Abstract
Influenza-related severe pneumonia and acute respiratory distress syndrome (ARDS) are severe threats to human health. The objective of this study was to assess the effects of systematic corticosteroid therapy in patients with pneumonia or ARDS. The PubMed, EMBASE, Web of Science and SCOPUS databases were searched up to July, 2019. Nineteen studies including 6637 individuals were identified, and fifteen studies (6427 patients) were included in the meta-analysis of mortality. Eighteen were observational studies and one was a randomized controlled trial (RCT). The meta-analysis results showed that corticosteroid therapy was associated with significantly higher mortality (OR 1.53, 95% CI [1.16, 2.01]) and incidence of nosocomial infection (OR 3.15, 95% CI [1.54, 6.45]). Subgroup analysis showed that among patients with unadjusted estimates, the odds of mortality were higher in patients receiving corticosteroid treatment (OR 1.98, 95% CI [1.23, 3.17]), however, among patients with adjusted estimates, the result showed no statistically significant difference between corticosteroid group and control group (OR 1.31, 95% CI [0.95, 1.80]). Current data do not support the routine use of corticosteroids in patients with influenza severe pneumonia or ARDS. RCTs are needed to provide more robust evidence.
Similar content being viewed by others
Introduction
Influenza is a viral infection that attacks the respiratory system. Rapidly progressing viral pneumonia and acute respiratory distress syndrome are pulmonary manifestations that are commonly observed in patients with influenza and are associated with considerable mortality1,2,3, representing a severe threat and imparting a substantial financial burden worldwide4.
Individuals with community-acquired pneumonia may benefit from systematic corticosteroid therapy, which may block the inflammatory cascade reaction5. Corticosteroids could improve the lung tissue damage induced by influenza pneumonia and decrease the risk of mortality in animal models with influenza infections6,7. Many clinicians administer corticosteroids as an anti-inflammatory treatment for patients with severe influenza-related pneumonia to stop disease progression and improve clinical outcomes. A large cohort study of patients admitted to 148 ICUs in Spain found that the frequency of corticosteroid treatment by study period was 34.9% in 2009, 39.6% in 2010, 29% in 2013, and 31.4% in 20148. Recently, some studies have shown that corticosteroids may not be beneficial for patients with severe influenza and may even increase mortality9,10,11. However, there is considerable uncertainty regarding whether patients with influenza-related ARDS or severe pneumonia can benefit from adjuvant corticosteroid therapy.
We aimed to systematically review all experimental and observational studies on corticosteroid use in patients with influenza-related ARDS and severe pneumonia. The effect of corticosteroid treatment on clinical outcomes was investigated.
Methods
Study eligibility criteria
This systematic review included studies fulfilling the following inclusion criteria: (a) the studies were RCTs, quasi-experimental studies, or observational studies; (b) patients had confirmed influenza-related pneumonia, ARDS (PaO2/FiO2 < 300 mmHg); (c) the intervention group used corticosteroids, and the comparison group did not, with no restriction set on the dose or duration of the intervention; and (d) the outcomes were mortality, nosocomial infection, length of stay or other clinical outcomes. A study was excluded if it met any of the following criteria: (a) the study was a review article, conference abstract, case report or case series, case-control study; (b) the majority of included patients were immunocompromised; (c) insufficient data were available; (d) overlapping population; (e) studies with fewer than 20 participants. There were no restrictions on influenza subtype, patient age or study setting. If only some of the individuals included in a study fit the eligibility criteria and these individuals had extractable results corresponding to the objective of this study, then the study was included.
Clinical outcomes including mortality, nosocomial infection, duration of mechanical ventilation, length of stay, time to fever alleviation and clinical stability and viral shedding were evaluated.
Search strategy and study selection
We comprehensively searched the PubMed, EMBASE, Web of Science and SCOPUS databases from inception to July 2019. The core search terms were defined as those related to influenza-related pneumonia, ARDS, acute respiratory failure and corticosteroid use (for details on the search strategy in EMBASE, refer to Supplementary Table S1). The references of eligible studies were screened, and two authors independently reviewed all citations that met the inclusion criteria. Study selection was performed in 2 stages: first, study title and abstract screening; second, full text examination.
Data extraction and quality assessment
Outcome data were independently extracted from the included studies by two investigators using a previously piloted standardized pro forma. We obtained the following data: (a) characteristics of studies (design, setting, country, period, methodological details for quality assessment); (b) characteristics of participants (demographics, co-morbid illnesses, disease severity, numbers in each group, influenza virus type); (c) characteristics of interventions (type, dose, timing and duration of corticosteroid use); and (d) outcomes.
