Abstract
Encapsulated bioactive agents applied to the Lactuca sativa L. present an innovative approach to stimulate the production of plant secondary metabolites increasing its nutritive value. Calcium and copper ions were encapsulated in biopolymeric microparticles (microspheres and microcapsules) either as single agents or in combination with biocontrol agents, Trichoderma viride spores, a fungal plant growth mediator. Both, calcium and copper ions are directly involved in the synthesis of plant secondary metabolites and alongside, Trichoderma viride can provide indirect stimulation and higher uptake of nutrients. All treatments with microparticles had a positive effect on the enhancement of plant secondary metabolites content in Lactuca sativa L. The highest increase of chlorophylls, antioxidant activity and phenolic was obtained by calcium-based microparticles in both, conventionally and hydroponically grown lettuces. Non-encapsulated fungus Trichoderma viride enhanced the synthesis of plant secondary metabolites only in hydroponics cultivation signifying the importance of its encapsulation. Encapsulation proved to be simple, sustainable and environmentally favorable for the production of lettuce with increased nutritional quality, which is lettuce fortified with important bioactive compounds.
Similar content being viewed by others
Introduction
Plant secondary metabolites (PSM) are natural sources of biologically active compounds used for a healthy diet, in traditional medicine and in a wide range of industrial applications1. The interest in enhancing PSM production is focused to obtain high yields suitable for commercial exploitation. Plant content of secondary plant metabolites is affected by genetic, environmental, and agronomic factors2. A variety of strategies (screening and selection of high-yielding cell lines, the culture of cells from various plant parts, suspension culture, induction by elicitors, metabolic engineering, optimizing media, plant growth regulators, etc.)3 as well as treatments with microspheres loaded with chemical and biological agents4 were used for enhancing PSM production in plant cell culture.
PSM such as polyphenols encompasses several classes of structurally diverse natural products biogenetically arising from the shikimate-phenylpropanoids-flavonoids pathways. Plants require these compounds for pigmentation, growth, reproduction, resistance to pathogens and for many other functions and they represent the adaptive characteristics that were subjected to the natural selection during evolution. In comparison to the animals, plants synthesize a broader spectrum of PSM because of the immobility and impossibility to escape predators, thus they evolved such a chemically based defense against predators5. The number of plant secondary metabolites in fresh lettuce can be improved with the addition of desirable compounds during the growth which is readily available for the plant root uptake. Higher PSM share would also have an important impact on human health by improving the antioxidant and nutrient intake through the human diet6,7.
With the broad spectrum of different secondary metabolites, plants can respond to diverse enemies and stressors. Since the production of the specific resistance traits can be extremely costly, new ways of defense enhancements should be employed. Methods involving increasing the expression of endogenous compounds could greatly influence plant resistance characteristics against plant attackers5.
Living microorganisms can be applied to the seeds, plant surfaces, or in the soil, in order to colonize the rhizosphere or the interior of the plant and promote growth by increasing the supply or availability of primary nutrients to the host plant8. Inoculation with Arbuscular mycorrhizal fungi enhances phenolics content and increases the antioxidant activity of lettuce leaves9, but efficient formulation demands a carrier material for living microorganism which must keep its functional properties after application.
One of the ways for site-specific delivery of living microorganisms is their encapsulation. Encapsulation is always developing technology that is superior to the other formulations in terms of living microorganisms protection from the harsh environment, with improved viability and the possibility of controlled and targeted release into the field10. Studies showed that Trichoderma species may induce changes in the microbiota composition of roots, enhance nutrient uptake, stabilize soil nutrients, promote root development, and increase root hair formation11. The dual roles of antagonistic activity against plant pathogens and the promotion of soil fertility make Trichoderma species a promising alternative to standard plant protection and nutrition methods.
Calcium ions are an essential part that plays an important role in the structure and permeability of cell membranes, plant cell division and elongation, carbohydrate translocation and N-metabolism12,13. Calcium cations also play a regulatory role in signal transduction and in the absorption of nutrients across the cell membranes13,14,15. Ca2+ has a role in signaling and helps in the upregulation of respective genes for polyphenols biosynthesis16. Ca2+ binds to the membrane phospholipids thus stabilizing the lipid bilayer and providing the structural integrity17,18 and is exhibited by the reduced malondialdehyde content in the plants treated with Ca2+ 14,19,20. Ca2+ is generally found in soil but it is relatively insoluble (e.g. CaCO3) in prevalent form. Trichoderma species acidify their surrounding environment by secreting organic acids and are able to solubilize phosphates, micronutrients and mineral cations21. From the other side, the simultaneous addition of calcium cations together with biocontrol agents improves the activity of biocontrol agents, that is, through a synergistic act22.
Copper ions show a stimulatory effect on the production of secondary metabolites in plants. They can induce synthesis of PSM with a positive effect on alkaloid production, synthesis of shikonin23,24, the production of digitalin25 and betalains26. Even though Cu2+ is a micro-constituent of growth media and is known to be essential for several biochemical and physiological pathways27 at higher concentrations it becomes toxic28. Therefore it is important to control the dosage of copper ions over the plant maturation time and to minimize the release into the environment which can be achieved by encapsulation.
Encapsulation results with more efficient use of chemicals and a convenient way of nutrients delivery for ecological and sustainable plant production29,30,31,32,33. Optimization of the encapsulation process is important to obtain microparticles with desirable traits. In our previous work, we have prepared microparticles for further applications29,30,31,32,33. This research introduces the application of optimized microparticles for the strategic delivery of active compounds to the plant (in this case lettuce) throughout the whole period of maturation. Not only with the intention to increase PSM to repel predators and pathogens but, consequently, also to obtain functional foods, lettuce fortified with important bioactive compounds.
