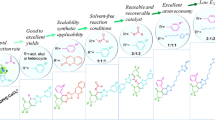
Abstract
A facile and efficient synthesis of tetrahydro-β-carbolines (tryptolines) in one step from tryptamine and aldehydes, in an environmentally friendly water solvent, has been investigated. This convenient and clean synthesis of various tryptolines was facilitated by l-tartaric acid, a natural compound, to obtain the desired products as clear crystals. Among the four crystalline products, the most substituted tryptoline 2 showed the best inhibitory activity against EJ cells and the least cytotoxicity, with an LC50 value of 1.49 mg/mL, against brine shrimp larvae.
Similar content being viewed by others
Introduction
The tetrahydro-β-carboline (tryptoline) moiety in the products of the Pictet-Spengler reaction has been observed in various synthetic and natural compounds. Tryptolines have been known to have many pharmacological and biological activities such as antimicrobial, antitumor, antiviral, and anticonvulsant activities, which has led to increased interest in the synthesis of these compounds1,2,3,4. Enantioselective tryptolines would be important for accessing useful chiral building blocks in complex alkaloid synthesis5. A number of enantioselective synthetic methods have been reported. Chirality can be introduced to the desired product by using asymmetric reduction methods using Noyori-type catalysis6. Better success has been achieved with Brönsted acid catalysts. Asymmetric catalysis of tryptoline products has been developed with N-acyliminium ions7 or N-sulfenyliminium ions8. More recently, chiral thiourea derivatives in combination with benzoic acid promote catalytic asymmetric Picted-Spengler reaction to provide unprotected tryptolines in high ee and yield9. However, most of the reported synthetic methods require organic solvents and expensive or hazardous catalysts10,11,12. Thus, the use of organic solvents in industrial organic reactions is limited because the presence of water in these solvents often results in low yields of the products and/or low reaction rates. Moreover, due to concerns over human health and the environment, environmentally friendly reactions have attracted greater attention from the viewpoint of green chemistry as compared to chemical processes that use organic solvents and toxic reagents. Thus, aqueous reactions are desirable since water is the safest and most inexpensive green solvent13,14,15,16. Aqueous reactions for the Pictet-Spengler cyclization are known, but they require harsh conditions or a large excess of strong acid catalysts. A variety of efficient catalytic systems, especially those based on Lewis or Brönsted acids, have been developed for these aqueous reactions, but most of the systems are expensive or hazardous7,17,18,19. Moreover, the products formed are racemic mixtures20.
In this study, we report a naturally abundant compound, l-tartaric acid, which facilitates the aqueous Pictet-Spengler reactions and affords the products as colorless crystals. l-tartaric acid facilitates the formation of the desired tryptolines 1–4 under mild conditions, in the absence of an organic solvent. This new method using water and l-tartaric acid is safe, inexpensive, and easy, and the tryptolines can be conveniently isolated by simple filtration as they are formed as crystals. The anticancer activity of tryptolines 1–4 against EJ cells was investigated, and their cytotoxicity was determined by the brine shrimp lethality assay21.
Results
We examined the substrate scope of the Pictet-Spengler reaction with four different aldehydes, as shown in Fig. 1. l-Tartaric acid facilitated the formation of colorless-crystalline tryptolines, as shown in Fig. 2. The optimum amount of l-tartaric acid was determined by carrying out the experiment with 0.1–1 equiv. of l-tartaric acid. We found that 0.5 equiv. of l-tartaric acid was sufficient to give the highest yield, as the yield did not improve when using more than 0.5 equiv. of l-tartaric acid. The reaction was carried out in water, which is a cheap and safe solvent. The reaction mixture was set still until the crystals formed, and the products were filtered without any additional workup. Tryptolines 1–4 were obtained in crystalline form and in fair yields (25–45%), and required no chromatography. The most substituted β-carboline 4 showed the best inhibitory activity against EJ cells.
Scope of the aqueous Pictet-Spengler reaction using tryptamine and four different aldehydes facilitated by l-tartaric acid. (1) 1-phenyl-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole, (2) 2,6-dimethoxy-4-(2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indol-1-yl)phenol, (3) 1-(4-methoxyphenyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]-indole, and (4) 1-cyclohexyl-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole.