The quality of each study was independently assessed by two individuals according to the Cochrane Risk of Bias tool for RCTs and the Newcastle-Ottawa Scale for nonrandomized trials and comparative observational studies. Three domains are assessed on the NOS for observational studies12: (1) “selection bias”, (2) “comparability bias”, and (3) “outcome bias”. Disagreements at any stage were resolved through discussion with the other authors until consensus was reached.
Sensitivity analysis
We performed sensitivity analysis to assess the effect of the study design on clinical outcomes using stratification if the number of studies was sufficient.
Data analysis
Odds ratios (ORs) and their corresponding 95% confidence intervals (CIs) were generated during the analysis of dichotomous outcome data, and mean differences or standardized mean differences and their corresponding 95% CIs were generated during the analysis of normally distributed continuous data. ORs or hazard ratios (HRs) for adjusted outcome estimates and their corresponding 95% CIs were obtained and are presented in the pooled analyses. Medians and interquartile ranges were generated in the analysis of continuous data that were not normally distributed.
The I² test for inconsistency was used to analyse heterogeneity. If I² > 50%, the heterogeneity across studies was significant, and a random-effects model was used in the meta-analysis; otherwise, a fixed-effects model was used. Subgroup analysis was performed in the following areas where possible: adult population versus child population; seasonal influenza versus outbreak influenza or pandemic influenza; ICU versus inpatient; adjusted estimates versus unadjusted estimates; and corticosteroid dose, timing and duration. All statistical analyses were performed using Cochrane systematic review software Review Manager (RevMan; Version 5.3.5; The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, 2014).
Main results
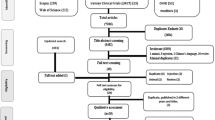
A total of 7771 relevant articles were identified during the initial search. After the removal of 2469 duplicates, 5302 articles remained. After screening the titles and abstracts of those articles, 5204 articles were excluded because of irrelevance. Of the 98 full-text articles reviewed, 81 were excluded for various reasons, and 19 articles remained. Details regarding the reasons for the exclusion of these studies are shown in Fig. 1 and Supplementary Table S2. Ultimately, 15 studies were included in the meta-analysis of mortality, while 4 studies reported outcomes other than mortality in association with corticosteroid use.
The characteristics of the participants in the included studies are summarized in Table 1 and Supplementary Table S3. The studies were published between 2010 and 2018. Eighteen of the studies had an observational design, while one had a randomized controlled trial design13. Outcome data were reported in 19 studies (6637 individuals, including 2675 in corticosteroid group and 3962 in the non-corticosteroid group), while mortality data were reported in 15 studies (6427 individuals). Eight studies (n = 2558) included only ICU patients. Fourteen studies assessed individuals with H1N1pdm09 virus infection, 1 study assessed individuals with H7N9 virus infection, and 4 study assessed individuals with inter-pandemic influenza virus infection. Eight studies (1956 individuals) had useable data related to patients with ARDS9,10,14,15,16,17,18,19. Fourteen studies (n = 6335) reported mortality associated with adults only.
The median ages varied from 2.5 to 60.1 years in all patients included. The proportion of male participants was higher than that of females (58.9% versus 41.1%) and range varied from 44.8% to 78.0% (15 studies, 2969 individuals). Obesity (BMI ≥ 30 kg/m2) was common (31.7%, 779/2454) in the included studies (7 studies, 2454 individuals), and the proportion of obese individuals ranged from 2.1% to 41.4%. Disease severity at baseline was reported in seven studies and in 4 studies (2166 individuals), the baseline disease severity was higher in individuals in the corticosteroid group than in those in the non-corticosteroid group19,20,21. Methylprednisolone (88.7%, 809/912) was the most common steroid used in the corticosteroid group (4 studies), and the median duration varied from 5.1 to 11.0 days. Almost all patients (97.0%, 5056/5211) received antiviral therapy (ranging from 96.0% to 100%, 12 studies), and 96.5% (2579/2673) of participants received antibiotic therapy (5 studies). The details of the therapies are described in Table 1.
Because all studies included in the meta-analysis of mortality were observational cohort studies, selection bias was inevitable. The risk of bias identified in the 19 included studies is shown in Supplementary Tables S4a,b. The studies’ NOS scores varied from 6 to 9, indicating that the quality of the included studies was high22. However, most included studies had substantial comparability bias because we could not adequately adjust for disease severity, and individuals with greater diseases severity tended to use corticosteroids.