Materials and Methods
Materials
Low-viscosity sodium alginate (CAS Registry No. 9005-38-3; A1112, Brookfield viscosity 4−12 cPs (1% in H2O at 25 °C)) and low molecular weight chitosan (CAS Registry No. 9012-76-4; 448869, molecular weight 100,000−300,000) were purchased from Sigma Aldrich (USA). All other chemicals were of analytical grade and used as received without further purification.
An indigenous isolate of T. viride originated from parasitized sclerotia of Sclerotinia sclerotiorum was used in all experiments34. To obtain spore suspensions, the fungus Trichoderma viride was grown in potato dextrose broth. Preparation of T. viride suspension was previously described29. Supplementary Fig. S1 presents macrophotograph of T. viride growing in a Petri dish (a), and microphotographs of T. viride mycelium (b) and spores suspension (c) taken under CLSM microscope29,30.
Preparation of microparticles, application in the field and growth conditions
A two-year research (2017 and 2018) on the ground field (conventional cultivation - CC) and a parallel one year research (2018) in the hydroponic type of cultivation (HC) of green lettuce (Lactuca sativa L. var. crispa cv. ‘Melina’) have been investigated with regards to the application of microparticles loaded with different active compounds. Our preliminary trial (2017) revealed no significant influence on the morphology of treated lettuces but significant influences on the chlorophyll’s content. The same procedure was repeated in 2018 and additionally, parallel research in hydroponics was performed to observe influence in two different environments under different conditions.
In accordance with our previous research29,30,31,32,33 after the optimization, eight different types of microparticles (microspheres and microcapsules) have been prepared and used in this research. Microspheres (Fig. 1) were prepared by dripping 1.5% sodium alginate (carrier) or a mixture of sodium alginate and T. viride spores into a cross-linking solution of 1% CaCl2 or CuSO4 × 5H2O. The production of microspheres was achieved with Encapsulator (Büchi-B390, BÜCHI Labortechnik AG, Switzerland) with the flow rate of carrier solution of 30 to 40 mL min−1 (determined by using encapsulator nozzle size of 1000 μm) at the vibration frequency of 40 Hz and the pressure of 0.3 bar. Microspheres were formed in the cross-linking solution under mechanical stirring, then washed several times with distilled water and filtered through Büchner funnel.
Microcapsules (Fig. 1) were prepared by dispersing microspheres in chitosan solution (0.5% chitosan in 1.0% CH3COOH) under constant stirring for 30 minutes. Obtained microcapsules were filtered, washed with distilled water and saline buffer. Microparticles were used the next day in the field. Suspension of T. viride spores in saline solution (0.85%) was used as side control (non-encapsulated), with control as non-treated samples. Two different types of cultivation were used, conventional in soil and in hydroponics, in order to compare the differences between cultivation type and to observe the influence on the same sample in different types of cultivation. Alginate-based microspheres containing either only chemical (Ca2+ or Cu2+) or both chemical and biological agents (T. viride spores - Tv) and microcapsules containing the aforementioned but with the addition of chitosan layer (suffix -c) are labeled and treatment samples are listed in Table 1.
The application was performed as weighing a 4 g of prepared microparticles and applying them directly near the root of a lettuce plant just before planting. Figure 2 shows the procedure and maturing time for two different types of cultivations. For the CC planting was performed at the Department of Vegetable Crops, Faculty of Agronomy, University of Zagreb during two years, 2017 and 2018. A single-factor assay with Melina lettuce was placed randomly in a block schedule in three repetitions (total of 540 seedlings). Field ground chemical analysis on the experimental area revealed: pH H2O – 7.5, nKCl – 6.86, Humus was 2.22%, N 0.2%, P2O5–41.1 and K2O – 25.5 as Al-mg/100 g. The distance between the seedlings in the row was 30 cm, while the spacing between the rows was also 30 cm. By planting the lettuces on these spaces, there was a complex of 11 plants × m−2 (18 plants per parcel). According to the available meteorological data from the Maksimir meteorological station, in the period from April to June, the average monthly air temperature ranged from 11.2–19.4 °C, which is optimal for outdoor lettuce growing. In all three months, 13 days were rainy and the average monthly precipitation was in the range from 61.5 mm in April to 96.8 mm in June.
Hydroponic cultivation of lettuce was carried out in 2018, in a protected area unsupplied with cooling system and energy curtains. The mono-factorial experiment was set up by the method of randomized complete block layout with four replications. Transplants of cv. ‘Melina’ with four developed leaves were planted on May 30 in growing pots filled with the inert substrate (perlite, 20 L per pot). During the planting, the microparticles were applied in the root-zone of transplants. Three transplants per pot were planted at a distance of 30 cm, and four pots per treatment were used. Growing pots were placed in pools tanked with a nutrient solution in composition recommended for leafy vegetables35. The nutrient solution was oxygen-enriched daily, but not supplemented or corrected. During the vegetation period, the abiotic parameters of the nutrient solution (temperature, pH and EC value, dissolved oxygen concentration) and air (minimum and maximum temperature and humidity) were monitored daily. Lettuce was harvested on July 9, and measurements carried out were: mass before and after primary finishing (g per rosette), diameter and height of rosette (cm), marketable yield (kg m−2).
Plant measurements, moisture determination and preparation of extracts
Plants were harvested after 44 days in conventional and after 41 days in hydroponics cultivation and morphological parameters were taken (total head weight, diameter, height and marketable yield). Fresh lettuce was cut in quarters, washed with tap water to remove the soil/insects and then with distilled water. Washed lettuce was blotted with the paper towels to remove adherent water. Root tip was completely removed with the precise knife, and fresh lettuce quarters were chopped to the size of 4–6 mm in FOSS homogenizer 2094 (Hillerød, Denmark). Homogenized lettuce (4–6 mm) was subjected to dry matter/water content determination. Briefly, 5.00 g of fresh homogenized lettuce was subjected to the drying in PMB 202 Moisture Analyzer (Adam Equipment Company, United Kingdom) at 130 °C until completely dried.
