Abstract
Malnutrition is common in patients with acute kidney injury (AKI) and the risk of mortality is high, especially if renal replacement therapy is needed. Between April 2013 through April 2014, we recruited critically ill adult patients (≥18 years) with severe AKI in two University hospitals in London, UK, and measured serial plasma concentrations of vitamin B1, B6, B12, C and D, folate, selenium, zinc, copper, iron, carnitine and 22 amino acids for six consecutive days. In patients receiving continuous renal replacement therapy (CRRT), the concentrations of the same nutrients in the effluent were also determined. CRRT patients (n = 31) had lower plasma concentrations of citrulline, glutamic acid and carnitine at 24 hrs after enrolment and significantly lower plasma glutamic acid concentrations (74.4 versus 98.2 μmol/L) at day 6 compared to non-CRRT patients (n = 24). All amino acids, trace elements, vitamin C and folate were detectable in effluent fluid. In >30% of CRRT and non-CRRT patients, the plasma nutrient concentrations of zinc, iron, selenium, vitamin D3, vitamin C, trytophan, taurine, histidine and hydroxyproline were below the reference range throughout the 6-day period. In conclusion, altered micronutrient status is common in patients with severe AKI regardless of treatment with CRRT.
Similar content being viewed by others
Introduction
Critically ill patients with acute kidney injury (AKI) are at risk of malnutrition due to several factors, including pre-existing malnourishment, chronic comorbidities, poor nutritional intake, uraemia and the effects of critical illness in general1,2,3,4. In patients treated with renal replacement therapy (RRT), unrecognised losses of nutrients may also contribute5,6,7,8,9,10,11,12,13. The exact degree of nutrient loss during modern RRT is unknown since most data stem from a few small studies with relatively short periods of RRT (i.e. 24 hours or less)6,7,8,9,10,11,12,14,15,16,17. In 1999, Story et al. found that eight patients treated with continuous renal replacement therapy (CRRT) had lower serum concentrations of selenium (Se), zinc (Zn), vitamin C and vitamin E than healthy volunteers7. In 2004, Berger et al. demonstrated significant concentrations of Cu, Se, Zn and vitamin B1 in effluent fluid after 8 hours of CRRT8. Amino acid losses up to 10–15 g/d during RRT have been reported14. A recent study showed that 80% of patients on CRRT had below-normal levels of at least one micronutrient i.e. thiamine, pyridoxine, ascorbic acid, folate, zinc or copper13.
It is unclear whether these alterations of nutrient status contribute to the poor outcomes of patients with AKI. Furthermore, it is not clear whether there is a role for routine micronutrient supplementation18. Guidance from official authorities and experts varies and clinical practice is variable16,19,20,21. More data, including prospective studies and intervention trials are urgently needed13,18,19. In preparation, more information about the micronutrient status of AKI patients receiving modern critical care is required.
We hypothesized that patients treated with CRRT had significantly lower plasma concentrations of water soluble nutrients than AKI patients without CRRT. Our objectives were:
- (i)
To perform serial measurements of plasma concentrations of amino acids, trace elements and vitamins for up to 6 days in critically ill patients with severe AKI and to compare CRRT patients with non-CRRT patients;
- (ii)
To investigate the types of nutrients that are removed during CRRT and to quantify the losses; and
- (iii)
To determine the proportion of patients with plasma nutrient concentrations below the reference range.
Results
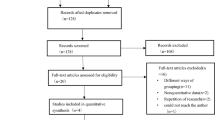
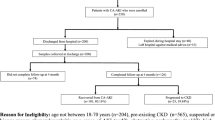
Between April 1, 2013, through April 1, 2014, 63 consecutive patients were recruited (Fig. 1). Following exclusion of eight patients [unexpected death within hours of enrolment (n = 3), transfer to another hospital (n = 1), withdrawal of consent (n = 1), and need to start TPN (n = 3)], 55 patients were analysed (33 patients in the CRRT group and 24 in the non-CRRT group). The main indications for CRRT were management or prevention of pulmonary oedema (57%) and severe metabolic acidosis (47%). During the 6-day follow-up period, 12 non-CRRT patients were commenced on CRRT, and 18 CRRT patients discontinued CRRT. As such, 12 patients had CRRT every day, 12 patients did not have CRRT on any day, and 19 patients had CRRT on some but not all 6 days.