Mortality
Overall mortality in the included studies
Mortality data were reported in 15 studies8,9,10,14,15,16,17,18,20,23,24,25,26,27. The pooled analysis of the crude results of the 15 included studies (6427 individuals) suggested that those who used corticosteroids had a significantly higher mortality rate (OR 2.30, 95% CI [1.68, 3.16], p < 0.01), and a moderate level of heterogeneity was observed (I² = 66%; Supplementary Fig. S1). Five studies8,10,14,15,26 reported adjusted effect estimates of 30-day or inhospital mortality (adjusted OR or adjusted hazard ratio (aHR), Table 2). The pooled analysis of the crude results of ten included studies and five adjusted effect estimates suggested that those who used corticosteroids had a significantly higher mortality rate (OR 1.53, 95% CI [1.16, 2.01], I2 = 53%, p < 0.002, Fig. 2). The subgroup analysis of unadjusted effect estimates showed a similar result (OR 1.98, 95% CI [1.23, 3.17], I2 = 32%, p = 0.005). However, the subgroup analysis of the five adjusted estimates showed no association between mortality and corticosteroid use (HR 1.31, 95% CI [0.95, 1.80], I2 = 70%, p = 0.1). The test for subgroup differences between adjusted and unadjusted mortality was not statistically significant (p = 0.06). There was no clear indication of publication bias in the funnel plot analysis (Supplementary Fig. S2).
Mortality in ARDS
Eight studies reported the mortality of patients with ARDS due to influenza (n = 1956, Supplementary Table S5). Meta-analysis of unadjusted and adjusted estimates suggested that the difference in mortality between the corticosteroid and control groups was not statistically significant (OR 1.68, 95% CI [0.94, 3.01], I2 = 69%, p = 0.08, Fig. 3). Li et al. reported that corticosteroid use was associated with a decreased risk of mortality in patients with ARDS (PaO2/FiO2 < 300 mmHg) (aHR 0.67, 95% CI [0.46, 0.98])15. However, Brun-Buisson et al.14 and Chawla et al.18 suggested that the risk of mortality was higher in corticosteroid group in the patients with ARDS. Cao et al. reported that a low-to-moderate dose of corticosteroids had no statistically significant association with the risk of mortality in patients with ARDS (OR 1.69, 95% CI [0.78, 3.64]), whereas a high dose was associated with greater mortality (OR 2.89, 95% CI [1.10, 7.56])10. The other four studies9,16,17,19 showed that corticosteroid use had no association with mortality (Fig. 3).
Sensitivity analysis
Considering the different management strategies for paediatric and adult patients, one study investigating paediatric patients was excluded27. The pooled analysis of 14 studies reporting adult individuals revealed a significant increase in the odds of mortality with corticosteroid use, with moderate statistical heterogeneity (OR 2.17 [1.59, 2.96], I2 = 65%).
Subgroup analysis
A subgroup analysis according to pure ICU patients (8 studies, 2558 individuals) and mixed patients (7 studies, 3878 individuals including both ICU and wards patients) was conducted. For the pure ICU subgroup, we found that corticosteroids were associated with an increased risk of mortality (OR, 1.71 [1.41, 2.06]) with low statistical heterogeneity (I2 = 7%), and a similar result was found for mixed patient groups (OR 3.14 [2.58, 3.83], I2 = 62%)(Supplementary Fig. S3).
Three studies (n = 3473) have reported the results of mortality excluding patients who had potential indications (e.g., asthma, COPD exacerbation, pregnancy/post-partum, shock or immunosuppressive conditions) for corticosteroid treatment that may have skewed the results8,14,15. A pooled analysis of these three studies showed no statistically significant association between corticosteroid use and mortality (OR 1.37, 95% CI [0.86, 2.18]), with a high level of heterogeneity (I² = 77%, P = 0.01).
Thirteen studies included adult patients with A/H1N1, A/H3N2 or B influenza. The pooled analysis of these studies found corticosteroid use to be associated with greater odds of mortality (OR 2.03, 95% CI [1.47, 2.79], with a moderate level of heterogeneity (I2 = 63%).
The number of studies was insufficient to perform subgroup analysis according to the various reported regimens. Two studies compared early versus later/no corticosteroid treatment; one defined early treatment as within three days of mechanical ventilation14, and the result suggested that early treatment with corticosteroids was associated with greater mortality (aHR 3.42, 95% CI [1.73, 6.75]); the other defined early treatment as within 72 h of NAIs17, and the result showed no statistically significant difference in mortality between patients receiving corticosteroids within 72 h of NAIs and those who did.