Further, 30 g of freshly homogenized lettuce sample was taken in 100 mL of 96% ethanol for further extraction with a laboratory mixer and was homogenized for 30 s. Suspensions were filtered through the Whatman No. 4 filter and the volume of extracts were adjusted to 100 mL with the addition of solvent. Extraction was performed in triplicates and all of the chemical analyses following were performed in duplicates or triplicates (per one sample, 6–9 analyses were performed for each method, respectively).
Chlorophylls analyses
The chlorophyll content was determined by extracting 1.0000 g of freshly homogenized lettuce with 25 mL of 80% (v/v) acetone by vortexing it for 2 minutes and filtering through the Whatman No. 4 filter paper. The final volume was set to 25 mL with the solvent. Absorbance was measured at 663 nm and 645 nm, and the chlorophylls content was calculated36. Results are expressed as mg of chlorophyll per dry weight of lettuce (µg/g d.w.).
Determination of total polyphenolic content (TPC)
The modified Folin Ciocalteu’s method37 was used to determine total polyphenolic content (TPC). Ethanolic lettuce extract (0.1 mL) was mixed with 7.9 mL distilled water and 0.5 mL Folin Ciocalteu’s reagent (diluted with distilled water in 1:2 ratio) and 1.5 mL 20% sodium carbonate. The suspension was vortexed and left for 2 h to react. Absorbance was measured at 765 nm, and the data are expressed as gallic acid equivalents per g of dry weight (mg GAE/g d.w.).
The total flavonoids (TF)
Total flavonoids content was determined by the modified spectrophotometric method38. Briefly, 1 mL of ethanolic lettuce extract was added in a 10 mL volumetric flask containing 4 mL of distilled water. Then, 300 μL of NaNO2 (0.5 g/L) solution was added. After 5 minutes, 300 μL of AlCl3 (1 g/L) solution was added and 6 minutes later, 2 mL of NaOH (1 mol/L) was added to the mixture. The final volume was set to 10 mL with the addition of distilled water. The solution was mixed and the absorbance was measured at 360 nm. Quercetin was selected as the standard and a seven-point standard calibration curve was plotted. The data are expressed as mg quercetin equivalents per dry weight of lettuce (mg QE/g d.w.).
Antioxidant potential measurements – ABTS and DPPH methods
The antioxidant potential of the ethanolic lettuce extracts was determined with the 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) reagent, according to the known procedures39,40. The data obtained are expressed as μmol Trolox equivalents per g of dry weight (μmol TE/g d.w.).
Statistical analysis
Statistical analysis was performed using Excel XLSTAT and IBM SPSS Statistics v22 software. One way ANOVA was performed and significant differences were observed based on the posthoc Tukey HSD test (p < 0.05). Principal component analysis (PCA) and agglomerative hierarchical clustering (AHC) were also performed. PCA type was set to Pearson (n) with filter factors a maximum number of 5. Bartlett’s test of sphericity was used to compare the correlation matrix with the matrix of zero correlations (significance level % was set to 5) The risk for the rejection of the null hypothesis H0, while it is true was <0.01%. Alpha was set to 0.05, and the p-value was <0.0001. The Keiser-Meyer-Olkin (KMO) measure of sampling adequacy was performed prior to the analysis. Further, Agglomerative hierarchical clustering (AHC) with Euclidean distance Dissimilarity and Agglomeration Ward’s method was performed.
Results and Discussion
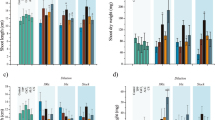
Due to the simpler results presentation in Figs. 3 and 4 and in Tables 1 to 4, the prefix CC denoting conventional cultivation is excluded, and for hydroponic cultivation, the prefix is denoted as H instead of HC.
Agglomerative hierarchical clustering (AHC) dendrogram of dissimilarities and main cluster formations (a) and dendrogram formation of clusters after the microparticles treatments and analysis (b). Abbreviations of investigated types of microparticles are presented in Table 1.
With regards to the morphological parameters (total head weight, diameter, height and marketable yield) there were no statistically significant differences found after the treatment with microparticles or T. viride spores suspended in saline solution when compared to the control or the treatments according to the posthoc Tukey HSD test. There was no significant influence (p < 0.05) of treatments with microparticles on the moisture content of the lettuces either. Even though somewhat lower percentage of water content was observed in the samples cultivated in hydroponics (lowest in H-Cu-c with 92.1% and highest for H-Cu samples with 92.9%) compared to the conventional (lowest for the Tv treatment with 92.9% and highest for the Cu/Tv-c treated sample with 94.3%), the results are in accordance with the literature where about 93% of water content was found in Batavia species41. No statistically significant changes reveal there was no effect of the treatments on the lettuce moisture content or morphology.
Changes in chlorophylls content
When comparing the cultivation type of lettuce, as conventional cultivation (CC) and hydroponics (HC), samples cultivated conventionally had a significantly higher content of chlorophylls (compared to the equal treatments in HC) which can be explained by the fact that hydroponically grown lettuces were in the greenhouse (Table 2), since more light directly affects photosynthesis, the production of more chlorophylls outside the greenhouse is expected. Light and heath conditions are the main factors affecting the plant physiology with the direct effect on the photosynthesis mechanisms. Exposure to the light determinates the quantity and quality of the energy available to the photosynthesis machinery to conduct its normal metabolic activities42.
