Abstract
It will get entirely unusual derivatives with gratifying chemical bonding schemes for boron clusters by doping with lithium, the lightest alkalis. The geometric structures and electronic properties of the LiBn0/− (n = 10−20) clusters have been studied through Crystal structure AnaLYsis by Particle Swarm Optimization (CALYPSO) structural search approach along with the density functional theory (DFT) calculations. The low-lying candidates of LiBn0/− (n = 10–20) are reoptimized at the B3LYP functional in conjunction with 6–311 + G(d) basis set. Three forms of geometric configurations are identified for the ground-state structures of LiBn0/− clusters: half-sandwich-type, quasi-planar and drum-type structures. The photoelectron spectra (PES) of the LiBn− clusters have been calculated through time-dependent density functional theory (TD-DFT). A promising LiB13 with tetrahedral-typed B13 ligand half-surround cluster and robust stability is uncovered. The molecular orbital and adaptive natural density partitioning (AdNDP) analysis show that B-B bonds in the B13 moiety combined with the interaction between the B13 shell and Li atom stabilize the C2v LiB13 cluster. Our results advance the fundamental understanding about the alkali metal doped boron clusters.
Similar content being viewed by others
Introduction
It is a significant milestone in the nanomaterials science that Kroto et al. discovered the C60 fullerene1, which aroused a surge of research activities about carbon. As the neighbor of carbon, boron has aroused much research2,3,4,5,6,7,8 because of its potential application and electron deficiency9,10. B4011, the boron analogue of C60, has also been experimentally observed12. Researches over the past few years manifested that the lowest-energy structures of the Bn−/0/+ clusters tend to be planar\quasi-planar. Detailly, the ground-state structures of the Bn− clusters keep to be planar\quasi-planar till n = 36 (B36−)13,14,15, n = 20 for the Bn clusters16 and n = 16 for the Bn+ clusters17. Soon after, the evolution from planar to fullerene-like structures, B39−18, for the Bn− clusters appeared when n approximately equal to 40. For the positive one, the cage-like structure which can be seen as a new borospherene contains 39 boron atoms19.
It will open a new chapter for boron clusters by doping with metal atom, which can get entirely unusual derivatives with gratifying chemical bonding schemes. In the last few years, there were great deals of reports about transition-metal-doped (TM-doped) boron clusters. These studies indicate that the configurations of the TM-doped boron clusters are contrast to that of pure boron clusters. TM-centered boron rings, Co@B8−, V@B9−, Nb@B9−, Ta@B9−, Ru@B9−, Ta@B10−20,21,22, have been experimentally observed. Subsequently, the Co-centered drum-type structure of CoB16− cluster23 and the half-sandwich-type structure of Co/Ru/IrB12− cluster24,25 have been found by Wang and coworkers. In addition, RhB18− cluster26 has been found that there are two different configurations for the ground state and both of the structures have been observed experimentally. In the year of 2017, a unique tubular molecular rotor has been observed for the global minimum of the TaB20− cluster27. The MnBn−/0/+ (n = 10−20) clusters have been systematically studied to reveal the geometric constructions of middle-sized boron clusters after doping of a manganese atom28. Not only the transition metals, other metallic elements also have been used as dopants. The Al2+[B73−] and Al+[B82−]29 clusters, both of which are umbrella-type structures, have been observed through experimental and theoretical studies. PrB7−, a rare-earth-atom-doped boron cluster, has been researched through photoelectron spectroscopy30. Doping of alkali metal atom into B3− cluster, which can get stable AM+[B3−] (AM = Li-Cs) cluster, has been theoretically predicted31. In addition, Alexandrova and coworkers32 found a stable C2v LiB6− cluster using the ab initio study. There is also a theoretical studies of the small-sized LiBn (n = 1–8) cluster33.
Although there are some studies about the Li-doped boron clusters, all the previous investigations are almost small-sized clusters except for LiB1234 and LiB2035. It is of crucial importance for us to have a systematical study of middle-sized Li-doped boron clusters. Here, extensive structure searches of the LiBn cluster were carried out using the CALYPSO structural search method. Subsequently, the predicted structures were reoptimized through the DFT calculations. Furthermore, we discussed the inherent stabilities of the lowest-energy structures of the LiBn clusters. Moreover, the theoretical PES of the ground-state anionic structures had been simulated. To interpret the stabilization mechanism, chemical bonding analyses were carried out.
