Abstract
The present work investigates biomass wastes and their ashes for re-use in combination with mineralised CO2 in cement-bound construction products. A range of biomass residues (e.g., wood-derived, nut shells, fibres, and fruit peels) sourced in India, Africa and the UK were ashed and exposed to CO2 gas. These CO2-reactive ashes could mineralise CO2 gas and be used to cement ‘raw’ biomass in solid carbonated monolithic composites. The CO2 sequestered in ashes (125–414 g CO2/kg) and that emitted after incineration (400–500 g CO2/kg) was within the same range (w/w). The CO2-reactive ashes embodied significant amounts of CO2 (147–424 g equivalent CO2/kg ash). Selected ashes were combined with raw biomass and Portland Cement, CEM 1 and exposed to CO2. The use of CEM 1 in the carbonated products was offset by the CO2 mineralised (i.e. samples were ‘carbon negative’, even when 10% w/w CEM 1 was used); furthermore, biomass ashes were a suitable substitute for CEM 1 up to 50% w/w. The approach is conceptually simple, scalable, and can be applicable to a wide range of biomass ashes in a closed ‘emission-capture’ process ‘loop’. An extrapolation of potential for CO2 offset in Europe provides an estimate of CO2 sequestration potential to 2030.
Similar content being viewed by others
Introduction
As the world’s population increases to 11.2 billion by the year 21001, the available resources to meet desired living standards must increase accordingly. By way of example, the supply of energy is projected to increase at an annual rate of 1.6%/yr, to 20302. Due to the increasing demand for energy, the Organisation for Economic Co-operation and Development (OECD) expect greenhouse gases (GHGs) to increase by 50% by 2050, and possibly to 750ppm by 2100, if no adequate management options are sought3.
The growing world population will drive the intensification of agricultural industrial activities, and as a consequence, larger quantities of waste/residues from both harvestable yield and non-harvestable biomass can be expected to be produced. The current global annual generation of all biomass waste including animal waste, is in the order of 140 Gt2,4, and when their disposal, utilisation and management are inappropriate, adverse environmental impacts arise.
In developing countries, most biomass residues are left in the field to decompose naturally or are burned in the open; impacting surface water and the atmosphere. By way of example, 1 tonne of landfilled dry, ash-free wood produces 0.73 ton CO25, whereas 1 tonne of fuel wood produces approximately 1.4 t or CO26.
Biomass burning contributes about 18% of total global emissions7,8, with 70% of this arising from use as domestic fuel, primarily by poor and agrarian communities9. The IEA projects that forestry and agriculture wastes will continue to increase10, with Asia and North America accounting for two-thirds of biomass wastes arising from crop production11.
If biomass residues have potential for other uses, their displacement should follow the “waste management hierarchy”, namely: prevention, re-use, recycling (including composting), energy recovery, and (only when no other options are available) disposal12. Low energy, low carbon management solutions that valorise waste are, therefore, a preferred option.
The valorised products from biomass ashes have potential to be significantly carbon negative in a ‘closed loop’ manufacturing process13. Biomass waste to energy plants emit 47 Mt CO2 each year14, and the ashes generated tend to be reactive to CO2 to a lesser or greater degree. By using ‘point source’ CO2 or flue gas capture in manufactured products made from ashes, this circular economic activity will reduce solid and gaseous emissions, landfilling and the extraction of virgin stone.
Concrete is the second most consumed material on Earth after water, with approximately 3.27 Gt currently produced, rising to 4.83 Gt by 203015. Andrews16 estimates that 39.3 ± 2.4 Gt CO2 were emitted by the cement industry during the period 1928–2016, with 90% being generated since 1990. As clinker production is currently growing at about 2.5% pa, generating 5–7% of total global CO2 emissions, technologies that reduce our reliance on cement or materials that can be used as a substitute are timely. Current estimates of the amount of CO2 emitted by cement production vary between 500–900 kg CO2/t cement produced, depending on the source of fuel and analysis methodology17,18.