Patient demographics
There were no clinically important differences in baseline characteristics between the CRRT and non-CRRT group on admission to the ICU (Table 1). In AKI patients receiving CRRT at enrolment, major surgery was the most common reason for admission (45%) compared to sepsis in patients with AKI not receiving CRRT (38%). ICU, hospital and 90-day mortality and length of stay were not significantly different between both groups (Table 1). There was no significant difference in protein and caloric intake between the CRRT and non-CRRT cohort during the first 24-hour period after enrolment (Table 2). The SOFA score was higher in those treated with CRRT but CRP and WBC were not significantly different.
Nutrient concentrations at 24 hours after enrolment
Twenty-four hours after enrolment, CRRT patients had significantly lower plasma concentrations of glutamic acid and carnitine than non-CRRT patients. There were no other statistically significant differences (Table 2).
Nutrient concentrations at day 6 after enrolment
Data were available for 43 patients. Intention-to-treat analysis confirmed significantly lower serum glutamic acid concentrations on day 6 in CRRT patients compared to non-CRRT patients (74.4 versus 98.2 μmol/L, respectively) (Table 3). Although the mean values of most other amino acids, trace elements and vitamins were lower in CRRT patients, the differences were statistically not significant. Analysis according to actual treatment (ie CRRT on all 6 days, CRRT on some days or no CRRT during 6 day period) received revealed no significant differences in nutrient concentrations between the three groups (Table 4).
Clearance of nutrients during CRRT
In CRRT patients, the mean dose of delivered RRT in the first 24 hours after enrolment was 46.1 L (SD 14.8) and the mean RRT dose during the whole period of RRT was 34.5 ml/kg/hr (SD 8.4). All amino acids, trace elements, and water soluble vitamins were detectable in effluent fluid (Table 5). Vitamin B1, B6, B12 and D were not detectable.
Nutrient concentrations below the reference range
In the majority of patients, serial plasma concentrations of vitamins and trace elements were below the reference range throughout the six-day observation period, especially Zn, Fe, Se, vitamin D3 and vitamin C, irrespective of CRRT. (Figs. 2 and 3 and Supplementary Table 2).
Apart from glutamic acid and tryptophan, the mean concentrations of all amino acids were within the reference range throughout the whole six-day follow-up period. (Supplementary Table 2) However, large proportions of patients had amino acid concentrations below the reference range, most notably trytophan, taurine, serine, glutamine, citrulline, histidine, hydroxyproline, lysine and arginine (Figs. 4 and 5 and Supplementary Table 3). There was no statistically significant difference between the CRRT and non-CRRT group.
Proportion of patients with plasma concentrationas of essential and conditionally essential amino acids below the reference range. CRRT patients in blue; severe AKI patients who were not treated with CRRT in red. Abbreviations: Arg = arginine; Glu = glutamine; Hist = histidine; Isoleuc = isoleucine; Leuc = leucine; Lys = lysine; Meth = methionine; Phenyl = phenylalanine; Threo = threonine; Trypt = tryptophan; Val = valine.
Proportion of patients with plasma concentrations of non-essential amino acids below the reference range. CRRT patients in blue; severe AKI patients who were not treated with CRRT in red. Abbreviations: Ala = alanine; Asp = aspartic acid; Citr = citrulline; Glyc = glycine; Hist = histidine; Hydro = hydroxyproline; Lys = Lysine; Orni = ornithine; Prol = proline; Ser = serine; Tau = taurine; Tyr = tyrosine.
