Abstract
Endometriosis is estrogen-dependent disorder. Two theories provide the explanations for the increased estrogen production. One is the feed-forward loop model linking inflammation and estrogen production. The more recent model evokes the tissue hypoxia resulting from endometrial debris detached and then regurgitated to the peritoneal cavity. Both models tacitly assume that everything occurs within the endometriotic stromal cells, seemingly without the need for exogenous factors. This study was undertaken to investigate as whether platelets may be responsible for local estrogen overproduction. We employed in vitro experimentation that evaluated the 17β-estradiol (E2) levels in endometriotic stromal cells treated with activated platelets, and the genes and protein expression levels of StAR, HSD3B2, aromatase, and HSD17B1, as well as their upstream genes/proteins such as NF-κB, TGF-β1, HIF-1α, SF-1 and phosphorylated CREB. In addition, we conducted 2 animal experimentations using platelet depletion/infusion and also neutralization of NF-κB and TGF-β1, followed by immunohistochemistry analysis of involved in StAR, HSD3B2, aromatase, and HSD17B1, as well as SF-1 and p-CREB. We found that treatment of endometriotic stromal cells by activated platelets increase the E2 production by 4.5 fold, and concomitant with increased gene and protein expression of StAR, HSD3B2, aromatase, and HSD17B1, the four genes/enzymes important to estrogen synthesis, along with their upstream genes HIF-1α, SF-1 and phosphorylated CREB. Moreover, platelets activate these genes through the activation of NF-κB and/or TGF-β1, and antagonism of either signaling pathway can abolish the induction of the 4 genes and thus increased estrogen production. The two animal experimentations confirmed these changes. Thus, platelets increase the E2 production in endometriotic stromal cells through upregulation of StAR, HSD3B2, aromatase, and HSD17B1 via the activation of NF-κB and/or TGF-β1. These findings provide a yet another compelling piece of evidence that endometriotic lesions are indeed wounds undergoing repeated tissue injury and repair. They strongly indicate that non-hormonal therapeutics for endometriosis is theoretically viable, with anti-platelet therapy being one promising avenue.
Similar content being viewed by others
Introduction
Endometriosis, defined as the deposition and growth of endometrium-like tissues outside the uterine cavity, is a common disorder affecting about 6–10% of women of reproductive age1. As a major contributor to pelvic pain and infertility2 impacting negatively on women’s quality of life3, a source of anxiety and depression4 and a leading cause of gynecological hospitalization in the United States5 and likely in many other parts of the world, endometriosis is frequently viewed as an enigmatic disease due to its elusive pathogenesis and pathophysiology6,7. Consequently, its effective treatment remains a challenge2,8.
Endometriosis has been viewed as a ultimate hormonal disease9, featuring estrogen-dependent growth and maintenance of ectopic endometrium as well as increased local production of estrogens due to molecular aberrations in steroidogenesis10. It is also commonly viewed as a pelvic inflammatory condition, featuring increased production of proinflammatory cytokines and chemokines11. So far perhaps the most comprehensive model that encompasses various mechanisms underlying both elevated 17β-estradiol (E2) production and increased of proinflammatory cytokines and chemokines is the feed-forward model proposed by Bulun et al.10, in which proinflammatory cytokines activate cyclooxygenase-2 (COX-2), resulting in increased production of prostaglandin E2 (PGE2), which, in turn, stimulates some key genes involved in the production of E2, such as steroidogenic acute regulatory protein (StAR), aromatase and 17β-hydroxysteroid dehydrogenase type-1 (HSD17B1), resulting in elevated production of E2, the most potent estrogen. The increased E2 further induces estrogen receptor β (ERβ), yielding further induction of COX-2. This positive feedback process, once started, supposedly perpetuates if untamed, resulting in increased inflammation due to elevated PGE2 levels and increased growth because of potent mitogenic effect of E210.
PGE2 activates the protein kinase A (PKA) signaling pathway via raising the intracellular levels of cyclic adenosine 3′,5′-monophosphate (cAMP)12,13,14, which can enhance the binding of steroidogenic factor-1 (SF-1) to promoters of these steroidogenic genes15, and induce phosphorylation of the transcriptional activator cAMP-response element-binding protein (CREB)16. The binding of SF-1 and CREB to the promoters of steroidogenic genes is responsible for inducing the expression levels and activity of these enzymes, thus promoting the estrogen biosynthesis in endometriotic stromal cells10,17,18.
One salient feature of this positive-feedback loop between inflammation and estrogen production in endometriosis is that everything occurs within the endometriotic lesions, or, more precisely, endometriotic stromal cells, seemingly without the need for extraneous factors. However, just as “it takes a village to raise a child”, an endometriotic lesion does not come into being completely on its own. Rather, its development and the subsequent manifestation of various symptomology require, by necessity, aid and assistance from many aiders and abettors. Indeed, accumulating evidence suggests that endometriotic lesions are essentially wounds undergoing repeated tissue injury and repair (ReTIAR)19,20,21,22, and, as such, many non-endometriotic cells in the lesional microenvironment, such as platelets21,23, macrophages19,24, and now nerve fibers25,26, are actively involved in facilitating lesional development.
In wound healing, estrogen has been well documented to be actively involved27,28. Numerous studies have shown that estrogen is important to wound healing, and its deficiency delays or impairs wound healing29,30,31,32,33. In fact, estrogen is found to be involved in all phases of wound healing27. One gene expression profiling study of wound tissues from young and elder men found that among genes that were differentially expressed, 78% of them being estrogen-regulated and only 3% being age-related34, suggesting that estrogen is more important than intrinsic aging in wound healing. Remarkably, in striking similarity to endometriotic lesions in which ERβ is shown to be overexpressed35,36, ERβ has been shown to play a critical role in wound healing37,38.
These findings in wound healing raise a question as whether culprits other than endometriotic cells in the lesional microenvironment could also be responsible for the increased local estrogen production. Recent studies have shown that hypoxia, occurred to menstrual debris that is devoid of blood supply when regurgitated into the peritoneal cavity, can dramatically turn endometrial stromal cells into endometriotic counterpart, causing completely different phenotypes39. This notion may explain as why endometrial debris may invade and then establish itself in ectopic sites. Since platelets are the first responder to injury, we wondered as whether activated platelets can induce a hypoxic state and indeed, we recent found that platelets can induce hypoxia-inducible factor 1α (HIF-1α) expression, effectively inducing a hypoxic sate in both endometriotic and endometrial stromal cells40. Thus, we hypothesized that activated platelets may activate genes involved in estrogen biosynthesis, resulting in increased local estrogen levels. This study was undertaken to test this hypothesis.
Results
Activated platelets induce increased E2 production in endometriotic stromal cells
To see whether activated platelets induce E2 production in endometriotic stromal cells, we first measured E2 concentration in the supernatant in human endometriotic stromal cells (HESCs) co-cultured for 48 hours with PBS, platelets alone, activated platelets, or thrombin alone. We found that, compared with those treated with PBS, the E2 concentration was significantly elevated in HESCs co-cultured with either platelets or activated platelets (both p-values = 0.018), but with thrombin alone (p = 0.87, Fig. 1A, left panel). Notably, HESCs co-cultured with platelets and activated platelets produced E2 that was 3.0 and 4.5 fold, respectively, higher than that treated with PBS (79.15 pg/mL and 119.16 pg/mL, respectively, vs. 26.32 pg/mL). In contrast, the E2 concentrations were all very low in the normal endometrial stromal cell line (ESCL), irrespective co-culture with or without activated platelets (Fig. 1A, right panel). To rule out the possibility that the E2 was synthesized by activated platelets, we also evaluated, in the absence of endometriotic or endometrial cells, the E2 concentration in the medium that was cultured with or without activated platelets for 48 hours and only found barely detectable E2 in all groups (data not shown), suggesting that the increased E2 came indeed from endometrial/endometriotic stromal cells only. The difference in E2 production between HESCs and ESCLs is consistent with what have been documented previously41,42,43,44,45.
Treatment of HESCs with activated platelets resulted in increased production of E2 through upregulation of steroidogenic genes. (A) Concentrations of E2 in the supernatant of HESCs (n = 7) and ESCL (n = 5) treated with PBS, platelets, activated platelets, and thrombin alone. Gene (B) and protein (D) expression levels of the genes involved in estrogen biosynthesis: StAR, CYP11A1, HSD3B2, CYP17A1, CYP19A1 and HSD17B1 in HESCs co-cultured with PBS, platelets, activated platelets and thrombin alone. (C) Representative Western blotting results for StAR, HSD3B2, aromatase and HSD17B1. Fold change for SF-1 (E) and p-CREB (F) protein expression in HESCs co-cultured with PBS, activated platelets and thrombin alone. “*” denotes that the p-value of the difference between the designated treatment and the PBS treatment is less than 0.05. Abbreviations used: PLT: platelets; A.PLT: activated platelets; THMB: thrombin. Paired Wilcoxon’s test was used.
Activated platelets upregulate key genes involved in estrogen biosynthesis in endometriotic stromal cells
Given increased E2 production in endometriotic stromal cells stimulated by activated platelets, we next evaluated the gene and protein expression levels in key genes critically involved in estrogen biosynthesis. Consistently, the mRNA levels of StAR, HSD3B2, aromatase and HSD17B1, but not CYP11A1 or CYP17A1, in HESC cells co-cultured with activated platelets, but not thrombin alone (all p’s > 0.20), were all significantly higher than those treated with vehicle (all p’s ≤ 0.028, Fig. 1B). The protein levels of StAR, HSD3B2, aromatase and HSD17B1 in HESCs co-cultured with platelets or activated platelets, but not thrombin alone, were also significantly higher than that that were co-cultured with vehicle (all p’s ≤ 0.017, Fig. 1C,D). Interestingly, the activated platelets increased the protein expression levels of StAR, HSD3B2, aromatase, and HSD17B1 by an average of 1.6, 1.3, 1.5, and 1.3 fold, respectively (Fig. 1C,D), and their product, i.e., 1.6 × 1.3 × 1.5 × 1.3 = 4.1, is uncannily close to 4.5 fold increase in E2 production as shown above.
To see whether platelets also affected the genes upstream of StAR, HSD3B2, aromatase and HSD17B1, we also evaluated the protein expression levels of SF-1 and p-CREB in HESCs in different co-culture conditions. We found that activated platelets, but not thrombin alone, resulted in significantly higher SF-1 and p-CREB protein expression levels as compared with controls (all p-values = 0.012, Fig. 1E,F), suggesting that activated platelets acted on the SF-1/CREB level, at least, and possibly higher level, in the E2 production.
