Abstract
Swine pneumonia is a great threat for pig industry around the world, which is usually accompanied with neutrophils infiltration in the airway. Although interleukin-8 (CXCL8) and its receptors, CXC chemokine receptor 1 and 2 (CXCR1/2) in human have been well documented, the expression and function of CXCR1/2 is still unknown in swine. To explore the feasibility to develop new veterinary anti-inflammatory drugs targeting porcine CXCR1/2, we detected CXCR1/2 expression in swine pneumonia through Real-Time PCR and immunohistochemistry for the first time. Two porcine CXCR1/2 antagonists, CXCL8(3–72)N11R/G31P (pN11R) and CXCL8(3–72)G31P (pG31P) were prepared and their anti-inflammatory effects were evaluated using cell chemotaxis assays and animal experiments. Our data showed that CXCR1/2 expression, which was closely related to neutrophil infiltration in the lung, was significantly up-regulated in swine pneumonia. The pN11R and pG31P could effectively inhibit the directional migration of neutrophils in vitro. In vivo data also indicated that both pN11R and pG31P significantly relieved LPS-induced pneumonia in mice through decreasing the expression of TNF-α, CXCL8, and IL-1β, and inhibiting neutrophil influx into the lung. pG31P was more efficient. Our study suggested that it is possible to develop new veterinary anti-inflammatory drugs targeting porcine CXCR1/2, and pG31P is a promising candidate.
Similar content being viewed by others
Introduction
The pig industry around the word has been suffering from respiratory diseases, especially pneumonia, which caused increasing mortality and decreasing production performance1. Swine pneumonia usually caused by co-infection with multiple pathogens, which bring a lot of obstacles to pneumonia treatment2,3. Treatment of swine pneumonia nowadays mainly relies on antibiotics or vaccine. However, many countries restrict the use of antibiotics since the drug residues in food have become a serious issue4. Therefore, it is urgent to develop a broad-spectrum, less residual anti-inflammatory drug for pneumonia treatment.
Swine pneumonia is always accompanied with migration of neutrophils in the interstitium and broncheoalveolar space. Therefore, neutrophils are considered to play a key role in pneumonia development and rational control of neutrophils exudation and migration represent a potential therapy for swine pneumonia5. Previous studies indicated that neutrophils infiltration primary mediated by interleukin-8 (CXCL8) and its specific receptors, CXCR1 and CXCR26,7. CXCL8 is a member of CXC chemokines, which was first discovered as a neutrophil chemotactic factor8. It can be can be produced by varieties of cells, such as neutrophils, monocytes, bronchial endothelial cells and airway smooth muscles cells9,10,11,12. CXCR1/2 belong to G-protein coupled receptor family, which are expressed on leukocytes, including neutrophil, monocytes, CD8+ T cells, etc.13,14. CXCL8 exerts its effects on neutrophils by binding with CXCR1/2. Currently, CXCL8 and CXCR1/2 have been proved to plays a central role in promoting neutrophils activation and recruitment to inflammatory site15. Therefore, successful blocking the interaction of CXCL8 and CXCR1/2 is a great therapy for inflammation control16.
During the past few decades, many antagonists target CXCL8-CXCR1/2, including Reparixin, SCH527123, SB22502 and CXCL8(3–73) K11R/G31P (hG31P) were developed17,18,19,20,21. Reparixin, (R) (-)-2-(4-isobutylphenyl) propionyl methansulfonamide), is a CXCR1/2 allosteric antagonist which mainly acts on the transmembrane domain of CXCR1/2 and induces the rearrangements of 3rd and 6th α-helix, thereby preventing downstream signal transduction and inhibiting neutrophil recruitment. SCH527123, 2-hydroxy-N,N-dimethyl-3-{2-[[(R)-1-(5-methylfuran-2-yl)propyl]amino]-3,4-dioxocyclobut-1-enylamino}benzamide, is an another allosteric antagonist of CXCR1/2, which could potently bind to CXCR1/2 and are difficult to dissociate, thereby inhibiting the interaction between CXCL1 or CXCL8 with CXCR1/2, affecting downstream signal transduction and controlling neutrophil infiltration22,23. Several studies have shown that Reparixin and SCH527123 could relieve acute lung injury, COPD and asthma etc19,24,25. SB22502, (N-(2-hydroxy-4-nitrophenyl)-N′-(2-bromophenyl)urea), the first non-peptide selective inhibitor of CXCR2, was reported to inhibit CXCL8 and GRO-α-mediated Ca2+ mobilization, thereby reducing neutrophil chemotaxis both in vitro and in vivo. hG31P, a CXCL8 mutant which shows higher affinity to CXCR1/2 than CXCL8, could inhibit about 50% neutrophils recruitment in airway26,27. It was reported that hG31P has a good efficacy in the treatment of multiple inflammatory diseases, such as acute lung injury, COPD, et al.18,27,28.
