrum disorder”[All Fields]). Moreover, in order to reduce the possibility of publication bias, we repeated the same searches in two preprint servers relevant for the field (PsyArXiv and BioRxiv) to identify unpublished articles on the topic.
In addition, we also performed a manual literature search which encompassed a search of the reference lists of the review articles and the original research papers retrieved via the Boolean queries. One paper was retrieved by using this method. Altogether the automatic and the manual literature searches resulted in 146 unduplicated published articles. No reviews, book chapters, master and doctoral theses or conference presentations were hereby included.
Screening process
The studies retrieved from the automatic and manual literature searches were independently assessed and screened by two authors (A.F. and L.Ra.). At the end of the first round of screening, they evaluated together the differences emerged from the two separate searches and discussed with the other authors the final set of papers to be included, based on the inclusion criteria as detailed below. The n reported represents the number of studies excluded at each step. Please, also refer to Fig. 1.
-
(1)
Screening: from the analysis of title and abstract, the empirical studies that did not include a task in which the processing of BM was assessed via PLD in both an ASD and a TD group were excluded from the initial paper count (n = 78).
-
(2)
Eligibility: the full text of the screened articles was read, and following this step, studies were further excluded if they did not actually meet the same criteria of the previous screening step (n = 20).
-
(3)
Qualitative assessment: from reading the whole publication, the articles were further excluded if they did not meet the following criteria:
-
If the study sample was the subset of a bigger sample presented in a different paper, the study with the smaller sample was discarded (n = 297,98).
-
If the study did not investigate behavioral measures such as accuracy, sensitivity (d’), threshold estimation, reaction times (RTs), or percentage of preferential looking (or first orienting) in a BM task. Thus, all the studies that use a passive view task in order to investigate BM perception using EEG or fMRI were discarded (n = 8).
-
If the dependent variables and/or task employed were not comparable to the majority of the studies included. Specifically, these studies were removed for the following reasons: (i) Krüger and colleagues99 used a confidence rating as the dependent variable, no accuracy or RTs were reported; (ii) Van Boxtel and colleagues100 used as the dependent variable the difference in the point of subjective equality (PSE) between the walking and running conditions, and thus the data related to BM performance could not be retrieved, for similar reasons, we also excluded the study conducted by Karaminis and colleagues101; (iii) Swettenham and colleagues102 used an attentional cuing paradigm in which cues were BM stimuli and reported RTs for valid and invalid conditions, which do not primarily map onto BM processes (n = 4).
-
If the study did not have sufficient statistical information (either present in the paper, Supplementary Materials or provided after contacting the authors) (n = 4).
-
Coding and reliability
The final sample of studies were coded based on the following rules:
-
1.
Level of BM processing, which led to the creation of three main categories: (1) first (2) direct, and (3) instrumental processing of BM;
-
2.
Type of scrambled stimulus used in tasks that assess the perception of BM vs. its scrambled version: (1) no scramble; (2) spatial scramble; (3) temporal scramble; (4) spatial and temporal scramble (see definition below for the details of both spatial and temporal scramble labels).
-
3.
Type of motion stimuli presented: (1) BM, when the data refer to the performance following the presentation of solely BM stimuli; (2) BM vs. non-BM, when the dependent variable refers to a task in which the perception of BM stimuli is contrasted with the perception of non-BM stimuli; (3) non-BM, when it is possible to separately extract data concerning only the performance of non-BM stimuli (i.e., the scrambled version of the BM stimulus or the motion of an object, such as a truck or a bicycle). Disagreement and inconsistencies between the two raters (A.F. and L.Ra., n = 7/114, 6% of all assessments) were questioned with all authors to gather consensus.
In addition, for all the studies we collected:
-
1.
the relevant sample sizes of both ASD and the TD groups;
-
2.
the percentage of male participants in each of the two groups;
-
3.
the mean age of each of the two groups;
-
4.
the test employed to assess general cognitive level of participants, and the relative mean score for each of the two groups;
-
5.
the type of behavioral measure collected: accuracy rates (including error rates) or percentage of looking time. Since RTs were present only in 5 of the studies included in this meta-analysis, we preferred to exclude them to increase the homogeneity and the comparability of measures;
-
6.
whether behavioral tasks are performed during an EEG or fMRI acquisition;
-
7.
a brief qualitative description of the task and the displayed stimuli.
All this relevant information about the studies to be included in the final analyses is reported in Table S1 as Supplementary Information. Moreover, we used the descriptive statistics or the associated test statistics of the performance from both the ASD and TD groups to calculate the Cohen’s ds. If further groups were tested in the study, the statistics of the other groups were not considered.
Effect size calculation
For each observation, using the descriptive or test statistics present in the papers selected, we calculated Cohen’s d as a measure of the size of the group effect (ASD vs. TD) on the variable of interest. Only two studies explicitly reported Cohen’s d. For all other studies, we extracted Cohen’s d from mean and standard deviations (or standard errors), or from the t-test value (or the associated p-value) of the reported statistical analyses. The mean and the standard deviation were available in most of the studies, therefore we used the following equation (1) to compute the Cohen’s d (see103,104):
where M1 is the mean of the dependent variable for the first group (e.g., ASD), M2 is the mean of the dependent variable for the second group (e.g., TD), SD1 and SD2 are respectively the standard deviations of the mean for the first and the second group, n1 and n2 refer to the number of participants in the first and the second group, respectively. We arbitrarily set the direction as positive indicating a better performance for the TD group as compared to the performance of the ASD group (e.g., lower error rate). According to the guidelines by Cohen105, an absolute effect size of 0.2–0.3 is considered a small effect, ~0.5 a medium effect and >0.8 a large effect. The variance associated to each Cohen’s d was computed following equation (2)103:
In some cases, two or more Cohen’s ds referring to the same study were included in the same meta-analysis. When more than one task was included in the same (sub)group of the meta-analysis, we pooled those Cohen’s ds in order to compensate for the possible underestimation of the variance of the mean effect size resulting from the inclusion of multiple effect sizes from a single study in the same meta-analysis106,107. The Cohen’s ds referring to the same study were pooled using the formulas reported by Borenstein and colleagues106, p. 230 (equations 3 and 4):
where \(\bar{d}\) and \(Var(\bar{d})\) are the average Cohen’s d and its variance, m is the number of Cohen’s ds that were pooled, \({d}_{j}\) and \({d}_{k}\) are the Cohen’s ds referring to measures j and k, and \({r}_{jk}\) is the correlation between measures j and k, which was arbitrarily set to 0.5. The maximum number of Cohen’s ds that were pooled is 10. This number varies depending on the meta-analysis performed.
In each of the four meta-analyses, multiple Cohen’s ds from the same study were pooled when they referred to tasks that were coded in the same manner. For instance, in Annaz and colleagues study108 there are two tasks, one measuring the sensitivity (d’) for the perception of BM in normal PLD vs. scrambled stimuli, and the other establishing the thresholds for the detection of a PLD walker in noise. Since both tasks were coded as direct processing, the two Cohen’s d were always pooled together. This was also the case of the “action”, “subjective state”, and “emotion” conditions in Hubert and colleagues109, that were all coded as tasks of instrumental processing. In some cases, multiple Cohen’s ds from the same study could be kept separate or pooled together depending on whether the categorical distinction between the tasks was relevant or irrelevant for the meta-analysis at hand. For instance, in Nackaerts and colleagues110 the Cohen’s ds referring to the task categorized as direct processing and the task categorized as instrumental processing were pooled in the first general meta-analysis [1 = All BM studies], but they were kept separate in the meta-analysis testing the effects of the level of BM processing [2 = Level of processing].
Mixed effects models
Statistical analyses were conducted in R, using the package metafor111. The meta-analysis performed was analyzed using a mixed effects model111, estimating the regression coefficients for each moderator included as well as the error between experiments. No within error estimate was included in the model, because we only included one Cohen’s d per study. The rationale for this choice was that the majority of data from the studies presented a single effect size and a traditional random-effect model would suffice106. A simple model with one moderator variable is given in equation (5):
for i ∈ {1,…,n}
yi is the Cohen’s d for the ith study
b0 is the fixed intercept for the regression model
b1 is the fixed slope for the regression model
xi is the predictor for the ith study
ei ∼ N(0, σ) is a Gaussian error term.
The variance components of the model including the predictor variables refer to the unexplained variance. To answer specific questions, we run four separate meta-analyses:
-
1.
