Abstract
Cardiovascular (CV) morbidity is the major cause of death in patients with Systemic Lupus Erythematosus (SLE). Previous studies on mannose-binding lectin (MBL) gene polymorphisms in SLE patients suggest that low levels of complement MBL are associated with cardiovascular disease (CVD). However, as large studies on MBL deficiency based on resulting MBL plasma concentrations are lacking, the aim of our study was to analyze the association of MBL concentrations with CVD in SLE patients. Plasma MBL levels SLE patients included in the Swiss SLE Cohort Study were quantified by ELISA. Five different CV organ manifestations were documented. Of 373 included patients (85.5% female) 62 patients had at least one CV manifestation. Patients with MBL deficiency (levels below 500 ng/ml or 1000 ng/ml) had no significantly increased frequency of CVD (19.4% vs. 15.2%, P = 0.3 or 17.7% vs. 15.7%, P = 0.7). After adjustment for traditional CV risk factors, MBL levels and positive antiphospholipid serology (APL+) a significant association of CVD with age, hypertension, disease duration and APL+ was demonstrated. In our study of a large cohort of patients with SLE, we could not confirm previous studies suggesting MBL deficiency to be associated with an increased risk for CVD.
Similar content being viewed by others
Introduction
Systemic lupus erythematosus (SLE) is an idiopathic, chronic and highly heterogeneous inflammatory disorder, driven by an immune response against self-antigens. The prevalence generally ranges from 20 to 70 per 100.0001 and affects both sexes of any age but predominates in women of childbearing age2 with a male-to-female ratio of about 9:13.
The etiology of SLE is multifactorial and the complexity of different factors (genetic, hormonal, immunological and environmental) matches the diversity of clinical manifestations in SLE patients4. Associated with the presence of immune complexes, ongoing complement system activation leads to inflammation and consumption of complement proteins5. This chronic inflammatory state predisposes SLE patients to premature cardiovascular disease (CVD) and infections.
Premature CVD, mostly related to accelerated atherosclerosis6, is acknowledged as the major cause of death in SLE patients, regardless of the time after diagnosis7,8, resulting in a bimodal mortality curve, first described by Urowitz et al. in the 1970s9. The 5-year survival after diagnosis nowadays exceeds 90%10,11,12, but this survival rate has not improved since the 1980s.
The prevalence of ischemic heart disease in SLE patients is estimated to be between 3.8 and 16%13,14,15,16,17,18, a 10-fold higher prevalence compared to the general population19. Moreover, the risk of myocardial infarction in young women with SLE has been found to be 50 times higher compared to women of similar age20 and the risk of stroke was found to be increased by 2–8 fold19,21,22.
As traditional Framingham risk factors (smoking, dyslipidemia, diabetes mellitus (DM), hypertension and overweight) cannot fully explain the high rates of ischemic events, SLE is nowadays considered an independent risk factor for CVD23.
Several studies suggest that components of the innate immune system, which includes pattern-recognition molecules of the complement system such as mannose-binding lectin (MBL), may play an additional role in the pathogenesis of atherosclerosis and CVD24,25,26. Among those, MBL is a liver-derived serum protein that binds certain sugars on the surface of pathogenic micro-organisms and cellular debris and, as it cannot directly opsonize pathogens, triggers complement activation via the lectin pathway27. MBL deficiency (<1000 ng/ml) is mostly caused by a three-point mutation (O alleles) in exon 1 of the MBL gene (in codon 52, 54, 57) that disrupts the assembly of the oligomers and also by a promotor polymorphism (LX) that is associated with low MBL production. However, despite carrying the same genotype, actual MBL levels can vary significantly between individuals27,28,29.
Previous studies indicate that genetic variability in MBL may be involved in the pathogenesis of SLE30, more precisely that functional MBL deficiency is associated with an increased susceptibility to SLE31. Furthermore, Panda et al. described that patients with low MBL-producing genotype have a predisposition to develop SLE32. However, data remains controversial as indicated by Losada Lopez et al. who only described a tendency to a higher allele B incidence in SLE patients33.
Independent of its association with SLE, MBL deficiency has been reported to be associated with an increased risk of atherosclerosis26 and coronary artery disease24 in the general population, and therefore, to be mostly disadvantageous in this regard24,25,26,34. Of note, Limnell et al. even described that MBL deficiency might lead to venous bypass graft occlusion in patients with coronary heart disease35. This observation is supported by another report on high MBL levels having a cardioprotective effect, i.e. being associated with a decreased risk for myocardial infarction (MCI) among patients with diabetes36. However, data are controversial as some studies show an association of cardiovascular (CV) manifestations with MBL deficiency in the general population but others found no such link37,38,39. This controversy has also recently been summarized by Larsen et al.40. In addition, an association of deficiency of complement MBL with reduced mortality in patients with MCI undergoing percutaneous coronary intervention41 as well as with smaller cerebral infarcts and a favorable outcome42 has been described previously. These studies suggest that MBL deficiency – being rather disadvantageous with regard to atherosclerosis and the occurrence of CVD - has an advantageous effect on the outcome after CV events.
