Abstract
Ovule-derived haploid culture is an effective and important method for genetic study and plant breeding. Gerbera hybrida is a highly heterozygous species, and the lack of homozygous lines presents a challenge for molecular genetic research. Therefore, we performed haploid induction through unpollinated ovule culture and evaluated the effects of several important factors on this culturing procedure in G. hybrida, including genotype, low temperature, and the development seasons of the ovules. Among 45 G. hybrida cultivars analyzed, 29 cultivars exhibited adventitious bud induction via in vitro unpollinated ovule culture with significant different responses, indicating that the genotype of donor plants was a vital factor for inducibility. Four cultivars with significantly different induction rates, including one non-induced cultivar, were selected to analyze seasonal effects. Ovules extracted in the summer consistently had the highest induction rates, and even the non-induced cultivar included in the analysis could be induced at low levels when ovules from summer were used. Low temperature treatment could also promote adventitious bud induction, and in particular, a strong and significant effect was detected after 7 days of cold treatment. Ploidy level measurements by flow cytometry revealed that 288 ovule-derived regenerants were haploid (55.17%) and 218 lines were diploid (41.76%). Moreover, genetic stability analysis of the regenerants indicated 100% similarity to the marker profile of the mother plant. This is the first report of ovule-derived haploids in G. hybrida, which may facilitate the development of homozygous lines for molecular research and plant breeding.
Similar content being viewed by others
Introduction
Gerbera hybrida is one of the most popular ornamental plants worldwide1. Since most of the commecial cultivars were bred from the crossing of wild-type G. jamesonii and G. viridifolia, the genome of G. hybrida is highly heterozygous2. Hundreds of G. hybrida varieties with extremely rich flower color patterns are available in the flower market, including white, yellow, red, pink, purple, and brown, and G. hybrida ranks fourth in cut flowers after rose, chrysanthemum, and tulip3. As a highly heterozygous species, G. hybrida naturally harbors genetic diversity, which is beneficial for hybridization breeding. However, the lack of a homozygous genome is challenging for molecular genetic research, including forward genetic screening and mutation mapping.
Ploidy breeding, the modification of the number of chromosome sets in a plant genome, is frequently used in ornamental plant breeding to induce novel variation and to create homogenous lines4. A commonly used protocol for ploidy breeding is haploid induction, which refers to haploid regeneration via a single gamete cell under specific conditions, using either pollen (anther or microspore culture) or egg cells (ovule culture)5. Essentially, the applicability of haploid induction is the main trigger for ornamental breeding, and some haploids of ornamentals have been commercially developed, including the horticultural variety Pelargonium ‘Kleine Liebling’6. Nevertheless, haploid induction, particularly induction via ovule culture, has only been reported in a limited number of ornamental species. An extensive review of haploidization in ornamental species listed 46 ornamental genera in which haploidization had been accomplished through one or multiple techniques, and ovule/ovary culture was reported in only six genera7. Moreover, the haploidization protocols are highly species specific, with genotype as a crucial factor for successful haploidization within a species8. In the Asteraceae family, different in vitro culture methods for haploid induction have been reported, such as haploidization from unfertilized ovules in G. jamesonii9,10,11 and Chrysanthemum morifolium12. However, similar strategies have not yet been reported in the most relevant ornamental species G. hybrida, even though this species has been used as an Asteraceae model plant for decades.
In this study, we conducted in vitro unpollinated ovule culture to induce haploidization in G. hybrida and evaluated the effects of the following factors: genotype, low temperature, and the development seasons of the ovules. First, we analyzed 45 G. hybrida commercial cultivars to measure the rate of adventitious bud induction and to assess the response to unpollinated ovule culture. Four G. hybrida cultivars with significantly different induction rates were selected, and for these cultivars, ovules from different seasons and 4 °C cold treatment were applied to optimize the conditions and ovule explants for unpollinated ovule culture. The eventual goal was to create regenerated haploids, which were confirmed by flow cytometry measurements. Our study provides a basic strategy for haploid induction via unpollinated ovule culture in G. hybrida, which may greatly facilitate the generation of homozygous lines for molecular research as well as doubled haploid lines for plant breeding.
