Abstract
In this article a new spent catalyst sample preparation method was implemented for the purpose of molybdenum leaching in a single step. Further molybdenum and vanadium in the leach liquor were separated and their concentrations were enriched using the solvent extraction and stripping techniques. The impervious sulfur (S0) layer of the spent catalyst sample was removed using carbon disulfide (CS2). The advantages of S0removal were evaluated by conducting different sets of the Mo leaching experiments and they were further examined by varying different conditions such as three lixiviants, hydrogen peroxide (H2O2) addition, and three leaching parameters. The leaching rate increased in an order, e.g. acetone washed < acetone-CS2 washed < acetone washed-H2O2 < acetone-CS2 washed-H2O2, for the experimental concentration range of different lixiviants with the maximum of 94.8%(w/w) Mo dissolution in a single step. Optimization of the pulp density was important as the interaction of lixiviant molecules with multiple reacting solid particles decreased the driving force of the chemical reactions. The solvent extraction followed by the stripping technique was found to be excellent as the concentration of vanadium and molybdenum enriched from 0.55 to 1.9 M and 0.0448 to 1.08 M, respectively.
Similar content being viewed by others
Introduction
Molybdenum (Mo) and vanadium (V), and their products are used in different applications like catalysis, alloying, aerospace industries, electrical connections, industrial motors, and filaments1,2,3. Generally they are extracted from their respective primary ores1,2. However, their reclamation from different spent catalysts (SCs) has been examined using hydrometallurgical and pyro-hydrometallurgical techniques4,5,6,7. There are different techniques, such as roasting followed by leaching, baking in acid followed by leaching and direct leaching, have been employed for their recovery8,9,10,11,12,13,14,15,16,17,18,19,20. Also bioleaching has been tried21,22,23,24,25,26,27,28,29,30. However, simultaneous leaching of the metals like Ni, V, and Mo has always been difficult due their individual chemical identity in the aqueous medium6,9,18,21,24. Therefore, a multi-step or sequential leaching process has been applied for their leaching6,18.
The sequential leaching is not a low-cost process as it undergoes different sub-processes and develops complexity during scale-up. Assembling the sub-processes is a headache during the pilot scale designing. Mishra et al.31 and Kim et al.32 have examined the problems associated with the SCs that lead to the sequential leaching, and found that it is Mo-matrix. The XRD analysis has revealed the presence of both sulfide and oxide form of Mo32. Olson and Clark33 have shown the refractory nature of Mo-sulfide which leads to its lower dissolution. The EDX analysis of the particular SC under this investigation is shown in Fig. 1 which shows that the association of S0 with the Mo-matrix within the SC matrix32. Since the S0 is a hydrophobic substance, it impedes the contact between Mo-matrix and lixiviants during the leaching31. Hence the mutual effect of the refractoriness of Mo-sulfide and the impervious S0 layer leads to the lower Mo dissolution31,32,33,34.
The impervious S0 layer should be removed in order to build the contact between Mo-matrix and lixiviant during the dissolution of Mo from the SC. It can be removed by dissolving in a suitable solvent31,35. Austin et al.35 has used three solvents such as liquid SO2, CS2, and CCl4 to determine the solubility of S0. They have found that the S0 dissolving capacity of SO2, CS2, and CCl4 are 0.015, 28.45 and 1.25%(w/v), respectively. Since the solubility of S0 is higher in CS2, Mishra et al.31 and Kim et al.32 have used it as the solvent to remove the S0 present in SC, though they have not studied its advantage on Mo leaching in details.
Issues related to the Mo leaching from a SC sample are sorted out on the basis of leaching experiments in this paper. The main objective of experiments is the complete Mo dissolution in a single step instead of a multi-step leaching process by addressing the obstructions. The extraction, separation, and concentration enrichment of Mo and V from the leach liquor is reported. Finally a process flow-diagram is suggested.
Experimental
Chemicals
Only analytical grade chemicals and deionized water were used in the experiments, unless otherwise specified.
Pretreatment of SC
The SC sample was obtained from the SK Incheon Petrochem, South Korea and used as the metallic resource for the leaching experiments. The as such SC accumulated different crude oil impurities and organic pollutants on its surface possibly during the refinery process. Since its surface contents are hydrophobic in nature, they should be washed before employing it in the leaching experiments. The boiled acetone was chosen over all other organic solvents for the washing30. The acetone washing of the SC was performed in a soxhlet for 2 hr. It was observed that the oily surface washed away completely during this washing period. Then the acetone washed SC (ASC) was removed from the soxhlet and first dried in the open air followed by drying in a hot oven at 50 °C until a constant mass obtained. The dried ASC was ground to powder using a porcelain mortar and pestle, and then sieved in to different size fractions. The particle size distribution (−106 + 45 µm)of the ASC powder was chosen for the leaching experiments as it was found suitable21. This specific sized ASC powder was stored in a desiccator and further used in the leaching studies.