Suppression of NF-κB activation abolishes platelet-induced upregulation of HIF-1α, COX-2, and VEGF
Given the documented role of prostaglandin E2 (PGE2) in estrogen biosynthesis in endometriosis16,45,46,47 and also of hypoxia in turning normal endometrial stromal cells into endometriotic stromal cell-like phenotype48,49, we wondered as whether activated platelets could activate NF-κB, the upstream of COX-2, which, in turn, is the gene encoding a rate-limiting enzyme in PGE2 production, and also HIF-1α, a key transcription factor that responds to hypoxia50, which is reported to be elevated51 and can upregulate COX-2 in endometriosis49. We found that, indeed, HESCs co-cultured with both platelets and activated platelets increased the protein expression levels of p-p65 (both p < 0.012, Fig. 2A,B). In addition, activated platelets induced significantly higher expression of HIF-1α, COX-2 and VEGF (which is downstream of HIF-1α) at both transcriptional and protein expression levels in endometriotic stromal cells (all p-values ≤ 0.012, Fig. 2C–E), consistent with our previous report40. Neutralization of NF-κB by JSH-23 nearly completely abolished this upregulation (all p’s ≤ 0.036, Fig. 2C–E). With the only exception of COX-2 mRNA abundance, which was still significantly higher than that of the HESCs treated with vehicle (p = 0.025, Fig. 2C), the gene and protein expression levels of these genes in HESCs co-cultured with platelets but pre-treated with JSH-23 were no different from controls (all p’s > 0.05, Fig. 2C,E).
Activated platelets activate NF-κB, and suppression of NF-κB activation abolishes platelet-induced upregulation of HIF-1α, COX-2, and VEGF. (A) Representative Western blotting results of phorphorylated-p65 in HESCs (n = 8) co-cultured with PBS (buffer), platelets, activated platelets, and thrombin. (B) Summary of Western blot results. (C) Fold change for HIF-1α, COX-2 and VEGF mRNA abundance in HESCs (n = 8) co-cultured with vehicle, activated platelets and activated platelets + JSH-23. (D) Representative Western blotting results and fold change for HIF-1α, COX-2, and VEGF in HESCs co-cultured with vehicle, activated platelets and activated platelets + JSH-23. (E) Summary of western blot results. “*” denotes that the p-value of the difference between the groups is less than 0.05. N or NS: p > 0.05. Abbreviation used: A.PLT: activated platelets. Paired Wilcoxon’s test was used.
NF-κB neutralization abolishes platelets-induced increase in E2 production through downregulation of StAR, HSD3B2, aromatase, HSD17B1, SF-1 and p-CREB in HESCs
Given the above finding that NF-κB neutralization abrogated platelets-induced upregulation of HIF-1α, COX-2, and VEGF, we evaluated the E2 levels in the culture medium of HESCs co-cultured with activated platelets with and without pre-treatment with JSH-23. We found that pre-treatment of HESCs with JSH-23 significantly reduced the E2 production in culture media as compared with that without the pre-treatment (p = 0.036) without reducing the cell viability (Supplementary Fig. S1), and, in fact, completely abolished the E2 production induced by platelets (p = 0.13, Fig. 3A). Consistently, NF-κB neutralization by pre-treatment with JSH-23 completely abolished platelets-induced expression of StAR, HSD3B2, aromatase and HSD17B1 at both transcriptional and protein levels (all p-values ≤ 0.025, Fig. 3B–D). In addition, NF-κB neutralization by pre-treatment with JSH-23 also abolished platelets-induced expression of SF-1 and p-CREB in HESCs, both at the protein levels (both p’s = 0.012, Fig. 3E,F). With the only exception of p-CREB protein levels, the gene and protein expression levels of these genes in HESCs co-cultured with platelets but pre-treated with JSH-23 were no different from controls (all p’s > 0.05, Fig. 3B,D–F).
Effect of suppression of NF-κB activation by JSH-23 on the production of E2 and expression of steroidogenic genes in HESCs. (A) Concentrations of E2 in the supernatant of HESCs (n = 8) treated with vehicle, activated platelets and activated platelets + JSH-23. (B) Gene expression levels of the genes involved in estrogen biosynthesis StAR, HSD3B2, CYP19A1 and HSD17B1 in HESCs co-cultured with vehicle, activated platelets and activated platelets + JSH-23. (C) Representative Western blotting results for StAR, HSD3B2, aromatase and HSD17B1 protein expression. (D) Summary of fold changes of protein expression levels of StAR, HSD3B2, aromatase and HSD17B1 in HESCs (n = 8) treated with vehicle, activated platelets and activated platelets + JSH-23. Summary of fold changes for SF-1 (E) and p-CREB (F) protein expression in HESCs (n = 8) treated with vehicle, activated platelets and activated platelets + JSH-23. “*” denotes that the p-value of the difference between the groups is less than 0.05. NS: p > 0.05. Paired Wilcoxon’s test was used.
TGF-β1 neutralization abolishes platelets-induced upregulation of HIF-1α, COX-2, and VEGF
We have previously shown that platelet-derived TGF-β1 activates TGF-β1/Smad3 signaling pathway in endometriotic epithelial and stromal cells21. We also have shown that activated platelets can induce upregulation of HIF-1α in HESCs40. We thus wondered as whether activated platelets could also induce upregulation of COX-2 and VEGF and whether neutralization of TGF-β1 could abrogate such an induction. We found that, at both transcriptional and protein expression levels, the expressions of HIF-1α, COX-2 and VEGF were significantly elevated after co-culture with activated platelets (all p’s ≤ 0.012, Fig. 4A–C), but the upregulation was completely abolished by TGF-β1 neutralization with pre-treatment with A83-01 (all p’s ≤ 0.012, Fig. 4A–C). Thus, TGF-β1 neutralization abolished platelets-induced upregulation of HIF-1α, COX-2, and VEGF in HESCs.
Effect of neutralization of TGF-β1 by A83-01 on platelet-induced gene and protein expression of HIF-1α, COX-2 and VEGF. (A) Fold change of HIF-1α, COX-2 and VEGF mRNA abundance in HESCs (n = 8) treated with vehicle, activated platelets and activated platelets + A83-01. (B) Representative Western blotting results for HIF-1α, COX-2 and VEGF protein expression in HESCs (n = 8) co-cultured with vehicle, activated platelets and activated platelets + A83-01, and (C) Summary of fold changes. “*” denotes that the p-value of the difference between the groups is less than 0.05. NS: p > 0.05. Paired Wilcoxon’s test was used.
TGF-β1 neutralization abolishes platelets-induced increase in E2 production through downregulation of StAR, HSD3B2, aromatase, HSD17B1, SF-1 and p-CREB in HESCs
Next, we evaluated the E2 levels in HESCs co-cultured with activated platelets with and without TGF-β1 neutralization through pre-treatment with A83-01 or vehicle. We found that pre-treatment of HESCs with A83-01 significantly reduced the E2 production in culture media as compared with that without the pre-treatment (p = 0.017) without reducing the cell viability (Supplementary Fig. S1), but the resultant E2 levels were still significantly higher than those pre-treated with vehicle (p = 0.012, Fig. 5A). Consistently, TGF-β1 neutralization by pre-treatment with A83-01 completely abolished platelets-induced expression of StAR, HSD3B2, aromatase and HSD17B1 at both transcriptional and protein levels (all p’s ≤ 0.025), with the only exception of the StAR protein levels which were still significantly higher than the control (p = 0.012, Fig. 5B–D). In addition, TGF-β1 neutralization by pre-treatment with A83-01 also completely abolished platelets-induced expression of SF-1 and p-CREB in HESCs, both at the protein levels (both p’s ≤ 0.05, Fig. 5E,F).
Effects of neutralization of TGF-β1 by A83-01 on the production of E2 and expression of genes involved in steroidogenesis in HESCs. (A) Concentrations of E2 in the supernatant of HESCs (n = 8) treated with vehicle, activated platelets and activated platelets + A83-01. (B) Gene expression levels of StAR, HSD3B2, CYP19A1 and HSD17B1 in HESCs (n = 8) treated with vehicle, activated platelets and activated platelets + A83-01. (C) Representative Western blotting results for StAR, HSD3B2, aromatase and HSD17B1 in HESCs under different treatments. (D) Summary of fold changes of protein expression levels of StAR, HSD3B2, aromatase and HSD17B1 in HESCs (n = 8) treated with vehicle, activated platelets and activated platelets + A83-01. Western blot analysis results for SF-1 (E) and p-CREB (F) proteins in HESCs treated with vehicle, activated platelets and activated platelets + A83-01. “*” denotes that the p-value of the difference between the groups is less than 0.05. NS: p > 0.05. Paired Wilcoxon’s test was used.
Platelet infusion increases, while platelet depletion reduces, the expression of steroidogenic proteins in endometriotic lesions in mice with induced endometriosis
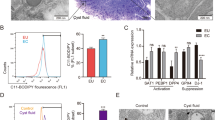
To further confirm the role of platelets in regulating genes involved in estrogen biosynthesis in endometriotic lesions, we carried out a mouse experiment using platelet depletion (PD) and platelet infusion (PI). We found that, while there was no difference in hotplate latency among the 3 groups of mice 2 days prior to the induction of endometriosis (p = 0.98, Fig. 6A), but 12 days after the induction the difference was highly significant (p = 0.0001, Fig. 6A). In particular, PI mice had significantly shorter (p = 0.018), while PD mice had significantly longer (p = 0.0055), latency than the control mice injected with a matched non-immune (NI) antibody. In addition, compared with the NI mice that received a mock antibody, the total lesion weight in PI mice increased by an average of 52.4% (p = 0.025, Fig. 6B), while that of PD mice was decreased by 40.2% (p = 0.017, Fig. 6B).
Effect of non-immune serum (NI), platelet infusion (PI) and platelet depletion (PD) on lesional development and lesional expression of steroidogenic proteins in mice with induced endometriosis. (A) Change in hotplate latency for mice in the NI, PD and PI groups tested at the time indicated. (B) Total lesion weight for mice in the 3 treatment groups. Boxplots of lesional staining levels in NI, PD and PI groups: StAR (C), HSD3B2 (D), aromatase (E), HSD17B1 (F), SF-1 (G), and p-CREB (H). The dotted horizontal line represents the median of all 3 groups. Symbols of statistical significance: *: p < 0.05, **:p < 0.01, ***: p < 0.001. n = 10 for each group. Except in (A), where the comparison was made using Kruskal’s test, all tests were made using Wilcoxon’s test in comparison with the NI group. (I) Representative photomicrographs of StAR, HSD3B2, aromatase, HSD17B1, SF-1 and p-CREB staining in endometriotic lesions (×400) in Balb/c mice with induced endometriosis in different treatment groups. Scale bar = 50 μm.