Inconsistencies among study results might in part be due to the retrospective character, the relatively small study populations or the focus on MBL2 gene polymorphisms but not on actual MBL plasma levels, which would implicate the MBL deficiency and which are also influenced by non-genetic factors such as inflammation43 and thyroid function44. Thus, as studies on MBL deficiency based on resulting MBL plasma concentrations were lacking, this study was undertaken to determine the potential association between low MBL levels and the risk for CV events in SLE.
Methods
Patients
373 SLE patients included in the Swiss Systemic Lupus Erythematosus Cohort Study (SSCS)45 were included in this study. SSCS is a nationwide, multicenter and ethics committee-approved longitudinal study of SLE patients living in Switzerland, conducted in 11 different institutions in 9 Swiss cities. All participants had to fulfill at least 3 out of 11 American College of Rheumatology (ACR) criteria for the classification as SLE at the time of inclusion and had to give written informed consent. In SSCS, the Systemic Lupus Erythematosus Disease Activity (SLEDAI) score with the Safety of Estrogens in Lupus Erythematosus National Assessment (SELENA) modification and Physician’s Global Assessment (PGA) keeps record of the disease activity, while the Systemic Lupus International Collaborating Clinics (SLICC) damage index is used to document chronic organic damage that occurred after diagnosis.
For the analysis of potential confounders, we included common disease activity scores (SELENA-SLEDAI and PGA) and CV risk factors (smoking status, diabetes mellitus (DM), hypercholesterolemia, arterial hypertension, overweight, and positive antiphospholipid serology (APL+)). Since data on packyears (PY) in some cases were missing we additionally compared ever-smokers with non-smokers. We assumed hypercholesterolemia if patients had any form of treated hyperlipidemia, either familial or acquired, and/or met the common laboratory analytic criteria (cholesterol > 5.2 mmol/l and/or low-density lipoprotein (LDL) > 3.4 mmol/l). Similarly, patients were classified as being hypertensive if any treatment with antihypertensive medication was documented. Overweight was classified using the body-mass-index (BMI) that was calculated using the information that was available at study visits but did not take weight changes over time into account. APL+ was defined based on the specification of the SSCS45, more specifically as positive finding of antiphospholipid antibody based on an abnormal serum level of IgG or IgM anticardiolipin antibodies or IgG or IgM beta 2-glycoprotein antibodies or a positive test result for lupus anticoagulant using a standard method.
This study was approved by the Ethical Committee of the Canton Basel, Switzerland (Ref. No. EK 262/06), all further involved ethical committees and Swissethics (Ethical Committee of the Canton Vaud, Switzerland Ref No 2017-01434). All subjects gave written informed consent in accordance with the Declaration of Helsinki.
Evaluation and ascertainment of inclusion criteria and endpoint
Patients solely were selected based on the availability of complete disease activity score (SELENA-SLEDAI, PGA), SLICC damage index, and available plasma sample at the time of study visit. Demographic (e.g. age, ethnic background, disease, and follow-up duration), clinical, laboratory and treatment information were extracted from the cohort database.
Patients were defined as having CVD if at least one CVD of the SLICC index was present. The following arterial thrombotic events were included: (a.) cerebrovascular ischemia (CVI), (b.) coronary heart disease (CHD), (c.) myocardial infarction (MCI), (d.) peripheral artery disease (PAD) and (e.) mesenteric insufficiency.
Determination of MBL levels and definition of cut-offs
Plasma samples from the 373 SLE patients were collected between 2008 and 2018, which had been exclusively stored at −70 °C and had never been thawed before use. MBL levels were quantified using a mannan-binding enzyme-linked immunosorbent assay (ELISA), as described previously46. This assay measures the ability of MBL to bind to a mannan-coated surface. Microtitre plates (Maxi sorp, Thermo Denmark) were coated with 10 µg/ml of mannan (M7504, Sigma-Aldrich USA) in fresh carbonate–bicarbonate buffer (C3041, Sigma-Aldrich USA), pH 9.6, and incubated overnight at 4 °C. Plasma samples were tested as in a sandwich ELISA but with Tris-buffered saline with 0.05% Tween-20 (TBST) (P5927, Sigma-Aldrich USA) and Calciumchlorid-dihydrate (CaCl2) (21101, Sigma-Aldrich USA), pH 7.5, as both the diluent and wash buffer. All incubations were carried out at 22 °C. First, the microtitre plates were incubated with samples at a 1:100 dilution for 90 minutes, followed by a biotinylated monoclonal anti-MBL antibody (HYB 131-01B, BioPorto Diagnostics, Denmark) for again 90 minutes to detect the bound MBL. After incubation with ExtrAvidin peroxidase conjugate (E2886, Sigma-Aldrich USA) for 40 minutes the plates were developed with BD TMB substrate solutions (555214, BD USA), stopped after 10 minutes with 0.5 M sulfuric acid (H2SO4) (1.00731, Merck Germany) and read immediately at 450 nm on a microplate biokinetics reader (BioTEK, Instruments, USA). MBL levels were calculated against a standard serum pool (ser101, BioPorto Diagnostics Denmark).
Taking the total number of CV events into account, we predefined two different cut-off levels for MBL deficiency, at 500 ng/ml47 and 1000 ng/ml48, respectively, as described previously.
As a control, measurement of MBL level was repeated in 120 of our 373 included patients using samples from different study visits and serum samples instead of plasma samples to investigate the long-term stability and intra-individual variability of MBL levels in our patients and the potential influence of biosampling on our study results.