Presence of S0
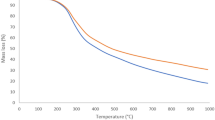
Previously our group has shown the association of S0 and Mo-matrix within the SC particle32. Further, its presence was confirmed by analyzing the thermo gravimetric analysis/differential scanning calorimetry (TG-DTA/DSC) analysis of the ASC using TG-DTA instrument (SHIMADZU). The temperature was increased from 20 to 700 °C with a linear heating rate of 10 °C/min during the analysis. Figure 2 shows the TG-DTA result of the ASC. There are total of five peaks in the Fig. 2. The exothermic peaks at 284 and 408 °C may be due to the loss of hydrocarbon and the transformation of sulfides to oxides of metals, respectively. The three endothermic peaks may correspond to the S0. The endothermic peaks at 79, 103 and 322 °C may be due to the α → β transition of S0, the melting of β-S0 and the complete sublimation of S0 36.
CS2 washing of SC
The solubility S0 is 241.6 g/L in CS2 at room temperature35. Therefore, the S0 was removed from the ASC sample using CS2 as the solvent at room temperature. For this purpose 100 g ASC was added to a beaker containing 500 mL CS2 and stirred gently with the help of an overhead stirrer for 30 min. Then the slurry was filtered through the Whatman filter paper and the residue was collected. The residue was then dried in the open air followed by in a hot air oven at 50 °C until a constant mass obtained. The residue was calledacetone + CS2 washed SC (ACD) sample which was stored in a desiccator and further used in the leaching experiments. The metal content of the ACD sample was analyzed by following the standard acid digestion process and ICP analysis. Previously our group has reported the metal content in the SC after different pretreatment methods30. The metal content of the ACD sample was further added to that table and is shown in Table 1.
Leaching
The leaching experiments were conducted in 250 mL conical flask containing 100 mL of lixiviants. The Mo leaching of both ASC and ACD was carried out using three different leaching reagents such as H2SO4, Na2CO3, and NaOH. Previously leaching of different SC samples was conducted using the above leaching reagents31,34. However, leaching of the SC samples after the removal of S0 was not reported in details. Further, the two-step leaching process was applied and reported31,34. Since this manuscript describes the improvement of Mo leaching rate after the S0 removal, the comparison of its dissolution was described on the basis of the above leaching mediums. The SC contains both sulfide and oxide of Mo. Direct dissolution of the Mo-oxides takes place in different acid and alkali solutions, however, the Mo-sulfides require an oxidant for the oxidation of S2− to S6+ with simultaneous dissolution of Mo. So 10 mL of H2O2 was added to different leaching reagents maintaining the lixiviant volume 100 mL. On the H2O2 aid, it further splitted to another two more sets, such as ASC + H2O2(AHP) and ACD + H2O2(ACHP). As a result four different SC conditions, such as ASC, AHP, ACD and ACHP, were developed for three lixiviants. The leaching reactions are shown in Eqs. 1–6 4,5,6,31,34. The experiments were conducted for 120 min. The time was counted after starting the agitation of content at 300 rpm with the help of a magnetic stirrer, which followed the addition of specified SC sample into the lixiviant. The experiments were conducted at ambient temperature i.e. 25 °C. The concentration of lixiviants and the pulp density were maintained as 1.0 M and 30%(w/v), respectively, unless and otherwise specified. For the analysis of metal concentration in the leach liquors, samples were collected in every 10 min interval and prepared with following precautions. About 2 mL of slurry was withdrawn from the running experiment using a 10 mL rubber head glass dropper without stopping the agitation and transferred into a 2 mL microcentrifuge tube, and then centrifuged using a microcentrifuge. Then exact 1 mL of supernatant was pipetted out using a micropipette and transferred into a 100 mL volumetric flask. Further it was acidified with 20 mL of 10%(v/v) HCl and the volume made up to mark by adding deionized water. The samples were analyzed by ICP-AES (JOBIN-YVON,JY38) after required dilutions.
The leaching rate was calculated using the formulae given in Eq. 7.
where,
Y = Leaching rate in %(w/w)
CL = Concentration of metal in leach liquor, mg/L
X = Metal content in the SC, %(w/w)
PD = Pulp density, weight of SC (in g) in 100 mL leaching media.