When comparing CC and HC with different microparticles or Tv treatments, a high correlation can be observed (rTotalChlorophylls = 0.75) which verifies the repeatability and feasibility of this experiment (since the same experiment is replicated in two different types of cultivation). Interestingly, statistically significant and the most pronounced effect on the lettuce treatment (in terms of chlorophylls) had microparticles containing Ca2+ ions, without the Tv presence. Additionally, calcium-based microcapsules had less effect than microspheres on the lettuces chlorophylls production due to the slower release of the ions and the lower cations availability to a plant. The least feasible effect on the production of the chlorophylls in the lettuces had the treatments with only a suspension of Tv (this is with the respect to the appropriate controls). This may be explained by the lower survivability of the non-encapsulated Tv, as well as the need for this fungus to uptake some of the nutrients from the surrounding media (ground) in order to survive (thus, the same or even negative impact on the plant). Respectively, the treatments with microparticles containing both chemical agents and Tv spores had a similar or lower effect on the production of chlorophylls in the lettuces compared to the same microparticles without the Tv. Similarly, we have previously found higher content of chlorophylls in the leaves of the Vitis vinifera L. plants treated with Ca2+ loaded microspheres as well as with the combination of Ca2+/Mg2+ loaded microspheres4. This can be ascribed to the electrostatic binding of the cations to negatively charged Tv spores which we confirmed in our previous research30,31,32.
Furthermore, the relative change in the total chlorophylls content was up to 45.3% for the CC and 75.8% for the HC treatments with Ca2+ microparticles, suggesting that the same microparticles can stimulate the production of more chlorophylls even with the less available light. The availability to uptake calcium ions is due to the fact that in the microparticles, calcium remains in the Ca2+ form, which is passively uptaken by the plant through the root system43. Our results are in accordance with the research with15 where they observed higher values of chlorophyll a, b and total chlorophylls in chickpea leaves supplemented with Ca2+. Even more, the pronounced effect was observed in the cadmium stressed plants where the application of Ca2+ improved synthesis and protection of the photosynthetic pigments. Furthermore, Ca2+ serves as a secondary messenger for cytokinin action in improving the synthesis of chlorophylls44.
Ca2+ ions are free to enter roots passively but the transfer to the shoots is limited by a metabolic barrier. Some of the metabolic inhibitors can stop the long-distance transport of Ca2+ to the shoots but cannot inhibit the uptake of the roots. Since Mg2+ and many other trace metals result in phytotoxicity when Ca2+ share is low, evidently the supply and availability of Ca2+ ions are important. When Ca2+ concentrations in the plants are low, phytotoxicities from trace metals are very common and even Mg2+ ions become extremely phytotoxic43,45. Since microparticles containing the Ca2+ ions are present throughout the period of the plant maturation, this has proved to be an efficient delivery system with a direct influence on the chlorophylls and the photosynthesis machine. Ca2+ ions control and modify the uptake of available Mg2+ and nitrogen, which are both important components of the chlorophyll structure46. Moreover, using the calcium-based microparticles, it is possible to eliminate the competition between magnesium and calcium ions thus increasing the availability of Mg2+ for plant uptake and at the same time preventing the occurrence of negative metabolic changes of the plant leaves. Accordingly, by increasing the number of magnesium ions to the plant, synthesis of more chlorophylls can be achieved in the leaves of the plant and thus decrease in the level of abiotic and biotic stress of the plant can occur.
Even though the chlorophyll a:b ratio was somewhat higher in the conventionally cultivated lettuces, there were no statistically significant differences found between the cultivation types under the different light conditions (the chlorophyll a:b ratio behaved similarly). The slightly higher chlorophyll a:b ratio in the conventional cultivation (average for CC 2.63 vs. average for HC 2.38) is in correlation with the literature, where an increase of the ratio is correlated with the available light. The ratio is physiologically flexible, and it is least influenced by the soil status or the water availability and is dissociated from patterns imposed by changes in leaf density47.
Total polyphenolic content (TPC) and total flavonoids (TF)
When comparing the “basic” metabolism, which comprises of anabolic/catabolic processes which are required for the cell maintenance and proliferation, PSM refers to the compounds that are present in the specialized cells which are not directly essential for the basic metabolism but are required for plant survival in the environment. PSM was considered simply as waste products of primary metabolism which accumulates in the plant cells because of the absence of an efficient excretion48,49,50, but that idea is not valid. Plant secondary metabolites (i.e. polyphenols) act as a defense (against herbivores, microbes, viruses or competing plants) and also as signal compounds (to attract pollinating or seed-dispersing animals) and they offer protection to the plants against ultraviolet radiation and oxidation processes51,52. These metabolites can, therefore, be acknowledged as adaptive characters that have been subjected to natural selection during the evolution. Compared to the animals, plants cannot escape from their biotic and abiotic stressors by being linked to the ground via their root system, thus because they are static the protection must be commenced in another way. Phytochemicals that they produce are to deter or kill pests and pathogens and this represents one point of self-protection5. Since polyphenols and flavonoids have high antioxidant activity and are electron-donating compounds53, they can scavenge reactive oxygen species. Polyphenolic compounds are generally synthesized through the signaling processes54,55. The role of Ca2+ in polyphenolic metabolism has been described by various authors and starting with a paper by56 demonstrated the direct role of Ca2+ in the synthesis of polyphenolic compounds. The application of Ca2+ increased phenylalanine ammonia-lyase activity, which ended with the accumulation of polyphenols and thus increasing the resistance to the infection by the specific fungus. Furthermore, Ca2+ indirectly activates peroxidase, as this cation induces the cross-linking of polygalacturonan chains into a structure that can be recognized by isoperoxidase57.
Different cultivation types did not significantly affect the accumulation of TPC in lettuces but the higher relative change was observed in the treatment of HC lettuces, respectively. Due to the fact that hydroponics is a more controlled environment (compared to conventional cultivation) the influence of the treatments was more clear. TPC of the treated samples was higher in H-Ca/Tv-c treated lettuces with the significant influence and the relative change of 47.6% compared to the control. From Table 3 it can be observed that treatments with Ca2+ based microparticles had a somewhat stronger effect on the synthesis of polyphenolic compounds compared to Cu2+ based microparticles.