As stated, a balance between positive and negative emissions is required in quest of meeting the commitments of 195 nations to hold the increase in the global average temperature to below 2 °C above pre-industrial levels under the Paris Agreement19,20. Technologies that transform waste and CO2 into products (e.g., construction materials, plastics and fuels) are now being developed and commercialised. The capture of CO2 from flue gas or the atmosphere as feedstock in value-added products is referred to as carbon capture and utilisation (CCU), including the mineralisation of CO2 in combination with solid waste21,22.
To date, the valorisation of biomass residues and their ashes has been largely overlooked, despite being ubiquitous, plentiful and rich in carbon. As developing countries, such as India, seek alternative resources to meet their infrastructure growth and emissions reduction needs23, valorised, sustainable biomass waste-based products may have a part to play.
World demand for construction aggregates will rise 5.2 percent annually to 51.7 Gt in 201924. In the UK for example, the construction industry accounts for 60% of all raw materials consumed25 and thus, low carbon waste-based construction materials are already available in the market.
The diversion of biomass wastes into construction products is being investigated in Europe26, as virgin materials resources are under increased pressure. However, the use of biomass ash with mineralised CO2 has not been explored for the manufacture of construction materials. The opportunity to combine biomass (waste) ash from energy plants with waste CO2 gas in an innovative CCU-based treatment step is another approach in the drive towards a sustainable materials supply chain for the construction industry.
In the present work, which is part of a wider study, we investigate the re-use potential of biomass waste for use in cement-bound construction products. Herein, we exploited the self-cementing property of biomass ashes when exposed to waste gaseous CO2. We applied a CO2 mineralization method to develop biomass waste (and their ashes) - based construction materials. As biomass ashes can self-harden as CO2 is mineralised, they could be used to replace cement in carbonated biomass-based construction materials; and potentially offset ‘carbon’. The utilisation of CO2 in waste-derived valorised products could help maintain a balance between point source emissions to the atmosphere and CO2 sequestrated in mineralised, valorised products.
Methods
Characterisation of biomass wastes
Biomass waste originating from agricultural and forestry activities, including wood, fruit peel, nut shell and fibre were sourced in India, Africa and the UK. These wastes were combusted into muffle furnace at 900 ± 25 °C. The temperature was raised gradually from 200 to 500 to 900 °C. Our primary aim was to produce a ‘mineral’ concentrate by fully combusting the biomass residues examined, and we used an uncontrolled heating rate to achieve this. The furnace specification stated that the tolerance was ±25 °C. The temperate was held at 900 °C for 4 h to ensure the full combustion of the biomass.
The resulting ashes were examined for their physical (e.g. particle size, bulk density, and ash content) and chemical (total carbon, elemental and phase) composition. The particle size distribution of ashes was measured by laser diffraction analysis (Malvern Mastersizer MS2000) and bulk density by loose compaction in cylindrical holders (expressed as kg/m3). Total carbon was analysed by the CHN analyser (FLASH EA 1112 Series), and the elemental composition was determined by X-ray fluorescence spectrometry (Philips LW1400 and XRFWIN software).
The BET surface area of biomass ashes was analysed with a surface area analyser (Micromeritics Gemini V2.00) using nitrogen adsorption measured as a function of relative pressure.
The biomass ashes were tested for their reactivity with CO2 under controlled moisture (20%) and pressure (~2 bars). The ashes were exposed to pure CO2 over four different cycles in a closed pressurised carbonation chamber. The first three cycles extended to one hour each, whereas the fourth cycle extended to 24 hours. The uptake of CO2 in ashes was determined by weight gain (% w/w).
Preparation and characterisation of products made from biomass wastes
-
(i)
Based on the mineralogy and CO2-uptake of biomass waste ashes, small cylindrical ash-only monolithic samples (7 mm × 7 mm) with 10–20% moisture were cast.
-
(ii)
These monoliths were exposed to pure CO2 for 24 hrs and evaluated for embodied carbon.
-
(iii)
CO2-reactive biomass ash was mixed with raw biomass and cast in larger (3.4 cm × 3.4 cm) cylinders. These cylinders also included biomass waste in combination, with Portland cement, CEM 1 (plus fine sand as a mineral filler) at 10–20% moisture to assess biomass ash as a substitute for CEM 1. Cylinders were exposed to pure CO2 for one week.