Solvent extraction
Solvent extraction (SX) experiments were conducted for a synthetic leach liquor whose concentration based on the composition of leach liquor obtained from the leaching condition (e.g. ACHP; NaOH, 2.0 M; pulp density, 30%(w/v)). The concentrations of nickel (Ni), V and Mo in the leach liquor were found to be 0, 0.554 and 0.0439 M, respectively. The concentration of leach liquor gives the representative solution for the SX experiments. Since the above mentioned condition is optimum for the leaching experiments, the representative solution for the SX experiments were prepared based on the concentration of leach liquor obtained from the above condition. Therefore, the composition of synthetic solution was maintained at 0.55 and 0.045 M for V and Mo, respectively, by adding appropriate amount of NaVO3 and Na2MoO4.2H2O to the deionized water. The pH of synthetic solution was maintained at 9.6 by adding NaOH solution, as it was the final pH of the original leach liquor. The quaternary amine salt such as Aliquat 336 diluted in toluene was used as the organic phase for the SX7. Aliquat 336 is a basic extractant suitable for the anionic loading. The SX experiments were conducted using the 60 mL separatory funnels containing the total volume of 24 mL for 30 min of agitation using a centrifugal shaker in ambient temperature. The volumetric ratio of aqueous to organic phase (A:O) were defined by keeping the total volume of the content 24 mL.The SX conditions were varied as specified. After completion of each SX run exactly 1 mL of sample was withdrawn from the aqueous phase and transferred into a 100 mL volumetric flask. The samples were prepared and analyzed accordingly (section 2.5). Followed by the SX, required stripping experiments were conducted using different solutions as specified. The stripping conditions and sampling were mostly similar to the SX experiments.
All the experimental runs were triplicated and the standard deviations of the results were within ±3%.
Results and Discussions
Effect of contact time and lixiviants
Acid leaching of Mo from four SC conditions, such as ASC, AHP, ACD and ACHP, was conducted using 1.0 M H2SO4 at different time interval and is shown in Fig. 3(a). Its leaching rate for the SC conditions did not show substantial deviation. The Mo forms either molybdic sulfate (MoO2SO4) or molybdic acid (H2MoO4) in the acidic medium. Also the MoO42− ion polymerizes in the acidic37. The solubility of metallic salts/ions formed in the leach liquor limits the leaching rate. Since different salts/ions of Mo were less soluble in the acidic medium, the H2SO4 leaching did not show the effective Mo dissolution37. The leaching rates for the SC conditions were just above 20%(w/w) before reaching the equilibrium. The maximum leaching rate and the corresponding equilibrium time for all set of experiments were evaluated and are given in Table 2. The equilibrium time of ASC was 40 min, and that for AHP, ACD and ACHP were 30 min. The lesser equilibrium time proved that those conditions favored the Mo dissolution; however, the solubility controlled the leaching rate. Since Mo is highly soluble in the alkali medium, its leaching was conducted for the SC conditions in the 1.0 M Na2CO3 medium. Figure 3(b) shows the effect of contact time on the Mo dissolution in the Na2CO3 medium. From Fig. 3(b), it can be observed that the leaching rate increased quickly for both ACD and ACHP within 20 min compared to their respective without CS2 washed conditions such as ASC and AHP. In the case of ASC and AHP, the impervious S0 was present in the Mo-matrix which prevented the contact between Mo-matrix and lixiviants resulting in the slower Mo diffusion31. When the impervious S0 was washed away in the case of ACD and ACHP, the sulfur voids developed within the SC particles which made the easy penetration of the lixiviants through the voids and they reacted with the underlying Mo-matrix32. The leaching rate and equilibrium time were 52.6%(w/w) and 50 min, respectively, in the case of ASC which may be due to dissolution of Mo-oxide easily approachable at the surface of SC particles. When the leaching rate of the ACD case compared to that of the ASC, it increased up to 65.2%(w/w) and the equilibrium time decreased to 40 min which were possible due to the S0 removal resulting in the easy approach of the lixiviants. In the case of AHP, the leaching rate and equilibrium time respectively increased up to 76.1%(w/w) and 80 min which may be due to the oxidation of both Mo-sulfide and S034. This was further confirmed by the leaching of ACHP condition. In the case of ACHP, the leaching rate increased up to 84.2%(w/w) and the equilibrium time decreased to 50 min, which were due to the mutual effect of the easy penetration of lixiviants in to the underlying Mo-matrix and the oxidation of Mo-sulfide present in the core of SC particles32,34. When the Na2CO3 leaching data compared to that of H2SO4 leaching, it was observed that the Mo leaching rate was much higher for the SC conditions which was possible due to the thermodynamic stability of Mo ions in the alkaline medium37. Although the Mo leaching rate increased when the lixiviant was switched from H2SO4 to Na2CO3, the complete Mo leaching was yet to be achieved. Therefore, further Mo leaching experiments were conducted using 1.0MNaOHfor the SC conditions. The effect of contact time on the Mo leaching rate using NaOH medium is shown in Fig. 3(c). The leaching rate in NaOH followed a similar contact time frame to that of NaCO3; however, the leaching rate increased by 2 to 4%(w/w) for different SC conditions. From Table 2, it can be observed that the equilibrium time for ASC, ACD and ACHP were same as those of Na2CO3 as the lixiviant, however, it decreased from 80 to 60 min in the case of AHP which proved that the oxidizing environment was more favoured by NaOH than NaCO3. Still the maximum leaching rate did not reach 90%(w/w) in the most favorable condition i.e. ACHP + 1.0 M NaOH. This may be due to the depletion of attacking species in the leaching medium for the complete Mo dissolution32. Therefore, further concentration of the lixiviants was varied keeping other parameters constant.