We next evaluated immunoreactivity against StAR, HSD3B2, aromatase, HSD17B1, SF-1 and p-CREB in the stromal component of endometriotic lesions (Fig. 6I) since that component has been traditionally regarded as the major site for increased local estrogen production in endometriosis17,47,52. We found that StAR and aromatase staining were primarily localized in the cell cytoplasm of stromal cells, but not epithelial cells. HSD3B2 and HSD17B1 stain were seen both in epithelial cells and stromal cells and localized in the cell cytoplasm. SF-1 and p-CREB stain were seen in both epithelial and stromal cells and localized in the cell nucleus. The changes of StAR, HSD3B2, aromatase, HSD17B1, SF-1 and p-CREB in NI group were obviously more as compared with PD group, but obviously less than in PI group.
Consistent with increased lesion weight and also with our in vitro findings, we found, by multiple linear regression analysis, significantly higher immunostaining levels of StAR, HSD3B2, aromatase, HSD17B1, SF-1 and p-CREB in the PI group but significantly lower staining levels of all these proteins in the PD group as compared with controls (all p’s < 0.032, R2 ≧ 0.38, Fig. 6C–H). The staining levels of all 6 markers were highly positively correlated (all r’s ≧ 0.52, all p’s < 0.0032). The lesion weight correlated positively with staining levels of all 6 markers involved in estrogen production (all r’s ≧ 0.50, all p’s < 0.0051).
Antagonism of either TGF-β1 or NF-κB reduces the expression of steroidogenic proteins in endometriotic lesions in mice with induced endometriosis
We also carried out a mouse experimentation to see whether antagonism of either TGF-β1 or NF-κB could reduce the genes/protein expression in endometriotic lesions in mice with induced endometriosis. Similar to the platelet infusion/depletion experiment as presented above, we found that, while there was no difference in hotplate latency among the 3 groups of mice 2 days prior to the induction of endometriosis, the difference was highly significant 12 days after the induction (p = 0.67 and p = 0.0046, respectively, Fig. 7A). In particular, mice treated with either SB431542 (a TGF-β1 inhibitor) or JSH-23 (an NF-κB inhibitor) had significantly longer (p = 0.008 and p = 0.004, respectively) latency than that treated with vehicle. In addition, the total lesion weight in mice treated with inhibitors of either TGF-β1 or NF-κB was reduced by an average of 65.7% and 34.3%, respectively (p = 0.014 and 0.004, respectively, Fig. 7B) as compared with that in those treated with vehicle.
Effect of antagonism of TGF-β1 (by SB431542) or of NF-κB (by JSH-23) on lesional development and lesional expression of steroidogenic proteins in mice with induced endometriosis. (A) Change in hotplate latency for mice treatment with vehicle (untreated, or U), antagonism of TGF-β1 (T), and antagonism of NF-κB (N) tested at the time indicated. (B) Total lesion weight in the 3 groups of mice. (C) Boxplots of the extent of lesional platelet aggregation using CD41 the 3 groups of mice. Boxplots of lesional staining levels in the 3 groups of mice: StAR (D), HSD3B2 (E), aromatase (F), HSD17B1 (G), SF-1 (H), and p-CREB (I). The dotted horizontal line represents the median of all 3 groups. Symbols of statistical significance: *: p < 0.05, **: p < 0.01, ***: p < 0.001. NS: p > 0.05. n = 10 for each group. Except in (A), where the comparison was made using Kruskal’s test, all tests were made using Wilcoxon’s test in comparison with the untreated group (U). (J) Representative immunoreactivity staining of CD41, StAR, HSD3B2, aromatase, HSD17B1, SF-1 and p-CREB in the endometriotic lesions (×400) in Balb/c mice with induced endometriosis which then treatment with vehicle, SB431542 (an antagonist for TGF-β1) and JSH-23 (an antagonist for NF-κB) for two weeks. Scale bar = 50 μm.
We also evaluated immunoreactivity against StAR, HSD3B2, aromatase, HSD17B1, SF-1 and p-CREB in the stromal component of endometriotic lesions, as well as the extent of platelet aggregation using CD41 as a marker (Fig. 7J), as platelets aggregated mostly in lesional stromal component23. We can see that, compared with mice treated with vehicle, mice treated with either SB431542 or JSH-23 had significantly reduced platelet aggregation, and immunostaining levels of StAR, HSD3B2, aromatase, HSD17B1, SF-1 and p-CREB in the stromal component of endometriotic lesions (all p’s < 0.007, Fig. 7C–I).
As expected, the extent of platelet aggregation correlated positively with staining levels of all 6 markers involved in estrogen production (all r’s ≧ 0.53, all p’s < 0.0027), confirming the roles of platelets in estrogen biosynthesis in endometriotic lesions. In addition, the lesion weight correlated positively with staining levels of all markers involved in estrogen production (all r’s ≧ 0.45, all p’s < 0.012) except SF-1 (r = 0.28, p = 0.14), which is upstream of aromatase.
Discussion
In this study, we have shown through in vitro and in vivo studies that activated platelets increase the E2 production in endometriotic stromal cells through upregulation of StAR, HSD3B2, aromatase, and HSD17B1. In addition, platelets activate these genes through the activation of NF-κB and/or TGF-β1, and antagonism of either signaling pathway can abolish the induction of the 4 genes and thus the estrogen production. Platelets also induce HIF-1α, SF-1 and p-CREB, suggesting that the platelet-induced estrogen overproduction can be achieved in multiple pathways. Remarkably, the product of the fold increase of the 4 proteins after platelet stimulation is nearly equal to the fold increase in E2 production in endometriotic stromal cells (4.1 vs. 4.5), suggesting that, first, the activated platelets are indeed responsible for the increased E2 production through activation of these 4 genes. Second, it suggests that other genes, such as CYP11A1 and CYP17A1 (Fig. 1), that are involved in the synthesis of E2 may also be involved but we were not able to detect them simply because of either the fold increase is small or there is lack of adequate statistical power due to small sample sizes used in our study.
While endometriosis has been traditionally viewed as an estrogen-dependent disease as well as an inflammatory condition, the notion that endometriotic lesions are wounds undergoing ReTIAR is only appreciated very recently21,22,53 despite the fact that endometriotic lesions exhibit dynamic changes in immune cell populations that are uncannily similar to that of a healing wound54. In particular, the roles of platelets in the development of endometriosis has been demonstrated only recently23, despite the disease is characterized conspicuously by cyclic bleeding55. Indeed, estrogen is very important to wound healing27,28 and so is ERβ37,38 in wound healing, another similarity that cannot be dismissed as just a pure coincidence. In essence, this study shows that, as wounds undergoing ReTIAR, platelets also participate in the increased local production of estrogens through various ways. We summarize the roles of platelets in estrogen biosynthesis in endometriotic lesions in Fig. 8.
A schematic diagram that depicts platelet-induced estrogen biosynthesis in endometriosis. Activated platelets induce the activation of the NF-κB signaling pathway and TGF-β1/Smad3 signaling pathway in endometriotic lesions21. The two signaling pathways regulate several genes coding for hypoxia and inflammatory molecules, such as HIF-1α, COX-2 and VEGF99,100,101, which are upregulated after platelets treatment in HESCs. Activated platelets induce hypoxia in endometriotic lesions as shown by elevated expression of HIF-1α and VEGF. Increased HIF-1α accumulation results in DUSP2 down-regulation, which results in prolonged activation of ERK and p38 MAPK and increased COX-2 expression in endometriotic stromal cells49, as well as increased ERβ expression66 which can also be induced by activated platelets. The COX-2 overexpression leads to increased production of PGE2 in endometriosis, further favor E2 synthesis in HESCs. High levels of local E2 (through ERβ) and PGE2 in turn may further amplify COX-2 expression83, which results in overexpression of steroidogenic genes, and continuous local production of E2 and PGE2 in endometriotic tissue. PGE2 increases intracellular cAMP levels via the receptors EP2 or EP4102. Elevated cAMP upregulates SF-1 and enhances its binding to the promoter of steroidogenic genes15, as well as PKA activation, resulting in the phosphorylation of CREB which facilitates its binding to a cAMP-response element (CRE) on the promoter region of the StAR, aromatase or some other steroidogenic genes45,103, and acts as an initiator to unfold the DNA-histone binding and then provides space for C/EBP binding to the promoter of these steroidogenic genes. PGE2-induced StAR promoter activity appears to be regulated by CREB and C/EBPβ in a cooperative manner in endometriotic stromal cells16. PGE2 negatively regulates AMPK via the PKA signaling pathway104, and CREB and related proteins are also the direct downstream targets for AMPK105. AMPK is crucial for the activation of CREB via phosphorylation and there is a negative regulatory role of AMPK in the expression of CREB106. CRTC2 is translocated into the nucleus, where it forms a transcription complex with CREB107. AMPK can directly phosphorylate CRTC family members. In the absence of AMPK activity, CRTC2 is dephosphorylated and translocated to the nucleus and associates with CREB, and then increases target gene expression108. Activated platelets also inhibit AMPK activation via downregulation the phosphorylation level of AMPK in HESCs (Qi et al., unpublished data), which can further activate the CREB binding to steroidogenic genes in HESCs. In addition, it reported that USF2 binds to the SF-1 promoter, and stimulates the expression of SF-1 and its target genes109, and recent research has shown that FXR competes with CREB in binding the promoter region of CYP19A1, which results in the downregulation of aromatase expression110. Progesterone induces the expression of epithelial HSD17B2 by activating stromal PR-B, which can mediate the formation of retinoid acid to bind to the promoter and thus activate HSD17B2. In endometriotic tissues, the decreased PR-B expression in stromal cells disrupts the paracrine action of progesterone. ERβ suppresses ERα and PR-B, leading to progesterone resistance and deficient inactivation of E2 in endometriotic lesions. Abbreviations used: AA: arachidonic acid, PGE2: prostaglandin E2, RA: retinoic acid, COX-2: cyclooxygenase-2, A: androstenedione, E1: estrone, E2: estradiol, P: progesterone, SF-1: steroidogenic factor-1, CREB: cAMP-response element-binding protein, StAR: steroidogenic acute regulatory, P450scc: cytochrome P450 side-chain cleavage, HSD3B2: 3-hydroxysteroid dehydrogenase type 2, P450c17: cytochrome P450 17α-hydroxylase/17, 20-lyase, HSD17B1: 17beta-hydroxysteroid dehydrogenase type 1, HSD17B 2: 17beta-hydroxysteroid dehydrogenase type 2. ER-α: Estrogen receptor-α; ER-β: Estrogen receptor-β. PR-B: progesterone receptor B, ERK: extracellular signal-regulated kinase, AMPK: AMP-Activated Protein Kinase, CRTC2: CREB-regulated transcription coactivator 2, C/EBPβ: CCAAT/enhancer binding protein β, USF2: Upstream stimulatory factor 2, FXR: Farnesoid X Receptor.