The CO2 uptake by both types of cylinders was calculated as CO2 equivalent from the total carbon analysed by CHN analysis. The strength of these monolithic products was evaluated by applying a force until the cylinders failed. The strength was calculated by using the Eq. 1:
where \({\sigma }_{c}\) is the compressive strength in megapascals, Fc is the fracture load in kilonewtons, Am is the mean area of the cylinder, and dm is the mean diameter of the cylinder.
For each sample, 5 cylinders were examined, and the average strength was calculated. The three axes of each cylinder were measured using digital callipers, and the load at failure determined (Mecmesin MFG250).
Assessment of CO2 uptake in valorised biomass products
The biomass ashes and resultant carbonate-cemented products were investigated by X-Ray diffractometry and back-scattered electron microscopy.
- (i)
X-Ray Diffractometry: The biomass ashes without and with CO2 exposure were analysed with a Siemens D500 diffractometer, fitted with a Siemens K710 generator using 40 kV voltage and 40 mA current, between 5–65° 2θ. The interpretation of diffractograms utilised DIFFRACplus EVA software (Bruker AXS).
- (ii)
SEM/EDAX analysis (JEOL JSM-5310LV, Oxford Instruments Energy Dispersive Spectrometer- EDAX) was performed upon polished resin blocks containing carbonated and un-carbonated biomass products.
Results
Reactivity of biomass ash with CO2
A range of biomass residues (including wood chips and shavings, nut shells, fibre, and fruit peel) obtained from Asia, Europe and Africa, were collected and characterised (Flash EA 1112 Series, CHN analyser). Following, they were thermally degraded, and the ‘mineral’ rich ashes were exposed to CO2 gas under controlled, static conditions. Four cycles of CO2 exposure were investigated (three 1-hour cycles, followed by a 24 hr fourth cycle of treatment) (see Table S2 in Supplementary Materials). The CO2 uptake, %w/w, was determined as a CO2 equivalent, derived from the total carbon observed in the ashes after each treatment step. A gradual increase in CO2 uptake was observed from the first to last CO2-exposure cycles, demonstrating that biomass ashes can be used to mineralise CO2 gas (via the formation of calcium carbonate cement) and thereby encapsulate the ‘raw’ biomass in solid carbonate-cemented monolithic composites.
An examination of ash mineralogy by X-ray diffraction (Siemens D500 diffractometer and DIFFRACplus EVA software- Bruker AXS) was used to examine the mineral phases present in the biomass ash and its carbonated counterpart. The results showed that the calcium oxide (CaO) in biomass ash reacted with the CO2 to produce calcium carbonate. This mineral induced strength development through carbonate-cementation.
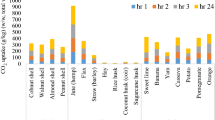
Incinerated biomass emitted 400–500 g CO2/kg, but when the ashes were carbonated, they sequestered –49-414 g of CO2/kg after 24 hrs of exposure to this gas (Fig. 1a; Table S3 in Supplementary Material). On a weight for weight basis, the CO2 ‘emissions’ and ‘uptakes’ were within the same range, however, the mass lost during thermal decomposition is far greater than that gained during carbonation of the ash.
(a) CO2 (equivalent) emission and capture potential (w/w%) of biomass residue ashes and ash cylinders, and their compressive strength in MPa (secondary vertical axis). (b) CO2 balance (g/kg), density (g/cm3), strength (KPa) and CO2 uptake (secondary vertical axis) (%) of valorised products from raw biomass with CO2-reactive ashes.
Some woody, fibre and nutshell-ash residues were observed to combine with significant amounts of CO2. Small cylindrical ‘ash-only’ monolithic samples (7 mm × 7 mm) were cast and exposed to CO2 for 24 hrs. The amount of CO2 up-taken varied between 147–424 g equivalent CO2/kg ash upon carbonation (Fig. 1a). These results show that all the biomass ashes investigated were CO2 reactive and produced mineral carbonates, which have potential to act as a carbonate-able binder, or as a partial substitute for hydraulic cements in certain applications involving Portland cement, CEM 1.