Results
Analysis of unpollinated ovule culture in 45 cultivars
First, we investigated the effect of genotype on unpollinated ovule culture. For this analysis, 45 different cultivars were used to obtain ovule explants from semi-open flowers. The unpollinated ovules were then cultured in vitro to determine whether adventitious buds could be induced. The results showed that 29 cultivars exhibited adventitious bud induction through unpollinated ovule culture, accounting for 64.44% of all tested varieties (Fig. 1). The rate of adventitious bud induction was over 5% in 12 cultivars, and the highest induction rate was detected in cultivar ‘Prince’ (18.19% ± 0.96, n = 3). These experimental data indicate that the ovules from different cultivars respond differently to unpollinated ovule culture, reflecting differences in their genetic backgrounds and genotypes, as each variety was derived from different hybrid lines.
Adventitious bud induction rate of ovules from different Gerbera hybrida cultivars. One-way ANOVA was used to assess statistical significance, and p values were calculated with Tukey’s HSD test (α = 0.05). The detailed data for this figure are available in Table S1.
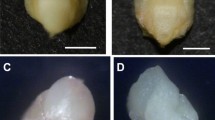
During the process of ovule induction, three distinct stages were identified from ovules to regenerants: (1) gradual expansion of the ovule, (2) callus formation, and (3) adventitious bud induction (Fig. 2). However, adventitious bud induction was not always preceded by callus formation, and we observed a small number of ovules that were directly induced to form adventitious buds (Fig. 2). When adventitious buds formed in this manner, the induction time was shorter than that of buds formed via callus formation.
The stages of ovule induce to form adventitious buds in Gerbera hybrida. (A) Gradual expansion of the ovule volume; (B) callus formation; (C) adventitious bud induction. (D) In some cases, an adventitious bud formed directly from an induced ovule without callus formation, and three distinguishable stages were displayed.
The effect different of seasons on unpollinated ovule culture
Based on the above analysis of ovule induction in 45 different cultivars of G. hybrida, four cultivars with significantly different adventitious bud induction rates (‘Prince’, ‘Sunshine’, and ‘Hongjixing’ along with the non-induced cultivar ‘Dolly’) were selected for further study. Ovule explants were obtained from different seasons (spring, summer, autumn, and winter) and used for unpollinated ovule culture. Our results indicated that ovule explants from summer had the highest adventitious bud induction rates and the shortest induction times (Table 1). Although the cultivar ‘Dolly’ was not induced in the preliminary analysis, adventitious buds were induced at a very low rate in this cultivar when ovules from spring and summer were used. Generally, there were no significant differences in the induction effect between ovules obtained in spring and autumn, but ovules obtained in winter had the lowest induction rate, indicating that temperature may also significantly influence unpollinated ovule culture.
Among the four cultivars, ‘Prince’ had the highest induction rate and the shortest induction time in summer, suggesting that it may be a suitable material for further analysis of G. hybrida unpollinated ovule culture. In contrast, it was difficult to induce adventitious buds in the cultivar ‘Dolly’, underscoring the impact of genotype on the induction of G. hybrida through unpollinated ovule culture.
The effect of low temperature on unpollinated ovule culture
Next, we investigated the effect of low temperature treatment on the same four cultivars using ovules extracted during the summer. Differences in the duration of low temperature treatment affected the response to unpollinated ovule culture, and for all cultivars, an optimal induction effect was observed with 7 days of treatment (Table 2). Specifically, ‘Prince’ had the highest induction rate (25.79% ± 1.44%, n = 3), which was approximately 5% greater than the induction rate of the control after 7 days of low temperature treatment. Consistent with the previous results, induction of ‘Dolly’ through unpollinated ovule culture was extremely limited, with some adventitious bud formation under low temperature treatment for 3 days and 7 days. Our results show that low temperature treatment could promote the induction of adventitious buds in G. hybrida, and the optimal treatment duration was 7 days.
Evaluation of regenerants
After the induction of adventitious buds, we cultivated each induction line into individual ovule-derived haploid regenerants. First, we examined the chromosome ploidy of ovule-derived haploids and control diploids by root tip chromosome counting. The ovule-derived haploids harboured 25 chromosomes (n = 25, Fig. 3), which was half of the chromosome number of the control diploids (2n = 50, Fig. 3), confirming that the unpollinated ovule culture was successful. Next, using flow cytometry and the leaf materials of ovule-derived haploids and control diploids, we determined the ploidy level of ovule-derived haploids from different diploid cultivars. The results showed that haploids, diploids, and mixoploids co-existed in 522 lines of the ovule-derived regenerated plant population. Specifically, 55.17% were haploids (288 lines), 41.76% were diploids (218 lines), and 16 strains were mixoploids. In terms of plant morphology, the regenerants of the ovule-derived haploids differed markedly from the control diploids and had weak, short, and narrow leaves (Fig. 4). This phenotype is consistent with those of other haploid plants.
