Abstract
Data from several large high-throughput drug response screens have become available to the scientific community recently. Although many efforts have been made to use this information to predict drug sensitivity, our ability to accurately predict drug response based on genetic data remains limited. In order to systematically examine how different aspects of modelling affect the resulting prediction accuracy, we built a range of models for seven drugs (erlotinib, pacliatxel, lapatinib, PLX4720, sorafenib, nutlin-3 and nilotinib) using data from the largest available cell line and xenograft drug sensitivity screens. We found that the drug response metric, the choice of the molecular data type and the number of training samples have a substantial impact on prediction accuracy. We also compared the tasks of drug response prediction with tissue type prediction and found that, unlike for drug response, tissue type can be predicted with high accuracy. Furthermore, we assessed our ability to predict drug response in four xenograft cohorts (treated either with erlotinib, gemcitabine or paclitaxel) using models trained on cell line data. We could predict response in an erlotinib-treated cohort with a moderate accuracy (correlation ≈ 0.5), but were unable to correctly predict responses in cohorts treated with gemcitabine or paclitaxel.
Similar content being viewed by others
Introduction
Drug response prediction based on genomic information is an active area of research with many practical applications including drug discovery, drug repurposing, patient selection for clinical trials, and personalized treatment recommendations (e.g. in a tumorboard setting). Several large-scale cell line drug sensitivity screens have been generated by the scientific community (e.g. CCLE1, CTRP2, GDSC3, gCSI4). These datasets contain molecular and drug response data on hundreds of cell lines, allowing for generation of predictive models. But despite the availability of such training data, our ability to accurately predict drug response still remains quite limited5,6. Reasons that make drug response prediction a hard problem include noise in the data, relatively low number of samples compared to the number of features (i.e. predictor variables), incomplete omics characterization, and the static nature of molecular data. Molecular data in such studies are usually acquired only before drug treatment7. Another important problem is the consistency of pharmacogenomics associations derived from different datasets. A number of studies examined agreement between the largest datasets and found that differences in experimental protocols and differences in data analysis likely contributed to the observed inconsistency8,9,10,11,12,13.
A broad spectrum of machine learning methods has been applied to the drug response prediction problem: regularized regression methods (e.g. lasso, elastic net, ridge regression)1,3,4,14,15,16,17, partial least squares (PLS) regression18, support vector machines (SVM)19, random forest (RF)3, neural networks and deep learning20,21, logical models3, or kernelised bayesian matrix factorization (KBMF)22,23. For a comprehensive recent review see Ali & Aittokallio (2018)24. However, no systematic exploration of model training strategies based on data from multiple large cell line screens has been reported so far. Also cell line-based models have not yet been compared to xenograft-based models. In the current study we attempt to close these gaps with the ultimate goal of improving accuracy of drug response prediction in cell lines and xenografts.
For our analyses we used data from the cancer cell line encyclopedia1 (CCLE), cancer therapeutics response portal2 (CTRP), genomics of drug sensitivity in cancer3 (GDSC) and the genentech cell line screening initiative4 (gCSI), together with the only publicly available xenograft screen ‒ Novartis Institutes for BioMedical Research PDX Encyclopedia25, NIBR PDXE (Table 1).
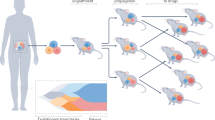
We first set out to systematically study the influence of different modelling parameters (e.g. response metric, number of features, type of features) on predictive performance in drug sensitivity testing. We included data from three large cell line-based datasets, CCLE, GDSC and CTRP, respectively (Fig. 1a). Only seven drugs were in overlap between these screens, and we predicted drug sensitivity for all of them in each dataset. Predictive performance was assessed by cross-validation. We then took the best parameter set and strategy from this analysis and checked whether drug sensitivity in animal xenograft systems could be predicted by training of models from cell line-based data (Fig. 1b). For this, we chose the gCSI and NIBR PDXE datasets, where only three drugs could be compared. Since in xenograft experiments each drug was tested on samples from a particular tissue type, we also restricted cell line samples accordingly, e.g. for erlotinib response prediction all xenograft samples belonged to the non-small-cell lung cancer type and all cell line samples used for this modelling task were lung cancer cell lines. We included, as a background, two additional variables to predict, tissue type and growth rate/doubling time, which should present comparatively easier prediction tasks.