Results and Discussions
Geometrical structures
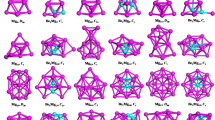
The calculated low-lying isomers of neutral and anionic LiBn (n = 10–20) clusters are exhibited in Figs. 1 and 2, respectively. Each isomers of the cluster is denoted by the labels: na/−, nb/− or nc/−. In brief, n is the number of B atoms of the corresponding cluster; a, b, c indicates the ground-state structure and the metastable structure, alphabetically. Beside the label, there is also the point group symmetry and relative energy (eV) of the corresponding structure. (The corresponding lateral views of the low-lying isomers are shown in Figs. S1 and S2 in the Supplementary Information.) As presented in Figs. 1 and 2, most of the structures, when n ≤ 15, grasp high point group symmetry. The lowest-energy structures of the LiBn0/− (10 ≤ n ≤ 15) clusters present in forms of half-sandwich type, while for n ≥ 16 turn out to be quasi-planar and drum-type structures. Both the anionic and neutral ground-state structures of LiB10–LiB15, except for LiB11, are presented as half-surrounded structures, where the Bn moiety half surround to the Li atom. For the a/a− structure of the LiB10 cluster, the B10 shell is a quasi-plan with two inner B atoms surround by an outer B8 ring. As exceptions, the structures of LiB110/− are plane structures. For the rest half-sandwich-type structures, the Bn fragments of 12a/a− and 14a are more flat than that of 13a/a−, 14a− and15a/a−. It should be noteworthy that the B13 ligand of the 13a/a− is no longer planar or quasi-planar but a tetrahedral-typed structure, which is in stark contrast to that of pure B13 cluster36 through doping of a Li atom. All of the 16a/a−, 17a/a− and 19a have a pattern that a quasi-planar Bn moiety with a doped Li atom connected to it, showing a quasi-planar structure. 19a− possesses an intermediate structure of quasi-planar and half-sandwich-type. Both the 20a and 20a− are beautiful drum-type structures. However, there are some distinctions between the structure of 18a/a− and 20a/a− where the Li atom is outside of the B18 drum. It is worth noting that the acquisition of an electron has little influence on the lowest-energy structures of LiBn0/− clusters except for LiB13 and LiB19. Diversely, there are some changes for the low-lying isomers due to the acquisition of an electron.
Photoelectron spectra
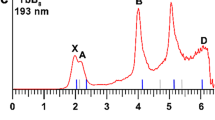
To deeply understanding the electronic structures of LiBn cluster, the simulated PES of LiBn− (n = 10–20) clusters are displayed in Fig. 3. The calculated PES together with the laboratorial PES of LiB6− cluster32, a smaller species than our LiBn cluster, are displayed in Fig. S3 in the Supplementary Information. For each spectrum, the location of the first peak (X peak) indicates the vertical detachment energy (VDE), which denotes electronic detachment transition from the ground-state anion to the corresponding ground-state or excited-state neutrals. The VDE values of the LiBn− clusters are presented in Table S1 in Supplementary Information. The calculated spectrum of LiB10− is sparse and the VDE is about 2.29 eV. Inversely, the spectrum of LiB11− expresses a compact spectral pattern except for the X peak, which located at 1.47 eV. There are four major peaks of the LiB12− PES and the VDE is about 2.48 eV, which corresponding to the position of the first peak. For LiB13−, there are five peaks and the VDE value is 2.44 eV. For the theoretical spectrum of LiB14−, the X Peak and A peak are very similar in shape and the X peak is located about 3.06 eV. The spectral pattern of LiB15− PES is crowded, of which the VDE value is 2.84 eV. There are five peaks of the LiB16− PES, of which the VED is 3.18 eV. The PES of LiB17− is compact and the value of VDE is about 2.76 eV. There are five peaks of the PES of LiB18− and the first peak is located at 2.73 eV. The VDE of LiB19− is 2.37 eV and the X peak is far from the other three peaks. There are four sharp peaks in the sparse PES of LiB20−, of which the VDE value is 2.50 eV.