It was demonstrated that CXCL8 is vital for inducing neutrophils migration to resist exogenous infection in swine29,30,31. However, the function of swine CXCR1/2 are largely unexplored. In this study, we aimed to explore the relationship between CXCR1/2 and swine pneumonia, and developed a promising anti-inflammatory drug candidate targeting porcine CXCR1/2. Our study hopes to provides a new idea for swine anti-inflammatory drugs development.
Results
CXCR1/2 expression in lung tissue of swine with pneumonia
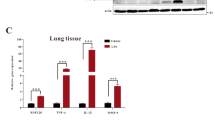
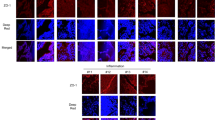
In this study, 127 cases of swine pneumonia were collected, including bronchopneumonia (17 cases), interstitial pneumonia (81 cases), serous pneumonia (8 cases) and suppurative pneumonia (21 cases). The alveolar septa in interstitial pneumonia were notably thickened by interstitial infiltration of numerous lymphocytes and few neutrophils. On the other hand, neutrophils infiltration in different levels in bronchioles and alveoli were observed in suppurative pneumonia, bronchopneumonia, and serous pneumonia. The expression of CXCR1 and CXCR2 was first examined by Real-Time PCR. Both CXCR1 and CXCR2 expression were up-regulated in all pneumonia samples. In addition, the expression of CXCR1 was significantly higher than CXCR2. Bronchopneumonia and suppurative pneumonia presented higher CXCR1/2 expression than interstitial pneumonia and serous pneumonia (Fig. 1b). The expression of CXCR1/2 was further examined by immunohistochemical staining of pneumonia samples. It was found that no expression of CXCR1/2 was observed in the normal lung tissues. However, the CXCR1/2 proteins were detected in neutrophils, which infiltrated in the lung in pneumonia (Fig. 1a). Additionally, the protein expression of CXCR1/2 were consistent with their mRNA expression pattern, which indicated that CXCR1/2 expression was significantly increased in swine pneumonia (Fig. 1c).
The expression of CXCR1/2 in swine pneumonia. Immunohistochemical staining showed that CXCR1/2 appeared undetectable expression in normal lung, while were positively expressed in neutrophils, which infiltrated in the lung in swine pneumonia (arrow) (a). Both mRNA (b) and protein (c) expression of CXCR1/2 were up-regulated in all above pneumonia, and the expression of CXCR1 was significantly higher than CXCR2 (P < 0.05). And the CXCR1/2 expression in bronchopneumonia and suppurative pneumonia are higher CXCR1/2 than that in interstitial pneumonia and serous pneumonia (P < 0.01). *P < 0.05, **P < 0.01, ***P < 0.001, unpaired t-test, two-tailed.
The impact of pN11R and pG31P on neutrophil migration in vitro
In this study, we also expressed antagonist pN11R and pG31P targeting porcine CXCR1/2 (Fig. 2) and evaluated their chemotactic effects on neutrophil in vitro at first. As showed in Fig. 3a, pCXCL8, pN11R, and pG31P could induce neutrophil migration. However, the ability of pN11R and pG31P inducing neutrophil migration was decreased by about 50% compare with pCXCL8 (Fig. 3b).
Recombinant PCXCL8(3–72)N11R/G31P and PCXCL8(3–72)G31P protein expression. The gene of porcine CXCL8(3–72)N11R/G31P and CXCL8(3–72)G31P were cloned into PGEX 6P-1, and then transformed into E. coli Rosetta (DE3) (a–c). Recombinant proteins CXCL8(3–72)N11R/G31P (pN11R) and CXCL8(3–72)G31P (pG31P) were induced by IPTG, treated with Prescission Protease, and then purified through GSTrapTM FF. Two proteins molecular weight of 10KD were finally obtained (d). The recombinant proteins could be identified by anti-human CXCL8 antibody (e).