Biological motion in ASD vs. TD [1 = All BM studies]. We included all the studies that were selected, and, for each of them, a single Cohen’s d was inserted (n = 29). Since cognitive functioning was assessed with different tests across studies (see Table S1 in Supplementary Information) we computed a Cohen’s d per study to have a comparable measure of the cognitive level to be used as a possible moderator. A positive Cohen’s d indicates that the cognitive level of the TD group is greater than the cognitive level of the ASD group. We included this moderator in the meta-analytic models as a way to assess if the ASD diagnosis has a specific effect on the processing of BM, which cannot simply be attributed to a reduction in cognitive level. Moreover, we insert also the mean age of ASD and of TD groups as moderators in order to control for the possible effect of age.
-
2.
Level of processing of biological motion in ASD vs. TD [2 = Level of processing]. We included one Cohen’s d per study in each of the three levels of BM processing identified (n = 7 first-order, n = 16 direct, n = 11 instrumental). ‘Level of processing’ was included in the meta-regression analysis as a categorical predictor, and no further moderating effects were considered here, due to the limited number of studies included in each group.
-
3.
Impact of low-level features of BM scrambled stimuli in ASD vs. TD [3 = Low-level features]. We included one Cohen’s d for each study that used the scrambled version of the BM stimulus (n = 7 spatial, n = 6 temporal). We considered two types of scrambled stimuli according to the manipulation, spatial or temporal, employed to generate them. To this aim, this meta-analysis includes a categorical moderator, ‘type of scramble’, which consists of two levels: (1) spatial scramble, the difference between the scrambled and the BM stimulus is limited to a difference in the spatial configuration. Specifically, in the scrambled version, each dot of the PLD was randomly displaced in a different spatial location; (2) temporal scramble, the difference between the scrambled and the BM stimulus is limited to a difference in the temporal phase. Specifically, each dot in the PLD scrambled stimulus was presented in the same spatial position but the motion of each single dot was temporally out of phase as compared to that of the BM stimulus. Studies with both spatial and temporal manipulations were excluded since they were not numerous enough to create a stand-alone group (n = 2). Considering the limited number of studies per level, we refrained from including other moderators.
-
4.
Non-biological motion in ASD vs. TD [4 = Non-BM]. This meta-analysis is limited to the studies that test, together with the perception of a BM stimulus, also the processing of another stimulus that can be defined as a non-BM stimulus (i.e., an object as a bike or a truck presented in PLD109,112 or of a scrambled version of the BM stimulus39,113,114 (n = 5). It is important to note that we do not consider this analysis to be ultimately conclusive, because an extensive analysis of this type should include also other types of non-BM tasks such as coherent motion, form-from-motion, etc. However, we still believe that this aspect is worth investigating to provide an initial characterization of possible dissociations between BM and non-BM processing in ASD vs. TD.
All tests were conducted with a significance level of 5%. The results are presented including the following measures: weighted mean effect sizes (Cohen’s d), 95% confidence intervals, I2 heterogeneity values, and p values. All error bars in forest plots are 95% confidence intervals and were generated by applying customized R scripts.
Moderator correlations
Being aware of the need to control for confounding moderators115, we opted to use ANOVAs (performed in R with the aov function) in order to evaluate possible systematic relationships between the categorical predictor (i.e. ‘level of processing’, ‘type of scramble’) and the quantitative predictor (i.e. ‘cognitive level’, ‘mean age of ASD’, ‘mean age of TD’). This procedure allowed us to define whether the cognitive level or the group age differed systematically across the levels of processing and type of scramble.
Publication bias
To determine the presence of publication bias for the main effect of each meta-analysis, we plot each study’s effect size (Cohen’s d ASD vs. TD) against its own standard error in a funnel plot. Publication bias is computed as a negative association between the sample size and the effect size across studies, since a result coming from a small sample must have a large effect size to be significant. More specifically, publication bias occurs when studies with small samples are more likely to be published when they report statistically significant results (i.e., a large effect size), as compared to when they do not (i.e., a small effect size). The publication bias implies an overestimation of the true effect size, for which appropriate correction methods are available.
Results
Biological motion in ASD vs. TD [1 = All BM studies]
The analysis including all papers contrasting the processing of BM in ASD vs. TD revealed a medium effect of the difference in performance between the two groups, Cohen’s d = 0.60, SE = 0.13, CI 95% = [0.36, 0.85], z = 4.77, p < 0.0001. In other words, there is a medium effect size indicating that individuals with ASD have more difficulties in BM processing than TD individuals (Fig. 2A). However, the I² coefficient reveals that 76% of the variability of the effect sizes can be attributed to the heterogeneity among the true effects (Test for Heterogeneity: Q(28) = 110.67, p < 0.0001). Cognitive level, mean age of ASD group and mean age of TD group proved to have no statistically significant effect on Cohen’s d (cognitive level: b = 0.19, SE = 0.17, CI 95% = [−0.14, 0.52], z = 1.13, p = 0.26; mean age of ASD group: b = 0.003, SE = 0.01, CI 95% = [−0.02, 0.02], z = 0.28, p = 0.78; mean age of TD group: b = 0.001, SE = 0.01, CI 95% = [−0.02, 0.02], z = 0.09, p = 0.93).
(A) Forest plot of the differences in the BM performance between the ASD and TD groups. Positive values indicate a better performance for the TD group as compared to the performance of the ASD group. (B) Funnel plot indicating the publication bias in studies of BM processing in ASD vs. TD. White circles represent the missing studies identified by the trim and fill test.
A visual inspection of the funnel plot resulting from this meta-analysis suggests a quite symmetrical distribution of the studies (Fig. 2B). To confirm whether or not a publication bias is present in this case, we computed a trim and fill test116. Such test estimates the absence of four studies on the left side (SE = 3.58) and none on the right side. When computing a new average effect size including the effect sizes of the three studies estimated to be absent, the average effect size was still significantly different from zero, Cohen’s d = 0.47, SE = 0.13, CI 95% = [0.22, 0.72], z = 3.63, p = 0.0003. Thus, since the effect size was still medium and significant even after the inclusion of the estimated missed studies, the difference in the processing of BM between ASD and TD groups cannot be considered dependent on publication bias.
Level of processing of biological motion in ASD vs. TD [2 = Level of processing]
To investigate the role of the level of processing needed to accurately perceive BM in ASD vs. TD, we computed a mixed effect model with ‘level of processing’ as a moderator. This moderator has three levels: (i) first-order processing; (ii) direct processing; (iii) instrumental processing, indicating the three subgroups in which tasks were divided. The I2 coefficient indicates that the heterogeneity among the true effects is ~71% (Test for Residual Heterogeneity: QE(31) = 103.85, p < 0.0001). The results reveal a significant effect of the moderator (QM(3) = 37.94, p < 0.0001). Thus, to further explore each level of the moderator we calculated separate meta-analyses for each level of processing. When analyzing the first-order processing level, we obtained Cohen’s d = 0.55, SE = 0.27, CI 95% = [0.02, 1.09], z = 2.04, p = 0.04, and the heterogeneity was comparable to that of the full model (I2 = 79%, Q(6) = 27.04, p < 0.0001, Fig. 3A). When analyzing the direct processing level, we obtained Cohen’s d = 0.40, SE = 0.18, CI 95% = [0.05, 0.74], z = 2.24, p = 0.03, and the heterogeneity was comparable to that of the full model (I2 = 76%, Q(15) = 61.29, p < 0.0001, Fig. 3B). Instead, the instrumental processing level is the one showing the greatest difference in the processing of BM between ASD and TD, Cohen’s d = 0.98, SE = 0.13, CI 95% = [0.73, 1.23], z = 7.56, p < 0.0001, and the heterogeneity was reduced as compared to that of the full model and not significant anymore (I2 = 34%, Q(10) = 15.52, p = 0.11, Fig. 3C).
(A) Forest plot of studies investigating the difference in first-order level of BM processing between ASD and TD group. (B) Forest plot of studies investigating the difference in direct level of BM processing between ASD and TD group. (C) Forest plot of studies investigating the difference in instrumental level of BM processing between ASD and TD group. In all plots, positive values indicate a better performance for the TD group as compared to the performance of the ASD group.
Moreover, in order to test if there is a significant difference between the magnitude of these estimated effect sizes, we performed three z-tests between the Cohen’s ds of the different levels of processing using the Bonferroni correction method. Only the difference between the direct and instrumental levels was statistically significant (direct vs. instrumental: z = −2.81, p = 0.015; first-order vs direct: z = −0.46, p ~ 1; first-order vs. instrumental: z = −1.45, p = 0.45).