Our results are consistent with our previous report that platelets induce the TGF-β1/Smad3 signaling pathway in endometriotic cells21. The result of 4.5 fold increase in estrogen production in endometriotic stromal cells stimulated with activated platelets vs. those unstimulated is remarkably close to the reported fold increase found between testosterone-added endometriotic and endometrial stromal cells (4.1 for follicular phase and 4.5 for luteal phase)41. Previous studies have reported that the TGF-β1/Smad3 signaling pathway is involved in the transcriptional regulation of aromatase in several cell types56,57,58, and particularly in endometriotic stromal cells59. Therefore, our finding is also consistent with these reports. Our results are also consistent with the report that activated platelets activate NF-κB in cancer cells60. The resultant hypercoagulability in women with endometriosis61,62 may be responsible for increased risk of preeclampsia63,64 due to inflammasome activation in trophoblasts induced by maternal extracellular vesicles and platelets65.
Our results are consistent with the finding that activation of HIF-1α and thus hypoxia can lead to increased estrogen production through depressing DUSP2 expression and thus COX-2 overexpression in endometriotic stromal cells49, as well as increased ERβ expression66. What is different, however, is that instead of invoking the assumption of endometrial fragments regurgitated into the peritoneal cavity that are deprived of oxygen66, our study made a simpler and more natural assumption that platelets that are activated due to ReTIAR and platelet activating factors secreted by endometriotic stromal cells67.
While endometriosis has always been defined as “the presence of endometrial glands and stroma outside of the normal location”68, the lesion does not develop, exist or even take its root totally on its own. Granted, while almost all past research focus has fixated on endometriotic epithelial and stromal cells nearly exclusively, there are many other cells in its microenvironment. Platelets21,23, neutrophils69, macrophages19,24, and recently nerve fibers25,26 all have been identified to be accessories to aid and abet the lesional development. Indeed, these cells are known to be irreplaceable partners in a successful wound healing70. The absence or over-abundance of any of these cell types would derail the normal healing process, leading healing astray and causing pathological healing such as fibrosis70.
Our findings give more credence to the notion that endometriotic lesions are wounds undergoing ReTIAR. Indeed, peripheral tissues produce estrogens71, and estrogens play a vital role in wound healing27,28. While normal endometrial stromal cells only produce negligible levels of estrogens41 likely due to a silenced aromatase because of hypermethylation41,42, endometriotic stromal cells, in contrast, do produce a much higher level of estrogens, due to demethylated aromatase42, and overexpression of StAR, HSD3B2 and HSD17B112,43,45,47. Moreover, it has been reported that estrogen promotes wound healing mainly through ERβ, not ERα38. Concomitant with declining wound healing ability, epidermal ERβ expression declines with age in both sexes72. And this may explain as why ERβ, but not ERα, is overexpressed in endometriosis35,36. Incidentally or not, we have shown previously that ERβ expression can also be upregulated by activated platelets73.
Our finding that platelets play an important role in estrogen production in endometriotic stromal cells has several important clinical implications. Currently, all existing drugs for treating endometriosis are hormonal74, with the aim of reducing estrogen production through induction of state of pseudo-pregnancy, pseudo-menopause, or chronic anovulation, thus creating an acyclic, hypoestrogenic environment75. In essence, this therapeutic approach is based on the idea of cutting off the fuel supply to lesion growth.
Along this line, there is a hypothesis of treatment window, that pharmacologically reduces the estrogen level to a certain level so that the growth of ectopic endometrium is curtailed or contained but the level is not too low to cause any detrimental side-effects due to hypoestrogenism76. The recent approval of the GnRH antagonists for treating endometriosis is more or less based on this notion. However, this notion only works if the following two implicit assumptions hold true: 1) the inter-individual variation in the treatment window is small, and 2) the inter-individual variation in response to drug treatment also is small. If either assumption is violated, this notion is unlikely to be therapeutically feasible.
However, the findings from this study justify the use of non-hormonal drugs for therapeutic purpose. They essentially show that, just by suppression of platelet activation either through anti-platelet treatment or neutralization of NF-κB or of TGF-β1, the lesional production of estrogen can be substantially curtailed or even minimized to achieve the desired therapeutic effect without messing up the hormonal system.
This study has several strengths. First, through highlighting the importance of platelets in lesional development, and, in this case, estrogen production, this study provides yet another piece of evidence that endometriotic lesions are indeed wounds undergoing ReTIAR. Second, through the use of both in vitro and in vivo studies, we have provided convincing evidence that platelets are an unindicted culprit responsible for increased local production of estrogen, and that this is mainly through the induction of NF-κB and TGF-β1 signaling pathways. Third, our study provides evidence that activated platelets can directly induce a hypoxia state in endometriotic lesions, which subsequently cause further phenotypic changes39. Lastly, this study further underscores the importance of lesional microenvironment in shaping its fate.
Our study also has some limitations. First, we did not evaluate all genes that are possibly involved in estrogen biosynthesis in endometriotic stromal cells, and focused mainly on StAR, aromatase, HSD3B2 and HSD17B1, in which the product of their fold increase seemingly accounted for most of the variation in estrogen production in endometriosis. In addition, we also evaluated their upstream genes, such as HIF-1α, SF-1 and CREB. However, there are other genes/enzymes that have been reported to be involved in the increased estrogen production in endometriosis, such as AMPK77. While this may be viewed as a deficiency, we note that any study, by necessity, needs to be limited by its aim and scope, and simply cannot evaluate all possible genes/enzymes involved in a complex process such as this in one single study. Hopefully, our study can pique the interest in the ultimate cause for estrogen overproduction in lesions in future studies.
Second, while we have identified NF-κB and TGF-β1 signaling pathways that are involved in lesional estrogen overproduction, we did not fully elucidate the exact molecular mechanisms in more detail, nor did we demonstrate just exactly how platelets activate NF-κB and/or TGF-β1. Again, this should await further investigation, and it is possible that platelet-activating factor (PAF) may activate NF-κB78. It is also possible that NF-κB is activated by platelets in a direct signaling manner as in cancer cells60. Future studies are warranted to clarify this mechanism.
Lastly, we only employed ovarian endometrioma tissue samples in the in vitro study. Given the known heterogeneity and complexity among different subtypes of endometriosis79, this raises the question whether the conclusions reached from this study apply to all subtypes of endometriosis. As we showed previously, however, the vast heterogeneity of endometriosis appears to be determined mostly by the lesional microenvironments80. Namely, regardless of subtypes, all endometriotic lesions appear to undergo identical molecular processes such as epithelial-mesenchymal transition (EMT), fibroblast-to-myofibroblast transdifferentiation (FMT), smooth muscle metaplasia (SMM), and fibrogenesis25,26,53, even in adenomyotic lesions81,82. Moreoever, increased local production of estrogen is common to all subtypes of endometriosis83 and even to adenomyosis84. Therefore, in this context, the conclusions reached by this study are likely to be true to all subtypes of endometriosis.
In summary, we have demonstrated that activated platelets increase the E2 production in endometriotic stromal cells through upregulation of StAR, HSD3B2, aromatase, and HSD17B1 via the activation of NF-κB and/or TGF-β1. In addition, the increased lesional estrogen production by activated platelets provides a compelling piece of evidence that endometriotic lesions are indeed wounds undergoing ReTIAR. Our findings strongly indicate that non-hormonal therapeutics for endometriosis is theoretically viable, with anti-platelet therapy being one promising avenue.
Materials and Methods
Patients and specimens
This study strictly adhered to the ethical principles outlined by the Helsinki Declaration and was approved by the institutional ethics review board of Shanghai OB/GYN Hospital, Fudan University. After informed consent, endometriotic tissue samples were obtained from 15 premenopausal patients (mean age = 32.2 ± 5.2 years) with surgically diagnosed and histologically confirmed ovarian endometriomas, who were admitted to the Shanghai OB/GYN Hospital, Fudan University, from May 2015 to March 2016. The harvested endometriotic tissue samples were used for deriving primary endometriotic stromal cells, which were then used for ELISA analysis, real-time PCR analysis and Western blotting analysis.
Cell culture and reagents
Endometriosis-derived primary human endometriotic stromal cells (HESCs) were isolated and cultured as reported previously23,73. Briefly, the endometriotic tissues were minced into small pieces of about 1 mm³ in size, after washing with DMEM/F-12 medium (Dulbecco’s modified Eagle’s medium/Ham’s F-12 medium, Hyclon, Logan, UT, USA) supplemented with 100 IU/mL penicillin and 100 mg/ml streptomycin (Invitrogen, Paisley, UK). After the enzymatic digestion of minced tissues with 0.2% collagenase II (Sigma, St Louis, MO, USA) in a shaking bed for about 1.5 hours at 37 °C, they were separated by filtration through a 149 μm and then a 37 μm (all pore sizes) nylon mesh. The stromal cells remaining in the filtrate were collected by centrifugation, re-suspended in DMEM/F12 (reconstituted with 10% charcoal-stripped fetal bovine serum (FBS, Gibco Laboratories, Invitrogen, Auckland, New Zealand) and 1% antibiotics), and seeded into 25 cm² cell culture flasks and incubated at 37 °C in humidified atmosphere of 5% CO2 in air. The purity and homogeneity of the stromal cell preparation (≥98%) were verified by immunocytochemistry with an antibody against vimentin (Abcam, Cambridge, UK), a specific marker of stromal cells, and an antibody against cytokeratin 7 (CK7) (CK-7, Abcam), a specific marker of epithelial cells, and, finally, an antibody against follicle-stimulating hormone receptor (FSHR, Abcam), a specific marker for granulosa cells (used to rule out the possibility of contamination with ovarian granulosa cells). After 3–4 passages, the vimentin staining was positive, while CK7 staining and FSHR staining were negative. Then, the isolated cells were seeded into cell culture plates for further analyses. The human endometrial stromal cell line (ESCL), established by Dr. Krikun and her colleagues85, was kindly provided by Dr. Asgi Fazleabas, and cultured in DMEM/F-12 medium (Hyclone) supplemented with 5% FBS and 1% antibiotics.