Statistical analysis
First, we examined the baseline characteristics of patients that developed or did not develop CVD using standard descriptive statistical tests. Data are presented as medians (interquartile range (IQR)) or frequencies with percentages when suitable. Beyond the use of predefined cut-offs for the definition of MBL deficiency, a ROC curve was established to explore the data for a potential cutoff level.
Analyzing the long-term stability and intra-individual variability of MBL levels we used Wilcoxon Signed-Ranks Test and Spearman rank correlation for the comparison of the two MBL concentrations that were measured in the same patients at two time points.
Pearson’s qui2 test and Fisher’s exact test, respectively, dependent on the group size, were used when comparing the occurrence of CVD and categorical variables. We used the Mann-Whitney U Test and Kruskal-Wallis Test, respectively, when comparing continuous with categorical variables as well as linear regression when comparing two continuous variables. The frequencies of CVD dependent on MBL cut-off levels and MBL levels itself were compared for disease activity index and common CV risk factors as well as APL+. Subsequently, we repeated the analysis for all CVD subcategory.
Last, we analyzed the association of possible risk factors with the presence of CVD using logistic regression models. A multiple logistic regression analysis was performed, taking the dichotomized (yes/no) CVD in total as the dependent variable and those as independent variables that reached statistical significance in the univariate analysis and all variables, corresponding to traditional CV risk factors.
Two-tailed tests and a 5% significance level were used in all analyses. All reported P values of less than 0.05 were considered to indicate statistical significance. The analysis was performed with the statistical package IBM SPSS Statistics 25 for MAC OS X, graphs were performed using the publicly available statistical program R.
In a first exploratory analysis of 177 SLE patients, a strong trend towards a significant difference in plasma MBL concentrations between patients with and without CVD was observed. We calculated an adequately powered study (80%) to require at least 362 SLE patients (alpha = 0.05 incidence of CVD 15% standard deviations as observed in the first 177 patients) in order to be able to detect a difference in MBL plasma levels of at least 450 ng/ml in patients with versus without CVD. Based on this calculation, we selected a SLE patient population of 373 patients to account for potentially missing data.
Statement
The abstract of this article is present on a university repository website and can be accessed on https://www.sgaim.ch/fileadmin/user_upload/Adaptionen/Congress/BilderHighres/Bilder_FK_2019/Abstracts_2019.pdf. This article is not published nor is under publication elsewhere.
Compliance with ethical standards
This study was approved by the Ethical Committee of the Canton Basel, Switzerland (Ref No EK 262/06), all further involved ethical committees and Swissethics (Ethical Committee of the Canton Vaud, Switzerland Ref. No. 2017-01434). All procedures performed in this study involving human participants were in accordance with the ethical standards of the research committee and with the 1964 Helsiniki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Results
Patients characteristics
The study population comprised 373 patients of whom 319 (85.5%) were female and 54 (14.5%) male. The median (Interquartile Range (IQR)) age at time of first clinical manifestation of SLE was 31.0 (21.6–43.9) years, the median age at the time of blood sampling 43.1 (32.2–54.3) years, the median age at SLICC index assessment 44.8 (34.6–57.5) years, and the median SLE duration at the time of damage assessment since first clinical manifestation was 9.5 (5.2–18.1) years. Of all patients 74.5% were Caucasian, 10% African, 9.7% Asian, 5.1% Native Americans and 0,3% Pacific Islanders. At the time of the study visit 44.2% had a SLEDAI score of 6 or more (median (IQR) SLEDAI score 4 (2–9)) and 53.4% had a PGA equal to 1 or higher.
The basic demographic and clinical characteristics of all patients are summarized in Table 1.
MBL - Baseline associations and long-term stability
The median (IQR) MBL level was 1131 (335–2344) ng/ml. 129 patients (34.6%) showed levels below 500 ng/ml and 175 patients (46.9%) below 1000 ng/ml (Table 1). MBL levels had no association with active disease (i.e. SLEDAI ≥ 6 or PGA ≥ 1) at the time of the study visit (p = 0.5 and p = 0.2, respectively). In addition, long-term stability and lack of significant intra-individual variability of MBL levels could be shown in 120 patients (32.2%) whose samples from different study visits were measured twice in different specimens (serum and plasma), at different time points (2014 and 2018) and by two different investigators.
MBL levels at the two different time points correlated significantly (Spearman’s rho Correlation Coefficient 0.946, p < 0.001, Fig. 1) and did not change over time (median (IQR) levels (1222 (372–2035) ng/ml vs. 1131 (336–2344) ng/ml) as determined by the Wilcoxon Signed Rank Test (Z = −1.717, p = 0.086).
Distribution data of MBL deficiency from healthy Swiss controls were not available. However, likewise to our study population, the frequency of MBL deficiency below 500 ng/ml in SLE patients of our study cohort (median (IQR) 1130 (340–2340) ng/ml, 34.6%) was similar (p = 0.22) to the frequency observed in Swiss scleroderma patients (median (IQR) 1290 (330–2300) ng/ml, 30.3%) as described previously49. This study showed a good sample size (=195) and is, therefore, comparable considering the close relationship between systemic sclerosis and SLE.
