Abstract
Previous studies have demonstrated effects of racial and socioeconomic factors on survival of adults with cancer. While less studied in the pediatric population, data exist demonstrating disparities of care and survival in pediatric oncology patients based on socioeconomic and racial/ethnic factors. Brain cancers recently overtook leukemia as the number one cause of childhood cancer fatalities, but demographic and socioeconomic disparities in these tumors have not been adequately studied. We obtained data from the SEER Program of the National Cancer Institute (NCI). We selected patients under 19 years of age with central nervous system (CNS) cancers diagnosed between 2000 and 2015. We included patient demographics, tumor characteristics, treatment, and socioeconomic characteristics as covariates in the analysis. We measured overall survival and extent of disease at diagnosis. We saw that Black and Hispanic patients overall had a higher risk of death than non-Hispanic White patients on multivariable analysis. On stratified analysis, Black and Hispanic patients with both metastatic and localized disease at diagnosis had a higher risk of death compared to White, non-Hispanic patients, although the difference in Black patients was not significant after adjusting for mediating factors. However, our findings on extent of disease at diagnosis demonstrated that neither Black race nor Hispanic ethnicity increased the chance of metastatic disease at presentation when controlling for mediating variables. In summary, racial and ethnic disparities in childhood CNS tumor survival appear to have their roots at least partially in post-diagnosis factors, potentially due to the lack of access to high quality care, leading to poorer overall outcomes.
Similar content being viewed by others
Introduction
Previous studies have demonstrated effects of racial and socioeconomic factors on survival of adults with cancer1,2,3. For instance, it has been shown that access to private insurance compared to Medicaid or no insurance positively affects the prognosis of adult glioblastoma patients leading to better overall survival4. While less studied in the pediatric population, data exist demonstrating disparities of care and survival in pediatric oncology patients based on socioeconomic and racial/ethnic factors5,6,7. In the U.S., malignant neoplasms are the leading cause of death by disease in children past infancy. Brain cancers recently overtook leukemia as the number one cause of childhood cancer fatalities, despite being less common8. This is due to improvements in leukemia treatment and reduction of mortality in these patients, as well as the relative stagnation of improved outcomes in brain cancer patients. Despite improvement of overall survival in leukemia patients, children of lower socioeconomic status have benefitted less than their higher income contemporaries9. Length of time to diagnosis and treatment modalities influence survival of children with various cancers10,11. Additionally, treatment factors such as extent of tumor resection, tumor location, age, and year of diagnosis have been shown to be associated with survival in pediatric glioblastoma patients12. However, little attention has been devoted to specific demographic and socioeconomic risk factors contributing to survival of childhood brain cancers as a group, and how these factors may influence survival. In this context, we hypothesized that demographic and socioeconomic disparities may impair access to care and/or quality of care in childhood brain cancers, leading to poorer survival. In this study, we examined the effect of demographic and socioeconomic factors on survival in pediatric brain tumors using the Surveillance, Epidemiology, and End Results (SEER) database. We analyzed overall survival and stage at diagnosis for subjects 0–19 years of age, based on race, ethnicity, socioeconomic status, and other demographic variables to determine risk factors and mechanisms of poorer outcomes in this group.
Methods
Data collection
Data were obtained from the SEER Program of the National Cancer Institute (NCI). SEER collects information from population-based cancer registries that currently cover approximately 28 percent of the U.S. population. Available data include information on patient demographics, tumor characteristics at diagnosis, treatment, survival time based on linkage to mortality data from the National Center for Health Statistics, and county-level socioeconomic information based on census data.
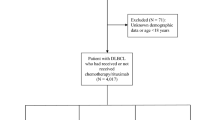
We selected patients between the ages of 0 and 19 years with central nervous system (CNS) tumors (International Classification of Diseases for Oncology 3rd edition (ICD-O-3)13 topography codes C70.0-C70.9, C71.0-C71.9, C72.0-C72.9, C75.1, and C75.3) diagnosed between 2000 and 2015 from the SEER 18 Regs Custom Data, Nov 2017 Submission using SEER*Stat Version 8.3.5. We selected only those tumors with malignant behavior and only the first occurrence of cancer. We limited the following International Classification of Childhood Cancer, Third edition (ICCC-3)14 site recodes to Grades III and IV: III(b) Astrocytomas, III(d.1) Oligodendrogliomas, III(d.2) Mixed and unspecified gliomas, III(a.2) Choroid plexus tumor, III(d.3) Neuroepithelial glial tumors of uncertain origin, III(e.4) Neuronal and mixed neuronal-glial tumors, III(e.5) Meningiomas, III(f) Unspecified intracranial and intraspinal neoplasms. For the remaining ICCC-3 CNS site recodes, all patients with known grade were included.