HESCs were added to the serum-free DMEM/F-12 medium at about 80% confluence and were starved for 24 hours before proceeding experiments. To see the effect platelets may have on endometriotic stromal cells, HESCs were incubated with 3.5 mL of different treatment media for 48 hours as follows: PBS group (standard medium + PBS, i.e. phenol red free DMEM/F-12 medium (Gibco Laboratories, Life Technologies, Grand island, NY, USA) with 10% charcoal-stripped FBS), platelets group (standard medium containing 107 human platelets), activated platelets group (standard medium containing 107 human platelets and human thrombin (1.49 NIH U (Sigma, St. Louis, MO, USA)), and thrombin group (standard medium containing human thrombin 1.49 NIH U). To examine the potential molecular mechanisms that underlie activated platelets-induced estrogen production, HESCs were pretreated with 30 μM JSH-23 compound (an NF-κB inhibitor, Santa Cruz, CA, USA) for 6 hours or with 1 μM A83-01 compound (a TGF-β receptor inhibitor, Santa Cruz) for 2 hours, and then incubated with activated platelets plus JSH-23 or A83-01 for 48 hours.
To ensure that the real-time PCR and Western blot analysis results refer to HESCs but not platelets, we washed the HESCs that co-cultured with platelets with sterile PBS three times to remove the platelets as reported previously86. The supernatant of HESC and ESCL, after treatment with PBS, platelets, activated platelets (by thrombin stimulation) and thrombin alone for 48 hours, was collected for ELISA analysis.
Preparation of platelets
Platelets, donated by healthy male volunteers who denied taking any medications for at least two weeks prior to donation after informed consent, were isolated from the whole blood samples by centrifugation at room temperature as reported previously23,73. The blood was first centrifuged at 150 g for 10 min, the supernatant platelet rich plasma (PRP) was then centrifuged at 300 g for 5 min, and the supernatant PRP was harvested again and then centrifuged at 1000 g for 14 min. Finally, the depositing platelets were suspended in DMEM/F12 culture medium with 10% FBS for cell culture experiments, yielding ~2 × 108 platelets from 20 mL of peripheral blood samples from each volunteer.
ELISA
To determine the concentration of E2 in the supernatants, the Human 17β-estradiol ELISA Kit (Abcam) was used according to the manufacturer’s protocol. Briefly, purified human E2 was used to coat the microtiter plate wells to form solid-phase antibody. The aqueous samples were added to individual wells. E2 present in the aqueous combined with horseradish peroxidase (HRP)-labeled antibody to form antibody-antigen-enzyme-antibody complex. After washing thoroughly with prepared washing solution, tetramethylbenzidine (TMB) substrate solution was added, turning the HRP-enzyme-catalyzed TMB substrate blue. The reaction was terminated by the addition of a sulphuric acid solution, and the color change was measured spectrophotometrically at a wavelength of 450 nm. The concentration of E2 in the samples was then determined by comparing the optical density (OD) of the samples to the standard curve. We plotted the OD of the standards against the standard concentration on graph, and draw the curve through these points to construct the standard curve (R2 = 0.99). The concentrations for unknown samples were determined from the standard curve. For each sample, the concentration of E2 was measured twice, and the average was used as the concentration of E2 in that sample.
RNA isolation and real-time PCR
After co-culture with different media for 48 hours, the total RNA was isolated from HESCs using Trizol reagent (Invitrogen, UK), and cDNA synthesis was performed using the reverse transcription kit (Takara, Otsu, Japan). We measured the expression level of the estrogen production related genes, StAR, HSD3B2, CYP19A1 and HSD17B1 and downstream molecules of NF-κB, HIF-1α, COX-2, and VEGF by RT-PCR using SYBR Premix Ex Taq (Takara, Otsu, Japan). The oligonucleotide primer sequences (synthesized by Biotnt Corporation, Shanghai, China) used this study are listed in Table 1. Melting curves of the products were obtained after cycling by a stepwise increase of temperature from 55 °C to 95 °C. At the end of 40 cycles, the reaction products were separated electrophoretically on an agarose gel and stained with ethidium bromide for visual confirmation of the PCR products. The expression values were normalized to the geometric mean of GAPDH measurements and the quantification of mRNA abundance was made using the method as described in87.
Western blot analysis
For total protein extraction, HESCs were scraped and extracted in commercialized RIPA buffer (Thermo, Waltham, MA, USA). The protein concentration, after co-cultured with different media for 48 hours, was determined using BCA protein quantitative analysis kit (P0010S, Beyotime, Shanghai, China). All proteins mixed with SDS-PAGE loading buffer (P0015, Beyotime) were heated for 10 min at 95 °C for denaturalization. The protein samples were loaded on a 10% SDS-PAGE, and electro-blotted onto PVDF membranes (Bio-Rad, Hercules, CA, USA). The membranes were blocked in Western blocking buffer (P0023B, Beyotime) for 1 hour at room temperature and subsequently incubated at 4 °C overnight with the primary antibodies as listed in Table 2. After the membranes were incubated with HRP-labeled secondary antibodies for 1 hour at room temperature, the signal was detected using ECL (Pierce, Thermo Scientific, Rockford, IL, USA) on Image Quant LAS 4000 mini. The protein expression levels were quantified by Quantity One software (Bio-Rad).
In Western blot analysis using different primary antibodies, we cropped the polyvinyl difluoride (PVDF) membranes since antibodies against different proteins may require slightly different treatment. However, when only a single primary antibody was used, no cropping was performed.
Mouse experiments
A total of 90 virgin female Balb/C mice, ~6 weeks old and about 16–18 g in bodyweight, were purchased from SLAC Experimental Animal Company (Shanghai, China) and used in two experiments. All mice were maintained under controlled conditions with a light/dark cycle of 12/12 hours and had access to food and water adlibitum. All our experiments were performed following the guidelines of the National Research Council’s Guide for the Care and Use of Laboratory Animals88 and approved by the institutional experimental animals review board of Shanghai OB/GYN Hospital, Fudan University.
We used an established mouse model of endometriosis89 by intraperitoneal (i.p.) injection of endometrial fragments as our previous studies23,90. Briefly, after one week of acclimatization, 7-week-old donor mice were intramuscular injected with 3 μg/mouse estradiol benzoate (Animal Medicine Factory, Hangzhou, China) to stimulate the growth of endometrium. One week later they were sacrificed and their uteri were harvested. The uterine tissues were seeded in a Petri dish containing warm sterile saline, and split longitudinally with a pair of scissors.
Two uterine horns from each mouse were minced with scissors, ensuring that the maximal diameter of the fragment was consistently smaller than 1 mm. The fragments were then injected i.p. to recipient mice. To minimize any individual variation, the endometrial tissue fragments from two donor mice were mixed together and then divided into three or four parts, each injected i.p. to one mouse each from one of the three or four groups, depending on the experiment design.
The first experiment evaluated the effect of platelet infusion and platelet depletion on the immunoreactivity against proteins involved in estrogen biosynthesis in endometriotic lesions. The second experiment, in contrast, evaluated the effect of NF-κB or TGF-β1 antagonism on the immunoreactivity against proteins involved in estrogen biosynthesis in lesions.
In the first experiment, 45 mice were used. Among the 45 mice, 15 were randomly selected as donors, and the remaining 30 were recipients who received endometrial tissues from donor mice. Ninety male mice of similar age and the same strain were used mainly for harvesting platelets. Briefly, two days (Day-2) before the induction of endometriosis (Day 0), all recipient mice were weighed and subjected to a baseline hotplate test. The mice were randomly divided into three groups of 10: PI, PD and NI. In the PI group, platelets (4 × 107/mouse) were given intravenously on Days −2, 3 and 8. In PD and NI groups, rat anti-mouse GPIba polyclonal IgG or rat anti-mouse isotope-matched non-immune polyclonal IgG respectively (both from Emfret Analytics, 1.5 mg/g bodyweight) were given intravenously on Days −2, 3 and 8. The concentration was determined following the manufacturer’s instructions and our previous study23.
JSH-23 and SB-431542 can suppress NF-κB and TGF-β1/Smad3 signaling pathways respectively and thus might inhibit platelet aggregation in endometriosis91,92. In the second experiment, 30 mice were randomly divided into three groups of equal size: control group (CT), NF-κB antagonism group (NA), and TGF-β1 antagonism group (TA). Mice in the NA, TA, and CT groups received i.p. injection of SB-431542 (10 mg/kg, MCE, Shanghai, China)93, JSH-23 (20 mg/kg, MCE)94, and equal volume of vehicle very other day (i.e. days −2, 0, 2, 4, ..., 10 and 12), starting from 2 days before the induction of endometriosis (day 0, the day when induction was performed)95. Two days after the first injection, endometriosis was induced as described above for all mice. Two days before and 12 days after induction, all mice were weighed and subjected to hotplate test. All mice were sacrificed by cervical dislocation and their lesions were excised, weighed, and processed for further evaluation or immunohistochemistry.
The hotplate test and lesion measurement
To evaluate endometriosis-associated hyperalgesia in mice, hotplate test was administrated to all mice using a Hot Plate Analgesia Meter (Model BME-480, Institute of Biomedical Engineering, Chinese Academy of Medical Sciences, Tianjin, China) as reported previously23. The extent of endometriosis was measured by measuring the dry weight of all lesions excised from mice as described in96. The endometriotic lesions were excised and carefully weighed and then fixed with 10% formalin (w/v) for IHC and histochemistry analyses.