Relative stabilities
In our recent work, the inherent stabilities of the LiBn0/− clusters are determined by three criteria: average binding energy (Eb), second-order differences of energy (Δ2E) and the energy gap (Egap). First, the Eb of LiBn0/− clusters are calculated according to the following formulas:
Among these, E express the energy of the matching atom or cluster. The values of Eb of the ground-state clusters are presented in Table 1 and visualized in Fig. 4(a). Larger Eb value is the representation of a stronger chemical stability. The anions LiBn clusters are more inert than the neutral ones for that the neutral Eb curve sits below the anionic curve. It is obviously that both the anionic and neutral curves roughly increase with augmentation of the cluster size n, indicating that it is easier for the formation of the larger cluster. It is worth noting that, there is a distinct heave in the neutral curve when n = 11.
As a sensitive argument to reflect the relative stability, the Δ2E of the lowest-energy LiBn0/− clusters is defined as:
Both the neutral and anionic Δ2E values can be fined in Fig. 4(b). Fig. 4(b) presents that the LiB11, LiB13, and LiB17 clusters along with the LiB12−, LiB14−, LiB16− and LiB18− clusters possess obviously higher Δ2E values which indicating their enhanced stability relative to the immediate neighbors.
The Egap between the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) is also a signature of the stability of the corresponding cluster. The higher Egap values the stronger chemical stability of the given cluster. The calculated Egap values of the lowest-energy LiBn0/− clusters are presented in Table 1 and Fig. 4(c). It is distinct to notice that both of the Egap curves express the even-odd oscillation behavior. There are two obvious outliers for LiB11 and LiB13 cluster suggesting that they are more inert than the others. Accordingly, we can determine that, from the results of Eb, Δ2E, and Egap above, the neutral LiB11 and LiB13 cluster can be viewed as “magic” clusters which are chemically inert.
Chemical bonding analysis
The molecular orbital (MO) and AdNDP analyses are presented to fully grasp the bonding mechanism of lithium-doped boron clusters. LiB13 (a half-sandwich-type structure, C2v) is chosen as a representation for its chemical stability and unusual geometric structure. There are eleven pictorial MOs near the HOMO of LiB13 along with their corresponding energy levels in Fig. 5. The LUMO of LiB13 cluster is a σ-hybrid orbital and primarily formed of 2p atomic orbital (AO) of boron atom. For the rest MO of LiB13, the nondegenerate HOMO, HOMO-8 and HOMO-9 orbital, which formed by p-type AO of B atom and s-type AO of Li atom, are σ orbital. However, as σ orbital, HOMO-1 is predominantly composed by B p-orbital of the B13 atoms moiety. The HOMO-a (a = 2–7) feature π orbital are primarily p-type AOs of the B atom. Based on the analyses, it has been found that the 2p AOs of boron atoms have made necessary contributions for these molecular orbital. Meanwhile, stabilize of the LiB13 structure is attributed to the mutual effect between the Li atom and the B13 fragment.
To have a further understanding of the bonding properties of LiB13 cluster, the chemical bonding analysis by the means of AdNDP code was made. AdNDP algorithm describe the chemical bonding in terms of nc−2e (1 ≤ n ≤ the maximum number of atoms), which are obtained by partitioning the valence electron density matrix. The visualized results of LiB13 cluster together with their occupation number (ON) are presented in Fig. 6. All of the ONs approximately equal to the ideals values which lending credence to the AdNDP results we obtained. We divided these bonds into two categories: one work for the partial stability in the B13 shell and the other provide overall stability between the B13 moiety and the impurity atom Li. First, there are seventeen bonds on the B13 moiety and all of them are σ bond. The interactions of the B atoms in the peripheral B8 ring and the middle B4 ring are visualized by twelve σ bonds (eight 3c–2e bonds with ON = 1.95–1.96|e| and four 6c–2e bonds with ON = 1.97|e|). Meanwhile, the two 4c–2e σ bonds (ON = 1.94|e|) reveal the interactions between apical B atom and the four B atoms in the middle. Among the rest bonds, 11c–2e σ bonds, 12c–2e σ bonds and 13c–2e σ bonds, all the occupation numbers are 2.00|e|, also describe the B-B interaction in the B13 fragment. Then, there are three bonds for the integrity stability between the Li atom and the B13 shell and all of the three ON are 2.00|e|. Both of the 9c–2e π bonds and 10c–2e π bonds, formed by the 2 s orbital of Li atom the 2p orbital of B atoms, visualize the π mutual effect between the Li atom and part of the B13 shell. The connection between the entire B13 fragment and the Li atom is presented by the delocalized 14c−2e σ bond. Overall, both of the two groups of bonds earn substantial stabilization for the LiB13 cluster.