Three studies categorized corticosteroid dose as low/low-to-moderate and high10,15. A large retrospective cohort study reported that a low-to-moderate dose (25–150 mg/d methylprednisolone or equivalent) was associated with a decreased risk of mortality in patients with PaO2/FiO2 < 300 mmHg (aHR 0.51, 95% CI [0.33–0.78]), whereas in patients with PaO2/FiO2 ≥ 300 mmHg (aHR 0.88, 95% CI [0.56, 1.39]), a low-to-moderate dose was associated with greater mortality (aHR 3.70, 95% CI [1.20–11.34])15. A high dose (>150 mg/d methylprednisolone or equivalent) showed no benefit in all patients15. A retrospective Chinese cohort study of 288 people with influenza A H7N9 virus infection suggested that compared to the controls, the mortality risk in patients receiving low-to-moderate doses of corticosteroids (25–150 mg/d methylprednisolone or equivalent), was not significantly different (aHR 1.64, 95% CI [0.79, 3.39]), whereas in participants treated with high-dose corticosteroids (defined as > 150 mg/d methylprednisolone or equivalent), the mortality risk was significantly greater (aHR 3.05, 95% CI [1.28, 7.25])10. Another study reporting the result of 62 patients with acute respiratory failure due to influenza showed no statistically significant difference between low dose and high dose corticosteroid therapy (8/19 versus 7/19, p > 0.05)16.
Two of the included studies reported outcomes related to children, but only one reported the risk of mortality (OR 8.12, 95% CI [2.44, 27.02]) related to corticosteroid use27. However, in that study, all children who received corticosteroids had ARDS, while the patients in the non-corticosteroid group had less severe disease conditions. Another retrospective cohort study of children with pneumonia caused by the 2009 H1N1 influenza virus only reported length of hospital stay and duration of fever28 and found a shorter length of stay and duration of fever in corticosteroid group (Table 3).
Nosocomial infection
Six studies reported an association between corticosteroid use and nosocomial infection. In four of these studies, corticosteroid use was associated with an increased risk of developing a nosocomial infection15,17,20,21, while the remaining two studies did not show a statistically significantly increased odds of developing infection10,14. Overall, the pooled results revealed that the odds of nosocomial infection were significantly higher in patients who were administered corticosteroids than in those who were not (OR 3.15, 95% CI [1.54, 6.45], p < 0.0001), but a high level of heterogeneity was observed (I² = 82%) (Fig. 4).
Three studies reported the common pathogens isolated from patients with nosocomial infection. One study reported that the most common bacteria isolated was Acinetobacter baumannii (28.2%)17. In a study of 2141 patients with severe influenza pneumonia15, 245 patients had nosocomial infection, and the most commonly isolated pathogens were Acinetobacter baumannii (35.0%), Pseudomonas aeruginosa (13.5%), and Staphylococcus aureus (11.0%), while in another cohort study of 1846 patients with severe influenza pneumonia8, Streptococcus pneumoniae (49.1%), Pseudomonas aeruginosa (10.1%), and Staphylococcus aureus (7.5%) were the most frequently isolated microorganisms.
Length of stay and length of MV
Seven studies reported length of stay according to corticosteroid use; all were unadjusted for disease severity (Table 3). Six studies found no statistically significant difference between the groups. One study19 showed a longer length of ICU stay associated with corticosteroid use, while the total length of hospital stays was not significantly different between the groups. Notably, one of the five studies analysed the duration of hospital stay in people with influenza pneumonia treated with corticosteroid versus those receiving placebo, and found no significant difference between the groups (adjusted difference −2.24 days, 95% CI [−9.61, 5.12])13.
Linko et al.19 reported a longer duration of mechanical ventilation in the group treated with corticosteroid therapy while Brun-Buisson et al.14 and Moreno et al.8 found no statistically significant difference between the groups. (Table 3).
Time to fever alleviation, time to clinical stability and viral shedding
Two studies reported the time to fever alleviation according to corticosteroid use28,29. Kudo et al.29 found no statistically significant difference between the groups, while another cohort study of children28 with severe influenza pneumonia reported a shorter time to fever alleviation. Notably, two studies found a shorter time to clinical stability in the corticosteroid group. The study of influenza A/H7N9 found a significantly longer duration of viral shedding associated with corticosteroid treatment10. The details of these outcomes are described in Table 3.