Furthermore, we investigated the potential association of erythrocyte sedimentation rate (ESR) as marker of inflammation with MBL levels. We observed a very weak correlation between MBL plasma concentration and the corresponding ESR (r = 0.12, p = 0.03) as well as the daily prednisolone (PDN) dose (r = 0.14, 0 = 0.04), respectively.
In addition, using a commonly used pathologic ESR cut-off level of 28 mm/h50, we saw a weak association between patients with an elevated ESR and a MBL deficiency below 1000 ng/ml (p = 0.047). However, this association was not found (p = 0.084) when using a 500 ng/ml cut-off. Further, we saw no such association between MBL deficiency and steroid intake, but as expected patients with a higher ESR were more likely to receive higher doses of PDN as part of the treatment (r = 0.3, p < 0.0001).
CVD
In total, 62 patients (16.6%) had at least one CV manifestation, of these patients 77.4% were Caucasians, 11.3% African, 6.5% Asians and 4.8% Native Americans. 20 patients (5.3%) had more than one CVD manifestation, leading to a total of 93 distinctive CVDs that were documented in the study population. Separated into subcategories, 10.2% of the patients had a CVI (38/373), 7.8% a CHD (20/373), 4.3% a MCI (16/373), 2.4% a PAD (9/373) and 1% (1/373) a documented mesenteric insufficiency. (supplemental Table 1)
Distribution of CVD risk factors in the study population and distribution of CVD according to age, disease duration, ethnicity, CV risk factors, and other confounders are shown in Tables 1 and 2, respectively.
Association of MBL deficiency cut-off levels and continuous MBL levels with CVD and confounders
Patients with MBL deficiency being defined as plasma concentrations below 500 ng/ml had no significantly increased, nor decreased, frequency of CVD (19.4% vs. 15.2%, P = 0.3). Similarly, MBL levels below 1000 ng/ml were not associated with an increased rate of CVD (17.7% vs. 15.7%, P = 0.6). In addition, MBL deficiency was not associated with the occurrence of any subcategory of CVD.
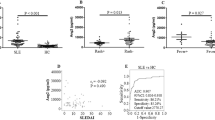
Furthermore, MBL levels were similarly distributed in CVD versus no-CVD patients (median (IQR) 1180 (339–2344) ng/ml vs. 955 (306–2389) ng/ml, p = 0.5, Fig. 2a). Last, an explorative receiver operating characteristic (ROC) analysis of MBL levels predicting CVD in SLE patients was performed, but could not identify a candidate cut-off level (area under the curve (AUC) 0.529, Fig. 3).
Distribution of MBL levels in 373 SLE patients (a) with at least one cardiovascular disease (CVD) and (b) with cardiovascular disease according to the subcategory. The horizontal lines depict the median values. (CVD - cardiovascular disease, CVI - cerebrovascular ischemia, CHD - coronary heart disease, MCI - myocardial infarction, PAD - peripheral artery disease).
Similarly, the distribution of MBL levels in subgroup analyses (Supplemental Table 1 and Fig. 2b) did not differ significantly. Of note, mesenteric insufficiency was recorded only once in the whole study population.
Furthermore, our analyses showed that MBL, neither below the deficiency cut-offs nor as a continuous parameter, does not associate with the included confounders including disease duration (data not shown). Likewise, ethnicity was not associated with the occurrence of CVD.
Association of CVD with risk factors and confounders
With regard to common CVD risk factors, more than half of the patients (52.3%) had hypercholesterolemia, 41.5% had arterial hypertension, 6,7% were diabetic, more than one-third (35.2%) were overweight with a BMI ≥ 25 kg/m2 and 42.4% had at least smoked once in their life. In addition, 43.7% of our patients had APL+.
As shown in Table 2, disease duration was longer in patients who had CVD than in those without (median 20.0 vs 8.9 years, p < 0.001). In addition, patients with CVD were older (median age 57.5 vs. 41.9 years, p < 0.001), more likely to be men (22.6% vs. 12.9%, p = 0.047), more frequently smokers (57.1% vs. 39.5%, p = 0.015), more likely to have comorbidities associated with CVD (hypertension 71.7% vs. 35.2%, p < 0.001, hypercholesteremia 92% vs. 42.7, p < 0.001, p = 0.1, APL+ 62.3% vs. 40.0%, p < 0.001, DM 11.3% vs. 5.8%) (Fig. 4) and accrued more damage overall (median SLICC index 2.8 vs. 1.8, p = 0.5). The same results in terms of significance pertained to the CVD subcategories.
Risk of CVD adjusted for common CV risk factors, APS and MBL levels by univariate and multivariate regression analysis. (a) The univariate logistic regression model was performed for MBL levels, sex, age, hypertension, smoking, hypercholesterolemia, BMI, disease activity scores as well as APS. (b) We included significant predictors into the multivariate analysis. Due to missing data, hypercholesterolemia had to be excluded to gain explanatory power.
The ESR median in patients with CVD was 18.5 mm/h vs. 16.0 mm/h in patients without CVD (p = 0.3). ESR or the dose of PDN were not associated with the incidence of cardiovascular events in the present study (data not shown).