Conclusion
As a conclusion, we have presented a systematical research of the neutral and anionic lithium-doped boron clusters through CALYPSO structural search approach and DFT calculations. The lowest-energy geometric structures of LiBn0/− (n = 10–20) clusters are determined. The evolution of the lowest-energy structures is that: half-surrounded structures for size n from 10 to 15 and quasi-planar or drum-type structures for the larger species (n ≥ 16). The inherent stabilities have been analyzed by the average binding energy, second-order differences of energy and HOMO–LUMO energy gaps. A new tetrahedral-typed B13 ligand half-surround LiB13 cluster with high stability is identified. The molecular orbital and AdNDP analysis of the neutral LiB13 cluster suggest that B-B σ bonds in the B13 fragment combined with the strong interaction between B13 shell and Li atom stabilize the C2v LiB13 cluster. This finding may provide guidance to future synthesis of boron-based nanomaterials.
Computational Details
Our structural search of neutral and anionic lithium-doped boron clusters are implemented by utilizing CALYPSO code37,38,39,40. The capability of this method has been successfully confirmed in structural prediction of several systems with only given the chemical composition41,42,43,44,45. We have followed fifty generations to achieve the global-minimum structures during the structural predictions. Every generation contains thirty structures. Thus, we can obtain about 1500 isomers for each cluster size n. The top fifty low-lying candidates are selected and re-optimized using the all-electron DFT theory via the Gaussian 09 package46. The optimization calculations are performed using the B3LYP functional47,48 with 6–311 + G (d) basis set49, which is chosen for both of the B and the Li atom. Multiple spin states up to sextet and quintet are fully involved for the structure optimization. Vibration frequencies calculations are performed to ensure all the isomers are global-minimum. The PES of the ground-state LiBn− clusters are calculated by means of the TD-DFT50. To further understanding the bonding mechanism of Li-doped boron clusters, the molecular orbital and AdNDP analyses51 are calculated utilizing the Multiwfn 3.3.8 program package52.
Data availability
The data in this manuscript is availability.
References
Kroto, H. W., Heath, J. R., Obrien, S. C., Curl, R. F. & Smalley, R. E. C60: Buckminsterfullerene. Nature 318, 162–163 (1985).
Moreno, D. et al. B18 2−: A quasi-planar bowl member of the Wankel motor family. Chem. Commun. 50, 8140–8143 (2014).
Jalife, S. et al. Dynamical behavior of boron clusters. Nanoscale 8, 17639–17644 (2016).
Saha, R. et al. A spinning umbrella: Carbon monoxide and dinitrogen bound MB12 − clusters (M = Co, Rh, Ir). J. Phys. Chem. A 121, 2971–2979 (2017).
Chen, X. et al. Lanthanides with unusually low oxidation states in the PrB3 − and PrB4 − boride clusters. Inorg. Chem. 58, 411–418 (2019).
Chen, T. T., Li, W. L., Li, J. & Wang, L. S. [La(ηx-Bx)La]− (x = 7–9): A new class of inverse sandwich complexes. Chem. Sci. 10, 2534–2542 (2019).
Cheung, L. F., Czekner, J., Kocheril, G. S. & Wang, L. S. High resolution photoelectron imaging of boron-bismuth binary clusters: Bi2Bn (n = 2−4). J. Chem. Phys. 150, 064304 (2019).
Pan, S. et al. Fluxional boron clusters: From theory to reality. Acc. Chem. Res. 52, 2732–2744 (2019).
Vast, N. et al. Lattice dynamics of icosahedral α-boron under pressure. Phys. Rev. Lett. 78, 693–696 (1997).
Fujimori, M. et al. Peculiar covalent bonds in α-rhombohedral boron. Phys. Rev. Lett. 82, 4452–4455 (1999).
Martinez-Guajardo, G. et al. Dynamical behavior of borospherene: A nanobubble. Sci. Rep. 5, 11287 (2015).
Zhai, H. J. et al. Observation of an all-boron fullerene. Nat. Chem. 6, 727–731 (2014).
Piazza, Z. A. et al. Planar hexagonal B36 as a potential basis for extended single-atom layer boron sheets. Nat. Commun. 5, 3113 (2014).