Immunohistochemistry (IHC) staining
Serial 4 μm sections were obtained from each paraffin-embedded tissue block, with the first resultant slide was stained with hematoxylin and eosin (H&E) to confirm pathologic diagnosis, and the subsequent slides for IHC analysis for StAR, HSD3B2, aromatase, HSD17B1, SF-1 and p-CREB. Routine deparaffinization and rehydration procedures were performed. The optimal concentrations of these primary antibodies were shown in Table 3. The slides were heated at 98 °C in a citrate buffer (pH 6.0) for a total of 30 min for antigen retrieval, cooled naturally to the room temperature, and were washed 3 times in PBS 0.5% goat serum for blocking nonspecific binding sites. Sections were then incubated overnight with the primary antibody at 4 °C. After slides were rinsed, the biotinylated secondary antibody, Supervision TM Universal (Anti-Mouse/Rabbit) Detection Reagent (HRP) (Shanghai GeneTech Company, Shanghai, China), was incubated for 30 min at room temperature. The bound antibody complexes were stained for 3–5 min or until appropriate for microscopic examination with diaminobenzidine and then counterstained with hematoxylin and mounted. Immunostaining results were evaluated using a semi-quantitative scoring system, as reported previously97. Briefly, images were obtained with the microscope (Olympus BX51, Olympus, Tokyo, Japan) fitted with a digital camera (Olympus DP70, Olympus) and exported as a TIFF-format digital file, and then used IPP (Image-Pro Plus, version 6.0, Media Cybernetics, Inc., Bethesda, MD, USA) to count the number or the intensity of positive stain, blind to which group the slide belonged to. A series of 4–5 images randomly selected from several sections per tissue sample were taken for each immunostaining parameter to obtain a mean value. The positive and negative controls are shown in Supplementary Fig. S2.
Statistical analysis
To compare the treatment effect in in vitro experimentations, paired Wilcoxon’s test was used. The comparison of distributions of continuous variables between the two groups was made using Wilcoxon’s test. Pearson’s correlation was used when evaluating correlations between two variables. To see whether factor is associated with the immunostaining levels of a given marker when more than one factor is involved (such as platelet infusion and depletion), multiple linear regression analysis was used. A p-value of less than 0.05 was considered statistically significant. All calculations were carried out using the software R (version 3.5.1)98 or SPSS (version 16.0).
References
Giudice, L. C. & Kao, L. C. Endometriosis. Lancet 364, 1789–1799, https://doi.org/10.1016/S0140-6736(04)17403-5 (2004).
Giudice, L. C. Clinical practice. Endometriosis. N. Engl. J. Med. 362, 2389–2398, https://doi.org/10.1056/NEJMcp1000274 (2010).
Vitale, S. G., La Rosa, V. L., Rapisarda, A. M. C. & Lagana, A. S. Impact of endometriosis on quality of life and psychological well-being. J. Psychosom. Obstet. Gynaecol. 38, 317–319, https://doi.org/10.1080/0167482X.2016.1244185 (2017).
Lagana, A. S. et al. Anxiety and depression in patients with endometriosis: impact and management challenges. Int. J. Womens Health 9, 323–330, https://doi.org/10.2147/IJWH.S119729 (2017).
Eskenazi, B. & Warner, M. L. Epidemiology of endometriosis. Obstet. Gynecol. Clin. North. Am. 24, 235–258, https://doi.org/10.1016/S0889-8545(05)70302-8 (1997).
Garry, R. Is insulin resistance an essential component of PCOS?: The endometriosis syndromes: a clinical classification in the presence of aetiological confusion and therapeutic anarchy. Hum. Reprod. 19, 760–768, https://doi.org/10.1093/humrep/deh147 (2004).
Lagana, A. S. et al. Unus pro omnibus, omnes pro uno: A novel, evidence-based, unifying theory for the pathogenesis of endometriosis. Med. Hypotheses 103, 10–20, https://doi.org/10.1016/j.mehy.2017.03.032 (2017).
Taylor, H. S. et al. Novel therapies targeting endometriosis. Reprod. Sci. 18, 814–823, https://doi.org/10.1177/1933719111410713 (2011).
Gurates, B. & Bulun, S. E. Endometriosis: the ultimate hormonal disease. Semin. Reprod. Med. 21, 125–134, https://doi.org/10.1055/s-2003-41319 (2003).
Bulun, S. E. et al. Regulation of aromatase expression in estrogen-responsive breast and uterine disease: from bench to treatment. Pharmacol. Rev. 57, 359–383, https://doi.org/10.1124/pr.57.3.6 (2005).
Vercellini, P., Vigano, P., Somigliana, E. & Fedele, L. Endometriosis: pathogenesis and treatment. Nat. Rev. Endocrinol. 10, 261–275, https://doi.org/10.1038/nrendo.2013.255 (2014).
Noble, L. S. et al. Prostaglandin E2 stimulates aromatase expression in endometriosis-derived stromal cells. J. Clin. Endocrinol. Metab. 82, 600–606, https://doi.org/10.1210/jcem.82.2.3783 (1997).
Yang, S. et al. Regulation of aromatase P450 expression in endometriotic and endometrial stromal cells by CCAAT/enhancer binding proteins (C/EBPs): decreased C/EBPbeta in endometriosis is associated with overexpression of aromatase. J. Clin. Endocrinol. Metab. 87, 2336–2345, https://doi.org/10.1210/jcem.87.5.8486 (2002).
Breyer, R. M., Bagdassarian, C. K., Myers, S. A. & Breyer, M. D. Prostanoid receptors: subtypes and signaling. Annu. Rev. pharmacology Toxicol. 41, 661–690, https://doi.org/10.1146/annurev.pharmtox.41.1.661 (2001).
Bulun, S. E. et al. Steroidogenic factor-1 and endometriosis. Mol. Cell Endocrinol. 300, 104–108, https://doi.org/10.1016/j.mce.2008.12.012 (2009).
Hsu, C. C., Lu, C. W., Huang, B. M., Wu, M. H. & Tsai, S. J. Cyclic adenosine 3′,5′-monophosphate response element-binding protein and CCAAT/enhancer-binding protein mediate prostaglandin E2-induced steroidogenic acute regulatory protein expression in endometriotic stromal cells. Am. J. Pathol. 173, 433–441, https://doi.org/10.2353/ajpath.2008.080199 (2008).
Sun, H. S., Hsiao, K. Y., Hsu, C. C., Wu, M. H. & Tsai, S. J. Transactivation of steroidogenic acute regulatory protein in human endometriotic stromalcells is mediated by the prostaglandin EP2 receptor. Endocrinol. 144, 3934–3942, https://doi.org/10.1210/en.2003-0289 (2003).
Zeitoun, K. M. & Bulun, S. E. Aromatase: a key molecule in the pathophysiology of endometriosis and a therapeutic target. Fertil. Steril. 72, 961–969, https://doi.org/10.1016/s0015-0282(99)00393-3 (1999).
Duan, J., Liu, X. & Guo, S.-W. The M2a macrophage subset may be critically involved in fibrogenesis of endometriosis in mouse. Reprod. Biomed. Online 37, 254–268, https://doi.org/10.1016/j.rbmo.2018.05.017. (2018).
Guo, S. W., Ding, D., Shen, M. & Liu, X. Dating Endometriotic Ovarian Cysts Based on the Content of Cyst Fluid and its Potential Clinical Implications. Reprod. Sci. 22, 873–883, https://doi.org/10.1177/1933719115570907 (2015).
Zhang, Q., Duan, J., Liu, X. & Guo, S. W. Platelets drive smooth muscle metaplasia and fibrogenesis in endometriosis through epithelial-mesenchymal transition and fibroblast-to-myofibroblast transdifferentiation. Mol. Cell Endocrinol. 428, 1–16, https://doi.org/10.1016/j.mce.2016.03.015 (2016).
Zhang, Q., Duan, J., Olson, M., Fazleabas, A. & Guo, S. W. Cellular Changes Consistent With Epithelial-Mesenchymal Transition and Fibroblast-to-Myofibroblast Transdifferentiation in the Progression of Experimental Endometriosis in Baboons. Reprod. Sci. 23, 1409–1421, https://doi.org/10.1177/1933719116641763 (2016).
Ding, D., Liu, X., Duan, J. & Guo, S. W. Platelets are an unindicted culprit in the development of endometriosis: clinical and experimental evidence. Hum. Reprod. 30, 812–832, https://doi.org/10.1093/humrep/dev025 (2015).
Bacci, M. et al. Macrophages are alternatively activated in patients with endometriosis and required for growth and vascularization of lesions in a mouse model of disease. Am. J. Pathol. 175, 547–556, https://doi.org/10.2353/ajpath.2009.081011 (2009).
Liu, X., Yan, D. & Guo, S.-W. Sensory nerve-derived neuropeptides accelerate the development and fibrogenesis of endometriosis. Hum. Reprod. 34, 452–468, https://doi.org/10.1093/humrep/dey392 (2018).
Yan, D., Liu, X. & Guo, S.-W. Neuropeptides substance P and calcitonin gene related peptide accelerate the development and fibrogenesis of endometriosis. Sci. Rep. 9, 2698, https://doi.org/10.1038/s41598-019-39170-w (2019).
Horng, H. C. et al. Estrogen Effects on Wound Healing. Int J Mol Sci 18, https://doi.org/10.3390/ijms18112325 (2017).
Wilkinson, H. N. & Hardman, M. J. The role of estrogen in cutaneous ageing and repair. Maturitas 103, 60–64, https://doi.org/10.1016/j.maturitas.2017.06.026 (2017).
Ashcroft, G. S. et al. Estrogen accelerates cutaneous wound healing associated with an increase in TGF-beta1 levels. Nat. Med. 3, 1209–1215 (1997).
Ashcroft, G. S. et al. Estrogen modulates cutaneous wound healing by downregulating macrophage migration inhibitory factor. J. Clin. Invest. 111, 1309–1318, https://doi.org/10.1172/JCI16288 (2003).
Hardman, M. J., Emmerson, E., Campbell, L. & Ashcroft, G. S. Selective estrogen receptor modulators accelerate cutaneous wound healing in ovariectomized female mice. Endocrinol. 149, 551–557, https://doi.org/10.1210/en.2007-1042 (2008).
Pepe, G. et al. Self-renewal and phenotypic conversion are the main physiological responses of macrophages to the endogenous estrogen surge. Sci. Rep. 7, 44270, https://doi.org/10.1038/srep44270 (2017).
Mukai, K. et al. 17beta-Estradiol administration promotes delayed cutaneous wound healing in 40-week ovariectomised female mice. Int. wound J. 13, 636–644, https://doi.org/10.1111/iwj.12336 (2016).
Hardman, M. J. & Ashcroft, G. S. Estrogen, not intrinsic aging, is the major regulator of delayed human wound healing in the elderly. Genome Biol. 9, R80, https://doi.org/10.1186/gb-2008-9-5-r80 (2008).
Brandenberger, A. W. et al. Oestrogen receptor (ER)-alpha and ER-beta isoforms in normal endometrial and endometriosis-derived stromal cells. Mol. Hum. Reprod. 5, 651–655, https://doi.org/10.1093/molehr/5.7.651 (1999).