Discussion
The overall findings of this meta-analysis indicated that patients with pneumonia or acute respiratory distress syndrome who were administered corticosteroids had significantly higher mortality and incidence of nosocomial infection but the use of corticosteroids did not influence the length of hospital stay.
Our studies suggested a deleterious effect of steroids on mortality and nosocomial infection. Several factors need to be accounted for in interpreting these findings. First, most studies did not adjust the clinical outcomes for potential confounding factors. Clinically, more severe cases tended to be treated with corticosteroids, which may obscure the real value of this treatment regarding mortality30,31. Therefore, in this study, we preferred the use of adjusted estimates of the effect to minimize potential confounding between the treatment groups. However, five studies reported adjusted estimates of mortality, and their inclusion in the meta-analysis still revealed a higher odds of mortality related to steroids use. Good evidences indicated that secondary bacterial pneumonia is an important cause of mortality related to influenza32,33. Therefore, increasing risk of nosocomial infection due to corticosteroid treatment may partly account for the potential harm from corticosteroid use. Two included studies8,15 found that secondary bacterial pneumonia such as due to Acinetobacter baumannii, Pseudomonas aeruginosa, Streptococcus pneumoniae, Staphylococcus aureus or invasive fungal infection, were more common in corticosteroid-treated patients. Several studies showed that prolonged viral shedding and delayed viral clearance were noted in corticosteroid-treated patients10,34, whereas slower clearance of virus loads was associated with higher mortality in patients with ARDS due to H1N1pdm09 virus infection35. Thus, prolonged viral shedding and delayed viral clearance may also contribute to higher mortality.
Second, most of the included observational studies did not explain why some patients received systemic corticosteroid therapy and others did not. The initial intentions of corticosteroid therapy were unclear (was it used as a rescue therapy or due to COPD/asthma exacerbation or due to pneumonia/ARDS?). Different indication may easily confound the effect of the corticosteroid. Some evidences supported the use of corticosteroids for asthma or COPD or septic shock in the context of influenza infection36,37,38. In order to minimize the influences of different indications, subgroup analysis of the mortality in three studies (n = 3347) was performed after excluding patients receiving corticosteroids as rescue therapy or due to COPD/asthma exacerbation, and found no statistically significant difference between the steroid therapy groups and control groups and the heterogeneity was high (I² = 77%). However, the high level of statistical heterogeneity may result in unstable estimates of the meta-analysis. Therefore, well-designed clinical trials should be conducted to decrease the heterogeneity of patients and to provide more robust evidence.
The results from clinical studies of corticosteroid therapy in patients with influenza are conflicting. Many studies have shown a significant association between corticosteroid treatment and mortality in patients with influenza; however, several studies have reported that corticosteroids can provide benefits to patients under certain conditions15,28,39,40. An RCT13 included in this review noted an association between adjuvant corticosteroid therapy (50 mg of prednisone given orally for 7 days) and decreased time to clinical stability. Low-to-moderate doses of corticosteroids are beneficial in people with hypoxia ((PaO2/FiO2) <300 mmHg), whereas high doses of corticosteroids showed no benefit in this group; however, low-to-moderate doses of corticosteroids may increase the 60-day mortality rate in those with PaO2/FiO2 > 300 mmHg15. Kil et al.28 reported that rapid (methylprednisolone, 10 mg/kg/d) and short-term (tapered off within a week) corticosteroid treatment for children with severe pneumonia halted clinical exacerbation and possibly prevented progression to ARDS. However, in another study, compared with no treatment, administration (steroid therapy was initiated at a median daily dose equivalent to 270 (IQR, 200–400) mg of hydrocortisone, and a median duration of 11 (IQR, 6–20) days within the first 3 days of MV was more strongly associated with an increased risk of death, whereas when administration was beyond the first 3 days of MV, the association was no longer significant14. Considering the findings of the aforementioned studies, the condition of the patients’ respiratory system and the dose, timing and duration of corticosteroids could be contributing factors that affect the effects of corticosteroids.
Several recent systematic reviews and meta-analyses concluded that corticosteroid therapy is significantly associated with mortality41,42,43. However, in these studies, there were no special limitations on subject inclusion criteria, which means that the patients were very diverse. Additionally, there was no subgroup analysis for these patients under different disease conditions. Compared to patients in those previous studies, we focused only on patients with pneumonia or ARDS, which is more specific and makes the outcomes more targeted. Our study observed a different outcome according to corticosteroid use in patients with ARDS due to influenza.