We excluded cases reported on death certificates or autopsies only, cases with zero days of survival, and cases for whom this was not the patient’s first primary malignant tumor. The remaining cases were categorized according to the ICCC-3 site recode into five tumor types: (1) ependymomas, (2) gliomas (including astrocytomas, oligodendrogliomas, and mixed and unspecified gliomas), (3) PNET/Pineal/ATRT (including primitive neuroectodermal tumors (PNET), atypical teratoid/rhabdoid tumors (ATRT), and pineal parenchymal tumors), and (4) medulloblastomas (including medulloblastomas and medulloepithelioma). Other/unspecified tumors (including chorioid plexus tumors, germ cell tumors, neuroepithelial glial tumors of uncertain origin, neuronal and mixed neuronal-glial tumors, meningiomas, and unspecified intracranial and intraspinal neoplasms) were included in the overall analysis, but because of the heterogeneity of this group, these tumors were not included in subgroup analysis.
We included year of diagnosis, patient demographics, tumor characteristics, treatment, and socioeconomic characteristics as covariates in the analysis. Patient characteristics included year of diagnosis, sex (male and female), race (White, Black, and Other), Hispanic ethnicity, age group (0 years, 1 to 4 years, 5 to 9 years, 10 to 14 years, and 15 to 19 years), and insurance status (private versus public or no insurance; note that insurance information was only available beginning in 2007). Tumor characteristics included SEER Summary Stage (localized versus regional or distant, which together composed the metastatic category) and tumor size (less than 6 cm, 6 cm or more, and unknown). Treatment variables were limited to treatment at initial diagnosis and included the use of radiation therapy and surgery at the primary site. Chemotherapy was not included as a variable because it is not a standard measure available through SEER and, when collected, is not captured as sensitively as data on radiation therapy15.
County-level socioeconomic characteristics were obtained from 2007–2011 Census American Community Survey data and included percent population with less than a high school degree, percent of families below the poverty line, and percent of households in language isolation. The Census Bureau considers a household to be linguistically isolated when all members above the age of 14 speak a non-English language and speak English less than “very well.” For these socioeconomic characteristics, patients were categorized by whether the patient’s county was in the most disadvantaged quartile for the study population.
Statistical analysis
We measured overall survival as the number of months from diagnosis to death due to any cause. Patients who were alive at the last follow-up were censored at the date of last follow-up, and patients surviving more than five years were censored at 60 months.
We performed statistical analyses using SAS software, Version 9.4 (SAS Institute Inc., Cary, NC), and significance was defined at a p-value <0.05. Chi-square tests were used to evaluate bivariate associations between covariates and tumor type. Multivariable logistic regression was used to evaluate the effects of demographic, socioeconomic, and tumor characteristics on metastatic disease at diagnosis and overall survival, as well as the use of radiation and surgery. Kaplan-Meier survival curves and the log-rank test were used to evaluate univariate effects on survival. Hazard ratios (HR) were obtained using Cox proportional hazards for univariate and multivariable survival analyses. The proportional hazards assumption was evaluated using Schoenfeld residuals and violations were addressed using time-dependent interaction terms in multivariable models.
Results
A total of 1,881 unique records were selected from the SEER database with a diagnosis of malignant tumors of the CNS, including both cranial and spinal neoplasms. Patient characteristics, demographics, and disease categories are shown in Table 1. Of the total number of patients, Whites comprise 78.15% (1,470/1,881) of the cohort, Blacks 13.18%, non-Hispanics 72.09%, and Hispanics 27.91%. To study the effect of socioeconomic factors on survival, patients in the highest quartile of the sample for percent with high school or less education, percent below poverty level, and percent language isolation were considered the most disadvantaged. Gliomas were the most common tumors (n = 788), followed by ependymomas and medulloblastomas.
Univariate analysis
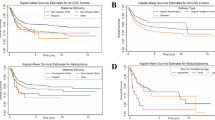
We first undertook a univariate analysis to determine which factors correlated with risk of death (Table 2). Age, race, ethnicity, tumor type, extent of disease, and living in areas with higher poverty all had a significant impact on risk of death. Survival was worse for Black compared to White patients (HR: 1.31, 95% CI: 1.08–1.58, p-value = 0.0064) and worse for Hispanic compared to non-Hispanic patients (HR: 1.25, 95% CI: 1.07–1.45, p-value = 0.0038). Areas of high poverty exhibited worse overall survival (HR: 1.26, 95% CI: 1.09–1.46, p-value = 0.0019). The risk of death was also greater in subjects with metastatic disease compared to localized, and ages 0 and 5–9 years compared to age 1–4 years. The risk of death was lower in all tumor subtypes compared to gliomas and lower for subjects who underwent surgery compared to those who did not. Tumor size, the remaining socioeconomic measures, and radiation administration did not show significant association with overall survival.