Risk stratification of CVD with potential confounders
Adjusting the risk of CVD for MBL levels, potential and traditional CV risk factors, revealed a significant association of CVD with male sex (p = 0.05), age (p < 0.001), disease duration (p < 0.001), APL+ (p < 0.001), hypertension (p < 0.001), hypercholesterolemia (p < 0.001) and history of smoking (p = 0.02) in the univariate analysis, while the association with MBL levels p = 0.34), disease activity (SLEDAI p = 0.9, PGA p = 0.56), BMI (p = 0.58) and DM (p = 0.12) did not reach statistical significance. The final multiple logistic regression model including significant variables in the univariate analysis (with the exception of hypercholesterolemia due to missing values in two third of the patients) revealed an independent significant association between CVD in SLE patients with age (p < 0.001), disease duration (p < 0.001), hypertension (p < 0.005) and APL+ (p < 0.003), while the association with male sex (p = 0.49) and smoking (p = 0.26) did not reach significance anymore (Fig. 4).
Discussion
Compared to the general population20 patients with SLE have an increased risk for CV morbidity and preclinical atherosclerosis51,52. Traditional CV risk factors cannot fully explain this observation19, suggesting that SLE disease-specific factors may play an additional role53. Previous studies described deficiency of complement MBL to be related to CVD in the general population36 and in SLE patients54,55.
With a focus on patients with SLE, previous studies on MBL gene polymorphisms suggest that low-producing MBL genotypes are associated with increased intima-media thickness56 and arterial thrombosis, more specifically with coronary events55. This is in accordance with another study in which MBL-deficient SLE patients were found to have a 3.3 fold increased risk of CVD54.
However, Calvo-Alen et al. found an association of MBL deficiency with cerebrovascular incidents (CVI) but not with ischemic heart disease57, and Jönsen et al. could show that classical risk factors (smoking, hypertension, low alcohol intake, elevated triglyceride concentration) were relatively more important for the development of CVD than MBL deficiency58.
Our study was designed to elucidate whether MBL deficiency, based on the resulting blood concentrations, is also associated with an increased incidence of CVD in SLE patients. To address this question, we measured MBL plasma levels and investigated their potential association with clinical CV manifestation and with the main CV risk factors.
Our results suggest that MBL deficiency is not a determinant of CVD in SLE patients, independent of other risk factors.
In part, our findings are in contrast to data reported by previous studies. These differences can be explained by a number of factors. First, our study focused on the determination of plasma MBL concentrations and not the genotypes54,55,57,58. With regard to the variability of MBL levels within one genotype27,29, MBL plasma levels seem to be more discriminative in terms of immunological functionality36. Second, we focused on all CVD out of the 12 organ systems that are recorded by the SLICC damage index, including CVI, CHD, MCI PAD and mesenteric insufficiency. Garred et al.59 were the first to describe a higher frequency of thrombotic disease in SLE patients with MBL low-producing genotypes and this analysis was limited to arterial thrombosis in a later study by ∅hlenschlaeger et al.55, while Calvo-Alen et al.57 restricted their analysis to CVI. Of interest, Jönsen et al.58 and Calvo-Alen et al.57 used a similar outcome as in our study, allowing a better direct comparison between the studies. While the latter one showed an association with CVI, the first one, similarly to our study, rather described traditional CV risk factors to be primarily associated with CVD.
In direct comparison with the study by Jönsen et al.58 our study population had a considerably shorter follow-up time (in years) (median (range) 14 (0–49) vs. median (IQR) 9.5 (5.2–18.1)) but a somewhat higher age (in years) of the patients (median (range) 40 (10–83) vs. median (IQR) 44.8 (34.6–57.5)).
Another parameter that needs to be considered is the limited number of patients and, as a consequence, the limited number of patients with CVD investigated in previous studies. In comparison, our study evaluated a larger study population than the studies by ∅hlenschlaeger et al.55, Jönsen et al.58 and Font et al.54 but slightly less patients than the study by Calvo et al.57 Although in our study MBL deficiency was numerically more common in the group of SLE patients with CVD than in the Non-CVD group (40.3% vs. 33.4% below 500 ng/ml and 50.0% vs. 46.3% below 1000 ng/ml), this difference was not significant, which could be due to the relatively small number of events in total. In this context, it is of interest to note that patients in the study by ∅hlenschlaeger et al.55 developed an arterial thrombosis in 26%, a frequency 10% higher than in our study population. Likewise, Calvo-Alen et al.57, Jönsen et al.58 and Font et al.54 showed a considerably higher rate of CV events, which might be related to the younger age of our SLE patients and the relatively short follow-up time in our study. As a consequence, in spite of the relatively large number of SLE patient, our study might be limited in power due to its comparatively small number of CVD in total.
A further limitation that needs to be mentioned is that the damage assessment was carried out using the SLICC index and not with a specific focus on CVD at the time of data collection. Last, risk stratification analyses could not be adjusted to the thyroid function, but, according to our database records, the vast majority of our patients had no evidence of thyroid disease.
In balance with these limitations, the strength of our study is the large cohort size of SLE patients being prospectively included in the Swiss SLE Cohort Study (SSCS). The study population included both genders in a typical distribution as observed in patients with SLE and was shown to have typical patient characteristics. In addition, we focused on the determination of plasma MBL concentrations which maybe best reflect functionality. MBL levels in our patients were found to be stable over time and not related to SLE disease activity.
In this setting, we could not find a significant association between MBL deficiency and the occurrence of CVD in SLE patients. However, large prospective studies with long follow-ups would be required to definitely exclude a role of MBL in SLE-associated CVD.