Effect of lixiviant concentration
In order to evaluate the effect of lixiviant concentration on the Mo leaching rate, the concentration of H2SO4, Na2CO3, and NaOH was varied as 0.1, 0.5, 1.0, 1.5 and 2.0 M. The leaching rates using different concentration of lixiviants are shown in Table 3. The variation of leaching rate for the SC conditions was noticeable at the lower concentration of H2SO4; however, it was not substantial for further study (Table 3). It was only 10.4%(w/w) in the case of ASC in 0.1 M H2SO4 and increased by just above 5%(w/w) in other three cases (e.g.AHP, ACD and ACHP). With the increase of H2SO4 concentration, there could hardly be any difference observed as the saturation of Mo in the acidic leach liquor. However, its difference was observed in the alkali leaching as it increased linearly with the increase of Na2CO3 concentration from 0.1 to 1.0 M (Table 3). Its increment was substantial from 1.0 to 1.5 M and on further increase from 1.5 to 2.0 M there could hardly any increase. With the increase of NaOH concentration 0.1 to 2.0 M for the SC conditions, it increased constantly. For a certain concentration of either Na2CO3 or NaOH its increment followed an order i.e. ASC < ACD < AHP < ACHP; however, the order was more contrast at higher concentration. It achieved the maximum of 94.88%(w/w) when the ACHP reacted with 2.0 M NaOH.
Effect of pulp density
The pulp density was maintained at 30%(w/v) for the above studies. In order to evaluate the Mo leaching rate at different pulp densities, further experiments were conducted by varying it as 30, 40, 50, 60, 70, 80, 90 and 100%(w/v). As the ACHP condition showed better leaching rate over other three SC conditions, the pulp density study was conducted for the ACHP using the lixiviants such as H2SO4, Na2CO3 and NaOH. However, the concentration of lixiviants increased to 2.0 M in order to maintain the adequate attacking species required for the elevated amount of Mo and other metals at the higher pulp densities. The Mo leaching rate plotted versus pulp density is shown in Fig. 4. The leaching rate decreased with the increase of pulp density for both acidic and alkaline medium which was obvious. Since the concentration of lixiviants was increased to maintain the chemical reaction environment at the higher pulp density, the leaching rate decreased may be due to vulnerable interactions between the reacting SC particles and the lixiviants38. The reacting SC particles did not experience any change of the lixiviants concentration at their interface when the lower pulp density was implemented. With the gradual increase of the pulp density, the suspension became more concentrated which made the reaction particles competitive among each other for the lixiviant molecules38. As a result it significantly reduced the driving force of the chemical reactions. The degree to which a chemical reaction slowed down by this interaction can be characterized by a dimension less parameter ‘η’ which can be expressed by Eq. 8 as follows38:
where,
a = number of mole of Mo in the balanced chemical equations (Eqs. 1–6);
b = number of mole of lixiviant in the balanced chemical equations (Eqs. 1–6), (for H2SO4 and NaOH, b = 1 and for Na2CO3, b = 2);
V = volume of suspension in L;
Cl = initial molar concentration of lixiviant;
Ms = initial number of mole of Mo in the SC sample.
The ‘η’ values were calculated from the experimental data and then the leaching rate in %(w/w) was plotted versus it for different lixiviants (Fig. 5). It can be observed that the Mo leaching rate decreased with the decrease of ‘η’ value which was due to the interaction between the lixiviant molecules and the multiple reacting SC particles at the higher pulp density decreased the driving force of the chemical reactions38.