1-Phenyl-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole (1)
Yield: 25% (crystalline). [α]25D = −0.48 (c = 0.5, MeOH). Mp. 240 ~ 243 °C. 1H NMR (500 MHz, MeOD) δ (ppm): 7.59 (d, 1H, J = 8.0 Hz), 7.56-7.53 (m, 3H), 7.48-7.46 (m, 2H), 7.33 (d, 1H, J = 8.0 Hz), 7.19 (t, 1H, J = 8.0 Hz), 7.12 (t, 1H, J = 8.0 Hz), 5.96 (s, 1H), 3.70-3.65 (m, 1H), 3.62-3.57 (m, 1H), 3.35-3.26 (m, 1H), 3.22-3.18 (m, 1H). 13C NMR (100 MHz, MeOD) δ (ppm): 137.1, 135.3, 130.6, 130.2, 129.3, 129.1, 126.3, 122.4, 119.5, 118.7, 112.1, 108.0, 56.0, 18.6. Microscope-FTIR (KBr pellet, cm−1) 3213 (s), 3051(w), 2904 (s), 2804 (s), 2748 (s), 2692 (s), 2675 (s), 2611 (s), 2571 (s), 2480 (w). LCMS [M + H]+ m/z calcd. for C17H17N2 249.1392; detected 249.1315.
2,6-Dimethoxy-4-(2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indol-1-yl)phenol (2)
Yield: 40% (crystalline). [α]25D = −2.76 (c = 0.5, MeOH). Mp. 206~210 °C.
1H NMR (500 MHz, MeOD) δ (ppm): 7.58 (d, 1H, J = 9.0 Hz), 7.34 (d, 1H, J = 8.0 Hz), 7.19 (t, 1H, J = 8.0 Hz), 7.12 (t, 1H, J = 8.0 Hz), 6.74 (s, 2H), 5.86 (s, 1H), 3.84 (s, 6H), 3.74-3.70 (m, 1H), 3.62-3.57 (m, 1H), 3.31-3.26 (m, 1H), 3.21-3.16 (m, 1H). 13C NMR (100 MHz, MeOD) δ (ppm):): 163.6, 139.3, 133.1, 129.7, 128.1, 128.0, 124.5, 121.5, 120.1, 116.6, 113.3, 109.7, 58.8, 56.8, 42.9, 20.4. Microscope-FTIR (KBr pellet, cm−1) 3433 (w), 3234 (w), 3059 (w), 2943 (w), 2902 (w), 2800 (s), 2646 (w), 1618 (s), 1523 (s), 1466 (s), 1427 (s), 1336 (s), 1238 (s), 1119 (s). LCMS [M + H]+ m/z calcd. for C19H21N2O3 325.1552; detected 325.1460.
1-(4-Methoxyphenyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]–indole (3)
Yield: 40% (crystalline). [α]25D = −2.04 (c = 0.5, MeOH). Mp. 242~244 °C.
1H NMR (500 MHz, MeOD) δ (ppm): 7.57 (d, 1H, J = 9.0 Hz), 7.33 (d, 1H, J = 8.0 Hz), 7.19 (t, 1H, J = 7.0 Hz), 7.12 (t, 1H, J = 7.0 Hz), 7.07 (d, 2H, J = 9.0 Hz), 5.89 (s, 1H), 3.86 (s, 3H), 3.67-3.63 (m, 1H), 3.59-3.54 (m, 1H), 3.11-3.07 (m, 1H), 3.30-3.24 (m, 1H), 3.20-3.15 (m, 1H). 13C NMR (100 MHz, MeOD) δ (ppm) 155.9, 137.1, 131.4, 130.3, 122.3, 120.2, 119.6, 119.2, 117.8, 115.2, 111.1, 107.6, 51.4, 39.8, 18.3. Microscope-FTIR (KBr pellet, cm−1) 3222 (s), 3014 (w), 2902 (s), 2790 (s), 2742 (s), 2567 (w), 1614 (s), 1516 (s), 1454 (s), 1430 9 s), 1307 (s), 1254 (s), 1180 (s), 741 (s). LCMS [M + H]+ m/z calcd. for C19H19N2O 279.1497; detected 279.1364.
1-Cyclohexyl-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole (4)
Yield: 45% (crystalline). [α]25D = −3.08 (c = 0.5, MeOH). Mp. 242~246 °C. 1H NMR (500 MHz, MeOD) δ (ppm): 7.51 (dd, 1H, J = 8.0 Hz, J = 2.5 Hz), 7.40 (d, 1H, J = 8.0 Hz), 7.18 (t, 1H, J = 7.0 Hz), 7.08 (td, 1H, J = 7.0 Hz, 0.5 Hz), 4.65 (d, 1H, J = 4.5 Hz), 3.79-3.74 (m, 1H), 3.47-3.42 (m, 1H), 3.18-3.11 (m, 1H), 3.11-3.07 (m, 1H), 2.35-2.29 (m, 1H), 1.95 (t, 2H, J = 10.5 Hz), 1.82 (t, 2H, J = 16.0 Hz), 1.53 (t, 1H, J = 10.0 Hz), 1.48-1.38 (m, 1H), 1.32-1.20 (m, 2H). 13C NMR (100 MHz, MeOD) δ (ppm): 137.3, 130.0, 126.8, 122.5, 119.8, 118.6, 112.3, 107.7, 58.2, 40.8, 30.2, 27.0, 26.7, 26.6, 18.9. Microscope-FTIR (KBr pellet, cm−1) 3219 (s), 3053 (w), 2933 (s), 2856 (m), 2763 (m), 1620 (s), 1454 (s), 1429 (s), 1307 (s), 1221 (s). LCMS [M + H]+ m/z calcd. for C17H23N2 255.1861; detected 255.1789.