Data availability
The data generated or analyzed during the current study are available from the corresponding author on reasonable request.
References
Pons-Estel, G. J., Alarcon, G. S., Scofield, L., Reinlib, L. & Cooper, G. S. Understanding the epidemiology and progression of systemic lupus erythematosus. Semin. Arthritis Rheum. 39, 257–268, https://doi.org/10.1016/j.semarthrit.2008.10.007 (2010).
Macedo, A. C. & Isaac, L. Systemic Lupus Erythematosus and Deficiencies of Early Components of the Complement Classical Pathway. Front. Immunol. 7, 55, https://doi.org/10.3389/fimmu.2016.00055 (2016).
Lisnevskaia, L., Murphy, G. & Isenberg, D. Systemic lupus erythematosus. Lancet 384, 1878–1888, https://doi.org/10.1016/S0140-6736(14)60128-8 (2014).
Font, J. et al. Clusters of clinical and immunologic features in systemic lupus erythematosus: analysis of 600 patients from a single center. Semin. Arthritis Rheum. 33, 217–230 (2004).
Crispin, J. C. et al. Pathogenesis of human systemic lupus erythematosus: recent advances. Trends Mol. Med. 16, 47–57, https://doi.org/10.1016/j.molmed.2009.12.005 (2010).
Bruce, I. N. ‘Not only…but also’: factors that contribute to accelerated atherosclerosis and premature coronary heart disease in systemic lupus erythematosus. Rheumatol. 44, 1492–1502, https://doi.org/10.1093/rheumatology/kei142 (2005).
Nossent, J. et al. Current causes of death in systemic lupus erythematosus in Europe, 2000–2004: relation to disease activity and damage accrual. Lupus 16, 309–317, https://doi.org/10.1177/0961203307077987 (2007).
Thomas, G. et al. Mortality associated with systemic lupus erythematosus in France assessed by multiple-cause-of-death analysis. Arthritis Rheumatol. 66, 2503–2511, https://doi.org/10.1002/art.38731 (2014).
Urowitz, M. B. et al. The bimodal mortality pattern of systemic lupus erythematosus. Am. J. Med. 60, 221–225 (1976).
Tucker, L., Menon, S., Schaller, J. & Isenberg, D. Adult- and childhood onset systemic lupus erythematosus: a comparison of onset, clinical features, serology, and outcome. Br. J. Rheumatol. 34, 866–872 (1995).
Abu-Shakra, M., Urowitz, M., Gladman, D. & Gough, J. Mortality studies in systemic lupus erythematosus. Results from a single center. II Predictor variables for mortality. J. Rheumatol. 22, 1265–1270 (1995).
Massardo, L. et al. Survival of Chilean Patients With Systemic Lupus Erythematosus. Semin. Arthritis Rheumatism 24, 1–11 (1994).
Fernandez-Nebro, A. et al. Cardiovascular Events in Systemic Lupus Erythematosus: A Nationwide Study in Spain From the RELESSER Registry. Med. 94, e1183, https://doi.org/10.1097/MD.0000000000001183 (2015).
Borchers, A. T., Keen, C. L., Shoenfeld, Y. & Gershwin, M. E. Surviving the butterfly and the wolf: mortality trends in systemic lupus erythematosus. Autoimmun. Rev. 3, 423–453, https://doi.org/10.1016/j.autrev.2004.04.002 (2004).
Petri, M., Perez-Gutthann, S., Spence, D. & Hochberg, M. C. Risk factors for coronary artery disease in patients with systemic lupus erythematosus. Am. J. Med. 93, 513–519 (1992).
Gladman, D. D. & Urowitz, M. B. Morbidity in systemic lupus erythematosus. J. Rheumatol. Suppl. 14(Suppl 13), 223–226 (1987).
Badui, E. et al. Cardiovascular manifestations in systemic lupus erythematosus. Prospective study of 100 patients. Angiology 36, 431–441, https://doi.org/10.1177/000331978503600705 (1985).
Gladman, D. et al. The development and initial validation of the systemic lupus international collaborating clinics American College of Rheumatology Damage Index for Systemic Lupus Erythematosus. Arthritis Rheumatism 39, 363–369, https://doi.org/10.1002/art.1780390303 (1996).
Esdaile, J. M. et al. Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis & Rheumatism 44, 2331–2337, https://doi.org/10.1002/1529-0131(200110)44:10<2331::AID-ART395>3.0.CO;2-I (2001).
Manzi, S., Meilahn, E., Rairie, J. & Conte, C. E. A. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with Framingham Study. Am. J. Epidemiol. 145, 408–415 (1997).
Avina-Zubieta, J. A. et al. Risk of Myocardial Infarction and Stroke in Newly Diagnosed Systemic Lupus Erythematosus: A General Population-Based Study. Arthritis Care Res. 69, 849–856, https://doi.org/10.1002/acr.23018 (2017).
Arkema, E. V., Svenungsson, E., Von Euler, M., Sjowall, C. & Simard, J. F. Stroke in systemic lupus erythematosus: a Swedish population-based cohort study. Ann. Rheum. Dis. 76, 1544–1549, https://doi.org/10.1136/annrheumdis-2016-210973 (2017).
Giannelou, M. & Mavragani, C. P. Cardiovascular disease in systemic lupus erythematosus: A comprehensive update. J. Autoimmun. 82, 1–12, https://doi.org/10.1016/j.jaut.2017.05.008 (2017).