This study has some limitations, including the lack of sufficient data on the dose, duration, timing and rationales of corticosteroid administration and the timing and duration of antiviral therapy. In addition, only one study included in this meta-analysis was an RCT, and 18 were observational in nature. Thus, it is possible that selection bias or comparability bias could have affected the quality of the analysed evidence. There is insufficient evidence in this meta-analysis to make a firm determination about the effectiveness of corticosteroids for people with influenza-related pneumonia or ARDS. The small number of included studies and the small number of patients in the included studies might also make the effect size of some outcome indicators insufficient, and we were unable to analyse the effect of some factors on the outcome indicators by meta-regression or subgroup analysis.
Conclusion
Current data do not support the routine use of corticosteroids in patients with influenza pneumonia or ARDS. However, the data assessed in this meta-analysis were extracted from 18 observational studies and only one RCT; therefore, the limitations associated with study design are important to consider. There is a need for more robust evidence on the role of corticosteroids in the treatment of influenza-related ARDS and severe pneumonia before a firm recommendation for clinical practice can be made.
References
Abdel-Ghafar, A. N. et al. Update on avian influenza A (H5N1) virus infection in humans. The New England journal of medicine 358, 261–273, https://doi.org/10.1056/NEJMra0707279 (2008).
Kumar, A. et al. Critically ill patients with 2009 influenza A(H1N1) infection in Canada. Jama 302, 1872–1879, https://doi.org/10.1001/jama.2009.1496 (2009).
Simonsen, L. et al. Global mortality estimates for the 2009 Influenza Pandemic from the GLaMOR project: a modeling study. PLoS Med 10, e1001558, https://doi.org/10.1371/journal.pmed.1001558 (2013).
Peasah, S. K., Azziz-Baumgartner, E., Breese, J., Meltzer, M. I. & Widdowson, M. A. Influenza cost and cost-effectiveness studies globally–a review. Vaccine 31, 5339–5348, https://doi.org/10.1016/j.vaccine.2013.09.013 (2013).
Torres, A. et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: a randomized clinical trial. Jama 313, 677–686, https://doi.org/10.1001/jama.2015.88 (2015).
Li, C. et al. Corticosteroid treatment ameliorates acute lung injury induced by 2009 swine origin influenza A (H1N1) virus in mice. PloS one 7, e44110, https://doi.org/10.1371/journal.pone.0044110 (2012).
Ottolini, M. et al. Combination anti-inflammatory and antiviral therapy of influenza in a cotton rat model. Pediatric pulmonology 36, 290–294, https://doi.org/10.1002/ppul.10320 (2003).
Moreno, G. et al. Corticosteroid treatment in critically ill patients with severe influenza pneumonia: a propensity score matching study. Intensive care medicine 44, 1470–1482, https://doi.org/10.1007/s00134-018-5332-4 (2018).
Kim, S.-H. et al. Corticosteroid Treatment in Critically Ill Patients with Pandemic Influenza A/H1N1 2009 Infection Analytic Strategy Using Propensity Scores. American journal of respiratory and critical care medicine 183, 1207–1214, https://doi.org/10.1164/rccm.201101-0110OC (2011).
Cao, B. et al. Adjuvant Corticosteroid Treatment in Adults With Influenza A (H7N9) Viral Pneumonia. Critical care medicine 44, e318–328, https://doi.org/10.1097/ccm.0000000000001616 (2016).
Delaney, J. W. et al. The influence of corticosteroid treatment on the outcome of influenza A(H1N1pdm09)-related critical illness. Critical Care 20, https://doi.org/10.1186/s13054-016-1230-8 (2016).
The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses, http://www.ohri.ca/programsclinicalepidemiology/oxford.asp (2014).
Wirz, S. A. et al. Pathogen- and antibiotic-specific effects of prednisone in community-acquired pneumonia. European Respiratory Journal 48, 1150–1159, https://doi.org/10.1183/13993003.00474-2016 (2016).
Brun-Buisson, C., Richard, J. C., Mercat, A., Thiebaut, A. C. & Brochard, L. Early corticosteroids in severe influenza A/H1N1 pneumonia and acute respiratory distress syndrome. American journal of respiratory and critical care medicine 183, 1200–1206, https://doi.org/10.1164/rccm.201101-0135OC (2011).
Li, H. et al. Effect of low-to-moderate-dose corticosteroids on mortality of hospitalized adolescents and adults with influenza A(H1N1)pdm09 viral pneumonia. Influenza and other respiratory viruses 11, 345–354, https://doi.org/10.1111/irv.12456 (2017).