To evaluate possible systematic differences in cognitive level across the levels of BM processing, we performed a one-way ANOVA on the Cohen’s ds referring to the cognitive level with ‘level of processing’ as a factor. No statistically significant differences were retrieved (F(1, 27) = 0.09, p = 0.76, ηp2 = 0.003). Moreover, two one-way ANOVAs showed that neither the mean age of the ASD groups nor that of the TD groups differed systematically across levels of BM processing (F(1, 31) = 1.71, p = 0.20, ηp2 = 0.05, and F(1, 32) = 1.94, p = 0.17, ηp2 = 0.06, respectively). In sum, the effects of level of processing on Cohen’s d cannot be explained in terms of systematic differences between cognitive level, mean age of the ASD groups, or mean age of the TD groups.
Impact of low-level features of BM scrambled stimuli in ASD vs. TD [3 = Low-level features]
With this third meta-analysis, we aim at further characterizing the low-level perceptual aspects potentially affecting the processing of BM in ASD vs. TD. Here, we focus only on studies that include both BM and non-BM scrambled stimuli. In other words, with this meta-analysis we inquire whether there are specific spatial or temporal features of the stimuli in motion that affect ASD and TD individuals differently.
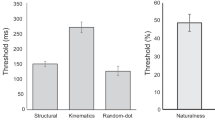
The I2 coefficient indicates that the heterogeneity among the true effects is ~58% (Test for Residual Heterogeneity: QE(11) = 25.95, p = 0.007). Interestingly, results indicate that the moderator is significant (QM(2) = 12.02, p = 0.003). To further investigate this finding, we calculated separate meta-analyses for each moderator level. When considering only the studies with a BM task that includes a spatially scrambled stimulus, results indicated no significant difference in the performance of ASD vs. TD (Cohen’s d = 0.38, SE = 0.23, CI 95% = [−0.07, 0.83], z = 1.67, p = 0.10, Fig. 4A). The confidence interval includes 0, suggesting that we cannot exclude that the effect size for this subgroup of studies is null, and the heterogeneity is still moderate and significantly different from zero (I2 = 60%, Q(6) = 14.84, p = 0.02).
(A) Forest plot of studies investigating the difference in BM performance between ASD and TD group that employed spatial scramble stimuli. (B) Forest plot of studies investigating the difference in BM performance between ASD and TD group that employed temporal scramble stimuli. In both plots, positive values indicate a better performance for the TD group as compared to the performance of the ASD group.
On the contrary, the model including the temporally scrambled stimulus showed a significant medium-large effect size (Cohen’s d = 0.67, SE = 0.21, CI 95% = [0.26, 1.08], z = 3.20, p = 0.001, Fig. 4B), and the included studies can be considered a homogenous group (I2 = 55%, Q(5) = 11.11, p = 0.05). These differences in the processing of scrambled stimuli were not confounded by the cognitive level, as it was shown by a one-way ANOVA on Cohen’s ds referring to cognitive level with ‘type of scramble’ as a factor (F(1, 9) = 0.009, p = 0.93, ηp2 = 0.001). Similarly, differences in the processing of scrambled stimuli were not confounded by the mean age of ASD groups (F(1, 12) = 2.29, p = 0.16, ηp2 = 0.16) nor by the mean age of TD groups (F(1, 13) = 4.29, p = 0.06, ηp2 = 0.25).
Non-biological motion in ASD vs. TD [4 = Non-BM]
We tested whether the deficit shown by individuals with ASD is selective to BM stimuli or it also generalizes to non-BM stimuli. Results indicate that no significant difference between the performance of individuals with ASD and TD is evident, (Cohen’s d = 0.26, SE = 0.29, CI 95% = [−0.31, 0.83], z = 0.89, p = 0.38, Fig. 5). The total heterogeneity estimated with I2 is ~75% (Q(4) = 19.80, p = 0.0005).
For a summary of the main results, please refer to Table 1 below.
Discussion
In this article, we conducted an original meta-analytic investigation examining the abilities of individuals with ASD in the processing of BM stimuli. The results of the general meta-analysis [1=All BM studies] showed that individuals with ASD present a moderate (d = 0.60) deficit in BM processing as compared to TD individuals. This deficit was not influenced either by the cognitive level or by the mean age of participants. Moreover, it was not due to publication bias. Nonetheless, we observed a high heterogeneity among studies (I² = 76%), and this is an interesting aspect because it suggests that possible (underestimated) sources of variability are present in the group of studies included. We believe that this result supports the idea that the perception of BM is a complex ability, which cannot always be reduced to a monolithic process.
The need for well-controlled meta-analytic approaches in evaluating BM processing in ASD is witnessed by the fact that, following our pre-print deposit on BioRxiv (January 24, 2019), two other groups have pursued similar investigations. Consistently with our results, both of these two meta-analyses have shown a general significative impairment in ASD to process BM117,118. However, behind the general deficit, the relevant question becomes which are the component(s) that can better explain the differences in behavioral performance between TD and ASD participants? Our work provides preliminary insights on this matter. By challenging the high heterogeneity reported in our general meta-analysis, we proposed meta-analytic investigations focusing on specific moderators.
Thus, we considered the distinct levels of processing defined in our three-level model (i.e., first-order, direct and instrumental BM processing), the type of manipulation used to create the comparison scrambled stimulus (spatial vs. temporal), and the selectivity of the impairment for biological as opposed to non-biological motion.
Is BM perception in ASD anomalous across all levels of processing?
A more precise analysis testing BM processing in ASD according to a three-level model that distinguished among first-order, direct and instrumental processing of BM confirmed that such a deficit was present independently of the specific level of information processing required in the BM task [2 = level of processing]. Nonetheless, this moderator revealed a significant effect on the observed effect size of the impairment in BM processing, suggesting that the magnitude of this impairment depends on the level of processing involved in the specific type of task. Individuals with ASD showed the largest deficit as compared to TD participants when the experimental task required the instrumental recognition of the BM (d = 0.98). Instead, the deficit was moderate (d = 0.55) for tasks requiring the first-order processing of BM and only small-to-moderate (d = 0.40) when tasks required the direct recognition of BM. It is worth pointing out two observations regarding the first- order level of processing: (i) first-order BM usually includes samples of younger children, which performed preferential looking tasks; (ii) the deficit for first-order BM is moderate, with a single study119 reporting a negative effect size. Such study tested preferential looking in children at the age of 6, rather than in younger children or infants. Post-hoc tests revealed that the effect sizes for direct and instrumental processing were significantly different. The differences between the ASD and TD groups across all levels of BM processing were not ascribable to differences in cognitive level or mean age.
The study of BM anomalies in ASD has so far been accumulating a conspicuous body of evidence, but often without putting the necessary effort in trying to understand the different sub-components required to efficiently process the distinct types of BM tasks. This meta-analysis shows that the most severe deficit in participants with ASD emerges when the recognition and categorization of a BM stimulus is serving a secondary purpose, such as when inferring someone else’s intentions, actions or emotional states; in other words, when correctly perceiving BM is a pre-requisite to disclose distinct (and possibly more complex) information. On the contrary, the least severe deficit is observable in participants with ASD when a more basic recognition of BM is tested, the level that we referred to as direct recognition of BM (e.g., BM vs. non-BM/scrambled stimulus; BM stimulus “A” vs. BM stimulus “B”, leftward vs. rightward moving stimulus, etc.). This less severe deficit could be due, at least in part, to compensatory mechanisms that can help participants with ASD to correctly process BM, when the task is not excessively complex. Interestingly, a recent study in which morphed PLD stimuli were created by BM prototypical action, found that individuals with ASD showed intact abilities to recognize actions, but weakened adaptation to BM100. This difference in the implicit processing of BM despite an intact explicit BM recognition suggests that individuals with ASD could develop compensatory mechanisms to perceive BM stimuli. Such compensation becomes more evident with increasing age. Accordingly, Rutherford and Troje120 have shown that, despite no group differences in sensitivity to BM or in the ability to identify the direction of motion were found between ASD and TD groups, only in the ASD group the IQ scores were significantly predictive of their performance. One possibility is that when participants with ASD are processing BM they use different strategies to compensate for their perceptual anomalies.
Overall, these results stress the need for a careful evaluation of what is at the core of BM processing and what are instead additional, secondary and higher-level information that can be extracted from a PLD depicting a stimulus moving in a biologically-plausible fashion. Persevering with such an ambiguity would undermine the study of this construct as a putative endophenotype for ASD with negative clinical repercussions. For this reason, to assess how different low-level features can affect BM perception in ASD is pivotal.