Solvent extraction
Variation of pH on SX
The SX of V and Mo from the synthetic leach liquor was conducted using Aliquat 336 diluted in toluene. In order to get the pH extraction isotherm curve, the V and Mo were extracted individually from the pure metallic solution at different pH range using 20%(v/v) Aliquat 336 by keeping the aqueous to organic phase ratio (A:O) constant at 1:1. The concentration of V and Mo were 0.55 and 0.045 M, respectively. Since the leach liquor was alkaline, the pH was varied from 12.0 to 2.0 for both V and Mo solutions by adding either concentrated H2SO4 or NaOH solution. The SX efficiency of V and Mo were evaluated and plotted versus pH, and the graph is shown in Fig. 6. The Fig. 6 shows that the V extraction increased with the decrease of pH from 12.0 to 8.0 and reached the maximum of 99.9% at pH 8.0. The maximum retained from pH 8.0 to 4.0 and on further decrease of the pH from 4.0 to 2.0 the V extraction decreased. For the Mo extraction, there was negligible extraction observed from pH 12.0 to 8.0. It increased with the decrease of pH from 8.0 to 5.0 and then remained at maximum (99.6%) from pH 5.0 to 2.0. The Fig. 6 shows that both V and Mo co-extracted below pH 8.0. Therefore, it was convenient to extract V at pH > 8.0 in the first step followed by the Mo extraction from the V-barren solution at pH < 5.0 in the second step. This was supported by the separation factor (SF) data given in Table 4. The pH variation data, such as concentration in organic phase, distribution coefficient (D), and SF, are given in Table 4. The maximum SF value of V over Mo was of 998001 at pH 8.0. With the superior SF value chances of the Mo co-extraction could be minimized during the V extraction at pH 8.0. For the remaining SX study, the V was extracted from the mixture solution at pH 8.0 followed by the Mo extraction from the V-barren liquor at pH 5.0.
The extraction isotherm curves for both V and Mo were developed by plotting log D versus equilibrium pOH, instead of versus pH, using the pH variation data for the better explanation of the equilibrium equations. It is shown in Fig. 7 which shows that the slopes of the curves were found to be 1.187 and 2.011 for V and Mo, respectively31. From the values of slopes, the following equilibrium equations are proposed for the SX of V (Eq. 9) and Mo (Eq. 10):
Extraction-stripping circuit of V
The V extraction isotherm was established by varying the A:O ratio as 1:23, 1:11, 1:7, 1:5, 1:3, 1:2, 1:1, 2:1, 3:1, 5:1, 7:1, 11:1, and 23:1 while other parameters (e.g. total volume, 24 mL; pH, 8.0; organic phase, 5%(v/v) Aliquat 336 in toluene; [V], 0.55 M; [Mo], 0.045 M) kept constant. In order to find out the number of stages required for its extraction, a McCabe-Thiele plot was established using the experimental data and is shown in Fig. 839. For the McCabe-Thiele plot, the operating line was based on the 70% V loading at the A:O ratio of 1:1.1. By putting the operating line, 0.55 M of V can completely be extracted from the leach liquor in a two-stage process. The co-extraction of Mo was very marginal as it was 4 × 10−5 M. The stripping of V from the loaded organic was conducted by using three different solutions, such as NaOH (2.0 M), NH4OH (2.0 M)and Na2CO3 (2.0 M), at the O:A ratio and the total volume of 1:1 and 24 mL, respectively. For the stripping experiments, the loaded organic was collected from the SX experiment with A:O of 1:1 where the V and Mo concentration was 0.38 and 4 × 10−5 M, respectively. The stripping solutions, such as NaOH, NH4OH and Na2CO3, respectively recovered 99.8, 94.2 and 82.6% of V from the loaded organic. Therefore, further stripping isotherm was evaluated using 2.0 M NaOH solution. In order to evaluate the number of stage required for the V stripping, the O:A ratio were varied as 1:23, 1:11, 1:7, 1:5, 1:3, 1:2, 1:1, 2:1, 3:1, 5:1, 7:1, 11:1 and 23:1while other parameters (e.g. total volume, 24 mL; [NaOH], 2 M; [V], 0.38 M; [Mo], 4 × 10−5 M) kept constant. The McCabe-Thiele plot for the V stripping was developed using the O:A variation data where the operating line based on the 70% stripping at the O:A ratio of 7.2:1 and is shown in Fig. 9. By putting the operating line, 0.38 M of V can completely be stripped from the loaded organic in a two-stage process. The V concentration enriched from 0.55 M in the leach liquor to 1.9 M in the stripped solution through the SX followed by the stripping whereas the negligible Mo co-extraction resulted in its concentration 2 × 10−4 M in the stripped solution.