Porcine CXCR1/2 antagonist pN11R and pG31P inhibit neutrophil migration in vitro. The under-agarose chemotactic test was used to detect the impact of pN11R and pG31P on neutrophil migration. The mean of distance for pCXCL8 was 1230.68 ± 35.56 μm, 591.35 ± 12.66 μm for pN11R and 573.04 ± 15.48 μm for pG31P (a). pN11R and pG31P treatments showed a decreasing ability on neutrophil migration in vitro (b). ***P < 0.001, unpaired t-test, two-tailed.
The impact of pN11R and pG31P on LPS-induced mouse pneumonia
We next assessed the impact of pN11R and pG31P on inflammatory response by using a mouse pneumonia model. After 24 h of induction, the saline-challenged mice showed normal histological appearance. However, all LPS-challenged mice suffered from pneumonia. Hemorrhage and an increased number of neutrophils infiltration in the lung were observed in LPS-challenged and saline-treated mice. Treatment with pCXCL8 enhanced hemorrhage and neutrophils infiltration in the lung, whereas treatment with pN11R and pG31P significantly reduced hemorrhage and neutrophils infiltration in the lung (Fig. 4). In addition, cells in BALF were collected and counted to determine the migration of both number and type of cells into alveoli. Almost all cells migrated into BAL were neutrophils. The neutrophils were almost invisible in BALF of saline-challenged mice, while 5.2 × 106 cells for LPS-challenged mice, 8.6 × 106 cells for LPS + pCXCL8-challenged mice, 4.1 × 106 cells for LPS + pN11R-challenged mice 3.4 × 106 cells for LPS + pG31P-challenged mice. It revealed that LPS challenge significantly increased the number of neutrophils in BAL. Treatment of LPS-challenged mice with pCXCL8 significantly increased the number of neutrophils. Conversely, treatment with pN11R/pG31P significantly reduced the number of neutrophils in BAL, although the number of neutrophils was still higher than control group (Fig. 5a). Additionally, the level of neutrophil degranulation marker, MPO, in the lung of pN11R/pG31P-treated mice was also dramatically lower than that in the saline-treated group (Fig. 5b). Since the inflammatory response is strongly related to the expression of inflammatory factors, we examined the impact of our antagonist on the expression of inflammatory factors, including TNF-α, IL-1β and CXCL8. As it was shown, treatment with pN11R or pG31P reduced the expression of TNF-α, IL-1β and CXCL8. Among them, the expression of CXCL8 in the pN11R/pG31P-treated group was even lower than that in the control group (Fig. 6).
pN11R and pG31P reduce hemorrhage and neutrophils infiltration in lung of LPS-challenged mice. H.E. staining of lung from mice treated with LPS + saline, LPS + pCXCL8, LPS + pN11R, LPS + pG31P, and saline. The saline-challenged (saline) mice showed normal histological appearance of lung. The LPS-challenged saline-treated mice (LPS + saline) showed grossly hemorrhage and an increasing number of neutrophils infiltration in the lung. Hemorrhage and neutrophils infiltration in the lung were enhanced after treated with pCXCL8 (LPS + pCXCL8), and inversely reduced after treated with pN11R (LPS + pN11R) and pG31P (LPS + pG31P).
pN11R and pG31P decrease neutrophils migration and MPO activity of lung. LPS challenge significantly increased the number of neutrophils in BALF (a) as well as MPO (b) activity of lung. The neutrophils exudation and MPO activity were increased after treated with pCXCL8 (LPS + pCXCL8), and significantly reduced after treated with pN11R (LPS + pN11R) and pG31P (LPS + pG31P). *P < 0.05, **P < 0.01, ***P < 0.001, unpaired t-test, two-tailed.
pN11R and pG31P reduce inflammatory factors expression. LPS-challenged mice (LPS + saline) appeared increasing TNF-α, IL-1β and CXCL8 expression compared to saline-challenged mice. The expressions of TNF-α, IL-1β and CXCL8 were also enhanced after treated with pCXCL8 (LPS + pCXCL8), inversely dramatically reduced after treated with pN11R (LPS + pN11R) and pG31P (LPS + pG31P). *P < 0.05, **P < 0.01, ***P < 0.001, unpaired t-test, two-tailed.