Discussion
Our study confirms that the majority of critically ill patients with AKI had an altered micronutrient status with a large proportion of patients having nutrient concentrations below the reference range, irrespective of CRRT. With the exception of glutamic acid, there were no significant differences in plasma nutrient concentrations between the CRRT and non-CRRT group during a six-day observational period. All water soluble nutrients were detectable in the effluent in variable amounts.
It is thought that nutrient losses during RRT play an important role in the development of malnutrition in AKI18. Indeed, several small, observational studies in patients receiving CRRT reported losses of vitamins and micronutrients into the effluent7,8,17. In each of these studies, RRT modality and study duration differed and the number of measured nutrients was limited. Our study is the first where nutrient concentrations were measured over a six-day period in patients receiving modern critical care. We showed that water soluble nutrients were indeed lost into the effluent in variable quantities and that a large proportion of patients had plasma nutrient concentrations below the reference range. However, there was no statistically significant difference between the CRRT and non-CRRT group. In addition, low nutrient concentrations were seen at baseline before CRRT was initiated. Therefore, losses from CRRT cannot be assumed to be the main reason for altered nutrient balance in severe AKI.
It is well known that Se, Zn, Fe and vitamin C homeostasis is altered in critical illness with redistribution from the blood to the tissues22. Vitamin D concentrations are also frequently low during critical illness, but less is known about the metabolism of vitamin D and other fat-soluble vitamins in AKI. Druml and colleagues measured plasma concentrations of vitamin A, E, D and K in AKI patients treated with haemodialysis and reported profound deficiencies with the exception of vitamin K.23 More research is ongoing24.
Besides their role as building blocks for proteins and hormones, amino acids are key regulators of metabolic pathways necessary for cell signaling, cell homeostasis, growth and immunity. Both, critical illness and AKI are pro-inflammatory conditions associated with an altered amino acid profile and increased protein catabolism14,25,26. Our study confirms that the concentrations of essential amino acids like arginine, histidine, lysine and tryptophan and glutamine were low in the majority of patients with severe AKI. We did not explore the effect on outcomes but previous studies in critically ill patients demonstrated an association between low glutamine concentrations and mortality27.
Carnitine, a 161 kDa amino acid derivate, has a key role in the transport of fatty acids from the mitochondrial intermembrane space into the mitochondrial matrix in skeletal and cardiac myocytes. In critically ill patients, carnitine deficiency has been associated with prolonged hospital stay28,29. Our study showed significantly lower carnitine concentrations in CRRT patients at 24 hours but not beyond.
Micronutrient deficiency has been associated with poor outcomes during critical illness 30. In vitro and animal studies suggest important roles for various nutrients. Recently, several investigators explored the role of vitamin c and thiamine supplementation during critical illness with particular emphasis on patients with sepsis31,32,33,34,35. However, the role of nutrient supplementation in patients with severe AKI has not been studied. With serial data of 33 different nutrients measured over a six-day period in 55 patients receiving modern critical care, this is the largest study to date investigating the nutrient profile in critically ill patients with AKI. Our results reject the commonly held view that losses during RRT are the main reason for altered micronutrient profile in patients with AKI.