Experimental design of the study. The study is organized in two parts. (a) We examine models for seven different drugs using data from 3 large cell line datasets (CCLE, CTRP and GDSC) by varying feature type, response metric and number of features used. (b) We compare models for 3 different drugs (in 4 tissues) trained on cell line data (gCSI) with the matching models trained on xenograft data (NIBR PDXE); for xenografts we also compare 4 different drug response metrics. Additionally we compare drug response models with tissue type and doubling time models in terms of accuracy.
Data and Methods
Data types and sources
For modelling we used molecular, drug response, tissue type, and division rate data from 4 large cell line sensitivity screenings ‒ CCLE1, CTRP2, GDSC3, gCSI4 and one xenograft screen ‒ NIBR PDXE25.
Cell line molecular data
Molecular data included gene expression, copy number information, and mutation information. Expression data comes from microarrays (except for gCSI dataset where it comes from RNA-seq), values are continuous. Copy number information comes from SNP arrays, it’s continuous. Mutation information is derived from whole exome sequencing and is represented by binary values per gene (1/0 for presence/absence of mutation).
Molecular information for CCLE, CTRP and GDSC datasets was obtained from the PharmacoGx package (version 1.8.3)26. Preprocessing details for each dataset are described in Safikhani et al.9. Molecular information for the gCSI dataset was taken from the supplementary data of the publication4.
Cell line drug response data
Drug response data included three metrics: IC50, AUC, and viability at 1 µM (Fig. s1). IC50 (half maximal inhibitory concentration) and AUC (area under the drug response curve) information was obtained from the PharmacoGx package; in particular we used values recomputed by the package from the raw data ‒ “ic50_recomputed” and “auc_recomputed”. In order to handle outlier values in the IC50 data, we truncated the distribution of IC50 values at the 85th percentile for each drug. Viability at 1 µM values were calculated from the raw drug response data in CCLE, CTRP and GDSC datasets.
Cell line tissue type and division rate data
For tissue type and division rate predictions in gCSI dataset we used tissue labels and division rate values from the cell line meta-information provided in the supplementary data of the publication4.
Xenograft molecular data
Molecular data included gene expression, copy number information, and mutation information. Expression data comes as continuous FPKM (fragments per kilobase of transcript per million mapped reads) values from RNA-seq. Copy number information comes from SNP arrays, it’s continuous. Mutation information has been derived from RNA-seq data and represented by binary values per gene (1/0 for presence/absence of mutation). All molecular data was taken from the supplementary data of the publication25.
Xenograft drug response data
We tested 4 different drug response metrics derived from raw drug response data i.e. volume change of the tumour during the treatment course between day 0 and day 21: tumour volume (at day 21), integral response (difference between the areas under the tumor growth curves from control and treated mice: AUCcontrol − AUCtreated), slope of the tumor growth curve, and differential slope (difference between the slopes under the tumor growth curves from control and treated mice: slopecontrol − slopetreated), see Fig. s2. Raw drug response data was taken from papers’ supplementary data25.
Xenograft tissue type and slope of growth curve data
For tissue type prediction in xenografts we used tissue labels from paper’s supplementary data25. Slope of the tumor growth curve rate for untreated mice was calculated from raw drug response data (control cases).
Sample sizes
The sample size for each modelling task is provided in the Supplementary Tables s2 and s3.
Modelling
Classes of supervised learning
Drug response prediction tasks and division rate/slope of growth curve prediction were regression tasks. Tissue type prediction tasks were classification tasks.
Modelling methods and hyperparameters
For regression tasks we used two modelling methods: support vector machine (svmRadial) and random forest (rf) in the first and second part of our analysis, respectively. For classification tasks we used xgBoost (xgbTree)27.
Each modelling method has its own set of hyperparameters: svmRadial ‒ sigma and C (cost); random forest ‒ mtry; xgbTree ‒ nrounds, max_depth, eta, gamma, colsample_bytree, min_child_weight, subsample.
Feature selection
In order to select a subset of all available features for modelling we performed feature selection using only data from the training set. We evaluated each feature individually with respect to the association between a feature vector and a vector with target variables (filter feature selection). For this evaluation we used two functions from the caret package ‒ gamScores for regression tasks and anovaScores for classification tasks.
The function gamScores fits a generalized additive model between a single predictor and the outcome using a smoothing spline basis function. For classification, anovaScores treats the outcome as the independent variable and the predictor as the outcome. In this way, the null hypothesis is that the mean predictor values are equal across the different classes. In each function a p-value for the whole model F-test is returned and is used as the score28.