Discussion
Pneumonia, the main disease for swine death, has caused huge losses to swine industry for a long time. Excessive neutrophil infiltration is usually found to be the primary pathological feature of pneumonia, which always leads to lung damage32,33. CXCL8 has been considered as an essential chemokine for neutrophil activation and recruitment. Blocking the binding of CXCL8 to its receptors, CXCR1/2, can effectively limit neutrophils recruitment and slow down inflammatory response34,35. In this study, the relationship between CXCR1/2 and swine pneumonia were determined using Real-Time PCR and immunohistochemical staining. The results revealed that the expression of CXCR1/2 in swine pneumonia were up-regulated. Interestingly, the expression of CXCR1/2 in bronchopneumonia and suppurative pneumonia were significantly higher than that in interstitial pneumonia and serous pneumonia (Fig. 1b,c). Immunohistochemical results showed that CXCR1/2 were expressed in neutrophil in pneumonia (Fig. 1a). Interstitial pneumonia is a type of proliferative pneumonia that is usually caused by viral infection. It is characterized by the proliferation of type II pneumocyte and less neutrophils infiltration. Bronchopneumonia, suppurative pneumonia and serous pneumonia belong to exudative pneumonia, which are mainly generated by bacterial infections or mixed infections and characterized by increasing neutrophils infiltration32. Histopathologically, the degree of neutrophil infiltration in suppurative pneumonia and bronchopneumonia were more obvious than that in serous pneumonia and interstitial pneumonia. Therefore, we concluded that the expression of CXCR1/2 in pneumonia was positively correlated with the number of neutrophil infiltrated in the lung. Our results demonstrated that CXCR1/2 expression was closely associated with swine pneumonia. Numerous studies showed that the expression of CXCR1 and/or CXCR2 increased in many inflammatory disease, such as ulcerative colitis and hyperoxia-induced lung injury, so that massive neutrophils could be recruited into the site of inflammation36,37. Reduction of CXCR1/2 expression or inhibition of CXCR1/2 binding would be an effective approach to inhibit neutrophil recruitment and relieve inflammation38,39,40. All these studies indicated that CXCR1/2 could be potential target for swine pneumonia therapeutic. Previously, a small molecular CXCL8 antagonist, hG31P was developed by Lee et al.41. They mutated Arg residue at site 11 of the main binding site of human CXCL8 to Lys11 and Pro residue at site 31 of the first β-turn of human CXCL8 to Gly to generate hG31P. It has a higher affinity to CXCR1/2 and was able to inhibit downstream signal transduction and, therefore, limit neutrophil migration42,43. Furthermore, it was also reported that hG31P can effectively inhibit neutrophil recruitment and reduce pathology of lung in mice models40,44. Porcine CXCL8 shares 73.6% homology on amino acid with hCXCL8, and has the same ELR+ sequence and cysteine site with hCXCL8. In this study, we substituted Arg and Pro for Lys11 and Gly31 to prepare pN11R, substituted Pro for Gly31 to prepare pG31P (Fig. 2a). Previous evidences showed that inhibiting the migration of neutrophils into the lung is critical in limiting lung inflammation45. Thereby, we firstly determined the impact of pN11R and pG31P on neutrophil migration in vitro. It was found that pN11R and pG31P could dramatically inhibit neutrophil migration in vitro individually. In addition, the in vivo effect on neutrophil migration response to LPS was also evaluated with the LPS-challenged mouse model. Histopathological results showed lower neutrophil infiltration in the lung of pN11R/pG31P-treated mice. MPO activity and neutrophils number in the BALF of LPS-challenged mice were significantly elevated compared to the control group. Treatment with pCXCL8 enhanced MPO activity and neutrophils migration in the lungs of LPS-challenged mice. By contrast, treatment with pN11R and pG31P reduced MPO activity and neutrophil migration. These results suggested that pN11R and pG31P could down-regulate neutrophil migration, therefore, contribute to limiting lung inflammation.
In addition, LPS stimulated the expression of TNF-α, IL-1β and CXCL8, however, treatment with pN11R/pG31P significantly decreased each of them. Cytokines, including IL-1β, TNF-α and CXCL8 were good indicators of lung inflammation46. In cases of pneumonia, inflammatory factors released in the lung could lead to increasing vascular permeability, thus resulting in rapid recruitment of neutrophils6. Neutrophils could express IL-1, TNF-α and CXCL847. Local secretion of these cytokines could induce more neutrophil recruitment and inflammatory mediator release15,48. Indeed, the levels at which neutrophils secreted these cytokines are closely related to their numbers. Decreasing expression of TNF-α, IL-1β and CXCL8 suggested slight neutrophils infiltration and inflammatory response. These demonstrated that pN11R and pG31P could reduce the expression of inflammatory mediator, thereby, inhibiting inflammatory response.