What are the low-level perceptual features linked to biological motion anomalies in ASD
After assessing the role of different levels of BM processing in influencing the strength of the BM processing deficit in individuals with ASD, we assessed another fundamental aspect of BM that is the role of low-level perceptual features [3 = Low-level features]. Indeed, in many studies, BM stimuli are compared to scrambled stimuli obtained by varying the spatial and/or temporal properties of the PLD. Experiments using a spatial scramble use comparison stimuli which maintain the original temporal phase, while all or part of the dots constituting the target PLD change spatial location. Experiments using a temporal scramble, instead, use comparison stimuli which maintain the original relative spatial position, while all or part of the dots constituting the target PLD change temporal phase. By testing the role of the type of scrambled stimuli used as a comparison, our aim was to inquire whether the deficits observable in individuals with ASD are dependent on the specific spatial or temporal properties of the target stimulus that are experimentally manipulated. We found that such a moderator had a significant influence in the effect size of the impaired BM processing in ASD. Studies employing a spatial scramble as a comparison stimulus revealed no significant difference in the performance between individuals with ASD and TD controls. On the contrary, a significant impaired performance was evident for studies employing a temporal scramble as a comparison stimulus, with a moderate-to-large effect size (d = 0.67). Although the sample size for each of this meta-analysis was limited (n = 7 spatial; n = 6 temporal), they furnish an initial interesting insight on the deficit underlying the perception of BM in ASD.
It is increasingly evident that simple spatio-temporal visual dynamics are essential building blocks for social behavior. A recent study in juvenile zebrafish showed that collective behavior, like shoaling, can be driven by simple specific motion cues that are constituted by black dots that mimic the precise zebrafish motion, while other naturalistic cues such as fish-like shape, pigmentation pattern, or non-visual sensory modalities are not required121. Anomalies in the processing stages connected to the tracking of low-level motion dynamics might be disruptive for detection, recognition and categorization of BM stimuli. Our results suggest that a candidate core mechanism playing a role in BM processing anomalies in ASD is linked to the temporal properties of moving dots embedded in a PLD. This is consistent with several experimental pieces of evidence and theoretical proposals suggesting that the temporal binding of unimodal and multisensory information is one of the core anomaly of individuals with ASD122,123,124,125. Additional evidence supports the idea that the aberration of simple spatio-temporal visual dynamics would be a common endophenotype for neurodevelopmental disabilities other than ASD124,125. Specifically, some evidence indicates that individuals with ASD show an extended temporal binding window (TBW; an epoch of time within which stimuli from different sensory modalities are highly likely to be bound), which would impair the temporal processing of sensory stimuli125. The majority of this evidence comes from audio-visual multisensory paradigms (for reviews see123,124,125), where researchers assess the temporal precision required to perceive auditory and visual stimuli as synchronous. Nonetheless, initial evidence for temporal processing anomalies in unisensory perception has been documented in individuals with ASD in both auditory48,126 and visual127,128 perception, suggesting a common underlying deficit in the fundamental coordination of brain networks that are responsible for the timing of sensory information processing129. It is also worth noting that the extension of TBWs has a high degree of malleability and they can shrink or expand as a function of the complexity and the low-level statistics of the incoming sensory information. TBWs change as a function of spatial and temporal relationship among stimuli. For example, when information from different sensory modalities originates from the same spatial position130 or can be temporally-predictable (e.g., rhythmic)131 is integrated across a larger temporal window (see also132). TBWs can shrink or expand also as a result of synchronization of brain rhythms (i.e. entrainment)133,134, or after few days of training in perceptual learning135,136,137. Intriguingly, the hypothesis of a temporal processing anomaly as one of the key aspects responsible for BM deficits observed in ASD would be consistent with the involvement of the cerebellum and its bidirectional connectivity with the right posterior superior temporal sulcus (STS), which represents an important part of the network involved in the processing of BM138,139,140,141,142. For more details, please refer to the subsection on the neural and oscillatory correlates of BM processing below.
Is the impairment observed in ASD specific to biological motion stimuli
We conducted a meta-analysis of the subgroup of studies that tested performance in tasks with BM vs. non-BM stimuli [4 = Non-BM]. The aim of this analysis was to assess whether the deficit shown by participants with ASD was selective for BM stimuli or generalized also to non-BM stimuli. We observed no evidence of impairment in the processing of non-BM stimuli in individuals with ASD. This seems prima facie suggest that suggests that the deficit is selective for BM stimuli and in particular, to their specific spatio-temporal pattern, whereas other types of complex stimuli in motion do not elicit a similar impairment. Among the non-BM tasks tested, we can find recognition/categorization of an object as a bike or a truck presented in PLD109,112 but also recognition/categorization of a scrambled version of the BM stimulus39,113,114. It is clear however that testing the scrambled version of BM stimuli is not ideal to claim that the deficit observed in individuals with ASD is specific to stimuli moving in a biological fashion. Consistently, there is evidence showing that only when neurotypical adults cannot extract a form from a non-BM stimuli, they find non-BM stimuli harder to discriminate than BM stimuli. In other words, the presence of a structure that can be extracted from non-BM stimuli seems to be a crucial aspect when comparing the recognition/categorization of BM and non-BM stimuli143. This would mean that BM is not generally easier to detect than equally-structured non-BM. Therefore, to claim that individuals with ASD show a deficit which is specific to BM, the processing of non-BM stimuli should involve a similar extraction and interpretation of a form-from-motion. In the present meta-analysis there were only two studies contrasting BM stimuli with structured non-BM stimuli109,112, and no clear evidence of impairment in participants with ASD was found, independently of the nature of the moving stimuli (BM vs. non-BM) employed.
We conclude that from the evidence available at the time of the present meta-analysis, it is still an open question whether anomalies observed in individuals with ASD are specific to BM. Thus, although interesting, the comparison between the processing of BM and non-BM stimuli needs a more careful investigation in future studies.
Neural and oscillatory correlates of BM processing in individuals with ASD
Support to the idea that BM is hardly conceivable as a monolithic process comes from the available literature on the neural correlates of BM processing in primates, neurotypical humans, and in individuals with ASD. Such literature highlights the brain networks responsible for BM processing are highly complex and probably not unitary. Neuroimaging studies in the typical population show that the activity of the posterior STS (pSTS) is specifically associated to BM stimuli presented as PLD. The pSTS involvement is often lateralized in the right hemisphere144,145,146,147. While this region is activated for the observation of a wide range of human movements148,149, it has not been revealed to be involved in studies examining the perception of static bodies or body parts, suggesting that pSTS is involved specifically in extracting features of human body motion rather than forms per se147. Congruently, studies in non-human primates have shown the presence of neurons that selectively respond to various biological actions (e.g. walking, turning of the head, bending of the torso, moving of the arms, etc150.). However, these neurons receive convergent information from the dorsal visual stream areas, middle temporal (MT) and medial superior temporal (MST), and also from the ventral stream area in the inferior temporal (IT) regions147. The homologue of pSTS in the primate brain is also connected with the inferior parietal cortex151. A recent meta-analytic investigation of human brain regions activated in different types of BM processing (i.e. whole body, hand and face movements) confirmed common convergent activations in the right pSTS region, independent of the specific motion category. However, the pattern of activations included also bilateral regions at the junction between middle temporal and lateral occipital gyri147.
Other evidence in favor of a highly complex network of brain regions supporting the processing of BM that goes well beyond pSTS comes from functional connectivity studies. For example, a study by Sokolov and colleagues139 that used fMRI to test healthy participants during a BM task, in combination with functional connectivity analysis and dynamic causal modeling, showed that the left lateral cerebellum (lobules Crus I and VIIB) exhibited increased activation and connectivity with right pSTS during the task.
More recently, Sokolov and colleagues142 claimed that BM processing is organized in a parallel, rather than in a hierarchical manner. Benefiting from innovative approaches that combine structural and effective connectivity, the authors confirmed the integrative role of the right STS, but they also observed that this area is not the single main gate that integrates the activity of the occipito-temporal and frontal regions. Also, other regions such as the fusiform gyrus and middle temporal cortex were found to be directly connected to right inferior frontal gyrus and insula during processing of BM stimuli. Moreover, both STS and insula have shown significant BM-specific effective connectivity with the cerebellum (cerebellar lobule Crus I). According to their conclusions, this parallel processing, dependent on the activity of different cortico-cortical and cortico-cerebellar pathways, may help to explain why BM processing is rather resilient to focal brain damage, whereas it is frequently impaired in neuropsychiatric conditions with distributed network alterations (e.g., ASD).
In addition, it has been suggested that the cerebellum uses a common computational algorithm not only upon motor but also non-motor functions, such as perceptual ones45,152. The idea that the cerebellum may play a critical role as internal “timing device” for motor and non-motor functions has been widely explored in the literature76. Notably, this idea may fit with the evidence indicating that the cerebellum is implicated in the pathophysiology of ASD14,15 and may also fit with our present results showing a specific role for temporal scrambled stimuli in explaining the anomalies in BM processing observed in individuals with ASD.