The greater the substitution on the benzyl ring of the product, the better its potency to inhibit the growth of the EJ cells. Therefore, the most substituted tryptoline 2 showed the highest inhibitory activity against the EJ cells, as shown in Fig. 3. We speculated that tryptolines may need a bulky substituent for better binding affinity to the hydrophobic pocket of the target protein. This assumption is consistent with the fact that the clinically used anticancer drugs vinblastine and vincristine are dimers of tryptolines and are thus bulkier than monomeric tryptolines.
The cytotoxicity results for tryptolines 1–4 against brine shrimp larvae are shown in Fig. 4. Tryptolines 1, 3, and 4 showed weak cytotoxicity, with LC50 values of 0.26 mg/mL, 0.30 mg/mL, and 0.43 mg/mL, respectively. However, 2, which showed the highest inhibitory activity against the EJ cells, exhibited the least cytotoxicity with an LC50 value of 1.49 mg/mL. Tryptoline 2 showed no significant cytotoxicity (LC50 > 1.0 mg/mL) against brine shrimp larvae, as opposed to the positive control (K2Cr2O7), which showed significant cytotoxicity with an LC100 value of 0.1 mg/mL, over incubation period of 24 h. Interestingly, 2 showed the highest potency against the EJ cells but the least cytotoxicity as compared to 1, 3, and 4.
Discussion
In conclusion, a facile method for the synthesis of biologically active tryptolines using l-tartaric acid, a simple and natural compound, is developed. l-tartaric acid facilitated aqueous Pictet-Spengler reactions with four different aldehydes. Moreover, the colorless crystalline tryptolines formed could be directly filtered to avoid further isolation steps such as extractions, column chromatography or HPLC. All of the major crystalline products showed (S)-conformer as a dominant species, based on the optical rotations we measured, although these reactions were not highly enantioselective. With respect to the anti-cancer activity of the crystalline products 1~4, compound 2 showed the best cytotoxicity against EJ cell line. We believe that this is one of the safest and easiest methods to prepare the key intermediates in the first step of the synthesis of biologically active vinca alkaloids, especially at the industrial-scale.
Methods
General procedure for the synthesis of tryptolines
l-Tartaric acid (0.5 equiv., 0.5 mmol) was added to a mixture of tryptamine hydrochloride (1.0 equiv., 1.0 mmol) and aldehydes (1.0 equiv., 1.0 mmol) in a 15 mL falcon tube. The reaction volume was adjusted to 4.0 mL with water, and the tube was sealed. The tube was placed in a hot water bath at about 60 °C and set still for 1–2 days until crystals were formed. The crystals were then filtered, followed by rinsing with cold water and ether.
High-resolution UPLC/QTOF/MS analysis
Ultra-performance LC/MS analysis was performed on an ultra-high-resolution Q-TOF LC-MS/MS system (Micro QTOF III, Bruker Co.) using a C18 column, with a particle size of 1.7 mm, dimensions of 2.1 × 100 mm, and flow rate of 0.6 mL min−1, and an electrospray ionization (ESI) source.
MTT assay
EJ cells (3 × 105 cells/well) were seeded in 6-well plates, followed by treatment with the indicated concentrations of the compounds. After 24 h, the used medium was removed, and a fresh medium containing 10 μL of MTT solution (5 mg/mL) was added to the wells, which were then allowed to stand for 1 h. Subsequently, the medium was removed, and the cells were treated with 0.1 mL of dimethyl sulfoxide (DMSO). The absorbance was measured at a wavelength of 540 nm using a microplate reader. The cell viability was expressed as the percentage of the absorbance value determined for the control cultures.
Brine shrimp lethality bioassay
The brine shrimp lethality bioassay was carried out to determine the cytotoxicity of the tryptolines (1–4). The concentration that killed 50% of the shrimps (LC50) was determined, followed by the known method [17]. Two sets of these experiments were carried out in triplicate.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request. Complete experimental procedures and characterization data for products are in Supporting Information.
References
Laine, A. E., Lood, C. & Koskinen, A. M. P. Pharmacological importance of optically active tetrahydro-β-carbolines and synthetic approaches to create the C1 stereocenter. Molecules 19, 1544–1567 (2014).
Lee, H. Y., Yerkes, N. & O’Connor, S. E. Aza-tryptamine substrates in monoterpene indole alkaloid biosynthesis. Chem. Bio. 16, 1225–1229 (2009).