It is important to acknowledge some potential limitations. First, in an intention-to-treat analysis, we compared patients who received CRRT on day of enrolment with non-CRRT patients. However, some patients recovered renal function during the study period, and similarly, a proportion of patients in the control group required CRRT later. While these confounding factors were unavoidable, we explored the influence of cross-over by comparing three patient groups based on treatment received. Second, we did not find significant differences between CRRT and non-CRRT patients but note that the mean and median values of most nutrients were lower in the CRRT cohort. This was an exploratory study and was not powered to be definitive. Adjusting for multiple testing will almost certainly render no differences statistically significant but this might be a type 2 error. Importantly, the absence of statistical significance should not be viewed as an indicator of lack of clinical significance. Third, we found low plasma concentrations of several different nutrients but are unable to comment on the underlying cause. Whilst we only included patients who were fully established on enteral nutrition, underfeeding may still have contributed to low nutrient concentrations in plasma. Fourth, we measured amino acid concentrations but did not measure any related proteins, peptides or hormones and cannot comment on the clinical impact of amino acid deficiency. Fifth, we did not control for underlying illnesses and acknowledge that plasma micronutrient concentrations may have been affected by systemic inflammation21. However, there were no significant differences in CRP, WBC and serum albumin between the CRRT and non-CRRT groups at 24 hours and at 6 days. Sixth, we measured vitamins B1, B6, B12, C and D and note that previous studies also reported deficiencies of vitamin A, B3, E and K22,36. Seventh, vitamin concentrations were evaluated on the basis of abundance in plasma or whole blood only. We did not assess vitamin utilization. Eighth, patients were enrolled in 2013/2014 but the laboratory and statistical analysis was delayed until sufficient funding was available for the detailed analyses. Nutrient concentrations were not affected by storage. We also believe that clinical practice and management of CRRT has not changed significantly since then and that our results remain highly relevant for current practice. Ninth, we analysed patients with severe AKI with and without CRRT but did not include a cohort of patients without AKI. When we conceptualised the study and drafted the study protocol, it was (and still is) common belief that CRRT is responsible for significant nutrient losses and nutrient deficiencies. We hypothesized that patients treated with CRRT had significantly lower plasma concentrations of water soluble nutrients than patients with similar degrees of AKI not treated with CRRT. To test this hypothesis, we compared two cohorts of patients who were similar apart from the intervention of interest. At that time, we had not expected that patients with AKI not treated with CRRT also had derangements of serum nutrient concentrations to such an extent as seen in our study. Finally, we are unable to make recommendations for changes in nutritional support for patients with AKI based on our observational data but feel that intervention studies are urgently required.
In conclusion, in critically ill patients with severe AKI, serum concentrations of important nutrients are commonly below the reference range, independent of treatment with CRRT. All amino acids, trace elements and water soluble vitamins were removed during CRRT but there were no statistically significant differences in plasma nutrient concentrations between the CRRT and non-CRRT group during a six-day follow-up period, except for glutamic acid. Future research should explore the role of nutrient supplementation in high risk patients with AKI.
Methods
Setting
This prospective observational study was conducted in multidisciplinary Intensive Care Units (ICUs) of two tertiary care hospitals in London, UK.
Patient population
Between April 1, 2013, through April 1, 2014, we recruited consecutive critically ill adult patients (≥18 years) with AKI stage 2 or 3 as defined by the Kidney Disease Improving Global Outcome classification21. Accordingly, AKI stage 2 was defined by 2.0–2.9 fold rise of serum creatinine from baseline and/or urine output <0.5 ml/kg/hr for ≥12 hours; AKI stage 3 was defined by either a ≥3-fold rise of serum creatinine from baseline, a serum creatinine rise to ≥353.6 µmol/L (4.0 mg/dL), initiation of renal replacement therapy, anuria for ≥12 hours or a fall in urine output to <0.3 ml/kg/hr for ≥24 hours21. Exclusion criteria were: AKI stage 2/3 for more than 36 hours, pre-existing dialysis dependent renal failure, receiving supplementation with intravenous multivitamins or trace elements, need for total parenteral nutrition (TPN), or expected life expectancy <48 hours. We also excluded patients in whom serial blood sampling was not desirable (ie. Jehovah’s witness, patients with haemoglobin <70 g/L). Patients who needed RRT were treated with continuous veno-venous haemofiltration or continuous veno-venous haemodialysis using citrate or heparin anticoagulation.