True-to-type clonal fidelity is the key prerequisite for in vitro-regenerated plantlets, since somaclonal variation may occur in the regenerants. Therefore, we used eight random amplified polymorphic DNA (RAPD) markers to evaluate the genetic stability of the in vitro-regenerated plantlets (five individual regenerants) of five G. hybrida cultivars (‘Prince’, ‘Sunshine’, ‘Hongjixing’, ‘Dolly’, and ‘Pretty Pink’, Fig. S1). The PCR results showed that all amplified bands from the ovule-derived haploids were monomorphic and similar to those of the mother plant. Moreover, a similarity matrix based on Jaccard’s coefficient showed that the pair-wise value between mother and regenerant plantlets was 1, indicating 100% similarity and no variability among the plantlets derived from unpollinated ovule culture. These findings indicate that ovule explants can be used for G. hybrida haploid induction without much risk of genetic instability.
Discussion
Multiple studies have demonstrated that genotype is a fundamental factor for successful haploid induction via in vitro unpollinated ovule culture8,13,14. Accordingly, the variety selected as the ovule donor for haploid induction is of utmost importance. The different responses to haploid induction reflect differences between species as well as differences among genotypes of an individual species. For example, genotype proved to be a key factor influencing in vitro gynogenesis and androgenesis in summer squash (Cucurbita pepo), cucumber (Cucumis sativus), winter squash (Cucurbita maxima), pumpkin (Cucurbita moschata), and watermelon (Citrullus lanatus)13,15,16,17. A previous study of Gerbera jamesonii including ten different Gerbera genotypes showed that genotype affected callus induction, which ranged from 5% to 43%, as well as the shoot regeneration rate, which ranged from 57% to 95%18. Several studies have also demonstrated that not all genotypes of G. jamesonii can produce haploid callus and shoots. For instance, Tosca et al. found that 12 out of 21 genotypes could induce haploid callus and only six could produce shoots19. Miyoshi and Asakura reported that 13 out of 17 genotypes could form callus in pot gerbera (G. jamesonii)11. In line with these previous findings, our analysis of 45 G. hybrida cultivars showed that 29 could be induced to form adventitious buds through unpollinated ovule culture, indicating that genotype has a significant effect on unpollinated ovule culture in G. hybrida. This difference in induction response may reflect the genetic diversity of the 45 cultivars, as each variety was derived from different genetic backgrounds.
In addition to genotype, other factors that critically affect unpollinated ovule culture include season and temperature. Seasonal effects on ovule culture have been observed in several species, but the available information is limited8. Cappadocia et al. reported a seasonal effect on G. jamesonii callus induction via unfertilized ovules and showed that callus produced in the spring had a higher morphogenetic capacity20. Tosca et al. also observed seasonal effects on unfertilized ovules in four genotypes of pot gerbera (G. jamesonii) from April to October in northern Italy; two genotypes had the highest callus formation in spring, the other two had the highest callus formation in autumn, and gynogenesis did not occur in winter21. In the present study, all four cultivars selected for further analysis had the highest rate of adventitious bud induction in summer. Notably, this included the cultivar ‘Dolly’, which did not exhibit adventitious bud induction when using ovules from autumn and winter. These findings indicate that season significantly affects ovule induction in G. hybrida, which is consistent with previous reports.
The positive effect of low temperature treatment (4 °C) for 4 to 5 days on in vitro androgenesis or gynogenesis has been reported in several species. In ornamental kale (Brassica oleracea L. var. acephala), cold treatment significantly improved plant regeneration from microspore-derived embryos to a rate of up to 79.0% under 4 °C for 2 days or 5 days22. Cold pretreatment (4 °C) of flower buds for 1–2 weeks also promotes effective induction of embryo-like structures in plant regeneration from unfertilized ovule culture of gentians (Gentiana spp.)23. Cold pretreatment also has a positive effect on direct embryo induction in pepper (Capsicum annuum L.)24. In our study, 4 °C cold pretreatment of flower buds for 7 days had the strongest effect on unpollinated ovule culture in vitro and led to the greatest increase in adventitious bud induction. This finding suggests that low temperature may promote adventitious bud induction in G. hybrida, consistent with the findings of previous studies. One possible explanation for this temperature effect is the diverting of normal gametophytic development into a sporophytic pathway, which would therefore promote the formation of haploid embryos25.