Extraction-stripping circuit of Mo
After the V recovery at pH 8.0, the concentrations of V and Mo in the raffinate (V-barren leach liquor) were 2 × 10−5 and 0.0448 M, respectively. The SX was conducted to recover Mo from the raffinate by varying the A:O ratio as 1:23, 1:11, 1:7, 1:5, 1:3, 1:2, 1:1, 2:1, 3:1, 5:1, 7:1, 11:1 and 23:1 while other parameters (e.g. total volume, 24 mL; pH, 5.0; organic phase, 5%(v/v) Aliquat 336 in toluene; [V], 2 × 10−5 M; [Mo], 0.0448 M) kept constant. The McCabe-Thiele plot was developed using the Mo extraction data and is shown in Fig. 10 39. The operating line was based on the 70% Mo extraction at the A:O ratio of 3.6:1. By putting the operating line, the 0.0448 M Mo can completely be extracted from the V-barren leach liquor in a three-stage process. The concentration of V and Mo in the loaded organic were 5 × 10−5 and 0.102 M, respectively. The Mo stripping from the loaded organic was conducted by using three different solutions, such as NaOH (2.0 M), NH4OH (2.0 M), and Na2CO3 (2.0 M), similar to the conditions of the V stripping. The NaOH, NH4OH and Na2CO3 respectively recovered 99.9, 96.8 and 92.5% of Mo from the loaded organic. Therefore, further stripping isotherm was evaluated using the 2.0 M NaOH solution. In order to evaluate the number of stage required for the Mo stripping, the O:A ratio were varied as 1:23, 1:11, 1:7, 1:5, 1:3, 1:2, 1:1, 2:1, 3:1, 5:1, 7:1, 11:1 and 23:1 while other parameters (e.g. total volume, 24 mL; [NaOH], 2 M; [V], 5 × 10−5 M; [Mo], 0.102 M) kept constant. The McCabe-Thiele plot was developed using the O:A variation data and is shown in Fig. 11. The operating line based on the 70% stripping of Mo from the loaded organic at the O:A ratio of 15.2:1. By putting the operating line, the 0.102 M of Mo can completely be stripped from the loaded organic in a three-stage process. The Mo concentration enriched from 0.0448 M in the V-barren leach liquor to 1.08 M in the stripped solution through the SX followed by the stripping whereas the negligible V co-extraction resulted in its concentration 1.2 × 10−4 M in the stripped solution.
From the above experimental evaluations, a flow-sheet was developed for the recovery of Mo and V from the SC sample and is shown in Fig. 12. The material balances starting from the leaching to stripping are mentioned in the flow-sheet. In the flow-sheet the final precipitation of Mo and V from respective stripped solutions were given on the basis of their standard precipitation processes40,41.
Conclusion
Both sulfide and oxide of Mo were present in Mo-matrix of the SC sample. The chemical properties of Mo and its association with the impervious S0 layer within the SC created difficulties during its leaching. The CS2 washing process successfully removed the S0 from the SC sample. When the impervious S0 was washed away, the sulfur voids developed within the SC particles which made the easy penetration of the lixiviants through the voids and they reacted with the underlying Mo-matrix resulting in the higher Mo dissolution. The leaching rate increased in an order, e.g. acetone washed < acetone-CS2 washed < acetone washed-H2O2 < acetone-CS2 washed-H2O2; however, the order was more contrast at the higher concentration of lixiviants. The maximum Mo dissolution was 94.8%(w/w) for the acetone-CS2 washed-H2O2 condition reacted with 2.0 M NaOH. The Mo leaching rate decreased with the increase of pulp density due to the interaction of lixiviant molecules with the multiple reacting solid particles decreased the driving force of the chemical reactions. The SX of V and Mo from the synthetic leach liquor was successful using Aliquat 336 diluted in toluene as the organic phase. The pH variation experiments of SX showed that the V should be extracted from the leach liquor at pH > 8.0 in the first step followed by the Mo extraction from the V-barren solution at pH < 5.0 in the second step. The V could completely be extracted from the leach liquor in a two-stage process using 5%(v/v) Aliquat 336 at the A:O ratio of 1:1.1. The V loaded organic could completely be stripped in a two-stage process using 2.0 M NaOH at the O:A ratio of 7.2:1. The Mo in the V-barren leach liquor could completely be extracted using 5%(v/v) Aliquat 336 in a three-stage process at the A/O ratio of 3.6:1. The Mo loaded organic could completely be stripped in a three-stage process using 2.0 M NaOH at the O:A ratio of 15.2:1. The laboratory scale experiments developed a model for the recovery of V and Mo from SC through the leaching and SX techniques; however, higher scale study should be conducted prior to development of the pilot scale operation.
References
Silvy, R. P. Future trends in refining catalyst market. Appl. Catal. 261, 247–52 (2004).
Marafi, M. & Stanislaus, A. Spent catalyst waste management: A review Part I. Developments in hydroprocessing catalyst waste reduction and use. Resour. Conserv. Recycl. 52, 859–873 (2008).
Dufresne, P. Hydroprocessing catalysts regeneration and recycling. Appl. Catal. A322, 67–75 (2007).