Following these findings, biomass ash was mixed with raw wood chips, nut shells or fruit peel and larger (3.4 cm × 3.4 cm) cylinders were cast and exposed to CO2. Strength was developed through carbonate cementation as previously observed. In addition, biomass ashes were investigated in combination with CEM 1 and fine sand as a mineral filler (Fig. 1b). Here, ash was used as a partial substitute for the cement during the production of the monolithic composites.
Impact of particle size and surface area
The biomass ashes had higher BET surface area than their raw counterparts (Table 1). The mineralisation of CO2 in biomass ashes is promoted by small particles as they have a higher surface area for reactions to proceed27,28. The addition of wood, nutshell and fruit peel-derived ashes to raw biomass enabled carbonation-induced cementation to produce a hardened composite.
Possan et al.29 reported that CO2 taken/used by mixed wood/coal ashes is largely regulated by surface area. However, particle size is not always the limiting factor for CO2 uptake. The findings of Nam et al.30 with municipal solid waste ash, showed the amount of CO2 sequestered increased as particle size decreased. This may well be valid for ashes with a similar chemistry, but where the amount of calcia varies in a feedstock, as seen in this work, particle size/surface area may be a secondary consideration.
The use of biomass residues in novel applications is of interest. Plant biomass, including dry miscanthus has been used for heavy metal removal from waste water31, but as a management strategy, large quantities of materials need to be utilised, such as in construction applications. The combination of biomass waste with cementitious binders to produce building materials has seen limited practice around the world32,33. Plant fibres derived from flax, hemp and straw are used to improve the mechanical properties of cement-based composites, but their durability performance is questionable. This results partly from the humid high pH environment (within the cemented product), as fibrous materials degrade via lime crystallisation and dissolution of cellulose and hemicellulose, and certain lignins34,35,36,37. Thus, the processing of biomass waste with mineralised CO2 as calcium carbonate may be expected to produce cemented product with a more favourable pH environment of <1038, thereby enhancing the durability performance of these fibrous residues.
The combined use of CEM 1 and ash at the different proportions up to 50% substitution was investigated. A comparison of the carbon dioxide ‘footprint’ of products enabled the embodied carbon in the ash-bound composites to be evaluated as a potential off-set to the use of CEM 1 (which is high in embodied carbon at ca. 670 kg eqCO2/t39).
The calculation of embodied CO2 involved all the materials employed, and the carbon dioxide gas uptaken showed the monoliths to be carbon negative, even when 10% w/w CEM 1 was used (Fig. 1b). Furthermore, the strength developed by the use of ash and a carbonation step were comparable to those of low density hydraulically-bound products used in construction applications40.
The major and minor elements including Ca, Fe, K, Mg, P, Na and Si in biomass were identified by X-ray fluorescence spectroscopy (XRF) (Philips LW 1400, with WIN Software) (see XRF results in Supplementary Material Table S2a–c). Selected key elements in biomass ashes are generally found in decreasing order of abundance as Ca > K > Si > Mg > Al (see Vassilev et al., for more information)41. The authors also reported a negative relationship between calcium and ash content in individual species of wood. We also observed a similar trend in wood (unpublished study of authors) and agricultural biomass. However, some of the soft peel residues had a high calcium and high ash content, which can be ascribed to their complex heterogeneous nature. For example, citrus fruit peel including orange has a high calcium content, and this seems to be a general finding with citrus fruit42.
The carbonation of biomass ashes was confirmed by X-Ray diffraction by the presence of calcite (Table 2; Fig. S1a–c in Supplementary Material). However, in some samples the presence of Ca(OH)2 and CaO indicated that complete carbonation was not always achieved.
Back scattered electron microscopy (Jeol JSM-5310 LV; Oxford Instruments EDS) was used to examine the microstructure of carbonated ashes and the distribution of key elements. The element maps obtained for Ca confirmed the presence of calcium carbonate, which gave rise to 3 distinct microstructures (see Fig. 2). Figure S2 in Supplementary Material gives the EDS spectra taken from the three carbonated samples. The 3 distinct microstructures observed comprised:
- (i)
Amorphous precipitates of carbonate often associated with relict (biomass) structures; forming a continuous cementing phase. Some of the relict structures were completely filled with carbonate. This microstructure was associated with well-cemented/hardened monolithic samples.