During the process of generating haploids from cultured ovules, most of the regenerants developed directly from callus, but we also observed a few adventitious buds that skipped the callus formation stage and developed directly from ovule tissues. This phenomenon has been observed previously in other haploid induction studies, and the adventitious buds may arise directly or be produced indirectly through a callus intermediate26. Since callus may form from somatic tissue associated with the egg cell, regenerants from callus may be haploid, diploid, or mixoploid8. In the present study, 55.17% of the 522 lines of ovule-derived regenerated plantlets were identified as haploid, and 41.76% were diploid. These results are consistent with a previous analysis of unfertilized ovule/ovary culture in gentians (Gentiana spp.), with 57.9% haploid and 34.3% diploid among the total of 1,515 regenerated plantlets23. Miyoshi and Asakura cultured unpollinated ovules of several genotypes of pot gerbera (G. jamesonii) and found that 80.4% were haploid, 15.2% were diploid, and 4.3% were mixoploid11. Cappadocia et al. similarly obtained 76% haploid plants using unpollinated ovules for G. jamesonii haploid production20. Among the unpollinated ovule-derived haploids in the present study, no genetic variation was detected by RAPD marker analysis, indicating that in vitro unpollinated ovule culture is a reliable method for haploid induction of G. hybrida without much risk of genetic instability. This observation is in line with the genetic stability of micropropagated plantlets of G. jamesonii, which showed no somaclonal variation during micropropagation27,28.
Our study of haploid induction via unpollinated ovule culture in G. hybrida demonstrates the applicability and reliability of this technique and provides basic knowledge for haploidization in G. hybrida. The findings show that this strategy can be applied to a wide range of G. hybrida genotypes. The haploids generated through this approach could potentially be used to create doubled haploids in breeding programs to reduce cultivar development time. The haploid lines could also facilitate molecular research in G. hybrida; for example, genome sequencing of haploids would reduce the cost and data volume due to the halved chromosome number.
Methods
Plant material and growth conditions
The following 45 commercial cultivars of Gerbera hybrida with different flower colors and genetic backgrounds were analyzed in this study: ‘Prince’, ‘Autumn’, ‘Sunshine’, ‘Colorful Clouds’, ‘Double Color’, ‘Golden Sunflower’, ‘Lucky’, ‘Red Hat’, ‘Yunjin Ziwei’, ‘Yunjin Snow’, ‘Wedding’, ‘Pretty Pink’, ‘Red Carpet’, ‘Hongjixing’, ‘Savanna’, ‘Qihua’, ‘Lady’, ‘Golden Phoenix’, ‘Red Enchantress’, ‘Chrysanthemum King’, ‘Beautiful Sha’, ‘Pure Heart’, ‘Flawless Clouds’, ‘Minister’, ‘Big Champagne’, ‘Winter’, ‘Dolly’, ‘Imperial Concubine’, ‘Over Fire’, ‘Red Gorgeous’, ‘Lalissa’, ‘Exquisite’, ‘Rose’, ‘Honey’, ‘Princess’, ‘Cosy Sweet’, ‘Sunshine Coast’, ‘Purple Queen’, ‘P2’, ‘GX23’, ‘Mi’, ‘W68’, ‘R45’, ‘GP51’, and ‘GR63’. The plant seedlings were obtained from Yuxi Yunxing Biological Technology Co., Ltd. (Yunnan Province, China). All seedlings were planted separately in individual pots (diameter = 14 cm, height = 12 cm), which were filled with commercial potting substrate (peat:perlite = 3:1) then cultivated in controlled-environment growth chambers in a lab at the Yunnan Academy of Agricultural Sciences. The conditions were as follows: 25 °C, 12 h photoperiod (240 μmol/m2s photosynthetic photon flux density), and 55% relative humidity3.