In tumor subtype analyses (Supplemental Table 1), significant effects included a greater risk for death in ependymoma and medulloblastoma patients with non-private insurance compared to privately insured subjects, and in Hispanic compared to non-Hispanic patients. In glioma patients, there was an increased risk of death in Black patients compared to White patients as well as for those living in areas of highest poverty. Ependymoma in less educated areas and areas with greater language isolation showed significantly increased risk of death compared with more educated and less language isolated areas.
Multivariable analysis
We then undertook multivariable analysis to determine which factors retained an effect on survival after controlling for mediating factors (Table 2). We started with a base model in which we controlled for demographic and tumor characteristics, then added treatment, and finally socioeconomic characteristics. In the base model, Black patients had worse survival compared to White patients (HR: 1.39, 95% CI: 1.14–1.70, p-value = 0.0014), and Hispanic patients had worse survival compared to non-Hispanic patients (HR: 1.36, 95% CI: 1.16–1.60, p-value = 0.0002). Other race showed no significant difference in survival compared with White race. After controlling for both treatment and socioeconomic characteristics, the HRs for both Black and Hispanic patients decreased, but remained significant (HR: 1.29, 95% CI: 1.04, 1.59, p-value = 0.0206; HR 1.29, 95% CI: 1.08, 1.53, p-value = 0.0051, respectively). Accounting for demographics, tumor type, and treatment modalities, patients in the highest quartile for county-level percent below poverty had poorer survival (HR: 1.27, 95% CI 1.03–1.57, p-value = 0.0282).
Overall, other tumor types had improved survival compared to gliomas, metastatic patients had poorer survival, patients receiving surgery had improved survival, and patients receiving radiation had improved survival initially, but survival worsened compared to those without radiation as follow-up time progressed (Supplemental Fig. 1).
When stratifying by tumor type and controlling for demographic, treatment, and socioeconomic characteristics (Supplemental Table 1), Black children diagnosed with ependymoma had a higher risk of mortality compared with White children, while the increased mortality risk for Hispanic children did not quite meet statistical significance. For medulloblastoma patients, increased poverty worsened survival, but a lower level of education was associated with improved survival. Glioma patients receiving surgery had a decreased HR compared with those not receiving surgery.
Extent of disease analysis
To determine whether survival disparities could be explained by a greater extent of disease at diagnosis, we examined the relationship of race and ethnicity with metastatic disease in a multivariable analysis, using models with and without consideration for socioeconomic characteristics (Table 3). In the base model, Hispanic patients had higher odds of being diagnosed with metastatic disease, but after controlling for socioeconomic characteristics, this difference was no longer significant. These results matched those found specifically in ependymoma, while in PNET/Pineal/ATRT tumors, the relationship remained significant even after controlling for socioeconomic characteristics. Black patients did not have an increased odds of metastatic disease overall or within any of the tumor types.
Stratified analysis by extent of disease
Next, to determine potential influences of our variables of interest on patient outcomes after diagnosis and during the treatment process, we performed a multivariable analysis stratified by extent of disease (Table 4). We used the same three survival models to examine mediation: a base model with demographic and tumor characteristics, one controlling for treatment, and finally controlling for treatment and socioeconomic factors. In the base model for localized disease, both Black patients and Hispanic patients had poorer survival (HR 1.35, p-value = 0.0131; HR 1.36, p-value = 0.0023). After controlling for treatment and socioeconomic factors, the HRs for both Black and Hispanic patients decreased, with the HR for Black patients no longer significant (HR: 1.24, p-value = 0.0954) while the HR for Hispanic patients remained significant (HR: 1.27, p-value=0.0304). A similar pattern for Black patients was seen in the models limited to metastatic disease. In the base model, Black patients had significantly poorer survival (HR: 1.53, p-value = 0.0323), and in the full model, the HR decreased and was no longer significant (HR: 1.41, p-value = 0.1026). Hispanic patients had significantly poorer survival even in the full model (HR: 1.38, p-value = 0.0462). Areas with high poverty were associated with decreased survival (HR:1.77, p-value = 0.0084), and higher language isolation was initially associated with better survival, but survival worsened in comparison to persons living in areas with lower language isolation as follow-up increased.