Although it is not within the scope of the present article to provide an extensive review of the neuroimaging literature that investigates the neural correlates of impaired BM processing in ASD, it is interesting to notice that consistent reports in the literature show that during the processing of BM stimuli individuals with ASD show hypoactivation in the pSTS153,154,155,156 and in regions of the prefrontal cortex98,156. However, only few studies so far have tested the effective connectivity among the key regions of the cortical and subcortical (i.e. cerebellum) network responsible of the processing of BM141,157,158. These studies suggest that a defective temporo-parietal158 and cerebellar-pSTS connectivity141 could contribute to the anomalies in the processing of BM reported in ASD and reviewed in the present meta-analysis. Although these studies provide initial important evidence, the results of the present meta-analysis suggest that distinct levels of BM processing can be differentially affected in participants with ASD, and that the manipulation of low-level perceptual features may result in markedly distinctive behavioral outcomes. These aspects should be taken into account in future neuroimaging studies that will address the neural correlates of the anomalies in the processing of BM in ASD. More generally, even from a neural perspective, BM should not be considered as a monolithic process, but as constituted by different components, that are likely supported by different parallel neural routes.
Limitations and future directions
Although not conclusive, we believe that the model and the cumulative findings reported in the present meta-analysis move the conceptualization of BM anomalies in ASD a step forward. To improve the explanatory power of this and other models proposed in the field of BM, we call for future studies to provide appropriate quantitative data to be summarized with metanalytic approaches. Specifically, we deem relevant to investigate whether the potential anomalies in BM processing in ASD are extended to non-BM stimuli, and whether they are selectively tied to temporal binding anomalies. To this end, testing multiple BM and non-BM stimuli (e.g., BM, non-BM, spatial scramble, temporal scramble) within the same ASD sample would be of paramount importance (please refer to meta-analysis 3 = Low-level features and 4 = Non-BM).
Although we would find extremely interesting to produce evidence on the specificity of the ASD impairment in BM vs. other motion tasks (e.g., coherent motion or form-from motion tasks), only two of the studies included in our meta-analysis used coherent motion as a control task98,159. Results indicated that in the ASD group the performance to BM and coherent motion tasks are positively correlated. Future studies on contrasting the performance to BM, coherent motion and form-from motion tasks will aid the clarification of whether the deficit in BM processing showed by ASD individuals is specific to BM or instead is common to other complex motion tasks. In accordance with the idea of general perceptual anomalies in ASD that are not specific to BM, a recent study160 has shown that infants that are less attracted to multisensory BM stimuli were diagnosed with ASD at 2 years of age. Future work in this direction will clarify how anomalies in BM processing in ASD, if any, should be appropriately conceived for clinical aims.
Conclusions
Taken together, this work represents a new assessment of the putative impairment in the processing of BM in individuals with ASD, broken down by specific sub-processes and sub-components (see Table 1). Despite some of our analyses are probably influenced by the limited sample of studies available and by the high heterogeneity of participants’ characteristics, we made an effort toward interpreting the multifaceted aspects of the vast literature that uses BM as a proxy for social perception deficits in ASD. While on the one hand a significant impairment in BM processing emerged in individuals with ASD, on the other hand the idea that this deficit is selective for BM and does not extend to non-BM stimuli is, at present, not properly supported by the literature. Moreover, we observed a high heterogeneity due to the different experimental protocols employed. A major source of heterogeneity can be individuated in the distinct levels (first-order, direct, instrumental) of BM processing, given that the most severe deficit in participants with ASD emerges when processing of a BM stimulus is serving a secondary purpose (e.g. inferring other’s intentionality, action or emotional state). How more severe difficulties reported in the instrumental recognition of BM may represent just a byproduct of more basic anomalies should be clarified in future studies. Finally, an initial result points toward the importance of low-level temporal perceptual features in determining the deficit in the perception of BM in ASD.
References
Johansson, G. Visual perception of biological motion and a model for its analysis. Percept. Psychophys. 14, 201–211 (1973).
Proffitt, D. R., Bertenthal, B. I. & Roberts, R. J. The role of occlusion in reducing multistability in moving point-light displays. Percept. Psychophys. 36, 315–23 (1984).
Neri, P., Morrone, M. C. & Burr, D. C. Seeing biological motion. Nature 395, 894–6 (1998).
Simion, F., Regolin, L. & Bulf, H. A predisposition for biological motion in the newborn baby. Proc. Natl. Acad. Sci. USA 105, 809–13 (2008).
Pavlova, M. Biological motion processing as a hallmark of social cognition. Cereb. Cortex 22, 981–95 (2012).
Thompson, J. & Parasuraman, R. Attention, biological motion, and action recognition. Neuroimage 59, 4–13 (2012).
Rosa Salva, O., Mayer, U. & Vallortigara, G. Roots of a social brain: developmental models of emerging animacy-detection mechanisms. Neurosci. Biobehav. Rev. 50, 150–68 (2015).
Buescher, A. V. S., Cidav, Z., Knapp, M. & Mandell, D. S. Costs of autism spectrum disorders in the United Kingdom and the United States. JAMA Pediatr. 168, 721–8 (2014).
Lord, C., Elsabbagh, M., Baird, G. & Veenstra-Vanderweele, J. Autism spectrum disorder. Lancet 392, 508–520 (2018).
American Psychiatric Association. DSM-V. American Journal of Psychiatry, https://doi.org/10.1176/appi.books.9780890425596.744053 (2013).
Robertson, C. E. & Baron-Cohen, S. Sensory perception in autism. Nature Reviews Neuroscience, https://doi.org/10.1038/nrn.2017.112 (2017).
Lai, M.-C., Lombardo, M. V. & Baron-Cohen, S. Autism. Lancet (London, England) 383, 896–910 (2014).
Constantino, J. N. & Charman, T. Diagnosis of autism spectrum disorder: reconciling the syndrome, its diverse origins, and variation in expression. Lancet. Neurol. 15, 279–91 (2016).
Wang, S., Kloth, A. & Badura, A. The cerebellum, sensitive periods, and autism. Neuron 83, 518–32 (2014).
Fatemi, S. H. et al. Consensus paper: pathological role of the cerebellum in autism. Cerebellum 11, 777–807 (2012).
Paul, L. K., Corsello, C., Kennedy, D. P. & Adolphs, R. Agenesis of the corpus callosum and autism: a comprehensive comparison. Brain 137, 1813–29 (2014).
Kucharsky Hiess, R. et al. Corpus callosum area and brain volume in autism spectrum disorder: quantitative analysis of structural MRI from the ABIDE database. J. Autism Dev. Disord. 45, 3107–14 (2015).
Di Martino, A. et al. The autism brain imaging data exchange: towards a large-scale evaluation of the intrinsic brain architecture in autism. Mol. Psychiatry 19, 659–67 (2014).
Ecker, C. & Murphy, D. Neuroimaging in autism–from basic science to translational research. Nat. Rev. Neurol. 10, 82–91 (2014).
Stoner, R. et al. Patches of Disorganization in the Neocortex of Children with Autism. N. Engl. J. Med., https://doi.org/10.1056/NEJMoa1307491 (2014).
Haar, S., Berman, S., Behrmann, M. & Dinstein, I. Anatomical abnormalities in autism? Cereb. Cortex 26, 1440–52 (2016).
Khan, A. J. et al. Cerebro-cerebellar Resting-State Functional Connectivity in Children and Adolescents with Autism Spectrum Disorder. Biol. Psychiatry 78, 625–34 (2015).
Lewis, J. D. et al. The emergence of network inefficiencies in infants with Autism Spectrum Disorder. Biol. Psychiatry 82, 176–185 (2017).
Richards, C., Jones, C., Groves, L., Moss, J. & Oliver, C. Prevalence of autism spectrum disorder phenomenology in genetic disorders: a systematic review and meta-analysis. The lancet. Psychiatry 2, 909–16 (2015).
Iakoucheva, L. M., Muotri, A. R. & Sebat, J. Getting to the cores of autism. Cell 178, 1287–1298 (2019).
Gottesman, I. I. & Gould, T. D. The endophenotype concept in psychiatry: Etymology and strategic intentions. American Journal of Psychiatry, https://doi.org/10.1176/appi.ajp.160.4.636 (2003).
Kendler, K. S. & Neale, M. C. Endophenotype: A conceptual analysis. Molecular Psychiatry, https://doi.org/10.1038/mp.2010.8 (2010).
Klin, A., Shultz, S. & Jones, W. Social visual engagement in infants and toddlers with autism: early developmental transitions and a model of pathogenesis. Neurosci. Biobehav. Rev. 50, 189–203 (2015).