Accuracy metrics
For regression tasks we used three accuracy metrics: root of mean squared error (RMSE), coefficient of determination (R2), and concordance index. The concordance index is the rank correlation between observed and predicted data29. RMSE and R2 were calculated using the postResample function from caret package, the concordance index was calculated using “concordance.index” function from survcomp package (version 1.28.5)30.
For classification tasks we used the percentage of correctly predicted samples as the accuracy metric.
Model training and evaluation procedure
The following procedure was used for model testing for each [drug, dataset, drug response metric] combination (see Fig. s3):
- 1.
We split the data into training (70%) and test (30%) sets.
- 2.
Using the training set we performed feature selection.
- 3.
Then we fitted the model with N (from 10 to 500) selected features (with lowest p-values) on the training set data. In order to select hyperparameters, 30 different combinations of them were tested on training data via cross-validation procedures, and then the combination of hyperparameters that provides the best accuracy was used for fitting the final model.
- 4.
We applied the model to the test set, and calculated the accuracy metrics.
- 5.
We repeated steps (1–4) ten times and got average values of accuracy metrics.
Results
Testing influence of different aspects of model training on prediction accuracy
In order to find an optimal strategy for training drug response models, we explored the model parameter space in terms of (1) molecular types of features: expression only vs. expression + copy number + mutation; (2) metrics of drug response: IC50, AUC, Viability_1 µM; and (3) number of features used in the model: 10, 50, 200, and 500. To test these options we built a number of models for seven drugs ‒ erlotinib (EGFR), paclitaxel (β-tubulin), lapatinib (EGFR), PLX4720 (RAF), sorafenib (RAF), nutlin-3 (MDM2) and nilotinib (ABL/BCR-ABL). These seven drugs were selected because they were in overlap between CCLE, CTRP, and GDSC screens.
Results (for tests with all genomic features) in terms of R2 and concordance index are shown in Figs. 2a and s4, respectively. Each plot shows results for a certain response metric combination, and within each plot there are results for each drug in each data set for the tests with 500 variables (for the results with other variable set sizes see Fig. s5).
(a) R2 for 7 drugs across all tests, number of variables = 500. (b) Average R2 values (across three datasets) for each drug separately (for models with AUC metric). Error bars depict ± one standard deviation. (c) Number of common features out of the top 500 features between 3 datasets (CCLE, CTRP and GDSC) for each drug within each drug response metric.
In order to provide a reference for R2 values we repeated the analysis for Lapatininb using a linear regression model with only one predictor ‒ expression of the ERBB2 gene, which is a known biomarker for Lapatinib response in breast cancer31. The resulting average R2 values were 0.04, 0.25 and 0.27 for IC50, AUC, and viability_1 µM metrics, respectively.
We plotted predictions against observed values for each drug response metric for Nutlin-3, Paclitaxel and Sorafenib. In each plot the data points correspond to a test set from one particular (random) train/test split (Figs. 3 and s6). A dotted line shows where data points should lie in the ideal case of 100% correct predictions. In cases where the accuracy of prediction is satisfactory, the data points are grouped around the dotted line, e.g. IC50, nutlin-3 (1st row, 1st column); AUC, nutlin-3 and paclitaxel (2nd row, 1st and 2nd columns); viability_1uM, paclitaxel(3rd row, 2nd column). In cases where prediction accuracy is low, predicted and observed values are hardly correlated and therefore data points are not grouped around the dotted line.
In these tests the average value of R2 for modelling with all genomics data (0.153) was just slightly higher than for modelling with only expression data (0.145). While these differences are small for most of the drugs, for nutlin-3 and PLX4720 they are a bit more pronounced (Fig. s7). Below, we will discuss only models that are based on all genomic features (expression, copy number and mutation values).
We have not observed correlation between the number of features selected for modelling and the accuracy of prediction within the tested range of features (Figs. s5 and s12).
We found differences in accuracy for models based on different drug response metrics, IC50, AUC, and Viability_1uM: R2IC50 = 0.111, R2AUC = 0.186, R2viability_1uM = 0.162 (These differences are significant for comparisons IC50 vs. AUC and IC50 vs. Viability_1uM, p-values from t-test are 2.4∙10−5 and 4.3∙10−3, respectively. Difference for the comparison AUC vs. Viability_1uM is not significant, p-value is 0.2).