- (ii)
A more discrete distribution of carbonate precipitates occurring mainly on the surface of relict structures, especially at the point of contact between individual ash particles. These observations were also associated with well-cemented monolithic specimens.
- (iii)
Isolated, finely divided carbonate precipitates (typically ≤1 µm), distributed throughout the ash, with occasional agglomerations of approximately 5 µm in size. This microstructure was associated with lower-strength monolithic specimens.
Back Scattered Scanning Electron Micrographs of CCU-treated ash cylinders: (Type i) - Mixed wood ash, showing relict-planty structures enveloped by massive carbonated precipitates; (Type ii)- Nut shell-derived ash cemented by interstitial carbonate; (Type iii)- Wood ash, with dispersed, discrete precipitates of carbonate. This ash was hygroscopic, displayed mircocracking and low strength (Note: Image 1 is taken at a slightly higher magnification for clarity).
Figure S3(a–c) in Supplementary Material gives further examples of the microstructure of carbonated ash monoliths/cylinders observed together with their element maps, showing the spatial distribution of specific elements.
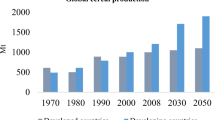
Our study has clearly demonstrated that the mineralisation of CO2 gas in biomass-based monolithic composites is possible, and that even when used as a 50% substitute for CEM 1, meaningful quantities of carbon can be mineralised. The indicative carbon savings that can be realised on a broader scale are illustrated in Fig. 3. It should be noted, however, that composite materials using selected biomass ash as a substitute for hydraulic cement will require rigorous selection and independent testing to ensure compliance with international materials-related standards before the approach outlined could be adopted commercially.
Our findings also suggest there may be further added value to be gained as it is possible to extract, for example, proteins and fibres from biomass, before these residues are ashed and used as a carbonate-able binder. This particular work, which is in progress, will be reported separately.
Discussion: A way forward
The utilisation of CO2 transformation technologies ideally involves stripping CO2 from a point source, or from the atmosphere before ‘storing’ it in a mineralised product. Our work shows this could be achieved by combining ‘raw’ biomass with their CO2-reactive ashes in a substitute for a hydraulic cement. The use of biomass ash in this way could help developing countries manage waste more effectively and reduce environmental impacts, whilst also providing for additional resources of building materials.
As an illustration of this potential, e.g., India is an emerging agro-based country with 159 M ha of arable land supporting ca. 800 Mt of agricultural/horticultural production, with 500–550 Mt/yr of waste arising7,34. There are significant surplus agricultural residues (raw biomass), estimated between 84–141 Mt per annum, with a further 90–140 Mt of ash, generated by burning on farm7,43.
As the Indian construction industry accounts for nearly 65% of total infrastructure investment, generating 11% of GDP (10640 billion INR, in 2016–17)44,45, the demand for construction materials in India is considerable. The use of aggregates alone is anticipated to reach 5 Gt by 202046.
Similarly, significant agricultural residues are generated in Europe. Approximately, 276 Mt/p.a. of residues are generated from major cereal and oil-based crops47. From our studies, we can assume that 70% of these residues produce CO2-reactive ashes. Thus, if the average ash content is 5% (w/w dry weight) of that burned, and the CO2 reactivity is of the order of 10% (w/w dry weight) there is potential to mineralise about 1.0 Mt of CO2 in 10 Mt of ash in Europe. Furthermore, these reactive ashes can be used to carbonate-cement the remaining 30% non-reactive biomass residues (utilising 83 Mt of raw residues from cereal and oil crops) into useful monolithic products. The available residue from these cereal and oil crops in Europe is projected to be 340 Mt by 2030, which will further mineralise 1.2 Mt CO2 directly in ash and also indirectly in raw residues replacing cement. On a global scale, the projections for 2050 indicate an increase in demand for all biomass, with a larger proportion from agricultural residues being used to produce energy48.