All patients who were enrolled were established on full enteral feed receiving Nutrison Multifibre (1000 kcal/L; 123 g carbohydrate, 39 g fat and 40 g protein per litre; Nutricia Ldt, Trowbridge, UK) as per departmental protocol. Patients were allowed additional oral food intake if appropriate. Following routine individualized nutrition assessment by the departmental dietetic team, some patients were changed to Nutrison Protein Plus Multifibre (1280 kcal/L; 141 g carbohydrate, 49 g fat and 63 g protein per litre; Nutricia Ltd, Trowbridge UK), Nepro (2000 kcal/L; 206 g carbohydrate, 96 g fat and 70 g protein per litre) or Nepro HP (1800 kcal/l; 147 g carbohydrate, 97.7 g fat and 81 g protein per litre; Abbott Nutrition, Berkshire, UK) during the course of the study. All enteral feeds provided similar amounts of amino acids, vitamins and trace elements per 100 kcal. Patients who had to be commenced on TPN for clinical reasons during the 6-day follow-up period after enrolment were excluded from the primary analysis.
Collection of samples
Blood samples were collected at baseline and 22–26 h, 46–50 h, 94–98 h and 142–146 h after enrolment for measurement of plasma concentrations of Se, Zn, Cu, iron (Fe), vitamins B12, C and D, folate, carnitine and 22 different amino acids (leucine, isoleucine, valine, glycine, alanine, methionine, arginine, citrulline, ornithine, proline, glutamic acid, glutamine, lysine, tryptophan, phenylalanine, tyrosine, hydroxyproline, threonine, serine, aspartic acid, taurine, histidine). Vitamin B1 and B6 concentrations were measured in whole blood.
In CRRT patients, effluent samples were collected at the same time points for measurement of the same nutrients. All samples were processed and stored in dedicated research freezers at −80 °C until batch analysis at the end of the study.
Collection of clinical data
We collected baseline demographics, admission diagnosis, Acute Physiology and Chronic Health Evaluation (APACHE) II and Sequential Organ Failure Assessment (SOFA) scores on admission to ICU, daily SOFA score, type and amount of nutrition, daily C-reactive protein (CRP), white cell count (WBC) and serum albumin concentration, and ICU, hospital and 90-day outcome. In CRRT patients, we calculated the total dose of RRT delivered per 24-hour period. The total daily amount of nutrients lost in the effluent was calculated by multiplying the nutrient concentration in the effluent with the total dose of RRT delivered in 24 hours.
Laboratory analyses
Se, Cu and Zn were measured by inductively-coupled mass spectrometry (ICP-MS) using the Thermo XSeries II instrument (Thermo Fisher Scientific, Hemel Hempstead, UK). Iron was measured using the colorimetric ferrozine method on the ADVIA 2400 analyser (Siemens Diagnostics, Frimley, UK).
Concentrations of vitamin B1 and B6 were determined by high-performance liquid chromatography (HPLC) with fluorescence detection (Chromsystems, Gafelfing, Germany), whilst vitamin C was measured by HPLC with ultra-violet (UV) detection. Folate and vitamin B12 were measured by immunoassay (Abbott Architect), and vitamin D3 concentrations were determined by liquid chromatography–mass spectrometry (LC-MS/MS) consisting of an Agilent 1260 LC system and a 6460 MS. Amino acids were measured underivatised by stable isotope dilution LC-MS/MS using an Agilent 1260/CTC-PAL LC system, with a Supelco Astec Chirobiotic T column (Sigma-aldrich, Poole, UK), and an ABSciex API6500 Qtrap MS/MS (ABSciex, Warrington, UK). The reference values of all nutrients are shown in Supplementary Table 1.
Statistics
Sample size calculations and statistical analysis
It was estimated that a pilot sample of 40 patients (20 CRRT and 20 non-CRRT patients) would provide sufficient precision with 95% confidence interval (CI) for the change in plasma nutrient concentrations between pre-CRRT and 24–144 hours later being estimated to approximately ±0.4 standard deviations (SDs) of the change. When possible, we presented the estimated differences between the two groups with 95% CI. If estimated differences could not be calculated due to small numbers, a p-value was given.