Fujimoto, J., Hirose, R., Sakaguchi, H. & Tamaya, T. Expression of oestrogen receptor-alpha and -beta in ovarian endometriomata. Mol. Hum. Reprod. 5, 742–747, https://doi.org/10.1093/molehr/5.8.742 (1999).
Merlo, S., Frasca, G., Canonico, P. L. & Sortino, M. A. Differential involvement of estrogen receptor alpha and estrogen receptor beta in the healing promoting effect of estrogen in human keratinocytes. J. Endocrinol. 200, 189–197, https://doi.org/10.1677/JOE-08-0442 (2009).
Campbell, L. et al. Estrogen promotes cutaneous wound healing via estrogen receptor beta independent of its antiinflammatory activities. J. Exp. Med. 207, 1825–1833, https://doi.org/10.1084/jem.20100500 (2010).
Wu, M. H., Hsiao, K. Y. & Tsai, S. J. Hypoxia: The force of endometriosis. J Obstet Gynaecol Res, https://doi.org/10.1111/jog.13900 (2019).
Qi, Q., Guo, S.-W. & Liu, X. Activated Platelets Induce Hypoxia-Inducible Factor-1α Expression Likely through Transforming Growth Factor-β1 in Human Endometrial Stromal Cells. Reprod Dev Med In press. (2019).
Izawa, M. et al. An epigenetic disorder may cause aberrant expression of aromatase gene in endometriotic stromal cells. Fertil. Steril. 89, 1390–1396, https://doi.org/10.1016/j.fertnstert.2007.03.078 (2008).
Izawa, M. et al. Demethylation of a nonpromoter cytosine-phosphate-guanine island in the aromatase gene may cause the aberrant up-regulation in endometriotic tissues. Fertil. Steril. 95, 33–39, https://doi.org/10.1016/j.fertnstert.2010.06.024 (2011).
Zeitoun, K. et al. Deficient 17beta-hydroxysteroid dehydrogenase type 2 expression in endometriosis: failure to metabolize 17beta-estradiol. J. Clin. Endocrinol. Metab. 83, 4474–4480, https://doi.org/10.1210/jcem.83.12.5301 (1998).
Xue, Q. et al. Transcriptional activation of steroidogenic factor-1 by hypomethylation of the 5′ CpG island in endometriosis. J. Clin. Endocrinol. Metab. 92, 3261–3267, https://doi.org/10.1210/jc.2007-0494 (2007).
Attar, E. et al. Prostaglandin E2 via steroidogenic factor-1 coordinately regulates transcription of steroidogenic genes necessary for estrogen synthesis in endometriosis. J. Clin. Endocrinol. Metab. 94, 623–631, https://doi.org/10.1210/jc.2008-1180 (2009).
Zeitoun, K., Takayama, K., Michael, M. D. & Bulun, S. E. Stimulation of aromatase P450 promoter (II) activity in endometriosis and its inhibition in endometrium are regulated by competitive binding of steroidogenic factor-1 and chicken ovalbumin upstream promoter transcription factor to the same cis-acting element. Mol. Endocrinol. 13, 239–253, https://doi.org/10.1210/mend.13.2.0229 (1999).
Tsai, S. J., Wu, M. H., Lin, C. C., Sun, H. S. & Chen, H. M. Regulation of steroidogenic acute regulatory protein expression and progesterone production in endometriotic stromal cells. J. Clin. Endocrinol. Metab. 86, 5765–5773, https://doi.org/10.1210/jcem.86.12.8082 (2001).
Hsiao, K. Y., Lin, S. C., Wu, M. H. & Tsai, S. J. Pathological functions of hypoxia in endometriosis. Front. Biosci. 7, 309–321, https://doi.org/10.2741/736 (2015).
Wu, M. H., Lin, S. C., Hsiao, K. Y. & Tsai, S. J. Hypoxia-inhibited dual-specificity phosphatase-2 expression in endometriotic cells regulates cyclooxygenase-2 expression. J. Pathol. 225, 390–400, https://doi.org/10.1002/path.2963 (2011).
Smith, T. G., Robbins, P. A. & Ratcliffe, P. J. The human side of hypoxia-inducible factor. Br. J. haematology 141, 325–334, https://doi.org/10.1111/j.1365-2141.2008.07029.x (2008).
Wu, M. H., Chen, K. F., Lin, S. C., Lgu, C. W. & Tsai, S. J. Aberrant expression of leptin in human endometriotic stromal cells is induced by elevated levels of hypoxia inducible factor-1alpha. Am. J. Pathol. 170, 590–598, https://doi.org/10.2353/ajpath.2007.060477 (2007).
Noble, F. et al. Pain-suppressive effects on various nociceptive stimuli (thermal, chemical, electrical and inflammatory) of the first orally active enkephalin-metabolizing enzyme inhibitor RB 120. Pain. 73, 383–391, https://doi.org/10.1016/S0304-3959(97)00125-5 (1997).
Guo, S. W. Fibrogenesis resulting from cyclic bleeding: the Holy Grail of the natural history of ectopic endometrium. Hum Reprod, https://doi.org/10.1093/humrep/dey015 (2018).
Lin, Y. J., Lai, M. D., Lei, H. Y. & Wing, L. Y. Neutrophils and macrophages promote angiogenesis in the early stage of endometriosis in a mouse model. Endocrinol. 147, 1278–1286, https://doi.org/10.1210/en.2005-0790 (2006).
Brosens, I. A. Endometriosis–a disease because it is characterized by bleeding. Am. J. Obstet. Gynecol. 176, 263–267, https://doi.org/10.1016/s0002-9378(97)70482-4 (1997).
Fiorelli, G. et al. Aromatase expression and activity in the human leukaemic cell line FLG 29.1. J. Steroid Biochem. Mol. Biol. 66, 105–112, https://doi.org/10.1016/s0960-0760(98)00050-8 (1998).
Shozu, M., Zhao, Y. & Simpson, E. R. TGF-beta1 stimulates expression of the aromatase (CYP19) gene in human osteoblast-like cells and THP-1 cells. Mol. Cell Endocrinol. 160, 123–133, https://doi.org/10.1016/S0303-7207(99)00233-6 (2000).
Liu, Y. et al. Effects of Smad3 on the proliferation and steroidogenesis in human ovarian luteinized granulosa cells. IUBMB life 66, 424–437, https://doi.org/10.1002/iub.1280 (2014).
Qu, J. et al. Smad3/4 Binding to Promoter II of P450arom So As to Regulate Aromatase Expression in Endometriosis. Reprod. Sci. 24, 1187–1194, https://doi.org/10.1177/1933719116681517 (2017).
Labelle, M., Begum, S. & Hynes, R. O. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell 20, 576–590, https://doi.org/10.1016/j.ccr.2011.09.009 (2011).
Ding, D., Liu, X. & Guo, S. W. Further Evidence for Hypercoagulability in Women With Ovarian Endometriomas. Reprod Sci, 1933719118799195, https://doi.org/10.1177/1933719118799195 (2018).
Wu, Q., Ding, D., Liu, X. & Guo, S. W. Evidence for a Hypercoagulable State in Women With Ovarian Endometriomas. Reprod. Sci. 22, 1107–1114, https://doi.org/10.1177/1933719115572478 (2015).
Baggio, S. et al. Delivery and pregnancy outcome in women with bowel resection for deep endometriosis: a retrospective cohort study. Gynecol. Surg. 12, 279–295, https://doi.org/10.1007/s10397-015-0901-9 (2015).
Lalani, S. et al. Endometriosis and adverse maternal, fetal and neonatal outcomes, a systematic review and meta-analysis. Hum. Reprod. 33, 1854–1865, https://doi.org/10.1093/humrep/dey269 (2018).
Kohli, S. et al. Maternal extracellular vesicles and platelets promote preeclampsia via inflammasome activation in trophoblasts. Blood 128, 2153–2164, https://doi.org/10.1182/blood-2016-03-705434 (2016).
Wu, M. H., Lu, C. W., Chang, F. M. & Tsai, S. J. Estrogen receptor expression affected by hypoxia inducible factor-1alpha in stromal cells from patients with endometriosis. Taiwan. J. Obstet. Gynecol. 51, 50–54, https://doi.org/10.1016/j.tjog.2012.01.010 (2012).
Guo, S. W., Du, Y. & Liu, X. Endometriosis-Derived Stromal Cells Secrete Thrombin and Thromboxane A2, Inducing Platelet Activation. Reprod. Sci. 23, 1044–1052, https://doi.org/10.1177/1933719116630428 (2016).
Beshay, V. E. & Carr, B. R. In Williams Gynecology. (eds B., Hoffman et al.) Ch. 10, 281–303. (McGraw-Hill Education, 2012).
Takamura, M. et al. Neutrophil depletion reduces endometriotic lesion formation in mice. Am. J. Reprod. Immunol. 76, 193–198, https://doi.org/10.1111/aji.12540 (2016).
Ellis, S., Lin, E. J. & Tartar, D. Immunology of Wound Healing. Curr. dermatology Rep. 7, 350–358, https://doi.org/10.1007/s13671-018-0234-9 (2018).
Labrie, F. All sex steroids are made intracellularly in peripheral tissues by the mechanisms of intracrinology after menopause. J. Steroid Biochem. Mol. Biol. 145, 133–138, https://doi.org/10.1016/j.jsbmb.2014.06.001 (2015).
Inoue, T. et al. The role of estrogen-metabolizing enzymes and estrogen receptors in human epidermis. Mol. Cell Endocrinol. 344, 35–40, https://doi.org/10.1016/j.mce.2011.06.015 (2011).
Zhang, Q., Ding, D., Liu, X. & Guo, S. W. Activated Platelets Induce Estrogen Receptor beta Expression in Endometriotic Stromal Cells. Gynecol. Obstet. Invest. 80, 187–192, https://doi.org/10.1159/000377629 (2015).
Groothuis, P. G. & Guo, S. W. Drug Development in Endometriosis and Adenomyosis: It Takes More Than Just Good Science. Reprod. Sci. 25, 1318–1329, https://doi.org/10.1177/1933719118785767 (2018).
Olive, D. L. & Pritts, E. A. Treatment of endometriosis. N. Engl. J. Med. 345, 266–275, https://doi.org/10.1056/NEJM200107263450407 (2001).
Barbieri, R. L. Hormone treatment of endometriosis: the estrogen threshold hypothesis. Am. J. Obstet. Gynecol. 166, 740–745, https://doi.org/10.1016/0002-9378(92)91706-g (1992).