Marafi, M. & Stanislaus, A. Spent hydroprocessing catalyst management: A review Part II. Advances in metal recovery and safe disposal methods. Resour. Conserv. Recycl. 53, 1–26 (2008).
Akcil, A., Vegliò, F., Ferella, F., Okudan, M. D. & Tuncuk, A. A review of metal recovery from spent petroleum catalysts and ash. Waste Manage. 45, 420–433 (2015).
Zeng, L. & Cheng, C. Y. A literature review of the recovery of molybdenum and vanadium from spent hydrodesulphurisation catalysts Part I: Metallurgical processes. Hydrometallurgy 98, 1–9 (2009).
Zeng, L. & Cheng, C. Y. A literature review of the recovery of molybdenum and vanadium from spent hydrodesulphurisation catalysts Part II: Separation and purification. Hydrometallurgy 98, 10–20 (2009).
Ramírez, S., Schacht, P., Quintana-Solórzano, R. & Aguilar, J. Leaching of heavy metals under ambient resembling conditions from hydrotreating spent catalysts. Fuel 110, 286–292 (2013).
Barik, S. P., Park, K. H., Parhi, P. K. & Park, J. T. Direct leaching of molybdenum and cobalt from spent hydrodesulphurization catalyst with sulphuric acid. Hydrometallurgy 111–112, 46–51 (2012).
Padh, B., Rout, P. C., Mishra, G. K., Suresh, K. R. & Reddy, B. R. Recovery of nickel and molybdate from ammoniacal leach liquors of spent HDS catalysts using chelating ion exchange resin. Hydrometallurgy 184, 88–94 (2019).
Xiaolei, Y. et al. Microwave sodium roasting (MWSR) spent HDS catalysts for recovery Mo and in situ sulfur fixation. J. Taiwan Inst. Chem. E. 97, 146–157 (2019).
Imam, D. M. & El-Nadi, Y. A. Recovery of molybdenum from alkaline leach solution of spent hydrotreating catalyst by solvent extraction using methyl tricaprylammonium hydroxide. Hydrometallurgy 180, 172–179 (2018).
Zhao, Z., Guo, M. & Zhang, M. Extraction of molybdenum and vanadium from the spent diesel exhaust catalyst by ammonia leaching method. J. Hazard. Mater. 286, 402–409 (2015).
Liu, J., Qiu, Z., Yang, J., Cao, L. & Zhang, W. Recovery of Mo and Ni from spent acrylonitrile catalysts using an oxidation leaching–chemical precipitation technique. Hydrometallurgy 164, 64–70 (2016).
Mahandra, H., Singh, R. & Gupta, B. Development of a hydrometallurgical route for the recovery of molybdenum from spent hydrodesulphurization catalyst using Cyphos IL 104. J. Ind. Eng. Chem. 65, 213–223 (2018).
Pinto, I. S. S. & Soares, H. M. V. M. Selective leaching of molybdenum from spent hydrodesulphurisation catalysts using ultrasound and microwave methods. Hydrometallurgy 129-130, 19–25 (2012).
Rout, P. C., Mishra, G. K., Padh, B., Suresh, K. R. & Reddy, B. R. Solvent extraction separation of molybdenum as thio-molybdate complex from alkaline tungsten leach liquor of spent HDS catalyst - A pilot study. Hydrometallurgy 174, 140–146 (2017).
Park, K. H., Mohapatra, D. & Nam, C. W. Two stage leaching of activated spent HDS catalyst and solvent extraction of Al using organo-phosphinic extractant, Cyanex-272. J. Hazard. Mater. 148, 287–295 (2007).
Park, K. H., Mohapatra, D. & Reddy, B. R. Selective recovery of molybdenum from spent HDS catalyst using oxidative soda ash leach/carbon adsorption method. J. Hazard. Mater. 138, 311–316 (2006).
Ding, Y. et al. Recovery of precious metals from electronic waste and spent catalysts: A review. Resour. Conserv. Recycl. 141, 284–298 (2019).
Pradhan, D. et al. Bioleaching kinetics and multivariate analysis of spent petroleum catalyst dissolution using two acidophiles. J. Hazard. Mater. 175, 267–273 (2010).
Asghari, I., Mousavi, S. M., Amiri, F. & Tavassoli, S. Bioleaching of spent refinery catalysts: A review. J. Ind. Eng. Chem. 19, 1069–1081 (2013).
Santhiya, D. & Ting, Y. P. Bioleaching of spent refinery catalyst using Aspergillusnigerwith high yield oxalic acid. J. Biotechnol. 116, 171–184 (2005).
Santhiya, D. & Ting, Y. P. Use of adapted Aspergillusnigerin the bioleaching of spent refinery catalyst. J. Biotechnol. 121, 62–74 (2006).