The primary outcome was the difference in plasma concentrations of essential nutrients between the CRRT and non-CRRT cohort at 24 hours after enrolment. For continuous data, differences in the baseline characteristics of the CRRT and non-CRRT group were tested using t-test, or Mann-Whitney U tests where data were non-normal and could not be transformed to normal. Categorical data were compared using Chi-square tests (χ²) or Fisher’s exact test. SPSS (version 21) was used for the data analysis.
Limited comparison of nutrients against reference ranges were made across a six-day period for all patient groups. Continuous measures were reported with mean, SD and difference and 95% CI for the difference between groups using the t method. When test assumptions were not justified, either Mann-Whitney U or Wilcoxon test were applied to provide an indicative p-value with median and interquartile range (IQR). Categorical data were presented with total number (%) unless specified and compared with Chi-square (χ²) test, or when test assumptions were not justified, Fisher’s exact test to give indicative p values. Treatment groups were compared using Analysis of Variance (ANOVA), Fisher’s exact or Kruskall Wallis test where appropriate. If the difference between groups was statistically significant, post hoc analysis was performed using the Fisher’s exact pairwise comparison, Mann-Whitney U, or the Tukey honest significant difference test.
We compared patients according to treatment at enrolment (CRRT versus no CRRT) at 24 hours and six days after enrolment. In addition, we differentiated between 3 groups based on the treatment with CRRT during the six-day study period: i) patients who received CRRT every day; ii) patients who did not receive any CRRT during the six day follow-up period; and iii) a mixed group of patients who had CRRT on some but no all days.
Ethics approval and consent to participate
The study was approved by the national Research Ethics Committee (REC) (REC number 13/LO/0064) and the institutional Research & Development Department. It was conducted in accordance with the Declaration of Helsinki and consent for participation was obtained. If possible, patients were asked to provide written informed consent prior to enrolment. If patients did not have capacity to consent, the opinion of a personal consultee was sought in accordance with section 32 of the Mental Capacity Act 2005 (UK). A personal consultee was a person who was engaged in caring for the participant (not professionally or for payment) and was interested in his/her welfare, and was prepared to be consulted. The personal consultee was asked to give an opinion as to whether the patient would object to taking part in medical research. If the personal consultee decided that the patient would have no objection to participating in the trial, he/she was asked to sign the Personal Consultee Declaration Form. As soon as the patient regained capacity, they were fully informed about the study and invited to give consent to continue participation. If the patient refused consent, all samples collected for research purposes were discarded and the patient’s data were not included in the analysis. If the patient did not regain capacity and/or died before consent could be sought, the REC felt it appropriate that the patient was included and that the samples were analysed provided the personal consultee did not indicate that the patient would not want to continue in this research project.
Clinical trial registration
The study was registered with ISRCTN (ISRCTN88354940) and ClinicalTrials.gov (Identifier: NCT02470520).
References
McClave, S. A. et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). J Parenter Enteral Nutr 40, 159–211 (2016).
Druml, W. Nutritional management of acute renal failure. J. Ren. Nutr. 15(1), 63–70 (2005).
Druml, W. The renal failure patient. World Rev. Nutr. Diet. 105, 126–135 (2013).
Cano, N. et al. ESPEN Guidelines on Enteral Nutrition: Adult Renal Failure. Clin. Nutr. 25, 295–310 (2006).
Oh, W. C., Gardner, D. S. & Devonald, M. A. Micronutrient and amino acid losses in acute renal replacement therapy. Curr. Opin. Clin. Nutr. Metab. Care. 18(6), 593–598 (2015).
Bellomo, R. & Boyce, N. Acute continuous haemodiafiltration: a prospective study of 110 patients and a review of the literature. Am. J. Kidney Dis. 21, 501–518 (1993).
Story, D. A., Ronco, C. & Bellomo, R. Trace element and vitamin concentrations and losses in critically ill patients treated with continuous venovenous haemofiltration. Crit. Care Med. 27(1), 220–223 (1999).