Deveau, A. M. et al. The synthesis of amino-Acid functionalized –carbolines as topoisomerase II inhibitors. Bioorg. & Med. Chem. Lett. 11, 1251–1255 (2001).
Mohr, J. T., Krout, M. R. & Stoltz, B. M. Natural products as inspiration for the development of asymmetric catalysis. Nature 455, 323–332 (2008).
Tayler, M. S. & Jacobsen, E. N. Highly enantioselective Catalytic Acyl-Pictet-Spengler reactions. J. Am. Chem. Soc. 126, 10558–10559 (2004).
Uematsu, N., Fujii, A., Hashiguchi, S., Ikariya, T. & Noyori, R. Asymmetric transfer hydrogenation of imines. J. Am. Chem. Soc. 118, 4916–4917 (1996).
Raheem, I. T., Thiara, P. S., Peterson, E. A. & Jacobsen, E. N. Enantioselective pictet-spengler-type cyclization of hydroxylactams: H-bond donor catalysis by anion binding. J. Am. Chem. Soc. 129, 13404–13405 (2007).
Wanner, M. J., van der Haas, R. N. S., de Cuba, K. R., van Maarseveen, J. H. & Hiemstra, H. Catalytic asymmetric Pictet-Spengler reactions via sulfenyliminium ions. Angw. Chem. Int. Ed. 46, 7485–7487 (2007).
Klausen, R. S. & Jacobsen, E. N. Weak brönsted Acid-thiourea co-catalysis: Enantioselective, catalytic protio-Pictet-Spengler reactions. Org. Lett. 11, 887–890 (2009).
Szawkalo, J. S. et al. Enantioselective syntheisis of some tetrahydroisoquinoline and tetrahydro-β-carboline alkaloids. Tetrahedron: Asym. 18, 406–413 (2007).
Allin, S. M., Gaskell, S. N., Elsegood, M. R. J. & Martin, W. P. A new asymmetric synthesis of the natural enantiomer of the indolizidino[8,7-b]indole alkaloid (+)-harmicine. Tetrahedron. Lett. 48, 5669–5671 (2007).
Huang, D., Xu, F., Lin, X. & Wang, Y. Highly enantioselective pictet-spengler reaction catalyzed by SPINOL-phosphoric acid. Chem. Eur. J. 18, 3148–3152 (2012).
Klijn, J. E. & Engberts, J. B. F. N. Fast reactions ‘on water’. Nature 435, 746–747 (2005).
Narayan, S. et al. On water: unique reactivity of organic compounds in aqueous suspension. Angw. Chem. Int. Ed. 44, 3275–3279 (2005).
Saha, B., Sharma, S., Sawant, D. & Kundu, B. Water as an efficient medium for the synthesis of tetrahydro-β-carbolines vis Pictect-Spengler reactions. Tetrahedron Lett. 48, 1379–1383 (2007).
Kitanosono, T., Masuda, K., Xu, P. & Kobayashi, S. Catalytic organic reactions in water toward sustainable society. Chem. Rev. 118, 679–746 (2018).
Desroses, M. et al. A facile and efficient synthesis of tetrahydro-β-carbolines. Tetrahedron Lett. 54, 3554–3557 (2013).
da Silva, W. A. et al. Novel supramolecular palladium catalyst for the asymmetric reduction of imines in aqueous media. Org. Lett. 11, 3238–3241 (2009).
Saeed, A. Oxa-pictet-spengler reaction in water. Synthesis of some (±)-1-aryl-6,7-dimethoxyisochromans. Chin. Chem. Lett. 21, 261–264 (2010).
Saito, A., Numaguchi, J. & Hanzawa, Y. Pictet-spengler reaction catalyzed by brönsted acid-surfactant-combined catalyst in water or aqueous media. Tetrahedron Lett. 48, 835–839 (2007).
Sasidharan, S., Darah, I. & Jain, K. In Vivo. and In Vitro. Toxicity study of Gracilaria changii. Pharm. Bio. 46, 413–417 (2008).
Acknowledgements
This work was supported by the National Research Foundation (NRF) of Korea, NRF-2017R1D1A1B03036334.
Author information
Authors and Affiliations
Contributions
H.J. Byun performed the experiments. K.H. Jung, G.S. Moon, S.K. Moon and H.Y. Lee interpreted and discussed the data. S.K. Moon and H.Y. Lee wrote the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Byeon, HJ., Jung, KH., Moon, GS. et al. A facile and efficient method for the synthesis of crystalline tetrahydro-β-carbolines via the Pictet-Spengler reaction in water. Sci Rep 10, 1057 (2020). https://doi.org/10.1038/s41598-020-57911-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-57911-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.