Xi, X. et al. Hospitalized adult patients with 2009 influenza A(H1N1) in Beijing, China: risk factors for hospital mortality. BMC infectious diseases 10, https://doi.org/10.1186/1471-2334-10-256 (2010).
Huang, S. F., Fung, C. P., Perng, D. W. & Wang, F. D. Effects of corticosteroid and neuraminidase inhibitors on survival in patients with respiratory distress induced by influenza virus. Journal of microbiology, immunology, and infection = Wei mian yu gan ran za zhi 50, 586–594, https://doi.org/10.1016/j.jmii.2015.08.016 (2017).
Chawla, R., Kansal, S., Chauhan, M., Jain, A. & Jibhkate, B. Predictors of mortality and length of stay in hospitalized cases of 2009 influenza A (H1N1): Experiences of a tertiary care center. Indian Journal of Critical Care Medicine 17, 275–282, https://doi.org/10.4103/0972-5229.120318 (2013).
Linko, R. et al. Corticosteroid therapy in intensive care unit patients with PCR-confirmed influenza A(H1N1) infection in Finland. Acta anaesthesiologica Scandinavica 55, 971–979, https://doi.org/10.1111/j.1399-6576.2011.02491.x (2011).
Viasus, D. et al. Effect of immunomodulatory therapies in patients with pandemic influenza A (H1N1) 2009 complicated by pneumonia. The Journal of infection 62, 193–199, https://doi.org/10.1016/j.jinf.2011.01.014 (2011).
Chien, Y. S. et al. Predictors and outcomes of respiratory failure among hospitalized pneumonia patients with 2009 H1N1 influenza in Taiwan. Journal of Infection 60, 168–174, https://doi.org/10.1016/j.jinf.2009.12.012 (2010).
Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in metaanalyses. Eur. J. Epidemiol. 25, 603–605, https://doi.org/10.1007/s10654-010-9491-z (2010).
Carrillo-Esper, R., Sosa-Garcia, J. O. & Arch-Tirado, E. Experience in the management of the severe form of human influenza A H1N1 pneumonia in an intensive care unit. Cirugia y cirujanos 79, 409–416 (2011).
Rios, F. G. et al. Lung function and organ dysfunctions in 178 patients requiring mechanical ventilation during the 2009 influenza A (H1N1) pandemic. Critical care (London, England) 15, R201, https://doi.org/10.1186/cc10369 (2011).
Sertogullarindan, B. et al. Clinical and prognostic features of patients with pandemic 2009 influenza a (H1N1) virus in the intensive care unit. African Health Sciences 11, 163–170 (2011).
Lee, N. et al. Neuraminidase inhibitors, superinfection and corticosteroids affect survival of influenza patients. European Respiratory Journal 45, 1642–1652, https://doi.org/10.1183/09031936.00169714 (2015).
Kinikar, A. A. et al. Predictors of mortality in hospitalized children with pandemic H1N1 influenza 2009 in Pune, India. Indian journal of pediatrics 79, 459–466, https://doi.org/10.1007/s12098-011-0578-7 (2012).
Kil, H. R. et al. Early corticosteroid treatment for severe pneumonia caused by 2009 H1N1 influenza virus. Critical Care 15, https://doi.org/10.1186/cc10082 (2011).
Kudo, K. et al. Systemic corticosteroids and early administration of antiviral agents for pneumonia with acute wheezing due to influenza A(H1N1)pdm09 in Japan. PloS one 7, e32280, https://doi.org/10.1371/journal.pone.0032280 (2012).
Balaganesakumar, S. R. et al. Risk factors associated with death among influenza A (H1N1) patients, Tamil Nadu, India, 2010. Journal of Postgraduate Medicine 59, 9–14, https://doi.org/10.4103/0022-3859.109481 (2013).
Dellinger, R. P. et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Critical care medicine 41, 580–637, https://doi.org/10.1097/CCM.0b013e31827e83af (2013).
Brundage, J. F. & Shanks, G. D. Deaths from bacterial pneumonia during 1918-19 influenza pandemic. Emerging infectious diseases 14, 1193–1199, https://doi.org/10.3201/eid1408.071313 (2008).
Morens, D. M., Taubenberger, J. K. & Fauci, A. S. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. The Journal of infectious diseases 198, 962–970, https://doi.org/10.1086/591708 (2008).