We checked the number of common features out of the selected top 500 features for each drug between 3 datasets (CCLE, CTRP and GDSC) within each drug response metric. The number of common features is relatively small for the IC50 metric (average = 10) and higher for AUC and viability at 1µM metrics (average = 47 and 83 respectively, see Fig. 2c). Independently of the response metric used, there is almost no common features across paclitaxel models and across nutlin-3 models.
The average R2 and concordance index values for each drug (for AUC models) are shown in Figs. 2b and s8. Five drugs ‒ PLX4720, paclitaxel, lapatinib, nutlin-3 and erlotinib had average R2 between 0.2 and 0.3, while nilotinib and sorafenib showed the lowest average predictability (R2 = 0.12 and 0.06, respectively). Average R2 for each tissue separately is shown in Fig. s9.
Comparison in accuracy between our method and methods from CCLE and DREAM Challenge
We compared our results for the CCLE dataset with the performance of elastic net models from the original CCLE study1 and with the performance from integrated (combined) random forest method (CRF)32, which was the second top performing method in the DREAM drug response prediction challenge33 (We are comparing our results with the second top performing method instead of the first one simply because both methods have quite similar accuracy score in the original paper, wpc-index equals to 0.583 and 0.577 correspondingly, but the second method to our convenience was already tested on the CCLE dataset with essentially the same accuracy metric that we are using in our analysis). Corresponding R2 values are shown in Table 2.
Tissue type, doubling time and drug response prediction in cell lines and xenografts
We used the gCSI study4 as an in-vitro training set. It is a high-quality pharmacogenomic dataset reasonably consistent with the CCLE and GDSC datasets. We used the NIBR PDXE25 data as an in-vivo validation set since this is the only publicly available xenograft screen. We assessed 6 modelling tasks in each set, i.e. prediction of tissue type, doubling time/slope of the tumor growth curve, erlotinib response (lung samples), gemcitabine response (pancreas samples), paclitaxel response (breast samples), and paclitaxel response (lung samples). Tissue type and doubling time prediction tasks serve as a positive controls ‒ we assume that these phenotypes should be explained by genomics data better than drug response.
For tissue prediction and doubling time/slope of the untreated tumor growth curve we used the top 400 features with the lowest p-values from F-test (see Feature selection subsection in Data and Methods section). For drug response prediction (since sample sizes were much lower) we used just the top 100 features.
In the xenograft tissue prediction we had 5 tissue classes with 27–50 samples per tissue. In the cell line tissue prediction we tried modelling with 13 tissue-classes with 10–68 samples per tissue, and with the 6 largest tissue classes (each tissue had at least 23 samples).
Prediction accuracy results for all prediction tasks are collected in Table 3. For tissue type prediction we report the percentage of correctly predicted samples, for doubling time/slope and drug response prediction we report R2 and concordance index values.
In addition to tissue prediction tests performed using all available cell lines and xenografts we made a test with equal number of samples per tissue (16 samples in training set and 7 samples in test set). A heatmap of the confusion table for the case of cell line predictions is shown in Fig. 4.
Confusion table heatmap for results of tissue classification in cell lines. Row labels depict true classes, column labels depict predicted classes. The test set contained 7 samples for each tissue, and the modelling procedure was repeated 10 times, so for each tissue class 70 predictions were made. The color shows the number of predicted classes per each true class.
While the differences in accuracies between tissue type prediction and doubling time/slope prediction are consistent for cell lines and xenografts, drug response prediction accuracies for the same drugs are not consistent between cell lines and xenografts. Particularly, the best performing drugs are gemcitabine and paclitaxel (breast) for cell lines and erlotinib, paclitaxel (lung) for xenografts. The level of consistency is also illustrated by the number of common molecular features between cell lines and xenografts out of the top features pre-selected for modelling. There are 31 common features out of the top 400 for tissue prediction between cell lines and xenografts, only 4 common features out of the top 400 for doubling time/slope prediction, and almost no common features out of the top 100 for drug response prediction.
In order to make the quality of prediction across different regression prediction tasks visually accessible, we plotted observed versus predicted values for different prediction tasks. In each task the data points correspond to a test set from one particular (random) train/test sets split (Fig. 5a).
Manual inspection of outlier cases from observed vs. predicted plots (Fig. 3a) shows that often a model’s inability to provide an accurate prediction for certain samples (outliers) is driven by under-representation of samples with similar molecular characteristics to these outliers in the training set.