A successful CCU-based treatment of biomass wastes, therefore, has potential to contribute to meeting this demand, especially if the residues can be used to replace carbon intensive materials, such as hydraulic cements (which may contribute 5% of the annual man-made carbon emissions worldwide40,49.
Our study indicates that a significant carbon positive ‘source’ of waste can be converted into a carbon negative ‘sink’, whilst producing products with potential value. The utilisation of biomass waste and their ashes combined with mineralised CO2 could help close the ‘emission-capture’ process ‘loop’, whilst also providing a sustainable management route for residues that have adverse environmental impacts. This method is conceptually simple, scalable, and potentially applicable to a wide range of biomass ashes produced in energy plants.
A diverse range of high-volume biomass residues appear to be suitable for processing with CO2 gas that would otherwise be emitted to atmosphere. The possibility of burning biomass for energy and then trapping the CO2 generated in a mineralisation step appears feasible. Thus, the processing of biomass waste via thermal destruction coupled with CO2 capture and a carbonation step, will reduce the environmental impacts and pestilence associated with these wastes, and help preserve virgin resources.
Data availability
Data is available as specified in the cited references. Data on research findings from this work is available in tables and figures.
References
UNEP (United Nations Environment Programme) Converting waste agricultural biomass into a resource. United Nations Environment Programme Division of Technology, Industry and Economics International Environmental Technology Centre, Osaka/Shiga, Japan, www.unep.org/ietc/Portals/136/Publications/Waste%20Management/WasteAgriculturalBiomassEST_Compendium.pdf (2015).
IEA. International Energy Agency, Extended Energy Balances, OECD/IEA, Paris, www.iea.org/w/bookshop/add.aspx?id=453 (2013).
OECD, OECD Environmental Outlook to 2050: The Consequences of Inaction, OECD Publishing, Paris, www.oecd.org/environment/outlookto2050 (2012).
Chun, A. M. S. Ground Rules for Humanitarian Design, A. M. S. Chun and E. Brisson Eds. (Wiley Publishers, London), ISBN 978-1-118-36159-7 (2015).
Lame, J. Carbon negative impacts from biomass conversion. Biof. Dig. http://www.biofuelsdigest.com/bdigest/2017/01/04/carbon-negative-impacts-from-biomass-conversion/ (2017).
MacCarty, N. et al. Laboratory comparison of the global-warming potential of six categories of bomass cooking stoves. Aprovecho Research Center, http://stoves.bioenergylists.org/stovesdoc/Aprovecho/gwp/Global_Warming_Potential_of_Six_Types_of_Stoves_Aprovecho_9-6-07.pdf.
FAO, World agriculture: towards 2015/2030 Summary Report, ISBN 92-5-104761-8, http://www.fao.org/3/a-y3557e.pdf (2002).
Jain, N., Pathak, H. & Bhatia, A. Sustainable management of crop residues in India. Current Adv. Agri. Sci. 6(1) ISSN: 0975-231, 51–9 (2014).
IEA. International Energy Agency, CCC World forest and agricultural crop residue resources for co-firing. ISBN 978-92-9029-571-6, https://www.usea.org/sites/default/files/042015_World%20Forest%20and%20agricultural%20crop%20residue%20resources%20for%20cofiring_ccc249.pdf (2015).
IEA, International Energy Agency, Sustainable Production of SECOND -Generation Biofuels Potential and perspectives in major economies and developing countries, https://www.iea.org/publications/freepublications/publication/second_generation_biofuels.pdf (2010).
Rose, S. et al. Bioenergy in energy transformation and climate management. Climatic Change. 123, 477–493 (2014).
UNEP, R Efficiency: Economics and Outlook for Asia and the Pacific, CSIRO Publishing, Canberra (2011).
Tripathi, N., Hills, C. D., Singh R. S., Atkinson, C. J. Biomass waste utilisation in low-carbon products: harnessing a major potential resource”, npj Climate and Atmospheric Science, 2(35), https://doi.org/10.1038/s41612-019-0093-5 (2019).
IEA Bioenergy. Mobilisation of agricultural residues for bioenergy and higher value bio-products: Resources, barriers and sustainability. Task 43, 2017:01, https://www.ieabioenergy.com/wp-content/uploads/2018/01/TR2017-01-F.pdf (2017).