Homberg, J. R. et al. Understanding autism and other neurodevelopmental disorders through experimental translational neurobehavioral models. Neurosci. Biobehav. Rev. 65, 292–312 (2016).
Gammer, I. et al. Behavioural markers for autism in infancy: scores on the Autism Observational Scale for Infants in a prospective study of at-risk siblings. Infant Behav. Dev. 38, 107–15 (2015).
Ronconi, L. et al. Weak surround suppression of the attentional focus characterizes visual selection in the ventral stream in autism. NeuroImage. Clin. 18, 912–922 (2018).
Ronconi, L., Devita, M., Molteni, M., Gori, S. & Facoetti, A. Brief Report: When large becomes slow: Zooming-out visual attention is associated to orienting deficits in autism. J. Autism Dev. Disord. 48, 2577–2584 (2018).
Ronconi, L., Gori, S., Ruffino, M., Molteni, M. & Facoetti, A. Zoom-out attentional impairment in children with autism spectrum disorder. Cortex 49, 1025–1033 (2013).
Robertson, C. E., Kravitz, D. J., Freyberg, J., Baron-Cohen, S. & Baker, C. I. Tunnel vision: sharper gradient of spatial attention in autism. J. Neurosci. 33, 6776–81 (2013).
Keehn, B., Müller, R.-A. & Townsend, J. Atypical attentional networks and the emergence of autism. Neurosci. Biobehav. Rev. 37, 164–83 (2013).
Vlamings, P. H. J. M., Jonkman, L. M., van Daalen, E., van der Gaag, R. J. & Kemner, C. Basic abnormalities in visual processing affect face processing at an early age in autism spectrum disorder. Biol. Psychiatry 68, 1107–13 (2010).
Ewing, L., Pellicano, E. & Rhodes, G. Reevaluating the selectivity of face-processing difficulties in children and adolescents with autism. J. Exp. Child Psychol. 115, 342–55 (2013).
Foss-Feig, J. H., Tadin, D., Schauder, K. B. & Cascio, C. J. A substantial and unexpected enhancement of motion perception in autism. J. Neurosci. 33, 8243–9 (2013).
Kröger, A. et al. Visual event-related potentials to biological motion stimuli in autism spectrum disorders. Soc. Cogn. Affect. Neurosci. 9, 1214–22 (2014).
Cattaneo, L. et al. Impairment of actions chains in autism and its possible role in intention understanding. Proc. Natl. Acad. Sci. USA 104, 17825–30 (2007).
Becchio, C., Pierno, A., Mari, M., Lusher, D. & Castiello, U. Motor contagion from gaze: the case of autism. Brain 130, 2401–11 (2007).
Rochat, M. J. et al. Impaired vitality form recognition in autism. Neuropsychologia 51, 1918–24 (2013).
Casartelli, L., Molteni, M. & Ronconi, L. So close yet so far: Motor anomalies impacting on social functioning in autism spectrum disorder. Neurosci. Biobehav. Rev. 63, 98–105 (2016).
Ansuini, C., Podda, J., Battaglia, F. M., Veneselli, E. & Becchio, C. One hand, two hands, two people: Prospective sensorimotor control in children with autism. Dev. Cogn. Neurosci. 29, 86–96 (2018).
D’Angelo, E. & Casali, S. Seeking a unified framework for cerebellar function and dysfunction: from circuit operations to cognition. Front. Neural Circuits 6, 116 (2012).
Parma, V., Bulgheroni, M., Tirindelli, R. & Castiello, U. Body odors promote automatic imitation in autism. Biol. Psychiatry 74, 220–6 (2013).
Collignon, O. et al. Reduced multisensory facilitation in persons with autism. Cortex. 49, 1704–10 (2013).
Kwakye, L. D., Foss-Feig, J. H., Cascio, C. J., Stone, W. L. & Wallace, M. T. Altered auditory and multisensory temporal processing in autism spectrum disorders. Front. Integr. Neurosci. 4, 129 (2011).
Rozenkrantz, L. et al. A Mechanistic Link between Olfaction and Autism Spectrum Disorder. Curr. Biol. 25, 1904–10 (2015).
Endevelt-Shapira, Y. et al. Altered responses to social chemosignals in autism spectrum disorder. Nat. Neurosci. 21, 111–119 (2018).
Moore, D. G., Hobson, R. P. & Lee, A. Components of person perception: An investigation with autistic, non-autistic retarded and typically developing children and adolescents. Br. J. Dev. Psychol. 15, 401–423 (1997).
Pelphrey, K. A. & Carter, E. J. Brain mechanisms for social perception: lessons from autism and typical development. Ann. N. Y. Acad. Sci. 1145, 283–99 (2008).
Redcay, E. The superior temporal sulcus performs a common function for social and speech perception: implications for the emergence of autism. Neurosci. Biobehav. Rev. 32, 123–42 (2008).
Giorgio, E. et al. Filial responses as predisposed and learned preferences: Early attachment in chicks and babies. Behav. Brain Res. J. 1–15 (2016).
Bertenthal, B. I., Proffitt, D. R. & Cutting, J. E. Infant sensitivity to figural coherence in biomechanical motions. J. Exp. Child Psychol. 37, 213–30 (1984).
Fox, R. & McDaniel, C. The perception of biological motion by human infants. Science (80-.). 218, 486–487 (1982).
Hadad, B. S., Maurer, D. & Lewis, T. L. Long trajectory for the development of sensitivity to global and biological motion. Dev. Sci. 14, 1330–1339 (2011).
Hadad, B. S., Maurer, D. & Lewis, T. L. Sparing of sensitivity to biological motion but not of global motion after early visual deprivation. Dev. Sci. 15, 474–481 (2012).
Bottari, D. et al. The neural development of the biological motion processing system does not rely on early visual input. Cortex 71, 359–367 (2015).
Lewis, T. L. et al. Sensitivity to global form in glass patterns after early visual deprivation in humans. Vision Res. 42, 939–48 (2002).
Le Grand, R., Mondloch, C. J., Maurer, D. & Brent, H. P. Impairment in holistic face processing following early visual deprivation. Psychol. Sci. 15, 762–8 (2004).
Robbins, R. A., Nishimura, M., Mondloch, C. J., Lewis, T. L. & Maurer, D. Deficits in sensitivity to spacing after early visual deprivation in humans: A comparison of human faces, monkey faces, and houses. Dev. Psychobiol. 52, 775–781 (2010).
Ronconi, L., Molteni, M. & Casartelli, L. Building Blocks of Others’ Understanding: A Perspective Shift in Investigating Social-Communicative Deficit in Autism. Front. Hum. Neurosci., https://doi.org/10.3389/fnhum.2016.00144 (2016).
Karmiloff-Smith, A. Development itself is the key to understanding developmental disorders. Trends Cogn. Sci. 2, 389–398 (1998).
Casartelli, L. & Parma, V. The functional and developmental role of imitation in the (a) typical brain. The Behavioral and brain sciences 40,, e387 (2017).
Rezlescu, C., Barton, J. J. S., Pitcher, D. & Duchaine, B. Normal acquisition of expertise with greebles in two cases of acquired prosopagnosia. Proc. Natl. Acad. Sci. USA 111, 5123–5128 (2014).
Rizzolatti, G. & Sinigaglia, C. The mirror mechanism: a basic principle of brain function. Nat. Rev. Neurosci. 17, 757–765 (2016).
Rizzolatti, G., Fogassi, L. & Gallese, V. Neurophysiological mechanisms underlying the understanding and imitation of action. Nat. Rev. Neurosci. 2, 661–70 (2001).
Trevarthen, C. & Delafield-Butt, J. T. Autism as a developmental disorder in intentional movement and affective engagement. Front. Integr. Neurosci. 7, 49 (2013).
Pavlova, M., Staudt, M., Sokolov, A., Birbaumer, N. & Krägeloh-Mann, I. Perception and production of biological movement in patients with early periventricular brain lesions. Brain 126, 692–701 (2003).
Krägeloh-Mann, I. et al. Brain lesions in preterms: origin, consequences and compensation. Acta Paediatr. 88, 897–908 (1999).
Casile, A. & Giese, M. A. Critical features for the recognition of biological motion. J. Vis. 5, 6 (2005).
Casile, A. & Giese, M. A. Nonvisual motor training influences biological motion perception. Curr. Biol. 16, 69–74 (2006).
Sokolov, A. A. et al. Recovery of biological motion perception and network plasticity after cerebellar tumor removal. Cortex 59, 146–152 (2014).