The significant effect on the TF accumulation with the microparticles treatments can be observed on the samples cultivated in hydroponics, where microparticles based on calcium-alginate (loaded with Ca2+ ions with or without T. viride spores) achieved an increase from 19.4% (H-Ca/Tv) to 42.2% (H-Ca/Tv-c). In this case, it seems that chitosan coating prevents the burst release of the Ca2+ (which is in accordance with our in vitro tests33) so the uptake to the root was slower (rate controlled) than those lettuces treated with microspheres. Due to the preparation conditions (to achieve the necessary solubility of the chitosan) microcapsules are more acidic. There is some literature dealing with the influence of the lower pH on the production of TF, where there was found a high correlation between the low pH and higher TF content in leaves. By this, the pH of the surrounding media strongly affects the accumulation of PSM which can lead to a significant enhancement in the productivity of flavonoids58. In both types of cultivation (CC and HC) calcium-alginate microparticles (Ca and H-Ca) treatment had the most pronounced effect and statistically significantly influenced, compared to the respective controls with the relative change of 15.2% for the CC and 39.0% for the HC. In general, somewhat lower changes were observed in CC compared to the HC, but this can be explained by the lower values of TF for the control in HC (7.0 mg QE/g d.w.) compared to the control from the CC (8.3 mg QE/g d.w.). Recently was proved that flavonoids in sprouts were accumulated more under light irradiation than under dark59. Therefore, CC is evidently more favorable for the production of lettuces with higher TF. Results of the influence on TPC are in correlation with regards to the TF content, but with no statistically significant changes against the control, except in the case of HC for the H-Ca/Tv-c treatment (relative change – 47.6%).
Tv spore suspension in HC had a significantly higher influence on the synthesis of TPC and TF with the high antioxidant activity of lettuces. Due to the fact that in CC Tv suspension was injected into the soil, which is already contaminated with other microorganisms, non-encapsulated Tv had no effect or somewhat negative effect on the production of PSM. Also, non-encapsulated Tv had no significant effect on the production of chlorophylls, in both types of cultivation, but significantly increased TPC and TF in HC. This can be explained because in hydroponics, when planting, the cleaner environment was achieved, with no competition for T. viride, thus it could thrive. The results of60 indicated that some Trichoderma sp. were able to increase the total amount of polyphenols and antioxidant activity in the grapes (Vitis vinifera L.). In our experiment, the treatment with non-encapsulated T. viride achieved significant influence, with the relative change of 28.1% compared to the control4.
Influence of the treatments on the antioxidant activity (AA)
Ahmad et al.15 show that supplementation with Ca2+ boosts antioxidant activity in plants. Again, as shown in Table 4, in CC and HC, Ca2+ microparticles had a significant effect on the antioxidant activity of lettuce. In general, Cu2+ based microparticles, with or without T. viride, had a lower effect on AA compared to the Ca2+ based, but this can be explained by lowering the activity of antioxidant enzymes. In higher concentrations, Cu2+ can indirectly act as pro-oxidants. This may increase free radical-mediated lipid peroxidation in plants. Since moderate-high dosages of Cu2+ may activate zymogen or trigger the defensive system by ROS, antioxidant enzymes activity decreases, thus limiting the elimination of ROS. Alongside, MDA concentration can get higher in plants exposed to moderate-high Cu2+ concentrations thus indirectly produce superoxide radicals, resulting in increased lipid peroxidative products and oxidative stress61,62. This might be partly the explanation of why Cu2+ based microparticles had less influence on the lettuces AA, even though compared to the control the AA was higher.
Accordingly, to the results of TPC and TF, Tv had a significant influence on the AA of lettuces grown in HC, whereas there was no significant effect on lettuces grown in CC. The results are in correlation, and with the increased TPC and TF, antioxidant activity is higher, suggesting that T. viride in the right conditions plays a significant role in the synthesis of polyphenols, probably indirectly by enhancing the nutrient availability and by inducing the plant resistance via activating or priming induced systemic resistance mechanism60.
Pearson correlations, principal component analysis (PCA) and agglomerative hierarchical clustering (AHC) analyses
High correlations with regards to the comparison of CC and HC for all of the methods with the highest correlation between the TF in CC and HC lettuces (\({r}_{TF(CC/HC)}=0.87)\) were found. The high correlation between the antioxidant activity methods was also observed (\({r}_{ABTS/DPPH)}=0.92)\). Furthermore, when observing the methods, high correlations were found for TPC, TF and antioxidant activity (r > 0.80) and chlorophylls content (r > 0.99). No correlation was found in the relationship between chlorophylls content and TPC/TF/AA.
PCA was used to indicate multivariate dependence between the selected variables. The weighting of the results was made for the moisture content, chlorophyll a:b, total chlorophylls, TPC, TF, ABTS, and DPPH. For Bartlett’s Sphericity test, the risk for the rejection of the null hypothesis H0 while it was true was <0.01%. Alpha was set to 0.05, and the p-value was <0.0001. The Kaiser-Meyer-Olkin (KMO) measure of sampling adequacy gave a value of 0.734. High factor loading scores mean a tighter association with the same principal component (Table S1). PCA revealed the two significant components which explain altogether 88.08% of the total variance between the studied variables (Fig. 3a). Factor 1 (F1) describes 50.60% of the total variance and was tightly associated with the TPC, TF, ABTS, and DPPH whereas Factor 2 (F2) was associated with moisture content, chlorophyll a:b and total chlorophylls content. Figure 3b presents the distribution of the data for F1 and F2 and the correlation between the measured variables. There was no correlation found between F1 (TPC, TF, ABTS, DPPH) and F2 (moisture content, chlorophyll a:b, and total chlorophylls).
The dendrogram (Fig. 4a) was obtained based on the same variables as PCA, and it can be seen that three main clusters were formed (green, pink and red). HC lettuce treatments were separated in 2 closely related clusters (pink and red) where Tv from CC was also involved. Based on the obtained results (Fig. 4b), Tv from had the least influence on the CC treatments compared to the other treatments and was similar to the control of the latter, but because of the lower values of investigated PSM, it clustered with the control of HC. In general, Cu2+ based microparticles in CC are closely related to the control. In the first cluster (green) it can be observed that H-Ca treatment is more similar to the values of the CC type, and this is explained by the fact that Ca2+ microparticles in HC had the significant and highest influence on the plant secondary metabolites. Biplot (Fig. 3b) reveals similar results, where CC lettuces were divided from HC.