Best, L. G. et al. Prospective analysis of mannose-binding lectin genotypes and coronary artery disease in American Indians: the Strong Heart Study. Circulation 109, 471–475, https://doi.org/10.1161/01.CIR.0000109757.95461.10 (2004).
Hegele, R. A., Ban, M. R., Anderson, C. M. & Spence, J. D. Infection-susceptibility alleles of mannose-binding lectin are associated with increased carotid plaque area. J. Investig. Med. 48, 198–202 (2000).
Madsen, H. O., Videm, V., Svejgaard, A., Svennevig, J. L. & Garred, P. Association of mannose-binding-lectin deficiency with severe atherosclerosis. Lancet 352, 959–960, https://doi.org/10.1016/S0140-6736(05)61513-9 (1998).
Garred, P., Larsen, F., Madsen, H. O. & Koch, C. Mannose-binding lectin deficiency–revisited. Mol. Immunol. 40, 73–84 (2003).
Fidler, K. J. et al. Increased incidence and severity of the systemic inflammatory response syndrome in patients deficient in mannose-binding lectin. Intensive Care Med. 30, 1438–1445, https://doi.org/10.1007/s00134-004-2303-8 (2004).
Steffensen, R., Thiel, S., Varming, K., Jersild, C. & Jensenius, J. C. Detection of structural gene mutations and promoter polymorphisms in the mannan-binding lectin (MBL) gene by polymerase chain reaction with sequence-specific primers. J. Immunol. Methods 241, 33–42 (2000).
Turner, M. W. & Mannose-binding, H. R. lectin: structure, function, genetics and disease associations. Rev. Immunogenet. 2, 305–322 (2000).
Monticielo, O. A., Mucenic, T., Xavier, R. M., Brenol, J. C. & Chies, J. A. The role of mannose-binding lectin in systemic lupus erythematosus. Clin. Rheumatol. 27, 413–419, https://doi.org/10.1007/s10067-008-0838-8 (2008).
Panda, A. K. et al. Low producer MBL genotypes are associated with susceptibility to systemic lupus erythematosus in Odisha, India. Hum. Immunol. 74, 114–119, https://doi.org/10.1016/j.humimm.2012.09.003 (2013).
Losada López, I. et al. Mannose binding lectin polymorphisms in systemic lupus erythematosus in Spain. Eur. J. Inflamm. 14, 78–85, https://doi.org/10.1177/1721727X16646385 (2016).
Charakida, M. et al. Endothelial response to childhood infection: the role of mannose-binding lectin (MBL). Atherosclerosis 208, 217–221, https://doi.org/10.1016/j.atherosclerosis.2009.07.055 (2010).
Limnell, V. et al. Association of mannan-binding lectin deficiency with venous bypass graft occlusions in patients with coronary heart disease. Cardiology 98, 123–126, https://doi.org/10.1159/000066313 (2002).
Saevarsdottir, S. et al. Mannan binding lectin as an adjunct to risk assessment for myocardial infarction in individuals with enhanced risk. J. Exp. Med. 201, 117–125 (2005).
Albert, M. A., Rifai, N. & Ridker, P. M. Plasma levels of cystatin-C and mannose binding protein are not associated with risk of developing systemic atherosclerosis. Vasc. Med. 6, 145–149 (2001).
Dahl, M., Tybjaerg-Hansen, A., Schnohr, P. & Nordestgaard, B. G. A population-based study of morbidity and mortality in mannose-binding lectin deficiency. J. Exp. Med. 199, 1391–1399, https://doi.org/10.1084/jem.20040111 (2004).
Hansen, T. K. et al. Association between mannose-binding lectin and vascular complications in type 1 diabetes. Diabetes 53, 1570–1576 (2004).
Larsen, J. B., Hvas, C. L. & Hvas, A. M. The Lectin Pathway in Thrombotic Conditions-A Systematic Review. Thromb. Haemost. 118, 1141–1166, https://doi.org/10.1055/s-0038-1654714 (2018).
Trendelenburg, M. et al. Influence of functional deficiency of complement mannose-binding lectin on outcome of patients with acute ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Eur. Heart J. 31, 1181–1187, https://doi.org/10.1093/eurheartj/ehp597 (2010).
Osthoff, M. et al. Mannose-binding lectin deficiency is associated with smaller infarction size and favorable outcome in ischemic stroke patients. PLoS One 6, e21338, https://doi.org/10.1371/journal.pone.0021338 (2011).
Petersen, S. V., Thiel, S. & Jensenius, J. C. The mannan-binding lectin pathway of complement activation: biology and disease association. Mol. Immunol. 38, 133–149 (2001).
Potlukova, E. et al. The production of mannan-binding lectin is dependent upon thyroid hormones regardless of the genotype: a cohort study of 95 patients with autoimmune thyroid disorders. Clin. Immunol. 136, 123–129, https://doi.org/10.1016/j.clim.2010.02.015 (2010).
Ribi, C. et al. The Swiss Systemic lupus erythematosus Cohort Study (SSCS) - Cross-sectional analysis of clinical characteristics and treatments across different medical disciplines in Switzerland. Swiss Med. Wkly. 144, 1–9, https://doi.org/10.4414/smw.2014.13990 (2014).