Cross-prediction between cell lines and xenografts
We tested how well the models trained on cell line data can explain drug response in xenografts. For that we 1) trained drug response models using cell line genomics and drug response data (AUC), 2) got model predictions using xenograft molecular data as inputs, 3) assessed the correlation of resulting predictions (in cell line AUC units) with the actual xenograft drug response. We tried two strategies with respect to training set composition ‒ training using all cell lines and training using only cell lines that match the tissue type of the corresponding set of xenograft samples. Results in terms of correlation coefficients as well as predicted vs. observed plots for this analysis (for the case where we used all cell lines for training) are shown in Figs. 5b and s10.
Among four xenograft response metrics used in this study, volume and slope are expected to be positively correlated with cell line AUC while integral response (ΔAUC) and differential slope are expected to be negatively correlated. For both training strategies (all cell lines or cell lines from one relevant tissue type) we managed only for erlotinib to get predictions with the right sign of correlation coefficient (between predictions and observed drug response) and substantial absolute value for all xenograft drug response metrics. Volume and slope metrics worked especially well in the case of erlotinib, with correlation about 0.5 in both cases.
Discussion
We have shown that, dependent on the cellular phenotype that we want to predict from genomic data, the accuracy of prediction varies substantially. Tissue type classification can be achieved with relatively high accuracy. The percentage of correctly predicted samples is 0.79 for the cell line set, and 0.89 for the xenograft set. The accuracy of prediction depends substantially on the size of the training set for each tissue class. When we additionally include tissues which have from 10 to 20 samples into our cell line modelling set, the average accuracy drops from 0.79 to 0.64.
While the accuracy of tissue type classification is high on average, it varies across tissues. In a series of tests where we use equal number of samples per tissue (16 samples in the training set and 7 samples in the test set), we found that we have the lowest accuracy for lung samples, higher accuracy for breast samples, and the highest accuracy for pancreas, colon, skin and blood samples (Fig. 4). Interestingly this ranking holds for both cell line and xenograft predictions. A fraction of lung samples is often misclassified as breast samples (and to a lesser extent the other way around) which results in comparatively lower accuracies for lung samples. This can be explained by the partial overlap between features that separate lung and breast samples from other samples, particularly expression of some transcription regulators genes and membrane protein genes (see Table s1 for details). On a PCA plot the cluster of lung samples has a big overlap with the cluster of breast samples (see Fig. s11). The ability to predict tissue type using cellular expression data assures that more complex phenotypes can be in principle predicted from expression data as well.
The second cellular phenotype we aimed to predict was the cell line doubling time, or slope of untreated tumor growth curve in case of xenografts. Here we got lower accuracy compared to the tissue type prediction. The average accuracy is quite consistent between cell lines and xenografts: R2cell lines = 0.17, R2xenografts = 0.19. The lower accuracy of prediction shows that, unlike for tissue type, there is less information about the rate of cell division in the static expression data, which can be due to the post-translational regulation of the activity of cell cycle proteins. Additionally the prediction problem is complicated by the fact that expression data originates from the pooled group of cells that were at different cell cycle stages prior to expression profiling.
The main phenotype of interest in our analysis is drug response. Analogously to the doubling time prediction case, the accuracy of drug response prediction is substantially lower than accuracy of tissue type prediction. A possible reason for that is (as in the case of doubling time) that the static gene expression data obtained prior to drug treatment doesn’t necessarily contain enough information to fully explain drug response (although high expression of a drug’s target gene is a good biomarker of response, in the case when high expression of this gene is essential for cell proliferation).
We found that accuracy of drug response prediction doesn’t strongly depend on a choice of machine learning algorithm. If we look at models for erlotinib response in cell lines, the average R2 for random forest models (tested on gCSI data) is comparable to average R2 for support vector machine models (tested on data from CCLE, CTRP and GDSC): R2RF = 0.23, R2SVM = 0.19. Also according to our results the number of top features selected for modelling (via filter feature selection), within the tested range of 10–5000 features, doesn’t influence the resulting accuracy of predictions, which rather depends on the strength of correlation between top feature(s) and the outcome (Figs. s5 and s12).
We see that the accuracy of drug response prediction varies across the drugs, e.g. in our tests based on CCLE, CTRP and GDSC datasets average R2 ranges from 0.06 for Sorafenib to 0.27 for PLX4720 (results for AUC metric).