Troje, N. F. & Westhoff, C. The inversion effect in biological motion perception: Evidence for a ‘Life Detector’? Curr. Biol. 16, 821–824 (2006).
Bareš, M. et al. Consensus paper: Decoding the Contributions of the Cerebellum as a Time Machine. From Neurons to Clinical Applications. Cerebellum https://doi.org/10.1007/s12311-018-0979-5 (2018).
Buckner, R. L. The cerebellum and cognitive function: 25 years of insight from anatomy and neuroimaging. Neuron 80, 807–815 (2013).
Baumann, O. et al. Consensus Paper: The Role of the Cerebellum in Perceptual Processes. 197–220, https://doi.org/10.1007/s12311-014-0627-7 (2015).
Casartelli, L., Riva, M., Villa, L. & Borgatti, R. Insights from perceptual, sensory, and motor functioning in autism and cerebellar primary disturbances: Are there reliable markers for these disorders? Neurosci. Biobehav. Rev. 95, 263–279 (2018).
Kaiser, M. D. & Pelphrey, K. A. Disrupted action perception in autism: behavioral evidence, neuroendophenotypes, and diagnostic utility. Dev. Cogn. Neurosci. 2, 25–35 (2012).
Whiten, A. & van de Waal, E. Social learning, culture and the ‘socio-cultural brain’ of human and non-human primates. Neurosci. Biobehav. Rev. 82, 58–75 (2017).
Elsabbagh, M. & Johnson, M. H. Autism and the Social Brain: The First-Year Puzzle. Biol. Psychiatry 80, 94–99 (2016).
Leslie, A. M., Friedman, O. & German, T. P. Core mechanisms in ‘theory of mind’. Trends Cogn. Sci. 8, 528–33 (2004).
Meunier, H. Do monkeys have a theory of mind? How to answer the question? Neurosci. Biobehav. Rev. 82, 110–123 (2017).
Premack, D. & Woodruff, G. Does the chimpanzee have a theory of mind? Behav. Brain Sci. 1, 515 (1978).
Schaafsma, S. M., Pfaff, D. W., Spunt, R. P. & Adolphs, R. Deconstructing and reconstructing theory of mind. Trends Cogn. Sci. 19, 65–72 (2015).
Di Giorgio, E. et al. Difference in Visual Social Predispositions Between Newborns at Low- and High-risk for Autism. Sci. Rep. 6, 26395 (2016).
Troje, N. F., & Basbaum, A. Biological motion perception. The senses: A comprehensive reference. 2, 231-238 (2008).
Baron-Cohen, S., Leslie, A. M. & Frith, U. Does the autistic child have a ‘theory of mind’? Cognition 21, 37–46 (1985).
Baron-Cohen, S., Campbell, R., Karmiloff-Smith, A., Grant, J. & Walker, J. Are children with autism blind to the mentalistic significance of the eyes? Br. J. Dev. Psychol. 13, 379–398 (1995).
Fishman, I., Keown, C. L., Lincoln, A. J., Pineda, J. A. & Müller, R. A. Atypical cross talk between mentalizing and mirror neuron networks in autism spectrum disorder. JAMA Psychiatry 71, 751–760 (2014).
Adolphs, R. The social brain: neural basis of social knowledge. Annu. Rev. Psychol. 60, 693–716 (2009).
Caspers, S., Zilles, K., Laird, A. R. & Eickhoff, S. B. ALE meta-analysis of action observation and imitation in the human brain. Neuroimage 50, 1148–1167 (2010).
Casartelli, L. & Molteni, M. Where there is a goal, there is a way: What, why and how the parieto-frontal mirror network can mediate imitative behaviours. Neurosci. Biobehav. Rev. 47, 177–193 (2014).
Lesourd, M. et al. Cerebral correlates of imitation of intransitive gestures: An integrative review of neuroimaging data and brain lesion studies. Neurosci. Biobehav. Rev. 95, 44–60 (2018).
Moher, D. et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 4, 1 (2015).
Klin, A. & Jones, W. Altered face scanning and impaired recognition of biological motion in a 15-month-old infant with autism: REPORT. Dev. Sci. 11, 40–46 (2008).
Koldewyn, K., Whitney, D. & Rivera, S. M. Neural correlates of coherent and biological motion perception in autism. Dev Sci 14, 1075–1088 (2011).
Krüger, B. et al. Perceived intensity of emotional Point–Light Displays is reduced in subjects with ASD. J. Autism Dev. Disord. 1–11, https://doi.org/10.1007/s10803-017-3286-y (2017).
van Boxtel, J. J. A., Dapretto, M. & Lu, H. Intact recognition, but attenuated adaptation, for biological motion in youth with autism spectrum disorder. Autism Res. 9, 1103–1113 (2016).
Karaminis, T., Arrighi, R., Forth, G., Burr, D. & Pellicano, E. Adaptation to the speed of Biological Motion in Autism. J. Autism Dev. Disord., https://doi.org/10.1007/s10803-019-04241-4 (2019).
Swettenham, J. et al. Perception of pointing from biological motion point-light displays in typically developing children and children with autism spectrum disorder. J. Autism Dev. Disord. 43, 1437–1446 (2013).
Borenstein, M., Hedges, L., Higgins, J. & Rothstein, H. Introduction to meta-analysis. (2009).
Lakens, D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front. Psychol. 4, 863 (2013).
Cohen, J. Statistical power analysis for the behavioral sciences. (Routledge, 2013).
Borenstein, M., Hedges, L. V., Higgins, J. P. & Rothstein, H. R. Introduction to meta-analysis (Wiley, 2009).
Tanner-Smith, E. E., Tipton, E. & Polanin, J. R. Handling Complex Meta-analytic Data Structures Using Robust Variance Estimates: a Tutorial in R. J. Dev. Life-Course Criminol. 2, 85–112 (2016).
Annaz, D. et al. Atypical development of motion processing trajectories in children with autism. Dev. Sci. 13, 826–838 (2010).
Hubert, B. et al. Brief report: Recognition of emotional and non-emotional biological motion in individuals with autistic spectrum disorders. J. Autism Dev. Disord. 37, 1386–1392 (2007).
Nackaerts, E. et al. Recognizing biological motion and emotions from Point-Light Displays in Autism Spectrum Disorders. PLoS One 7, 1–12 (2012).
Viechtbauer, W. & Cheung, M. W.-L. Outlier and influence diagnostics for meta-analysis. Res. Synth. Methods, https://doi.org/10.1002/jrsm.11 (2010).
Wright, K., Kelley, E. & Poulin-Dubois, D. Schematic and realistic biological motion identification in children with high-functioning autism spectrum disorder. Res. Autism Spectr. Disord. 8, 1394–1404 (2014).
Herrington, J. D. et al. The role of MT+/V5 during biological motion perception in Asperger Syndrome: An fMRI study. Res. Autism Spectr. Disord. 1, 14–27 (2007).
Murphy, P., Brady, N., Fitzgerald, M. & Troje, N. F. No evidence for impaired perception of biological motion in adults with autistic spectrum disorders. Neuropsychologia 47, 3225–3235 (2009).
Field, A. P. & Gillett, R. How to do a meta-analysis. Br. J. Math. Stat. Psychol. 63, 665–94 (2010).
Taylor, S. & Tweedie, R. A Non-parametric ‘Trim and Frill’ Method of Assessing Publication Bias in Meta-analysis. (1998).
Van der Hallen, R., Manning, C., Evers, K. & Wagemans, J. Global motion perception in Autism Spectrum Disorder: A Meta-Analysis. J. Autism Dev. Disord. 49, 4901–4918 (2019).
Todorova, G. K., Hatton, R. E. M. B. & Pollick, F. E. Biological motion perception in autism spectrum disorder: A meta-analysis. Mol. Autism 10 (2019).
Wright, K., Kelley, E. & Poulin-Dubois, D. Biological motion and the animate-inanimate distinction in children with high-functioning Autism Spectrum Disorder. Res. Autism Spectr. Disord. 25, 1–11 (2016).
Rutherford, M. D. & Troje, N. F. IQ predicts biological motion perception in autism spectrum disorders. J. Autism Dev. Disord. 42, 557–565 (2012).
Larsch, J. & Baier, H. Biological motion as an innate perceptual mechanism driving social affiliation. bioRxiv 347419, https://doi.org/10.1101/347419 (2018).
Brock, J., Brown, C. C., Boucher, J. & Rippon, G. The temporal binding deficit hypothesis of autism. Dev. Psychopathol. 14, 209–24 (2002).
Feldman, J. I. et al. Audiovisual multisensory integration in individuals with autism spectrum disorder: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 95, 220–234 (2018).