In survival analyses stratified by tumor type and extent of disease (Supplemental Table 3), Black patients had worse survival than White patients for metastatic ependymoma and medulloblastoma. PNET/Pineal/ATRT patients with metastatic disease and living in high poverty areas showed worse survival when compared with those living in lower-poverty areas. Patients with medulloblastoma living in areas of lower education had better survival than those living in higher educated areas.
Treatment modality analysis
Finally, we assessed the association of our variables of interest with the likelihood of patients being treated with surgery and/or radiation, the two treatment modalities measured in standard SEER data (Fig. 1). Overall, race or ethnicity were not predictive as to whether patients received surgery or radiation. When the analysis predicting surgery was stratified by extent of disease, Black patients with localized disease were less likely than White patients to undergo surgery (OR: 0.54, 95% CI: 0.33–0.88, p-value = 0.0132), and Black patients with metastatic disease were more likely than White patients to undergo surgery (OR: 3.38, 95% CI: 1.12–10.17, p-value = 0.0304). When the analysis predicting radiation was stratified by extent of disease, Hispanic patients with metastatic disease were more likely to receive radiation (OR: 2.13, 95% CI: 1.18–3.83, p-value = 0.0121).
Discussion
In this study, we used SEER data to evaluate associations of demographic and socioeconomic variables with extent of disease at diagnosis and survival outcomes in childhood brain tumors. Black race and Hispanic ethnicity were both significantly associated with decreased overall survival after adjustment for mediating factors. Independently, those patients in areas of highest poverty had decreased overall survival when compared with areas of lower poverty. Our findings in pediatric brain tumors are consistent with previous studies showing disparities in outcomes for racial and ethnic minorities in other adult and pediatric cancers3,6,7. These prior studies have shown Black race and Hispanic ethnicity to be associated with poorer survival and/or later stage disease at presentation, although one study showed some mitigation by accounting for socioeconomic measures7.
Our findings on extent of disease at diagnosis suggest that Black race did not increase the chance of metastatic disease at presentation, and while Hispanic ethnicity did, it was in part explained by socioeconomic status. When we looked at survival stratified by extent of disease, we saw that Black and Hispanic patients with both metastatic and localized disease at diagnosis showed significant differences in survival likelihood compared to their White, non-Hispanic counterparts. However, once we controlled for treatment and socioeconomic factors, the survival difference was no longer significant for Black patients. Additionally, we saw evidence of differences in treatment by race and ethnicity when stratified by extent of disease, although this was somewhat limited by the lack of chemotherapy data. These data suggest that racial and ethnic disparities appear to be partially explained by post-diagnosis mediating factors that may fall in the pathway between race/ethnicity and poorer survival.
These post-diagnosis disparities depend in part on socioeconomic status, potentially implying lack of access to high quality care, leading to poorer overall outcomes, a root cause of disparities that has been described in other cancers16. Other factors on the patient/family side may also contribute to poorer outcomes post-diagnosis, such as language proficiency, stress about the cost of care, inability to take off time from work, and ability to secure transportation to treatment17. Socioeconomic factors also appeared to mediate the ethnic differences in the extent of disease at presentation overall. These findings overall are concordant with previous studies that have shown a correlation between disadvantaged socioeconomic status and survival outcome for both leukemias and solid tumors2,9. We had access to patients’ insurance status for only a subset of our population, and due to the smaller sample size, it was not included in multivariable analysis. As additional years of insurance data are collected, future studies may elucidate the association of insurance with extent of disease and survival. While potential biological differences in tumors between groups cannot be excluded and need to be further investigated, contributions to disparities based on socioeconomic status independent of race and ethnicity argue against an explanation based on racial/ethnic differences in tumor biology alone. To better understand underlying causes that contribute to the disparity of outcomes in pediatric brain tumors, patient-level data should be utilized in future studies to investigate both biological factors and pre/post-diagnosis treatment gaps in the care of children diagnosed with CNS tumors in the hopes of improving outcomes.
References
Parikh, R. R., Grossbard, M. L., Green, B. L., Harrison, L. B. & Yahalom, J. Disparities in survival by insurance status in patients with Hodgkin lymphoma. Cancer 121, 3515–3524, https://doi.org/10.1002/cncr.29518 (2015).
Nathan, S. S. & Healey, J. H. Making a case for the socioeconomic determinacy of survival in osteosarcoma. Clin. Orthop. Relat. Res. 471, 784–791, https://doi.org/10.1007/s11999-012-2575-1 (2013).
Grubb, W. R. et al. Racial and Ethnic Disparities in the Pediatric Hodgkin Lymphoma Population. Pediatr. Blood Cancer 63, 428–435, https://doi.org/10.1002/pbc.25802 (2016).