Lee, N. et al. Viral loads and duration of viral shedding in adult patients hospitalized with influenza. The Journal of infectious diseases 200, 492–500, https://doi.org/10.1086/600383 (2009).
To, K. K. W. et al. Delayed clearance of viral load and marked cytokine activation in severe cases of pandemic H1N1 2009 influenza virus infection. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America 50, 850–859, https://doi.org/10.1086/650581 (2010).
Ernst, P., Ariel, A. & Suissa, S. Differences between asthmatics and nonasthmatics hospitalised with influenza A infection. The European respiratory journal 41, 772–774, https://doi.org/10.1183/09031936.00137812 (2013).
Rhodes, A. et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive care medicine 43, 304–377, https://doi.org/10.1007/s00134-017-4683-6 (2017).
Bekkat-Berkani, R. et al. Seasonal influenza vaccination in patients with COPD: a systematic literature review. BMC pulmonary medicine 17, 79–79, https://doi.org/10.1186/s12890017-0420-8 (2017).
Quispe-Laime, A. M. et al. H1N1 influenza A virus-associated acute lung injury: response to combination oseltamivir and prolonged corticosteroid treatment. Intensive care medicine 36, 33–41, https://doi.org/10.1007/s00134-009-1727-6 (2010).
Athauda, D., Andrews, T. C., Holmes, P. A. & Howard, R. S. Multiphasic acute disseminated encephalomyelitis (ADEM) following influenza type A (swine specific H1N1). J. Neurol. 259, 775–778, https://doi.org/10.1007/s00415-011-6258-8 (2012).
Ni, Y.-N., Chen, G., Sun, J., Liang, B.-M. & Liang, Z.-A. The effect of corticosteroids on mortality of patients with influenza pneumonia: a systematic review and meta-analysis. Critical Care 23, https://doi.org/10.1186/s13054-019-2395-8 (2019).
Lansbury, L., Rodrigo, C., Leonardi-Bee, J., Nguyen-Van-Tam, J. & Lim, W. S. Corticosteroids as adjunctive therapy in the treatment of influenza. The Cochrane database of systematic reviews 2, Cd010406, https://doi.org/10.1002/14651858.CD010406.pub3 (2019).
Rodrigo, C., Leonardi-Bee, J., Nguyen-Van-Tam, J. & Lim, W. S. Corticosteroids as adjunctive therapy in the treatment of influenza. The Cochrane database of systematic reviews 3, Cd010406, https://doi.org/10.1002/14651858.CD010406.pub2 (2016).
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (81672005, 81001271, 81721091), the Mega-Project of National Science and Technology for the 12th and 13th Five-Year Plan of China (2018ZX10715-014-002, 2014ZX10004008, 2013ZX10004901, 2013ZX10004904 and 2011ZX10004-901), the Key Joint Project for Data Center of the National Natural Science Foundation of China (U1611264), and the Fundamental Research Funds for the Central Universities (2017FZA7004).
Author information
Authors and Affiliations
Contributions
Shigui Yang designed the study. Yuqing Zhou, Xiaofang Fu, Xiaoxiao Liu, Chenyang Huang, Guo Tian, Cheng Ding, Jie Wu, Lei Lan collected the data. Yuqing Zhou, Xiaofang Fu, Xiaoxiao Liu, Guo Tian, Cheng Ding and Jie Wu analyzed the data. Yuqing Zhou and Shigui Yang interpreted the results. Yuqing Zhou wrote the manuscript. Yuqing Zhou and Shigui Yang revised the manuscript from preliminary draft to submission. Shigui Yang supervised the study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhou, Y., Fu, X., Liu, X. et al. Use of corticosteroids in influenza-associated acute respiratory distress syndrome and severe pneumonia: a systemic review and meta-analysis. Sci Rep 10, 3044 (2020). https://doi.org/10.1038/s41598-020-59732-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-59732-7
This article is cited by
-
Differences of respiratory mechanics in mechanical ventilation of acute respiratory distress syndrome between patients with COVID-19 and Influenza A
Respiratory Research (2024)
-
Immune response in influenza virus infection and modulation of immune injury by viral neuraminidase
Virology Journal (2023)
-
Host-directed immunotherapy of viral and bacterial infections: past, present and future
Nature Reviews Immunology (2023)
-
Flu-IV score: a predictive tool for assessing the risk of invasive mechanical ventilation in patients with influenza-related pneumonia
BMC Pulmonary Medicine (2022)
-
No evidence of harmful effects of steroids in severe exacerbations of COPD associated with influenza
Infection (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.