Selection of ovule explants
Gerbera hybrida flower development can be divided into five stages: flower buds, sepal open, ray florets colored, ray florets open, and flower semi-open. In this study, the ovule explants were obtained from semi-open stage flowers (Fig. 5). First, the semi-open flowers were washed with detergent and rinsed with deionized water. On an ultra-clean workbench, the ray florets at the edge of the inflorescence were cut and removed using a sharp surgical blade, followed by disinfection with 0.1% HgCl2, 2% NaClO, and deionized water for 15 minutes, 10 minutes, and 5 minutes, respectively. Finally, the ovules of disc florets at the center of the inflorescence were dissected and peeled off under a microscope. Ovules with a full appearance and no mechanical damage were used as explants.
In vitro unpollinated ovule culture
The unpollinated ovules were cultured in vitro in MS medium (0.6 mg/L BA, 0.1 mg/L NAA, 30 g/L sugar, 7 g/L agar, pH = 5.8) in controlled-environment chambers at 22 °C in the dark for 7 days then transferred to a 10 h photoperiod and 50 μmol/m2s photosynthetic photon flux density. The ovules were transferred to fresh medium every 30 days. After 90 days, the adventitious bud induction rate was calculated using the following formula: induction rate (%) = number of ovules with induced adventitious buds/number of cultured ovules. Induction time was quantified as the average time for adventitious bud formation from three replicates.
Ovule explants from different seasons and low temperature treatment
The Gerbera hybrida cultivars ‘Prince’, ‘Sunshine’, ‘Hongjixing’, and ‘Dolly’ had significantly different adventitious bud induction rates and were selected to further study the effect of different seasons on unpollinated ovule culture. Seedlings of these four cultivars were planted in a greenhouse under natural conditions. Semi-open flowers of each cultivar were collected in spring (April), summer (July), autumn (October), and winter (January) to extract the ovules that developed in different seasons. Three individual plants were used as replicates for each treatment.
These four cultivars were also used to investigate the effects of low temperature treatment on unpollinated ovule culture during the summer season. The plants were subjected to low temperature treatment (4 °C) for 0 days, 3 days, 7 days, and 10 days in controlled-environment chambers, followed by ovule extraction. Three individual plants were used as replicates for each treatment.
Identification of ovule-derived haploid regenerants
The ovule culture-derived haploid plants were identified using a flow cytometer (CyFLOW Cube, Partec GmbH, Germany), equipped with a UV lamp emitting at 358 nm and a TK 420 filter, together with chromosome ploidy analysis software. Young leaf tissue samples (10 cm2) from the tested plants were placed in a clean culture dish with 500 μL extraction lysis buffer (HR-A, Partec High Resolution Staining Kit). The leaf tissue was rapidly chopped using a sharp surgical blade then completely immersed in the extraction lysis buffer for 5 minutes in the dark. Next, 1600 μL DAPI staining buffer (HR-B, Partec High Resolution Staining Kit) was added and mixed, followed by a 5 minute incubation in the dark. Lastly, the leaf samples were processed through a filter with a pore size of 20 μm (Partec Cell Trics), and the leaf cell suspensions were transferred into a U-shaped tube for flow cytometry analysis under an excitation wavelength of 350 nm.
The leaves of haploid and diploid plants that had been confirmed by root tip chromosome counting were used as standards for the flow cytometry analysis. Specifically, the fluorescence intensity of the chromosome separation peak was adjusted to 50 for haploid samples; for diploid samples with double the chromosome number, a fluorescence intensity peak occurred at 100 (Fig. 6). Three leaves from each tested plant were measured as three samples, and each plant was measured three times. The fluorescence intensity of the chromosome separation peak was used to distinguish haploid, diploid, and mixoploid.