We found that the average accuracy of models based on all genomic data (expression + mutation + copy number data) is just marginally higher than the average accuracy of models based on expression data only (Fig. s7), which shows that expression data can explain most of the variation in drug response. This is consistent with the finding from the DREAM drug response prediction challenge33. However, the use of additional omics profiles, e.g. methylation and proteomics data can still improve the accuracy of prediction34. Also it was recently shown that transcriptional perturbation signatures (e.g. from the LINCS-L1000 project35) can be successfully used for drug response prediction36.
The choice of a drug response characterization metric has a serious impact on the accuracy of predictions. Having compared three drug response metrics for cell lines we found that the area under the drug response curve (AUC) provides the highest predictive performance. AUC combines information about drug efficacy and potency into a single value, and it was reported to be the most robust metric previously37. Additionally we compared these three metrics with a recently proposed GR_AOC metric which takes into account the difference in growth rates between cell lines38. GR_AOC performed slightly worse than AUC and viability_1uM but better than IC50 (Fig. s13). Comparing performance of different xenograft drug response metrics we found that simple metrics like “tumor volume (day 21)” and “slope” perform better (average R2 = 0.23 and 0.25) than those which additionally take into account data from untreated controls ‒ “Integral response” and “Differential slope”, with an average R2 = 0.095 and 0.145, respectively.
We also showed that the size of the training set is an important determinant for the accuracy of a model. In our tests based on gCSI data the average R2 for models trained on cell lines from all tissues (n = 329) was 0.267, while for models that used only cell lines from a certain tissue for each drug (n = 26–68), the average R2 was 0.102. Low number of samples makes it harder to distinguish the true signal from noise33, especially in the case of multi-omics datasets which are high-dimensional. To deal with high-dimensionality in our modelling tests, we employed filter-based feature selection (i.e. selected a subset of features that have high association with a target variable). Alternative options for combating high-dimensionality include literature based feature selection (e.g. using the list of cancer census genes from COSMIC for modelling24), and common dimensionality reduction methods like PCA. Creating aggregated features using pathway information39 can help with dimensionality reduction, however in our preliminary tests we found that combining pathway features with simple genomic features (selected via feature selection) can improve resulting accuracy only marginally (see Fig. s14).
Finally we assessed our ability to predict drug response in xenografts using models trained on cell line data. We tested our predictions in four cohorts ‒ NSCLC treated with erlotinib, PDAC treated with gemcitabine, BRCA and NSCLC treated with paclitaxel. Only for the NSCLC cohort treated with Erlotinib our predictions were moderately accurate, i.e. positively correlated (r = 0.5) with the tumor volume and the slope of the tumor growth curve. Performance of the models built and tested on xenograft and cell line data separately can’t explain why this type of prediction worked only for erlotinib-treated cohort. Indirectly it shows that pharmacogenomic associations were consistent between cell line and xenograft dataset only in the case of erlotinib, which has only one target-molecule, EGFR, but not in the cases of more pleiotropically acting drugs such as gemcitabine and paclitaxel which block DNA synthesis and cell division, respectively. Also differences in cell growth and differences in drug response quantification between 2D and 3D model systems probably contributed to the inconsistency in pharmacogenomics associations between cell lines and xenografts.
Conclusions
While more and more data from high-throughput drug sensitivity screenings become available, accurate drug response prediction remains challenging. As we show in the present study, we cannot expect that accuracy of drug response prediction in cell lines (or xenografts) will be equal (or even close) to accuracy of tissue type prediction. Nevertheless it is possible to fit models of drug response which will provide moderate accuracy. Here we explored the parameter complexity one faces when fitting these models. We found that generally the choice of a particular machine learning algorithm doesn’t influence accuracy, and the number of variables included in the model doesn’t matter much. What matters, though, is the degree of association between the top predictor variables and outcome. Among the parameters that do influence accuracy are class of molecular predictors ‒ we see that expression data has the highest explanatory power, response metric (AUC provide the best results in cell lines, Volume and Slope of tumor growth curve demonstrate the best results in xenografts) and, quite importantly, the number of samples in the training set.
Mastering cell line drug response models will make it ultimately possible to perform personalized prediction of drug sensitivities for individual cancer patients40. In the present study we tried to predict drug response in xenografts using models trained on cell line data, which can be seen as an approximation to the patient prediction scenario. We managed to get reasonably accurate predictions only in the NSCLC xenograft cohort treated with erlotinib, but not in other cohorts treated with either gemcitabine or paclitaxel. With more xenografts and patient material screens publically available in the future, it will be possible to understand in which cases drug response associations are transferable between cell lines and xenografts/patients and in which cases they are not. This understanding, combined with the wealth of available high-throughput data, will bring closer the era of personalised cancer medicine.