Ca (top right quadrant) and H-Ca/Tv-c (bottom right quadrant) treatments can be seen as extremes on the right side of the biplot (Fig. 3b), and Tv alongside H-control on the left side. In both cases, control is significantly distanced from the Ca and H-Ca/Tv-c treatments, signifying the influence of these treatments on the lettuces development of PSM. Cu2+ microparticles formulations are in general grouped together (respectively to the appropriate type of cultivation). It seems that Ca/Tv-c, Ca-c and Ca/Tv had a somewhat similar and significant effect on the synthesis of PSM, and Cu/Tv-c, Cu/Tv and Cu-c are more closely related to the control. This is in correlation to the HC where H-Cu/Tv, H-Cu-c, H-Cu and H-Cu/Tv are closer to the control, compared to their Ca2+ counterparts microparticles, H-Ca/Tv, H-Ca, H-Ca-c and H-Ca/Tv-c. The only exception in two cases of cultivation types is Tv, where Tv in CC had no significant influence, but H-Tv in HC had a significant influence on the TPC, TF, ABTS and DPPH and is closely related to the extreme in HC (H-Ca/Tv-c). PCA and AHC revealed the significant influence of some treatments (i.e. Ca2+ based microparticles) on lettuces in both conventional and hydroponic cultivation.
Conclusions
Delivery of biological and chemical agents by means of encapsulation presents an innovative approach to stimulate the production of PSM. This way, it is possible to fortify the plants’ defense system against pests and increase the resistance to harsh environmental conditions.
Results revealed that microparticles treatments significantly stimulated the synthesis of PSM without a significant impact on the lettuce morphology and moisture content. The controlled release was achieved and the plant can uptake ions passively through the root system during the whole period of maturation. Evidently, non-encapsulated T. viride spores had significantly less influence on the lettuces compared to the encapsulated ones. Ca2+ based microparticles had a higher effect on the synthesis of PSM compared to their counterparts Cu2+ based microparticles, even though significant changes occurred in all treatment types, respectively.
The final product (microparticles) is commercially very affordable and further is necessary only to upscale the production process. Bioencapsulation proved to be an efficient way to deliver both chemical and biological agents for plant nutrition/protection and the production of functional foods. Our method may be applied in the production of plants (in this case lettuce) with improved nutritional quality and as a potential dietary source of natural phenolic antioxidants.
References
Tiwari, R. & Rana, C. S. Plant secondary metabolites: a review. Int. J. Engin. Res. Gen. Sci. 3, 661–670 (2015).
Neugart, S. et al. The intrinsic quality of brassicaceous vegetables: How secondary plant metabolites are affected by genetic, environmental, and agronomic factors. Scient. Hortic. 233, 460–478 (2018).
Anand, S. Various approaches for the secondary metabolite production through plant tissue culture. Pharmacia 1, 1–7 (2010).
Vinceković, M. et al. The enhancement of bioactive potential in Vitis vinifera leaves by application of microspheres loaded with biological and chemical agents. J. Plant Nutr. 42, 543–558 (2019).
Lattanzio, V., Lattanzio, V. M. T. & Angela Cardinali, A. Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects In Phytochemistry: Advances in Research (ed. Imeprato, F.), 23–67 (Research Signpost, 2006).
Mampholo, B. M., Sivakumar, D., Beukes, M. & van Rensburg, W. J. Effect of modified atmosphere packaging on the quality and bioactive compounds of Chinese cabbage (Brassica rapa L. ssp. chinensis). J. Sci. Food Agr. 93, 2008–2015 (2013).
Mampholo, B. M., Maboko, M. M., Soundy, P. & Sivakumar, D. Phytochemicals and Overall Quality of Leafy Lettuca (Lactuca sativa L.) Varieties Grown in Closed Hydroponic System. J. Food Quality 39, 805–815 (2016).
Banerjee, M. R., Yesmin, L. & Vessey, J. K. Plant-Growth-Promothing Rhizobacteria As Biofertilizers and Biopesticides In Handbook of Microbial Biofertilizers (ed. Rai, M.), 140–141 (Food Products Press, 2006).
Avioa, L., Sbrana, C., Giovannetti, M. & Frassin, S. Arbuscular mycorrhizal fungi affect total phenolics content and antioxidant activity in leaves of oak leaf lettuce varieties. Scient. Hortic. 224, 265–271 (2017).
John, R. P., Tyagi, R. D., Brar, S. K., Surampalli, R. Y. & Prévost, D. Bio-encapsulation of microbial cells for targeted agricultural delivery. Crit. Rev. Biotechnol. 31, 211–226 (2011).
Harman, G. E. Overview of mechanisms and uses of Trichoderma spp. Phytopathology 96, 190–194 (2006).
White, P. J. Calcium channels in higher plants. Biochim. Biophys. Acta Biomembr. 1465, 171–189 (2000).
El-Beltagi, H. S. & Mohamed, H. I. Alleviation of cadmium toxicity in Pisum sativum L. seedlings by calcium chloride. Not. Bot. Horti Agrobot. Cluj-Napoca. 41, 157–168 (2013).
Talukdar, D. Exogenous calcium alleviates the impact of cadmiuminduced oxidative stress in Lens culinaris Medic. seedlings through modulation of antioxidant enzyme activities. J. Crop. Sci. Biotechnol. 15, 325–334 (2012).
Ahmad, P. et al. Calcium and Potassium Supplementation Enhanced Growth, Osmolyte Secondary Metabolite Production, and Enzymatic Antioxidant Machinery in Cadmium-Exposed Chickpea (Cicer arietinum L.). Front. Plant Sci. 7, 513, https://doi.org/10.3389/fpls.2016.00513 (2016).