Genetic stability evaluation of ovule-derived haploid regenerants
Since the plantlets were derived through callus, somaclonal variation may have occurred. To evaluate the genetic fidelity of the ovule-derived haploid regenerants, eight random amplified polymorphic DNA (RAPD) markers were used to analyze the in vitro-regenerated plantlets of five G. hybrida cultivars (‘Prince’, ‘Sunshine’, ‘Hongjixing’, ‘Dolly’, and ‘Pretty Pink’). Five ovule-derived haploid regenerants were chosen randomly from the population and compared with the mother plant from which the ovule explants were taken. The genomic DNA of mother plants and regenerants were extracted from young leaves using the CTAB method29. The RAPD primers were synthesized by the Beijing Genomics Institute (BGI TECH, Shenzhen, China) (Table 3). The 25 μL PCR reaction volume included the following: 2 μL genomic DNA template, 2.5 μL 10 × buffer, 0.5 μL dNTPs, 1 μL primer, 0.3 μL DNA Taq polymerase, 1.5 μL MgCl2 (2 mM), and 17.2 μL nuclease-free water. PCR amplification was performed on a thermal cycler (RePure-A, Bio-Gener, China) using the following PCR reaction conditions: 5 min at 95 °C; followed by 40 cycles of 60 s at 95 °C, 60 s at the optimal annealing temperature, and 120 s at 72 °C; and a final extension of 5 min at 72 °C. The PCR products were separated by 1% agarose gel electrophoresis and stained with ethidium bromide. Each amplified fragment in the size range of 100 bp to 5 kb was manually scored by the following rules according to Jaccard’s similarity coefficient: the presence or absence of bands in the gel were denoted by ‘1’ and ‘0’, respectively28.
Statistical analysis
Data analysis and statistics were performed using Microsoft Excel 2016 and Data Processing System30. One-way ANOVA with Tukey’s HSD post-hoc test was used to compare multiple samples at the 5% significance level. The data were obtained from three replicates and presented as mean value ± standard deviation in the tables.
References
Wani, M. A. et al. In Sustainable Agriculture Reviews 27 (ed. Eric Lichtfouse) 91–127 (Springer International Publishing, 2018).
Deng, X. et al. Virus-induced gene silencing for Asteraceae - a reverse genetics approach for functional genomics in Gerbera hybrida. Plant. Biotechnol. J. 10, 970–978 (2012).
Li, F., Li, S. & Shan, Q. The Effect of Temperature on Plant Growth in Four Gerbera hybrida Cultivars. HortScience 54, 1164–1167, https://doi.org/10.21273/hortsci13919-19 (2019).
Eeckhaut, T. et al. In Ornamental Crops (ed. Johan Van Huylenbroeck) 145–173 (Springer International Publishing, 2018).
Kiełkowska, A., Adamus, A. & Baranski, R. In Plant Cell Culture Protocols (eds Víctor M. Loyola-Vargas & Neftalí Ochoa-Alejo) 301–315 (Springer New York, 2018).
Daker, M. ‘Kleine Liebling’, a haploid cultivar of Pelargonium. Nat. 211, 549–550 (1966).
Ferrie, A. M. & Caswell, K. L. Review of doubled haploidy methodologies in ornamental species. Propag. Ornam. plants 11, 63–77 (2011).
Hadziabdic, D., Wadl, P. A. & Reed, S. M. In Plant Tissue Culture, Development, and Biotechnology (eds Robert N. Trigiano & Dennis J. Gray) 385–396 (CRC Press, 2016).
Meynet, J. & Sibi, M. Haploid plants from in vitro culture of unfertilized ovules in Gerbera jamesonii. J. plant. Breed. 36, 1774–1786 (1984).
Sitbon, M. Production of haploid Gerbera jamesonii plants by in vitro culture of unfertilized ovules. Agronomie 1, 807–812 (1981).
Miyoshi, K. & Asakura, N. Callus induction, regeneration of haploid plants and chromosome doubling in ovule cultures of pot gerbera (Gerbera jamesonii). Plant. Cell Rep. 16, 1–5 (1996).
Wang, H. et al. Characterization of in vitro haploid and doubled haploid Chrysanthemum morifolium plants via unfertilized ovule culture for phenotypical traits and DNA methylation pattern. Frontiers in Plant Science, 5, https://doi.org/10.3389/fpls.2014.00738 (2014).
Zou, T., Su, H.-N., Wu, Q. & Sun, X.-W. Haploid induction via unfertilized ovary culture in watermelon. Plant. Cell, Tissue Organ. Cult. 135, 179–187 (2018).
Van Huylenbroeck, J. Ornamental Crops. (Springer, 2018).
Diao, W.-P. et al. Efficient embryo induction in cucumber ovary culture and homozygous identification of the regenetants using SSR markers. Sci. horticulturae 119, 246–251 (2009).
Shalaby, T. A. Factors affecting haploid induction through in vitro gynogenesis in summer squash (Cucurbita pepo L.). Sci. horticulturae 115, 1–6 (2007).
Kurtar, E. S., Balkaya, A. & Kandemir, D. Evaluation of haploidization efficiency in winter squash (Cucurbita maxima Duch.) and pumpkin (Cucurbita moschata Duch.) through anther culture. Plant. Cell, Tissue Organ. Cult. 127, 497–511 (2016).