Rong, X. et al. Influence of insurance status on survival of adults with glioblastoma multiforme: A population-based study. Cancer 122, 3157–3165, https://doi.org/10.1002/cncr.30160 (2016).
Shen, C. J. et al. Socioeconomic factors affect the selection of proton radiation therapy for children. Cancer 123, 4048–4056, https://doi.org/10.1002/cncr.30849 (2017).
Jacobs, A. J., Lindholm, E. B., Levy, C. F., Fish, J. D. & Glick, R. D. Racial and ethnic disparities in treatment and survival of pediatric sarcoma. J. Surg. Res. 219, 43–49, https://doi.org/10.1016/j.jss.2017.05.031 (2017).
Cooney, T., Fisher, P. G., Tao, L., Clarke, C. A. & Partap, S. Pediatric neuro-oncology survival disparities in California. J. Neurooncol 138, 83–97, https://doi.org/10.1007/s11060-018-2773-0 (2018).
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer Statistics, 2017. CA Cancer J. Clin. 67, 7–30, https://doi.org/10.3322/caac.21387 (2017).
Petridou, E. T. et al. Socioeconomic disparities in survival from childhood leukemia in the United States and globally: a meta-analysis. Ann. Oncol. 26, 589–597, https://doi.org/10.1093/annonc/mdu572 (2015).
Baker, J. M. et al. Influence of length of time to diagnosis and treatment on the survival of children with acute lymphoblastic leukemia: a population-based study. Leuk. Res. 38, 204–209, https://doi.org/10.1016/j.leukres.2013.11.014 (2014).
Ferrari, A. et al. The Sooner the Better? How Symptom Interval Correlates With Outcome in Children and Adolescents With Solid Tumors: Regression Tree Analysis of the Findings of a Prospective Study. Pediatr. Blood Cancer 63, 479–485, https://doi.org/10.1002/pbc.25833 (2016).
Lam, S., Lin, Y., Zinn, P., Su, J. & Pan, I. W. Patient and treatment factors associated with survival among pediatric glioblastoma patients: A Surveillance, Epidemiology, and End Results study. J. Clin. Neurosci. 47, 285–293, https://doi.org/10.1016/j.jocn.2017.10.041 (2018).
Fritz A, P. C., et al. International Classification of Diseases for Oncology, third edition. (World Health Organization, 2000).
Steliarova-Foucher, E., Stiller, C., Lacour, B. & Kaatsch, P. International Classification of Childhood Cancer, third edition. Cancer 103, 1457–1467, https://doi.org/10.1002/cncr.20910 (2005).
Noone, A. M. et al. Comparison of SEER Treatment Data With Medicare Claims. Med. Care 54, e55–64, https://doi.org/10.1097/MLR.0000000000000073 (2016).
Esnaola, N. F. & Ford, M. E. Racial differences and disparities in cancer care and outcomes: where’s the rub? Surg. Oncol. Clin. N. Am. 21, 417–437, https://doi.org/10.1016/j.soc.2012.03.012 (2012). viii.
Kullgren, J. T. & McLaughlin, C. G. Beyond affordability: the impact of nonfinancial barriers on access for uninsured adults in three diverse communities. J. Community Health 35, 240–248, https://doi.org/10.1007/s10900-010-9230-0 (2010).
Acknowledgements
Dr. Cockburn and Ms. Eguchi were supported in part by P30CA046934.
Author information
Authors and Affiliations
Contributions
R.F. and A.L.G. conceptualized the study. R.F. and S.Z. led the data collection. M.E. led the data analysis, with contributions from R.F., S.Z., A.L.G., and M.C. R.F. led the manuscript writing, with contributions from S.Z., M.E., and A.L.G. M.E. created the tables, with contributions from S.Z. S.Z. led the manuscript revisions, with data analysis by M.E. M.H., M.E., and A.L.G. contributed to manuscript revisions. All authors approved the final submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fineberg, R., Zahedi, S., Eguchi, M. et al. Population-Based Analysis of Demographic and Socioeconomic Disparities in Pediatric CNS Cancer Survival in the United States. Sci Rep 10, 4588 (2020). https://doi.org/10.1038/s41598-020-61237-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-61237-2
This article is cited by
-
Disparities in Neuro-Oncology
Current Neurology and Neuroscience Reports (2023)
-
Facility patient volume and survival among individuals diagnosed with malignant central nervous system tumors
Journal of Neuro-Oncology (2023)
-
Population-based analysis of CNS tumor diagnoses, treatment, and survival in congenital and infant age groups
Journal of Neuro-Oncology (2022)
-
Racial and ethnic disparities among children with primary central nervous system tumors in the US
Journal of Neuro-Oncology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.