Statista, Major countries in worldwide cement production from 2012 to 2017 (in million metric tons), https://www.statista.com/statistics/267364/world-cement-production-by-country/ (2018).
Andrews, R. Global CO2 emissions from cement production. Earth Syst. Sci. Data 10, 195–217, https://doi.org/10.5194/essd-10-195-2018 (2018).
Dietz, S., French E. & Jackson, P. Carbon performance Assessment of Cement Producers; Note on Methodology. Transition pathway Initiative. 19th September, http://www.lse.ac.uk/GranthamInstitute/tpi/wp-content/uploads/2017/09/Methodology-note-for-cement-8-Aug.pdf accessed on 25thNovember 2018 (2017).
Savage, N. Carbicrete: Steel in, carbon out. Nature. 545(S15), 18 May (2017).
Rogelj, J. et al. Zero emission targets as long-term global goals for climate protection. Environ. Res. Lett. 10, 105007 (2015).
Sanderson, B. M., O’Neill, B. C. & Tebaldi, C. What would it take to achieve the Paris temperature targets? Geophys. Res. Lett. 43, 7133–7142 (2016).
Gunning, P. J., Hills, C. D. & Carey, P. J. Accelerated Carbonation Treatment of Industrial Wastes. Waste Manag. 30, 1081–90 (2010).
Flannery, T.A. ‘Third Way’ to Fight Climate Change. The opinion pages. New York Times, http://www.nytimes.com/2015/07/24/opinion/a-third-way-to-fight-climate-change.html?_r=0 (2015).
Press Release- Indian Standards for alternative materials to natural sand and other natural resources, www.bis.org.in/other/PR_NSNR.pdf.
World Construction Aggregates- Demand and Sales Forecasts, Market Share, Market Size, Market Leaders. Freedonia group study no. 3389, https://www.freedonia group.com/World-Construction-Aggregates.html (2016).
https://www.theengineer.co.uk/building-materials-organic-waste/Building materials made from organic waste could help boost bio-economy.
Klimesch, D. S. & Ray, A. Autoclaved cement-quartz pastes: the effects on chemical and physical properties when using ground quartz with different surface areas part ii: results of accelerated carbonation. Cement and Concrete Research 27, 1073–1078 (1997).
Arandigoyen, M., Bicer-Simsir, B., Alvarez, J. I. & Lange, D. A. Variation of microstructure with carbonation in lime and blended pastes. Applied Surface Science 252, 7562–7571 (2006).
Possan, E., Thomaz, W. A., Aleandri, G. A., Felix, E. F. & Santos, A. C. P. CO2 uptake potential due to concrete carbonation: A case study. Case Studies in Construction Materials 6, 147–161 (2017).
Nam, S. Y., Seo, J., Thriveni, T. & Ahn, J. W. Accelerated carbonation of municipal solid waste incineration bottom ash for CO2 sequestration. Geosystem Engineering 15(4), 305–311 (2012).
Osman, A. I., Ahmed, A. T., Johnston C. R., Rooney, D. W. Physicochemical characterization of miscanthus and its application in heavy metals removal from wastewaters. Environmental progress and Sustainable Energy (AIChE), https://doi.org/10.1002/ep.12783 (2017).
Amziane, S., Arnaud, L. Bio-aggregate-based building materials: Applications to hemp concretes. Wiley-ISTE (2013).
Chevanan, N. et al. Bulk density and compaction behaviour of knife mill chopped switchgrass, wheat straw, and corn stover. Bioresour. Technol. 101, 207–214 (2010).
Guéguen, M., Moscardelli, S., VanSchoors, L., Nour, I. & Marceau, S. Study of the microbial development impact on bio-based building materials.Proc 1st Int Conf on Bio -Based Build Mater, Amziane, S., Sonebi, M., Clermont -Ferrand Ed. (France), 21–24 June 2015, RILEM Publications. 166–169 (2015).
Sonebi, M., Wana, M., Amziane, S. & Khatib, J. Investigation of the mechanical performance and weathering of hemp concrete. Proc 1st Int Conf on Bio -Based Build Mater, Amziane, S., Sonebi, M., Clermont -Ferrand Eds. (France), 21–24 June 2015, RILEM Publications. 166–169 (2015).