Data availability
Our work complies with the guidelines for research reproducibility from Gentleman et al.41. The analysis code and its documentation is open-source and freely available from URLs https://github.com/RomaHD/DrugRespPrediction and https://doi.org/10.5281/zenodo.3626896. The in vitro pharmacogenomic data can be obtained from the PharmacoGx package26 directly. In vivo pharmacogenomic data are available from the Journal’s website25.
References
Barretina, J., et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature 483.7391, 603 (2012)
Seashore-Ludlow, B. et al. Harnessing connectivity in a large-scale small-molecule sensitivity dataset. Cancer discov. 5.11, 1210–1223 (2015).
Iorio, F. et al. A landscape of pharmacogenomic interactions in cancer. Cell 166.3, 740–754 (2016).
Haverty, P. M. et al. Reproducible pharmacogenomic profiling of cancer cell line panels. Nat. 533.7603, 333 (2016).
Papillon-Cavanagh, S. et al. Comparison and validation of genomic predictors for anticancer drug sensitivity. J. Am. Med. Inf. Assn 20.4, 597–602 (2013).
Jang, I. S., Neto, E. C., Guinney, J., Friend, S. H., & Margolin, A. A. Systematic assessment of analytical methods for drug sensitivity prediction from cancer cell line data. Biocomputing 2014, pp. 63–74 (2014)
Kalamara, A., Tobalina, L. & Rodriguez, J. S. How to find the right drug for each patient? Advances and challenges in pharmacogenomics. Curr Opin Syst Biol (2018)
Haibe-Kains, B. et al. Inconsistency in large pharmacogenomic studies. Nat. 504.7480, 389 (2013).
Safikhani, Z., et al. Revisiting inconsistency in large pharmacogenomic studies. F1000Research 5 (2016)
Cancer Cell Line Encyclopedia Consortium, and Genomics of Drug Sensitivity in Cancer Consortium. Pharmacogenomic agreement between two cancer cell line data sets. Nature 528.7580, 84 (2015)
Geeleher, P., Gamazon, E. R., Seoighe, C., Cox, N. J. & Huang, R. S. Consistency in large pharmacogenomic studies. Nat. 540.7631, E1 (2016).
Bouhaddou, M. et al. Drug response consistency in CCLE and CGP. Nat. 540.7631, E9 (2016).
Mpindi, J. P. et al. Consistency in drug response profiling. Nat. 540.7631, E5 (2016).
Geeleher, P., Cox, N. J. & Huang, R. S. Clinical drug response can be predicted using baseline gene expression levels and in vitro drug sensitivity in cell lines. Genome biol, 15(3), p.R47 (2014)
Fang, Y. et al. DISIS: prediction of drug response through an iterative sure independence screening. PLoS one 10.3, e0120408 (2015).
Falgreen, S. et al. Predicting response to multidrug regimens in cancer patients using cell line experiments and regularised regression models. BMC cancer 15.1, 235 (2015).
Aben, N., Vis, D. J., Michaut, M. & Wessels, L. F. TANDEM: a two-stage approach to maximize interpretability of drug response models based on multiple molecular data types. Bioinforma. 32(17), i413–i420 (2016).
Li, B. et al. Development of a drug-response modeling framework to identify cell line derived translational biomarkers that can predict treatment outcome to erlotinib or sorafenib. PLoS one 10.6, e0130700 (2015).
Dong, Z. et al. Anticancer drug sensitivity prediction in cell lines from baseline gene expression through recursive feature selection. BMC cancer 15.1, 489 (2015).
Menden, M. P. et al. Machine learning prediction of cancer cell sensitivity to drugs based on genomic and chemical properties. PLoS one 8.4, e61318 (2013).
Ding, M. Q., Chen, L., Cooper, G. F., Young, J. D. & Lu, X. Precision Oncology beyond Targeted Therapy: Combining Omics Data with Machine Learning Matches the Majority of Cancer Cells to Effective Therapeutics. Mol. Cancer Res. 16.2, 269–278 (2018).
Ammad-Ud-Din, M. et al. Integrative and personalized QSAR analysis in cancer by kernelized Bayesian matrix factorization. J. Chem. Inf. Model. 54.8, 2347–2359 (2014).