Amiri, F., Yaghmaei, S. & Mousavi, S. M. Bioleaching of tungsten-rich spent hydrocracking catalyst using Penicilliumsimplicissimum. Bioresour. Technol. 102, 1567–1573 (2011).
Bharadwaj, A. & Ting, Y. P. Bioleaching of spent hydrotreating catalyst by acidophilic thermophile Acidianusbrierleyi: Leaching mechanism and effect of decoking. Bioresour. Technol. 130, 673–680 (2013).
Pradhan, D., Kim, D. J., Ahn, J. G., Chaudhury, G. R. & Lee, S. W. Kinetics and statistical behavior of metals dissolution from spent petroleum catalyst using acidophilic iron oxidizing bacteria. J. Ind. Eng. Chem. 16, 866–871 (2010).
Pradhan, D. et al. Comparison of bioleaching kinetics of spent catalyst by adapted and unadapted iron oxidizing bacteria - effect of pulp density; particle size. temperature. Korean J. Met. Mater. 49, 956–966 (2011).
Pradhan, D., Kim, D. J., Chaudhury, G. R. & Lee, S. W. Bio-dissolution of Ni, V and Mo from spent petroleum catalyst using iron oxidizing bacteria. J. Environ. Sci. Health A45, 476–482 (2010).
Pradhan, D., Mishra, D., Kim, D. J., Chaudhury, G. R. & Lee, S. W. Dissolution kinetics of spent petroleum catalyst using two different acidophiles. Hydrometallurgy 99, 157–162 (2009).
Mishra, D., Chaudhury, G. R., Kim, D. J. & Ahn, J. G. Recovery of metal values from spent petroleum catalyst using leaching-solvent extraction technique. Hydrometallurgy 101, 35–40 (2010).
Kim, D. J., Pradhan, D., Ahn, J. G. & Lee, S. W. Enhancement of metals dissolution from spent refinery catalysts using adapted bacteria culture - Effects of pH and Fe(II). Hydrometallurgy 103, 136–143 (2010).
Olson, G. J. & Clark, T. R. Bioleaching of molybdenite. Hydrometallurgy 93, 10–15 (2008).
Pradhan, D., Patra, A. K., Kim, D. J., Chung, H. S. & Lee, S. W. A novel sequential process of bioleaching and chemical leaching for dissolving Ni, V, and Mo from spent petroleum refinery catalyst. Hydrometallurgy 131–132, 114–119 (2013).
Austin, J. M., Jensen, D. & Meyer, B. Solubility of sulfur in liquid sulfur dioxide, carbon disulfide, and carbon tetrachloride. J. Chem. Eng. Data 16, 364–366 (1971).
Vinayan, B. P. et al. Performance study of magnesium-sulfur battery using a graphene based sulfur composite cathode electrode and a non-nucleophilic Mg electrolyte. Nanoscale 8, 3296–3306 (2016).
Lee, M., Sohn, S. & Lee, M. Ionic equilibria and ion exchange of molybdenum(VI) from strong acid solution. Bull. Korean Chem. Soc. 32, 3687–3691 (2011).
Sohn, H. Y. & Wadsworth, M. E. Rate Process of Extractive Metallurgy (Plenum, NewYork, 1979).
Parhi, P. K., Panigrahi, S., Sarangi, K. & Nathsarma, K. C. Separation of cobalt and nickel from ammoniacalsulphate solution using Cyanex 272. Sep. Purif. Technol. 59, 310–317 (2008).
Nasyrov, G. Z. &Ravdonikas, I. V. Method of preparing vanadium pentoxide. United States Patent US4039582A (1977).
Castagna, H., Gravey, G. & Roth, A. Process for recovering molybdenumvalues from spentcatalysts. United States Patent US4075277A (1978).
Acknowledgements
The authors are grateful to Prof. (Dr.) M.R.Nayak, President, SOA (Deemed to be University), for providing the infrastructures and encouragement throughout.
Author information
Authors and Affiliations
Contributions
Debabrata Pradhan: Done all experimental works independently, all interpretations, and writing the manuscript. Dong-Jin Kim: Supported the work from his project and partly guided the leaching experiments. Lala Behari Sukla: Partly guided the leaching experiments. Archana Pattanaik: Formatted the manuscript. Seoung-Won Lee: Partly guided the pretreatment of spent catalyst.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pradhan, D., Kim, DJ., Sukla, L.B. et al. Evaluation of molybdenum recovery from sulfur removed spent catalyst using leaching and solvent extraction. Sci Rep 10, 1960 (2020). https://doi.org/10.1038/s41598-020-58972-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-58972-x
This article is cited by
-
Spent sulfuric acid plant catalyst: valuable resource of vanadium or risky residue? Process comparison for environmental implications
Environmental Science and Pollution Research (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.