Chen, Q. et al. Quasi-planar aromatic B36 and B36 − clusters: All-boron analogues of coronene. Phys. Chem. Chem. Phys. 16, 18282–18287 (2014).
Wang, L. S. Photoelectron spectroscopy of size-selected boron clusters: From planar structures to borophenes and borospherenes. Int. Rev. Phys. Chem. 35, 69–142 (2016).
Kiran, B. et al. Planarto-tubular structural transition in boron clusters: B20 as the embryo of single-walled boron nanotubes. Proc. Natl. Acad. Sci. USA 102, 961–964 (2005).
Oger, E. et al. Boron cluster cations: Transition from planar to cylindrical structures. Angew. Chem., Int. Ed. 46, 8503–8506 (2007).
Chen, Q. et al. Experimental and theoretical evidence of an axially chiral borospherene. ACS Nano 9, 754–760 (2015).
Zhao, X. Y. et al. Cage-like B39 + clusters with the bonding pattern of σ+π double delocalization: new members of the borospherene family. Phys. Chem. Chem. Phys. 19, 10998–11003 (2017).
Romanescu, C., Galeev, T. R., Li, W. L., Boldyrev, A. I. & Wang, L. S. Geometric and electronic factors in the rational design of transition-metal-centered boron molecular wheels. J. Chem. Phys. 138, 134315 (2013).
Romanescu, C., Galeev, T. R., Li, W. L., Boldyrev, A. I. & Wang, L. S. Transition-metal-centered monocyclic boron wheel clusters (M©Bn): A new class of aromatic borometallic compounds. Acc. Chem. Res. 46, 350–358 (2013).
Pan, S. et al. Boron nanowheel with an axle containing noble gas atoms: Viable noble gas bound M©B10 − clusters (M = Nb, Ta). Chem. Eur. J. 24, 3590–3598 (2018).
Popov, I. A., Jian, T., Lopez, G. V., Boldyrev, A. I. & Wang, L. S. Cobalt- centred boron molecular drums with the highest coordination number in the CoB16 − cluster. Nat. Commun. 6, 8654 (2015).
Popov, I. A., Li, W. L., Piazza, Z. A., Boldyrev, A. I. & Wang, L. S. Complexes between planar boron clusters and transition metals: A photoelectron spectroscopy and ab initio study of CoB12 − and RhB12 −. J. Phys. Chem. A 118, 8098–8105 (2014).
Liu, L. et al. Structure and bonding of IrB12 −: converting a rigid boron B12 platelet to a Wankel motor. RSC Adv. 6, 27177–27182 (2016).
Jian, T. et al. Competition between drum and quasi-planar structures in RhB18 −: Motifs for metallo-boronanotubes and metallo-borophenes. Chem. Sci. 7, 7020–7027 (2016).
Li, W. L. et al. Observation of a metal-centered B2-Ta@B18 − tubular molecular rotor and a perfect Ta@B20 − boron drum with the record coordination number of twenty. Chem. Commun. 53, 1587–1590 (2017).
Zhao, L. Q. et al. Effects of manganese doping on the structure evolution of small-sized boron clusters. J. Phys.: Condens. Matter 29, 265401 (2017).
Galeev, T. R., Romanescu, C., Li, W. L., Wang, L. S. & Boldyrev, A. I. Valence isoelectronic substitution in the B8 − and B9 − molecular wheels by an Al dopant atom: Umbrella-like structures of AlB7 − and AlB8 −. J. Chem. Phys. 135, 104301 (2011).
Chen, T. T. et al. PrB7−: A praseodymium-doped boron cluster with a PrII center coordinated by a doubly aromatic planar η7-B7 3− ligand. Angew. Chem. 135, 1–6 (2017).
Kuznetsov, A. E. & Boldyrev, A. I. Theoretical evidence of aromaticity in X3 − (X = B, Al, Ga) species. Struct. Chem. 13, 141–148 (2002).
Alexandrova, A. N., Boldyrev, A. I., Zhai, H. J. & Wang, L. S. Photoelectron spectroscopy and ab initio study of the doubly antiaromatic B6 2− dianion in the LiB6 − cluster. J. Chem. Phys. 122, 054313 (2005).