Castel, Y., Amaziane, S. & Sonebi, M. Durabilité du béton de chanvre: résistance aux cycles d’immersionhydrique et séchage. IéreConférenceEuroMaghrébine des BioComposites,BioComposites, 28–31 March 2016, Marrakech (2016).
Filho, R. D. T., Silva, F. D. A., Fairbairn, E. M. R. & Filho, J. D. A. M. Durability of compression molded sisal fiber reinforced mortar laminates. Const. Build. Mat. 23(6), 2409–2420 (2009).
Bertos, M. F., Simons, S. J. R., Hills, C. D. & Carey, P. J. A review of accelerated carbonation technology in the treatment of cement-based materials and sequestration of CO2. J Haz. Mat. 112, 193–205 (2004).
Cement industry counts its carbon. Sustainable Business Magazine, 25 Sept, 2008. https://www.edie.net/library/Cement-industry-counts-its-carbon/4797
Gunning, P. J., Antemir, A. C. D., Hills & Carey, P. J. Secondary aggregate from waste treated with carbon dioxide. Proc. Institution of Civil Engineers: Construction Materials 164(CM5), 231–239, https://doi.org/10.1680/coma.1000011 (2011).
Vassilev, S. V., Vassileva, C. G., Song, Y. C., Li, W. Y. & Feng, J. Ash contents and ash-forming elements of biomass and their significance for solid biofuel combustion. Fuel 208, 377–409 (2017).
Sweitzer, J. Five calcium rich fruits for healthy teeth. Cirocco Dental Center, https://www.ciroccodentalcenterpa.com/food/5-calcium-rich-fruits-for-healthy-teeth/ (2018).
NAAS. Management of Crop Residues in the Context of Conservation Agriculture”. Policy Paper No. 58, National Academy of Agricultural Sciences, New Delhi 12 p. (2012).
Indian Mirror, Indian Construction Industry at a glance in 2011–2012, http://www.indianmirror.com/indian-industries/2012/construction-2012.html (2012).
Statistics Times, Ministry of Statistics and Programme Implementation, Planning Commission, Government of India, Sector-wise contribution of GDP of India, http://statisticstimes.com/economy/sectorwise-gdp-contribution-of-india.php (2017).
Aggregates Business International, Booming Indian aggregates market, http://www.aggbusiness.com/sections/market-reports/features/booming-indian-aggregates-market/ (2013).
https://www.theengineer.co.uk/building-materials-organic-waste/Buildingmaterials made from organic waste could help boost bio-economy.
ICCT. Availability of cellulosic residues and wastes in the EU. White paper, International Council on Clean Transportation. Washington, http://theicct.org/sites/default/files/publications/ICCT_EUcellulosic-waste-residues_20 131022.pdf (2013).
Baruya, P. World forest and agricultural crop residue resources for co-firing. IEACCC, CCC/249. ISBN: 978-92-9029-571-6 (2015).
Acknowledgements
The authors acknowledge the University of Greenwich and CSIR-Central Institute of Mining and Fuel Research for laboratory resources and technical assistance enabling to conduct the research. NT and CDH thank Prof. Chris Atkinson for help in sourcing the UK and African biomass wastes; and to Andrew Hurt and Zoltan Hiezl for technical support.
Author information
Authors and Affiliations
Contributions
N.T., C.D.H. and R.S.S. conceptualised and designed the research work; N.T. performed the experiments; N.T., C.D.H., R.S.S. and J.S.S. jointly wrote the paper. C.D.H. and R.S.S. are considered “co-first author”.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tripathi, N., Hills, C.D., Singh, R.S. et al. Offsetting anthropogenic carbon emissions from biomass waste and mineralised carbon dioxide. Sci Rep 10, 958 (2020). https://doi.org/10.1038/s41598-020-57801-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-57801-5
This article is cited by
-
Design strategy for effective passion fruit waste bioconversion with crude fungal enzyme extracts
Biologia (2023)
-
Valorisation of agricultural biomass-ash with CO2
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.