Nhut, D. T. et al. Effect of genotype, explant size, position, and culture medium on shoot generation of Gerbera jamesonii by receptacle transverse thin cell layer culture. Sci. horticulturae 111, 146–151 (2007).
Tosca, A., Lombardi, M., Marinoni, L., Conti, L. & Frangi, P. 280 edn 337–340 (International Society for Horticultural Science (ISHS), Leuven, Belgium).
Cappadocia, M., Chrétien, L. & Laublin, G. Production of haploids in Gerbera jamesonii via ovule culture: influence of fall versus spring sampling on callus formation and shoot regeneration. Can. J. Botany 66, 1107–1110, https://doi.org/10.1139/b88-158 (1988).
Tosca, A., Arcara, L. & Frangi, P. Effect of genotype and season on gynogenesis efficiency in Gerbera. Plant. Cell, Tissue Organ. Cult. 59, 77, https://doi.org/10.1023/a:1006418619992 (1999).
Wang, Y. et al. High frequency plant regeneration from microspore-derived embryos of ornamental kale (Brassica oleracea L. var. acephala). Sci. horticulturae 130, 296–302 (2011).
Doi, H. et al. Efficient haploid and doubled haploid production from unfertilized ovule culture of gentians (Gentiana spp.). Breed. Sci. 63, 400–406 (2013).
Popova, T. et al. Effects of low temperature, genotype and culture media on in vitro androgenic answer of pepper (Capsicum annuum L.). Acta Physiologiae Plant. 38, 273 (2016).
Trigiano, R. N. & Gray, D. J. Plant tissue culture, development, and biotechnology. (CRC Press, 2016).
Keller, E. R. J. & Korzun, L. In In Vitro Haploid Production in Higher Plants: Volume 1 — Fundamental Aspects and Methods (eds. S. Mohan Jain, S. K. Sopory, & R. E. Veilleux) 217–235 (Springer Netherlands, 1996).
Bhatia, R., Singh, K. P., Jhang, T. & Sharma, T. R. Assessment of clonal fidelity of micropropagated gerbera plants by ISSR markers. Sci. Horticulturae 119, 208–211 (2009).
Bhatia, R., Singh, K., Sharma, T. & Jhang, T. Evaluation of the genetic fidelity of in vitro-propagated gerbera (Gerbera jamesonii Bolus) using DNA-based markers. Plant. Cell, Tissue Organ. Cult. 104, 131–135 (2011).
Li, F. Meiotic recombination suppressors of Arabidopsis thaliana, Ghent University, (2018).
Tang, Q. Y. & Zhang, C. X. Data Processing System (DPS) software with experimental design, statistical analysis and data mining developed for use in entomological research. Insect Sci. 20, 254–260 (2013).
Acknowledgements
This work was funded by the National Natural Science Foundation of China (No. 31960608), the Basic Research Program of Yunnan Province - Youth Project (2019FD030), the Joint Project of Agricultural Basic Research of Yunnan Province - Youth Project (2018FG001-075), the Ten-thousand Talents Program of Yunnan Province – Yunling Scholar of Industrial Technology Leading Talent Project (Yun Fagai Renshi [2018] No. 212), Project of ministry-province co-construct (2018BSHJ0108), and the Science and Technology Talents and Platform Program of Yunnan Province (2018HB117).
Author information
Authors and Affiliations
Contributions
F.L. wrote the manuscript. F.L., L.W., and Q.S. completed the experiments. F.L. and X.Z. conducted genetic stability analysis of the regenerants. Y.C. and R.Y. performed data analyses. L.W. and H.L. conducted the flow cytometry measurements. L.W., S.L., and Q.S. jointly provided supervision. All authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, F., Cheng, Y., Zhao, X. et al. Haploid induction via unpollinated ovule culture in Gerbera hybrida. Sci Rep 10, 1702 (2020). https://doi.org/10.1038/s41598-020-58552-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-58552-z
This article is cited by
-
Double-haploid plant production through anther and ovule culture of wild Cyclamen persicum Mill. and Melody F1 cyclamen cultivar
In Vitro Cellular & Developmental Biology - Plant (2023)
-
Breeding of ornamentals: success and technological status
The Nucleus (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.