Xu, W. et al. Effect of calcium on strawberry fruit flavonoid pathway gene expression and anthocyanin accumulation. Plant. Physiol. Bioch. 82, 289–298 (2014).
Hirschi, K. D. The calcium conundrum. Both versatile nutrient and specific signal. Plant Physiol. 136, 2438–2442 (2004).
Yousuf, P. Y., Ahmad, A., Hemant Ganie, A. H., Aref, I. M. & Iqbal, M. Potassium and calcium application ameliorates growth oxidative homeostasis in salt-stressed indian mustard (Brassicajuncea). Pak. J. Bot. 47, 1629–1639 (2015).
Siddique, M. H., Al-Whaibi, M. H., Sakran, A. H., Basalah, M. O. & Ali, H. M. Effect of calcium and potassium on antioxidant system of Vicia faba L. under cadmium stress. Int. J. Mol. Sci. 13, 6604–6619 (2012).
Li, P. et al. Calcium alleviates cadmium-induced inhibition on root growth by maintaining auxin homeostasis in Arabidopsis seedlings. Protoplasma 253, 185–200 (2016).
Grondona, I. et al. Physiological and biochemical characterization of Trichoderma harzianum, a biological control agent against soilborne fungal plant pathogens. Appl. Environ. Microb. 63, 3189–3198 (1997).
El-Mougy, N. S., Abdel-Kader, M. M., Aly, M. D. E. & Lashin, S. M. Application of fungicides alternatives as seed treatment for controlling root rot of some vegetables in pot experiments. Life Sci. Adv. 2, 57–64 (2012).
Mizukami, H., Konoshima, M. & Tabata, M. Effect of nutritional factors on shikonin derivative formation in Lithospermum callus cultures. Phytochemistry 16, 1183–1186 (1977).
Fujita, Y., Hara, Y., Suga, C. & Morimoto, T. Production of shikonin derivatives by cell suspension cultures of Lithospermum erythrorhizon. II. A new medium for the production of shikonin derivatives. Plant Cell Rep. 1, 61–63 (1981).
Ohlsson, A. B. & Berglund, T. Effect of high MnSO4 levels on cardenolide accumulation by Digitalis lanata tissue cultures in light and darkness. J. Plant Physiol. 135, 505–507 (1989).
Trejo-Tapia, G., Jimenez-Aparicio, A., Rodriguez-Monroy, M., De Jesus-Sanchez, A. & Gutierrez-Lopez, G. Influence of cobalt and other microelements on the production of betalains and the growth of suspension cultures of Beta vulgaris. Plant Cell Tiss. Organ. Cult. 67, 19–23 (2001).
Murashige, T. & Skoog, F. A revised medium for rapid growth and bioassay with tobacco tissue culture. Physiol. Plantarum 15, 473–493 (1962).
Narula, A., Kumar, S. & Srivastava, P. S. Abiotic metal stress enhances diosgenin yield in Dioscorea bulbifera L. cultures. Plant Cell Rep. 24, 250–254 (2005).
Vinceković, M. et al. Encapsulation of Biological and Chemical Agents for Plant Nutrition and Protection: Chitosan/Alginate Microcapsules Loaded with Copper Cations and Trichoderma viride. J. Agric. Food Chem. 64, 8073–8083 (2016).
Vinceković, M., Jurić, S., Đermić, E. & Topolovec-Pintarić, S. Kinetics and Mechanisms of Chemical and Biological Agents Release from Biopolymeric Microcapsules. J. Agri. Food Chem. 65, 9608–9617 (2017).
Vinceković, M. et al. Release of Trichoderma viride Spores from Microcapsules Simultaneously Loaded with Chemical and Biological Agents. Agric. Conspec. Sci. 82, 395–401 (2017).
Jurić, S., Đermić, E., Topolovec-Pintarić, S., Bedek, M. & Vinceković, M. Physicochemical properties and release characteristics of calcium alginate microspheres loaded with Trichoderma viride spores. J. Integr. Agr. 18, 3–16 (2019).
Jurić, S., Šegota, S. & Vinceković, M. Influence of surface morphology and structure of alginate microparticles on the bioactive agents release behavior. Carbohydr. Polym. 218, 234–242 (2019).
Topolovec-Pintarić, S., Žutić, I. & Đermić, E. Enhanced growth of cabbage and red beet by Trichoderma viride. Acta Agr. Slov. 101, 87–92 (2013).
Pimpini, F., Giannini, M., & Lazzarin, R. Ortaggi da foglia da taglio in Origine, caratteri botanici, biologia e fisiologia (ed. Tadiotto, A. & Lavezzo, I.) 13–15 (Veneto Agricoltura, 2005).
Huang, Y., Sheng, J., Yang, F. & Hu, Q. Effect of enzyme inactivation by microwave and oven heating on preservation quality of green tea. J. Food Eng. 78, 687–692 (2007).
Singleton, V. L., Orthofer, R. & Lamuela-Raventós, R. M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Method. Enzymol. 299, 152–178 (1999).
Ivanova, V., Stefova, M. & Chinnici, F. Determination of the polyphenol contents in Macedonian grapes and wines by standardized spectrophotometric methods. J. Serb. Chem. Soc. 75, 45–59 (2010).
Brand-Williams Cuvelier, M. E. & Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT – Food Sci. Techn. 28, 25–30 (1995).
Re, R. et al. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Bio. Med. 26, 1231–1237 (1999).
Heimler, D., Vignolini, P., Arfaioli, P., Isolani, L. & Romani, A. Conventional, organic and biodynamic farming: differences in polyphenol content and antioxidant activity of Batavia lettuce. J. Sci. Food Agr. 92, 551–556 (2012).
Latasa, M. Pigment composition of Heterocapsa sp. and Thalassiosira weissflogii growing in batch cultures under different irradiances. Sci. Mar. 59, 25–37 (1995).