Xu, J. N. et al. Metformin inhibits StAR expression in human endometriotic stromal cells via AMPK-mediated disruption of CREB-CRTC2 complex formation. J. Clin. Endocrinol. Metab. 99, 2795–2803, https://doi.org/10.1210/jc.2014-1593 (2014).
Ye, R. D., Kravchenko, V. V., Pan, Z. & Feng, L. Stimulation of NF-kappa B activation and gene expression by platelet-activating factor. Adv. Exp. Med. Biol. 416, 143–151, https://doi.org/10.1007/978-1-4899-0179-8_24 (1996).
Nisolle, M. & Donnez, J. Peritoneal endometriosis, ovarian endometriosis, and adenomyotic nodules of the rectovaginal septum are three different entities. Fertil. Steril. 68, 585–596, https://doi.org/10.1016/s0015-0282(97)00191-x (1997).
Liu, X., Zhang, Q. & Guo, S. W. Histological and Immunohistochemical Characterization of the Similarity and Difference Between Ovarian Endometriomas and Deep Infiltrating Endometriosis. Reprod. Sci. 25, 329–340, https://doi.org/10.1177/1933719117718275 (2018).
Liu, X., Shen, M., Qi, Q., Zhang, H. & Guo, S. W. Corroborating evidence for platelet-induced epithelial-mesenchymal transition and fibroblast-to-myofibroblast transdifferentiation in the development of adenomyosis. Hum. Reprod. 31, 734–749, https://doi.org/10.1093/humrep/dew018 (2016).
Shen, M., Liu, X., Zhang, H. & Guo, S. W. Transforming growth factor beta1 signaling coincides with epithelial-mesenchymal transition and fibroblast-to-myofibroblast transdifferentiation in the development of adenomyosis in mice. Hum. Reprod. 31, 355–369, https://doi.org/10.1093/humrep/dev314 (2016).
Bulun, S. E. Endometriosis. N. Engl. J. Med. 360, 268–279, https://doi.org/10.1056/NEJMra0804690 (2009).
Yamamoto, T., Noguchi, T., Tamura, T., Kitawaki, J. & Okada, H. Evidence for estrogen synthesis in adenomyotic tissues. Am. J. Obstet. Gynecol. 169, 734–738, https://doi.org/10.1016/0002-9378(93)90654-2 (1993).
Krikun, G. et al. A novel immortalized human endometrial stromal cell line with normal progestational response. Endocrinol. 145, 2291–2296, https://doi.org/10.1210/en.2003-1606 (2004).
Tsujii, M. et al. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell 93, 705–716, https://doi.org/10.1016/s0092-8674(00)81433-6 (1998).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408, https://doi.org/10.1006/meth.2001.1262 (2001).
In Guide for the Care and Use of Laboratory Animals (1996).
Somigliana, E. et al. Endometrial ability to implant in ectopic sites can be prevented by interleukin-12 in a murine model of endometriosis. Hum. Reprod. 14, 2944–2950, https://doi.org/10.1093/humrep/14.12.2944 (1999).
Long, Q., Liu, X. & Guo, S. W. Surgery accelerates the development of endometriosis in mouse. American journal of obstetrics and gynecology, https://doi.org/10.1016/j.ajog.2016.02.055 (2016).
Shin, H. M. et al. Inhibitory action of novel aromatic diamine compound on lipopolysaccharide-induced nuclear translocation of NF-kappaB without affecting IkappaB degradation. FEBS Lett. 571, 50–54, https://doi.org/10.1016/j.febslet.2004.06.056 (2004).
Halder, S. K., Beauchamp, R. D. & Datta, P. K. A specific inhibitor of TGF-beta receptor kinase, SB-431542, as a potent antitumor agent for human cancers. Neoplasia 7, 509–521, https://doi.org/10.1593/neo.04640 (2005).
Sato, M. et al. Differential Proteome Analysis Identifies TGF-beta-Related Pro-Metastatic Proteins in a 4T1 Murine Breast Cancer Model. PLoS one 10, e0126483, https://doi.org/10.1371/journal.pone.0126483 (2015).
Ozkok, A., Ravichandran, K., Wang, Q., Ljubanovic, D. & Edelstein, C. L. NF-kappaB transcriptional inhibition ameliorates cisplatin-induced acute kidney injury (AKI). Toxicol. Lett. 240, 105–113, https://doi.org/10.1016/j.toxlet.2015.10.028 (2016).
Guo, S. W., Ding, D. & Liu, X. Anti-platelet therapy is efficacious in treating endometriosis induced in mouse. Reprod. Biomed. Online 33, 484–499, https://doi.org/10.1016/j.rbmo.2016.07.007 (2016).
Somigliana, E. et al. Use of knockout transgenic mice in the study of endometriosis: insights from mice lacking beta(2)-microglobulin and interleukin-12p40. Fertil. Steril. 75, 203–206, https://doi.org/10.1016/s0015-0282(00)01659-9 (2001).
Shen, F., Liu, X., Geng, J. G. & Guo, S. W. Increased immunoreactivity to SLIT/ROBO1 in ovarian endometriomas: a likely constituent biomarker for recurrence. Am. J. Pathol. 175, 479–488, https://doi.org/10.2353/ajpath.2009.090024 (2009).
R: A Language and Environment for Statistical Computing. (R Foundation for Statistical Computing, Vienna, Austria, 2016).
Konstantinopoulos, P. A. et al. EGF-R is expressed and AP-1 and NF-kappaB are activated in stromal myofibroblasts surrounding colon adenocarcinomas paralleling expression of COX-2 and VEGF. Cell. oncology: Off. J. Int. Soc. Cell. Oncol. 29, 477–482, https://doi.org/10.1155/2007/831416 (2007).
van Uden, P., Kenneth, N. S. & Rocha, S. Regulation of hypoxia-inducible factor-1alpha by NF-kappaB. Biochemical J. 412, 477–484, https://doi.org/10.1042/BJ20080476 (2008).
Chang, H. J. et al. Transforming growth factor (TGF)-beta1-induced human endometrial stromal cell decidualization through extracellular signal-regulated kinase and Smad activation in vitro: peroxisome proliferator-activated receptor gamma acts as a negative regulator of TGF-beta1. Fertil. Steril. 90, 1357–1365, https://doi.org/10.1016/j.fertnstert.2007.09.010 (2008).
Sacco, K., Portelli, M., Pollacco, J., Schembri-Wismayer, P. & Calleja-Agius, J. The role of prostaglandin E2 in endometriosis. Gynecol. endocrinology: Off. J. Int. Soc. Gynecol. Endocrinol. 28, 134–138, https://doi.org/10.3109/09513590.2011.588753 (2012).
Urata, Y. et al. Interleukin-4 and prostaglandin E2 synergistically up-regulate 3beta-hydroxysteroid dehydrogenase type 2 in endometrioma stromal cells. J. Clin. Endocrinol. Metab. 98, 1583–1590, https://doi.org/10.1210/jc.2012-3475 (2013).
Funahashi, K. et al. Prostaglandin E2 negatively regulates AMP-activated protein kinase via protein kinase A signaling pathway. Prostaglandins Other Lipid Mediat. 88, 31–35, https://doi.org/10.1016/j.prostaglandins.2008.09.002 (2009).
Thomson, D. M. et al. AMP-activated protein kinase phosphorylates transcription factors of the CREB family. J. Appl. Physiol. 104, 429–438, https://doi.org/10.1152/japplphysiol.00900.2007 (2008).
Huang, W. et al. AMPK Plays a Dual Role in Regulation of CREB/BDNF Pathway in Mouse Primary Hippocampal Cells. J. Mol. Neurosci. 56, 782–788, https://doi.org/10.1007/s12031-015-0500-2 (2015).
Altarejos, J. Y. & Montminy, M. CREB and the CRTC co-activators: sensors for hormonal and metabolic signals. Nat. Rev. Mol. Cell Biol. 12, 141–151, https://doi.org/10.1038/nrm3072 (2011).
Brown, K. A. et al. Subcellular localization of cyclic AMP-responsive element binding protein-regulated transcription coactivator 2 provides a link between obesity and breast cancer in postmenopausal women. Cancer Res. 69, 5392–5399, https://doi.org/10.1158/0008-5472.CAN-09-0108 (2009).
Utsunomiya, H. et al. Upstream stimulatory factor-2 regulates steroidogenic factor-1 expression in endometriosis. Mol. Endocrinol. 22, 904–914, https://doi.org/10.1210/me.2006-0302 (2008).
Wu, P. L. et al. Farnesoid X Receptor Agonist GW4064 Inhibits Aromatase and ERbeta Expression in Human Endometriotic Stromal Cells. Reprod Sci, 1933719118808912, https://doi.org/10.1177/1933719118808912 (2018).
Acknowledgements
This research was supported in part by grants 81530040 (SWG), 81771553 (SWG) 81671436 (XSL) and 81871144 (XSL) from the National Science Foundation of China, and a grant for Shanghai Medical Center for Female Reproductive Disease (2017ZZ01016) from the Science and Technology Commission of Shanghai Municipality. This research was supported in part by grants 81530040 (SWG), 81771553 (SWG), 81671436 (XSL), and 81871144 (XSL) from the National Science Foundation of China, and a grant for Shanghai Medical Center for Female Reproductive Disease (2017ZZ01016) from the Science and Technology Commission of Shanghai Municipality.
Author information
Authors and Affiliations
Contributions
S.W.G. conceptualized the study and carried out its design, analyzed and interpreted data, drafted the manuscript, and prepared Figures. 6 and 7. Q.M.Q. performed all the experiments except NF-κB activation experiment, which was done by Q.Z., carried out data analysis together with S.W.G., and prepared all figures, and XSL recruited patients and secured specimens. All participated in the writing and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Qi, Q., Liu, X., Zhang, Q. et al. Platelets induce increased estrogen production through NF-κB and TGF-β1 signaling pathways in endometriotic stromal cells. Sci Rep 10, 1281 (2020). https://doi.org/10.1038/s41598-020-57997-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-57997-6
This article is cited by
-
The Role of Abnormal Uterine Junction Zone in the Occurrence and Development of Adenomyosis
Reproductive Sciences (2022)
-
Mechanisms and Pathogenesis of Adenomyosis
Current Obstetrics and Gynecology Reports (2022)
-
Evaluation of safety and effectiveness of gestrinone in the treatment of endometriosis: a systematic review and meta-analysis
Archives of Gynecology and Obstetrics (2022)
-
Regulation of aromatase in cancer
Molecular and Cellular Biochemistry (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.