Tai, T. B. & Nguyen, M. T. Thermochemical properties, electronic structure and bonding of mixed lithium boron clusters (BnLi, n = 1–8) and their anions. Chem. Phys. 375, 25–45 (2010).
Dong, X. et al. Li2B12 and Li3B12: prediction of the smallest tubular and cage-like Boron structures. Angew. Chem. Int. Ed. 57, 4627–4631 (2018).
Liang, W. Y., Das, A., Dong, X. & Cui, Z. H. Lithium doped tubular structure in LiB20 and LiB20 −: A viable global minimum. Phys. Chem. Chem. Phys. 20, 16202–16208 (2018).
Zhai, H. J., Kiran, B., Li, J. & Wang, L. S. Hydrocarbon analogues of boron clusters−planarity, aromaticity and antiaromaticity. Nat. Mater. 2, 827–833 (2003).
Lv, J., Wang, Y. C., Zhu, L. & Ma, Y. M. Particle-swarm structure prediction on clusters. J. Chem. Phys. 137, 084104 (2012).
Wang, Y. C., Lv, J., Zhu, L. & Ma, Y. M. CALYPSO: A method for crystal structure prediction. Comput. Phys. Commun. 183, 2063–2070 (2012).
Wang, Y. C., Lv, J., Zhu, L. & Ma, Y. M. Crystal structure prediction via particle-swarm optimization. Phys. Rev. B: Condens. Matter Mater. Phys. 82, 094116 (2010).
Gao, B. et al. Interface structure Prediction via CALYPSO method. Sci. Bull. 64, 301–309 (2019).
Li, Y. W., Hao, J., Liu, H. Y., Li, Y. L. & Ma, Y. M. The metallization and superconductivity of dense hydrogen sulfide. J. Chem. Phys. 140, 174712 (2014).
Zhu, L., Liu, H. Y., Pickard, C. J., Zou, G. T. & Ma, Y. M. Reactions of xenon with iron and nickel are predicted in the earth’s inner core. Nat. Chem. 6, 644–648 (2014).
Zhu, L. et al. Substitutional alloy of Bi and Te at high pressure. Phys. Rev. Lett. 106, 145501 (2011).
Lv, J., Wang, Y. C., Zhu, L. & Ma, Y. M. Predicted novel high-pressure phases of lithium. Phys. Rev. Lett. 106, 015503 (2011).
Wang, H., Tse, J. S., Tanaka, K., Iitaka, T. & Ma, Y. M. Superconductive sodalite-like clathrate calcium hydride at high pressures. Proc. Natl. Acad. Sci. USA 109, 6463–6466 (2012).
Frisch, M. J. et al. Gaussian 09 (Revision C.0), Gaussian, Inc., Wallingford, CT, (2009).
Becke, A. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652 (1993).
Lee, C., Yang, W. & Parr, R. Development of the colic-salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B: Condens. Matter Mater. Phys. 37, 785–789 (1988).
Krishnan, R., Binkley, J. S., Seeger, R. & Pople, J. A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 72, 650–654 (1980).
Casida, M. E., Jamorski, C., Casida, K. C. & Salahub, D. R. Molecular excitation energies to high-lying bound states from time-dependent density-functional response theory: Characterization and correction of the time-dependent local density approximation ionization threshold. J. Chem. Phys. 108, 4439–4449 (1998).
Zubarev, D. Y. & Boldyrev, A. I. Developing paradigms of chemical bonding: adaptive natural density partitioning. Phys. Chem. Chem. Phys. 10, 5207–5217 (2008).
Lu, T. & Chen, F. Multiwfn: a multifunctional wavefunction analyzer. J. Comput. Chem. 33, 580–590 (2012).
Acknowledgements
This work is supported by the National Natural Science Foundation of China (Nos. 11574220 and 11874043).
Author information
Authors and Affiliations
Contributions
X.Y.K. and C.L. conceived the idea. H.X.S. and C.L. performed the calculations. H.X.S. wrote the manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shi, H., Kuang, X. & Lu, C. LiB13: A New Member of Tetrahedral-Typed B13 Ligand Half-Surround Cluster. Sci Rep 10, 1642 (2020). https://doi.org/10.1038/s41598-020-57769-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-57769-2
This article is cited by
-
Probing the Structural Evolution and Stabilities of LiBn− (n=2–12) Clusters
Journal of Cluster Science (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.