Wallace, A. & Mueller, R. T. Calcium uptake and distribution in plants. J. Plant Nutr. 2, 247–256 (1980).
Lechowski, Z. & Bialczyk, J. Calcium mediated cytokinin action on chlorophyll synthesis in isolated embryo of Scots pine. Biol. Plant. 35, 53–62 (1993).
Haynes, R. J. Ion Exchange Properties of Roots and Ionic Interactions within the Root Apoplasm: Their Role in Ion Accumulation by Plants. Bot. Rev. 46, 75–99 (1980).
Pal, R. N. & Laloraya, M. M. Effect of calcium levels on chlorophyll synthesis in peanut and linseed plants. Biochem. Physiologie Pfl. 163, 443–449 (1972).
Dale, M. P. & Causton, D. R. Use of the chlorophyll a:b ratio as a bioassay for the light environment of a plant. Funct. Ecol. 6, 190–196 (1992).
Whiting, D. A. Natural phenolic compounds 1900-2000: a bird’s eye view of a century’s chemistry. Nat. Prod. Rep. 18, 583–606 (2001).
Fraenkel, G. S. The raison d'ĕtre of secondary plant substances; these odd chemicals arose as a means of protecting plants from insects and now guide insects to food. Science 129, 1466–1470 (1959).
Kliebenstein, D. J. Secondary Metabolites and Plant/Environment Interactions: A View through Arabidopsis Thaliana Tinged Glasses. Plant Cell Environ. 27, 675–684 (2004).
Swain, T. Secondary Compounds as Protective Agents. Annu. Rev. Plant Physi. 28, 479–501 (1977).
Kutchan, T. M. Ecological Arsenal and Developmental Dispatcher. The Paradigm of Secondary Metabolism. Plant Physiol. 125, 58–60 (2001).
Michalak, A. Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Pol. J. Environ. Stud. 15, 523–530 (2006).
Bais, H. P., Walker, T. S., Schweizer, H. P. & Vivanco, J. M. Root specific elicitation and antimicrobial activity of rosmarinic acid in hairy rootcultures of Ocimum basilicum. Plant Phys. Biochem. 40, 983–995 (2002).
Li, W., Koike, K., Asada, Y., Yoshikawa, T. & Nikaido, T. Rosmarinic acid production by Coleus forskohlii hairy root cultures. Plant Cell Tiss. Org. Culture 80, 151–155 (2005).
Castaneda, P. & Perez, L. M. Calcium ions promote the response of citrus lemon against fungal elicitors or wounding. Phytochemistry 42, 595–598 (1996).
Penel, C., Van Cutsem, P. & Greppin, H. Interactions of a plant peroxidase with oligogalacturonides in the presence of calcium ions. Phytochemistry 51, 193–198 (1999).
Radić, S., Vujčić, V., Glogoški, M. & Radić-Stojković, M. Influence of pH and plant growth regulators on secondary metabolite production and antioxidant activity of Stevia rebaudiana (Bert). Period. Biol. 118, 9–19 (2016).
Nam, T. G., Kim, D.-O. & Hyun, S. Effects of light sources on major flavonoids and antioxidant activity in common buckwheat sprouts. Food Sci. Biotechnol. 27, 169–176 (2018).
Pascale, A. et al. Trichoderma and its secondary metabolites improve yield and quality of grapes. Crop Prot. 92, 176–181 (2017).
Meng, Q., Zou, J., Zou, J., Jiang, W. & Liu, D. Effect of Cu2+ concentration on growth, antioxidant enzyme activity and malondialdehyde content in garlic (Allium sativum L.). Acta Biol. Cracov. Bot. 49, 95–101 (2007).
Rahman, M. M., Chongling, Y., Rahman, M. D. M. & Islam, K. S. Effects of copper on growth, accumulation, antioxidant activity and malondialdehyde content in young seedlings of the mangrove species Kandelia candel (L.). Plant Biosyst. 146, 47–57 (2012).
Acknowledgements
This work was supported by the Croatian Science Foundation [Project: UIP-2014-09-6462]. Publication was supported by the OpenAccess Publication Fund of the University of Zagreb Faculty of Agriculture.
Author information
Authors and Affiliations
Contributions
M.V. designed the research work and supervised the experiments. S.J. performed all of the experiments, statistical analysis, and interpretation of results. Ż.K.-K. and K.S. carried out chemical analysis. S.F.U. and I.Ž. designed the cultivation procedures and cared for plants. E.Đ. and S.T.-P. provided biological agents and helped design research work. M.V. and S.J. wrote the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jurić, S., Sopko Stracenski, K., Król-Kilińska, Ż. et al. The enhancement of plant secondary metabolites content in Lactuca sativa L. by encapsulated bioactive agents. Sci Rep 10, 3737 (2020). https://doi.org/10.1038/s41598-020-60690-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-60690-3
This article is cited by
-
Innovative Applications of Electrospun Nanofibers Loaded with Bacterial Cells Towards Sustainable Agri-Food Systems and Regulatory Compliance
Food Engineering Reviews (2024)
-
Transcriptome Analysis Reveals Candidate Genes Involved in Calcium Absorption of Rosa roxburghii Plants and their Effects on the Bioactive Substance Accumulation in Fruit
Journal of Soil Science and Plant Nutrition (2024)
-
Gene clustering and co-expression analysis for the identification of putative transcription factors associated with the genes of secondary metabolic pathways in Plantago ovata Forsk. and its wild allies
Plant Biotechnology Reports (2024)
-
Elicitation: “A Trump Card” for Enhancing Secondary Metabolites in Plants
Journal of Plant Growth Regulation (2024)
-
Comparative responses of sulforaphene contents between radish (Raphanus sativus L.) and Baemuchae (xBrassicoraphanus) during seed development
Horticulture, Environment, and Biotechnology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.