Osthoff, M. et al. Association Study of Mannose-Binding Lectin Levels and Genetic Variants in Lectin Pathway Proteins with Susceptibility to Age-Related Macular Degeneration: A Case-Control Study. PLoS One 10, e0134107, https://doi.org/10.1371/journal.pone.0134107 (2015).
Peterslund, N. A., Koch, C., Jensenius, J. C. & Thiel, S. Association between deficiency of mannose-binding lectin and severe infections after chemotherapy. Lancet 358, 637–638, https://doi.org/10.1016/S0140-6736(01)05785-3 (2001).
Gordon, A. C. et al. Mannose-binding lectin polymorphisms in severe sepsis: relationship to levels, incidence, and outcome. Shock. 25, 88–93 (2006).
Osthoff, M. et al. Role of lectin pathway complement proteins and genetic variants in organ damage and disease severity of systemic sclerosis: a cross-sectional study. Arthritis Res. Ther. 21, 76, https://doi.org/10.1186/s13075-019-1859-1 (2019).
Dima, A., Opris, D., Jurcut, C. & Baicus, C. Is there still a place for erythrocyte sedimentation rate and C-reactive protein in systemic lupus erythematosus? Lupus 25, 1173–1179, https://doi.org/10.1177/0961203316651742 (2016).
El-Magadmi, M. et al. Systemic lupus erythematosus: an independent risk factor for endothelial dysfunction in women. Circulation 110, 399–404, https://doi.org/10.1161/01.CIR.0000136807.78534.50 (2004).
Lima, D. S., Sato, E. I., Lima, V. C., Miranda, F. Jr. & Hatta, F. H. Brachial endothelial function is impaired in patients with systemic lupus erythematosus. J. Rheumatol. 29, 292–297 (2002).
Roman, M. J. et al. Prevalence and correlates of accelerated atherosclerosis in systemic lupus erythematosus. N. Engl. J. Med. 349, 2399–2406, https://doi.org/10.1056/NEJMoa035471 (2003).
Font, J. et al. Association of mannose-binding lectin gene polymorphisms with antiphospholipid syndrome, cardiovascular disease and chronic damage in patients with systemic lupus erythematosus. Rheumatol. 46, 76–80, https://doi.org/10.1093/rheumatology/kel199 (2007).
Øhlenschlaeger, T., Garred, P., Madsen, H. O. & Jacobsen, S. Mannose-binding lectin variant alleles and the risk of arterial thrombosis in systemic lupus erythematosus. N. Engl. J. Med. 351, 260–267, https://doi.org/10.1056/NEJMoa033122 (2004).
Troelsen, L. N. et al. Double role of mannose-binding lectin in relation to carotid intima-media thickness in patients with rheumatoid arthritis. Mol. Immunol. 47, 713–718, https://doi.org/10.1016/j.molimm.2009.10.021 (2010).
Calvo-Alen, J. et al. Systemic lupus erythematosus in a multiethnic US cohort: XXXIV. Deficient mannose-binding lectin exon 1 polymorphisms are associated with cerebrovascular but not with other arterial thrombotic events. Arthritis Rheum. 54, 1940–1945, https://doi.org/10.1002/art.21787 (2006).
Jonsen, A. et al. Genetically determined mannan-binding lectin deficiency is of minor importance in determining susceptibility to severe infections and vascular organ damage in systemic lupus erythematosus. Lupus 16, 245–253, https://doi.org/10.1177/09612033070160040201 (2007).
Garred, P. et al. Mannose-binding lectin polymorphisms and susceptibility to infection in systemic lupus erythematosus. Arthritis Rheum 42, 2145–2152, https://doi.org/10.1002/1529-0131(199910)42:10<2145::AID-ANR15>3.0.CO;2-%23 (1999).
Acknowledgements
We thank all clinical centers and coordinators of the Swiss SLE cohort study. Our thanks also go to all patients and medical representatives who supported the collection of blood samples. Prof. Dr. Marten Trendelenburg is recipient of a project grant of the Swiss National Science Foundation (grant No. 310030_172956/1) and has research collaborations with Roche, Novartis and Idorsia (all Switzerland).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation and analysis were performed by A.K.G., S.V. and D.D. C.R., in charge of the SSCS in Lausanne and SSCS database manager in Switzerland, extracted and organized all data needed for the analyzes and proofread the manuscript. C.C., in charge of the SSCS in Geneva, helped to complete missing data and proofread the manuscript. U.H.D., in charge of the SSCS in Bern, helped to complete missing data and proofread the manuscript. M.O. was a major contributor in proofreading and writing the manuscript. M.T., in charge of the SSCS in Basel, contributed with his knowledge and expertise to the idea and realization of the project, besides, he was a major contributor in proofreading and writing the manuscript. The first draft of the manuscript was written by A.K.G. All authors commented on previous versions of the manuscript as well as read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kieninger-Gräfitsch, A., Vogt, S., Ribi, C. et al. No association of complement mannose-binding lectin deficiency with cardiovascular disease in patients with Systemic Lupus Erythematosus. Sci Rep 10, 3693 (2020). https://doi.org/10.1038/s41598-020-60523-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-60523-3
This article is cited by
-
Association of mannose-binding lectin 2 gene polymorphisms with Guillain-Barré syndrome
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.