Ammad-Ud-Din, M. et al. Drug response prediction by inferring pathway-response associations with kernelized Bayesian matrix factorization. Bioinforma. 32.17, i455–i463 (2016).
Ali, M. & Aittokallio, T. Machine learning and feature selection for drug response prediction in precision oncology applications. Biophys Rev, pp.1–9 (2018)
Gao, H. et al. High-throughput screening using patient-derived tumor xenografts to predict clinical trial drug response. Nat. Med. 21.11, 1318 (2015).
Smirnov, P. et al. PharmacoGx: an R package for analysis of large pharmacogenomic datasets. Bioinforma. 32.8, 1244–1246 (2015).
Tianqi, C. & Guestrin, C. Xgboost: A scalable tree boosting system. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, 785–794 (2016)
Kuhn, M. Variable selection using the caret package, http://cran.cermin.lipi.go.id/web/packages/caret/vignettes/caretSelection.pdf (2012)
Harrell, F. E., Lee, K. L. & Mark, D. B. Tutorial in biostatistics multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat. Med. 15(4), 361–387 (1996).
Schroeder, M. S., Culhane, A. C., Quackenbush, J. & Haibe-Kains, B. survcomp: an R/Bioconductor package for performance assessment and comparison of survival models. Bioinforma. 27(22), 3206–3208 (2011).
Montemurro, F. et al. Potential biomarkers of long‐term benefit from single‐agent trastuzumab or lapatinib in HER2‐positive metastatic breast cancer. Mol. Oncol. 8.1, 20–26 (2014).
Wan, Q. & Pal, R. An ensemble based top performing approach for NCI-DREAM drug sensitivity prediction challenge. PLoS one 9.6, e101183 (2014).
Costello, J. C. et al. A community effort to assess and improve drug sensitivity prediction algorithms. Nat. Biotechnol. 32.12, 1202 (2014).
Ghandi, M. et al. Next-generation characterization of the Cancer Cell Line Encyclopedia. Nature 569.7757, 503 (2019)
Subramanian, A. et al. A next generation connectivity map: L1000 platform and the first 1,000,000 profiles. Cell 171.6, 1437–1452 (2017).
Szalai, B. et al. Signatures of cell death and proliferation in perturbation transcriptomics data—from confounding factor to effective prediction. Nucleic Acids Res. 47(19), 10010–10026 (2019).
Fallahi-Sichani, M., Honarnejad, S., Heiser, L. M., Gray, J. W. & Sorger, P. K. Metrics other than potency reveal systematic variation in responses to cancer drugs. Nat. Chem. Biol. 9(11), 708 (2013).
Hafner, M., Niepel, M. & Sorger, P. Alternative drug sensitivity metrics improve preclinical cancer pharmacogenomics. Nat. Biotechnol. 35.6, 500 (2017).
Schubert, M. et al. Perturbation-response genes reveal signaling footprints in cancer gene expression. Nat. Commun. 9.1, 20 (2018).
Zhao, C., Li, Y., Safikhani, Z., Haibe-Kains, B., & Goldenberg, A. Using Cell line and Patient samples to improve Drug Response Prediction. bioRxiv, 026534 (2015)
Gentleman, R. & Temple Lang, D. Statistical analyses and reproducible research. J. Comput. Graph. Stat. 16(1), 1–23 (2007).
Acknowledgements
The authors thank D. Weese, T. Klein, D. Juraeva, T. Zenz, W. Huber, and M. Kapushesky for discussions on various aspects of the project. R. Kurilov was supported by SAP Health.
Author information
Authors and Affiliations
Contributions
B.B. conceived and supervised the project, B.H.K. co-supervised the project, R.K. performed the analysis and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kurilov, R., Haibe-Kains, B. & Brors, B. Assessment of modelling strategies for drug response prediction in cell lines and xenografts. Sci Rep 10, 2849 (2020). https://doi.org/10.1038/s41598-020-59656-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-59656-2
This article is cited by
-
Translating transcriptomic findings from cancer model systems to humans through joint dimension reduction
Communications Biology (2023)
-
A performance evaluation of drug response prediction models for individual drugs
Scientific Reports (2023)
-
A machine learning-based gene signature of response to the novel alkylating agent LP-184 distinguishes its potential tumor indications
BMC Bioinformatics (2021)
-
Using mathematical modeling to estimate time-independent cancer chemotherapy efficacy parameters
In Silico Pharmacology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.