Berger, M. M. et al. Copper, selenium, zinc and thiamine balances during continuous venovenous hemodiafiltration in critically ill patients. Am. J. Clin. Nutr. 80, 410–416 (2004).
Nakamura, A. T., Btaiche, I. F., Pasko, D. A., Jain, J. C. & Mueller, B. A. In vitro clearance of trace elements via continuous renal replacement therapy. J. Ren. Nutr. 14, 214–219 (2004).
Davenport, A. & Roberts, N. B. Amino acid losses during continuous high-flux haemofiltration in the critically ill patient. Crit. Care Med. 17, 1010–1013 (1989).
Davies, S. P., Reaveley, D. A., Brown, E. A. & Kox, W. J. Amino acid clearances and daily losses in patients with acute renal failure treated by continuous arteriovenous hemodialysis. Crit. Care Med. 19, 1510–1515 (1991).
Mokrzycki, M. H. & Kaplan, A. A. Protein losses in continuous renal replacement therapies. J. Am. Soc. Nephrol. 7, 2259–2263 (1996).
Kamel, A. Y. et al. Micronutrient alterations during continuous renal replacement therapy in critically ill adults: a retrospective study. Nutr. Clin. Pract. 33(3), 439–446 (2018).
Scheinkestel, C. D. et al. Impact of increasing parenteral protein loads on amino acid levels and balance in critically ill anuric patients on continuous renal replacement therapy. Nutrition. 19, 733–740 (2003).
Berg, A. et al. Glutamine kinetics during intravenous glutamine supplementation in ICU patients on continuous renal replacement therapy. Intensive Care Med. 33, 660–666 (2007).
Morena, M. et al. Convective and diffusive losses of vitamin C during haemo-diafiltration session: a contributive factor to oxidative stress in haemodialysis patients. Nephrol. Dial. Transplant. 17, 422–427 (2002).
Fortin, M. C., Amyot, S. L., Geadah, D. & Leblanc, M. Serum concentrations and clearances of folic acid and pyridoxal-5-phosphate during venovenous continuous renal replacement therapy. Intensive Care Med. 25, 594–598 (1999).
Ostermann, M. & Macedo, E. Oudemans-van Straaten, H.M. How to feed a patient with acute kidney injury. Intensive Care Med. 45, 1006–1008 (2019).
Druml, W. et al. Metabolic management and nutrition in critically ill patients with renal dysfunction: Recommendations from the renal section of the DGIIN, ÖGIAIN, and DIVI. Med. Klin. Intensivmed. Notfmed. 113(5), 393–400 (2018).
Baydock, T., Bector, S., Taylor, L. M. & Hansen, G. Survey of Nutrition Practice in Patients with Severe Sepsis among Canadian Registered Dieticians. Can. J. Diet. Pract. Res. 80(1), 8–13 (2019).
Kidney Disease Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. 2, 1–138 (2012).
Thurnham, D. I. & Northrop-Clewes, C. A. Inflammation and biomarkers of micronutrient status. Curr. Opin. Clin. Nutr. Metab. Care. 19(6), 458–463 (2016).
Druml, W., Schwarzenhofer, M., Apsner, R. & Hörl, W. H. Fat-soluble vitamins in patients with acute renal failure. Min. Electrolyte Metab. 24(4), 220–226 (1998).
Cameron, L. K. et al. Vitamin D levels in critically ill patients with Acute Kidney Injury: a protocol for a prospective cohort study (VID-AKI). BMJ Open. 7(7), e016486 (2017).
Wu, G. Amino acids: metabolism, functions, and nutrition. Amino Acids. 37, 1–17 (2009).
Druml, W., Bürger, U., Kleinberger, G., Lenz, K. & Laggner, A. Elimination of amino acids in acute renal failure. Nephron. 42(1), 62–67 (1986).