Wallace, M. T. & Stevenson, R. A. The construct of the multisensory temporal binding window and its dysregulation in developmental disabilities. Neuropsychologia, https://doi.org/10.1016/j.neuropsychologia.2014.08.005 (2014).
Zhou, H.-Y. et al. Multisensory temporal binding window in autism spectrum disorders and schizophrenia spectrum disorders: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 86, 66–76 (2018).
Foss-Feig, J. H., Schauder, K. B., Key, A. P., Wallace, M. T. & Stone, W. L. Audition-specific temporal processing deficits associated with language function in children with autism spectrum disorder. Autism Res. 10, 1845–1856 (2017).
Nakano, T., Ota, H., Kato, N. & Kitazawa, S. Deficit in visual temporal integration in autism spectrum disorders. 1027–1030, https://doi.org/10.1098/rspb.2009.1713 (2010).
Thompson, J. I. R. et al. Temporal processing as a source of altered visual perception in high autistic tendency. Neuropsychologia 69, 148–53 (2015).
Burr, D. & Morrone, C. Time Perception: Space–Time in the Brain. Curr. Biol. 16, R171–R173 (2006).
Zampini, M., Guest, S., Shore, D. I. & Spence, C. Audio-visual simultaneity judgments. Percept. Psychophys. 67, 531–44 (2005).
Denison, R. N., Driver, J. & Ruff, C. C. Temporal structure and complexity affect audio-visual correspondence detection. Front. Psychol. 3, 619 (2012).
Casartelli, L. Stability and flexibility in multisensory sampling: insights from perceptual illusions. J. Neurophysiol. 121, 1588–1590 (2019).
Ronconi, L., Busch, N. A. & Melcher, D. Alpha-band sensory entrainment alters the duration of temporal windows in visual perception. Sci. Rep. 8, 11810 (2018).
Ronconi, L. & Melcher, D. The Role of Oscillatory Phase in Determining the Temporal Organization of Perception: Evidence from Sensory Entrainment. J. Neurosci. 37, 10636–10644 (2017).
Powers, A. R., Hevey, M. A. & Wallace, M. T. Neural correlates of multisensory perceptual learning. J. Neurosci. 32, 6263–74 (2012).
Powers, A. R., Hillock, A. R. & Wallace, M. T. Perceptual training narrows the temporal window of multisensory binding. J. Neurosci. 29, 12265–74 (2009).
Stevenson, R. A., Wilson, M. M., Powers, A. R. & Wallace, M. T. The effects of visual training on multisensory temporal processing. Exp. Brain Res. 225, 479–489 (2013).
Sokolov, A. A., Gharabaghi, A., Tatagiba, M. S. & Pavlova, M. Cerebellar engagement in an action observation network. Cereb. Cortex 20, 486–91 (2010).
Sokolov, A. A. et al. Biological motion processing: the left cerebellum communicates with the right superior temporal sulcus. Neuroimage 59, 2824–30 (2012).
Sokolov, A. A., Erb, M., Grodd, W. & Pavlova, M. A. Structural loop between the cerebellum and the superior temporal sulcus: evidence from diffusion tensor imaging. Cereb. Cortex 24, 626–32 (2014).
Jack, A., Keifer, C. M. & Pelphrey, K. A. Cerebellar contributions to biological motion perception in autism and typical development. Hum. Brain Mapp. 38, 1914–1932 (2017).
Sokolov, A. A. et al. Structural and effective brain connectivity underlying biological motion detection. Proc. Natl. Acad. Sci. 201812859, https://doi.org/10.1073/pnas.1812859115 (2018).
Hiris, E. Detection of biological and nonbiological motion. J. Vis. 7(4), 1–16 (2007).
Brancucci, A., Lucci, G., Mazzatenta, A. & Tommasi, L. Asymmetries of the human social brain in the visual, auditory and chemical modalities. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 364, 895–914 (2009).
Grossman, E. et al. Brain areas involved in perception of biological motion. J. Cogn. Neurosci. 12, 711–20 (2000).
Pelphrey, K. A. et al. Brain activity evoked by the perception of human walking: controlling for meaningful coherent motion. J. Neurosci. 23, 6819–25 (2003).
Grosbras, M.-H., Beaton, S. & Eickhoff, S. B. Brain regions involved in human movement perception: a quantitative voxel-based meta-analysis. Hum. Brain Mapp. 33, 431–54 (2012).
Grosbras, M.-H. & Paus, T. Brain Networks Involved in Viewing Angry Hands or Faces. Cereb. Cortex 16, 1087–1096 (2006).
Thompson, J. C., Hardee, J. E., Panayiotou, A., Crewther, D. & Puce, A. Common and distinct brain activation to viewing dynamic sequences of face and hand movements. Neuroimage 37, 966–973 (2007).
Jellema, T. & Perrett, D. I. Cells in monkey STS responsive to articulated body motions and consequent static posture: A case of implied motion? Neuropsychologia 41, 1728–1737 (2003).
Seltzer, B. & Pandya, D. N. Parietal, temporal, and occipital projections to cortex of the superior temporal sulcus in the rhesus monkey: a retrograde tracer study. J. Comp. Neurol. 343, 445–63 (1994).
Knolle, F., Schröger, E. & Kotz, S. A. Cerebellar contribution to the prediction of self-initiated sounds. Cortex 49, 2449–2461 (2013).
Herrington, J. D., Nymberg, C. & Schultz, R. T. Biological motion task performance predicts superior temporal sulcus activity. Brain Cogn. 77, 372–81 (2011).
Freitag, C. M. et al. Perception of biological motion in autism spectrum disorders. Neuropsychologia 46, 1480–94 (2008).
Ahmed, A. A. & Vander Wyk, B. C. Neural processing of intentional biological motion in unaffected siblings of children with autism spectrum disorder: An fMRI study. Brain and Cognition (2013).
Kaiser, M. D. et al. Neural signatures of autism. Proc. Natl. Acad. Sci. USA 107, 21223–8 (2010).
Alaerts, K., Swinnen, S. P. & Wenderoth, N. Neural processing of biological motion in autism: An investigation of brain activity and effective connectivity. Sci. Rep. 7, 5612 (2017).
McKay, L. S. et al. Do distinct atypical cortical networks process biological motion information in adults with Autism Spectrum Disorders? Neuroimage 59, 1524–33 (2012).
Atkinson, A. P. Impaired recognition of emotions from body movements is associated with elevated motion coherence thresholds in autism spectrum disorders. Neuropsychologia 47, 3023–3029 (2009).
Falck-Ytter, T. et al. Reduced orienting to audiovisual synchrony in infancy predicts autism diagnosis at 3 years of age. J. Child Psychol. Psychiatry Allied Discip. 59, 872–880 (2018).
Acknowledgements
This work was supported by the award fellowship granted by SISSA to V.P., by a grant from the Italian Ministry of Health (RC 2016–2018 to L.C.), by “5per1000” funds for biomedical research (Scientific Institute IRCCS Medea; 2016, 2017) to L.C., and also by a grant from MIUR (Dipartimenti di Eccellenza DM 11/05/2017 n. 262) to the Department of General Psychology, University of Padova to M.V. The funders did not participate in the conception and development of this work. We additionally want to warmly thank the authors of the papers included in the meta-analysis who sent us the data we required along the process.
Author information
Authors and Affiliations
Contributions
A.F., V.P., L.C. and L.Ro. have conceived the idea of the paper. A.F. and L.Ra. executed the literature search, coded the papers and calculated the effect sizes, independently. A.F. and M.V. defined the analytical protocol and performed the analyses. A.F., V.P., L.C. and L.Ro. drafted the article, and all authors critically revised it. All authors approved the final version of this article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Federici, A., Parma, V., Vicovaro, M. et al. Anomalous Perception of Biological Motion in Autism: A Conceptual Review and Meta-Analysis. Sci Rep 10, 4576 (2020). https://doi.org/10.1038/s41598-020-61252-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-61252-3
This article is cited by
-
Evaluations of dyadic synchrony: observers’ traits influence estimation and enjoyment of synchrony in mirror-game movements
Scientific Reports (2024)
-
Impaired Biological Motion Processing and Motor Skills in Adults with Autistic Traits
Journal of Autism and Developmental Disorders (2023)
-
Associations Between Autism Spectrum Quotient and Integration of Visual Stimuli in 9-year-old Children: Preliminary Evidence of Sex Differences
Journal of Autism and Developmental Disorders (2023)
-
Atypical gaze patterns in autistic adults are heterogeneous across but reliable within individuals
Molecular Autism (2022)
-
Attentional influences on neural processing of biological motion in typically developing children and those on the autism spectrum
Molecular Autism (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.