Oudemans-van Straaten, H. M., Bosman, R. J., Treskes, M., van der Spoel, H. J. & Zandstra, D. F. Plasma glutamine depletion and patient outcome in acute ICU admissions. Intensive Care Med. 27(1), 84–90 (2001).
Zhou, Z. et al. Related factor of serum carnitine deficiency and influence of its deficiency on the length of hospital stay in critical ill patients. Zhonghua Wei Zhong Bing. Ji Jiu Yi Xue. 26, 890–894 (2014).
Sgambat, K. & Moudgil, A. Carnitine deficiency in children receiving continuous renal replacement therapy. Hemodial. Int. 20(1), 63–67 (2016).
Berthon, B. S. & Wood, L. G. Nutrition and Respiratory Health – Feature Review. Nutrients. 7(3), 1618–1643 (2015).
Carr, A. C. Vitamin C administration in the critically ill: a summary of recent meta-analyses. Crit. Care. 23(1), 265 (2019).
Marik, P. E. Vitamin C for the treatment of sepsis: The scientific rationale. Pharmacol. Ther. 189, 63–70 (2018).
Fowler, A. A. III. et al. Effect of vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure: The CITRIS-ALI Randomized Clinical Trial. JAMA. 322(13), 1261–1270 (2019).
Marik, P. E. Hydrocortisone, Ascorbic Acid and Thiamine (HAT Therapy) for the treatment of sepsis. Focus on Ascorbic Acid. Nutrients. 10(11), E1762 (2018).
Moskowitz, A. et al. Ascorbic acid, corticosteroids, and thiamine in sepsis: a review of the biologic rationale and the present state of clinical evaluation. Crit. Care. 22(1), 283 (2018).
Mehr, A. P. et al. De novo NAD+ biosynthetic impairment in acute kidney injury in humans. Nat. Med. 24(9), 1351–1359 (2018).
Acknowledgements
We would like to thank the patients for participating in this study. We wish to thank the research nurses who helped collecting samples and Dr Kishor Raja for reviewing the manuscript and suggesting changes for improvement. We are grateful to the European Society of Intensive Care Medicine for awarding us a research grant for this study. JP is supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. JP is an NIHR Senior Investigator. The study was partially funded through a research grant from the European Society of Intensive Care Medicine.
Author information
Authors and Affiliations
Contributions
M.O. led the research project. K.L. recruited patients and collected the samples. D.C. and D.H. performed the laboratory measurements of vitamins. N.D. and C.T. measured amino acid concentrations and RS measured trace element concentrations. D.B. collected all clinical and laboratory data. J.S. and J.P. performed the statistical analyses. All authors contributed to the interpretation of the data, the drafting of the manuscript and gave their final approval of the paper.
Corresponding author
Ethics declarations
Competing interests
D.B. has received speaker honoraria from Fresenius Kabi. All other authors declare that they have no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ostermann, M., Summers, J., Lei, K. et al. Micronutrients in critically ill patients with severe acute kidney injury – a prospective study. Sci Rep 10, 1505 (2020). https://doi.org/10.1038/s41598-020-58115-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-58115-2
This article is cited by
-
Vitamin D metabolism in critically ill patients with acute kidney injury: a prospective observational study
Critical Care (2024)
-
Advances in pediatric acute kidney injury pharmacology and nutrition: a report from the 26th Acute Disease Quality Initiative (ADQI) consensus conference
Pediatric Nephrology (2024)
-
Serum metabolomic signatures of fatty acid oxidation defects differentiate host-response subphenotypes of acute respiratory distress syndrome
Respiratory Research (2023)
-
Nutritional management of children with acute kidney injury—clinical practice recommendations from the Pediatric Renal Nutrition Taskforce
Pediatric Nephrology (2023)
-
Evaluation of thiamine as adjunctive therapy in COVID-19 critically ill patients: a two-center propensity score matched study
Critical Care (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.