Furthermore, we also compared the different impact on neutrophil migration between pN11R and pG31P, and found that although there was no difference in inducing neutrophil migration in vitro, the pG31P-treated mice showed lower MPO activity, neutrophils exudation in BALF and TNF-α expression (Figs. 5a and 6), it suggested that pG31P may be a candidate for the further research of swine antagonist. Fang Li et al. developed hG31P based on CXCL8(3–73) K11R, a high agonist of neutrophil CXCR1/249. However, our research demonstrated that it was possible to reduce the activity of porcine CXCL8 on inducing neutrophil migration by only mutating Pro into Gly31, and substituting Arg for Asn11 in pN11R might not increase the anti-inflammatory effect.
In summary, our study indicated that it is possible to develop veterinary anti-inflammatory drug by targeting CXCR1/2. The pG31P, which can significantly reduce neutrophils infiltration and relieve lung inflammation, is a promising drug candidate to treat swine pneumonia.
Materials and Methods
Ethics statement
All animals were treated in strict accordance with the Guidelines for Laboratory Animal Use and Care, which was approved by the Laboratory Animal Monitoring Committee of Huazhong Agricultural University.
Sample collection
Totally, 127 cases of swine pneumonia were collected in Hubei province, China from March 2017 to December 2018. The samples of lung were fixed with 10% formalin for histopathological analysis. For molecular biological analysis, samples were stored at -80 °C. The types of pneumonia were confirmed by gross and histopathological observation.
CXCR1/2 mRNA expression
Total RNA was extracted from lung samples using TRIzolTM Reagent (Invitrogen, Carlsbad, CA, U.S.A.). The cDNA of CXCR1/2 were synthesized from 1 μg total RNA using Prime ScriptTM RT Kit with gDNA Eraser (Takara, Japan). Then Real-Time PCR was performed using SYBR-Green Real-Time PCR Master Mix (TaKaRa, Japan) according to the manufacturer’s instructions. Primers used in Real-Time PCR reactions were listed in Table 1. Cycling parameters were 95 °C for 10 min; 40 cycles at 94 °C for 30 sec; 56 °C for 30 sec; 72 °C for 1 min.
Immunohistochemistry
Since porcine CXCR1/2 specific antibodies were not commercially available, CXCR1 and CXCR2 were used individually for mouse immunization to prepare polyclonal antibodies. Ten 6-week-old female BALB/c mice were respectively immunized with 100 μg of recombinant porcine CXCR1 (1–90 amino acid) and CXCR2 (1–88 amino acid) in Freund’s adjuvant (Sigma, ST Louis, MO, U.S.A.) four times at biweekly intervals. One week after the final immunization, mice were bled and sera were obtained and fractionated into IgG by a protein A agarose column (GE, Piscataway, NJ, U.S.A.)50. The purified sera were further detected by indirect enzyme-linked immunosorbent assay (ELISA) and western blot, and finally used to immumohistochemical staining. For immunohistochemistry, the slides were deparaffinized, rehydrated, and treated with 3% H2O2. After rinsing in PBS, the slides were blocked with normal goat serum followed by incubating with anti-CXCR1 and CXCR2 antibodies (1:100) at 4 °C overnight. After rinsing with PBS, the slides were incubated with HRP-conjugated goat anti-mouse IgG (Dako, Glostrup, Denmark) at room temperature for 40 min and then were visualized with DAB Detection Kit (GK500710, Gene Tech, Shanghai, China). Six views in different fields were randomly collected from slice under microscope (Nikon, Japan). The positive rate of each slice was analyzed by Leica Image Scope software (Wetzlar, Germany).
Preparation of porcine CXCR1/2 antagonist
The gene of porcine CXCL8(3–72)N11R/G31P and CXCL8(3–72) G31P (Fig. 2a) were synthesized at Sangon Biotech (Shanghai) Co., Ltd. and amplified by PCR (5′-TAGGATCCGCAGAACTTCGATGCCAGTG-3′ and 5′-CGCTCGAGTTATTGCTTCTCAGTTCTCTTCAAAAA-3′). Cycling parameters were 95 °C for 10 min; 32 cycles at 94 °C for 30 sec; 56 °C for 30 sec; 72 °C for 1 min. The PCR products were digested with BamH I and Xho I (Takara, Japan) and cloned into pGEX 6P-1. The generated plasmids were transformed into E. coli Rosetta (DE3) cells separately. Protein expression was induced with 0.8 mmol/L isopropy1-β-D-1-thiogalactopyranoside (IPTG) (Biosharp, Hefei, China) at 18 °C for 20 h. The bacteria were collected by low-speed centrifugation and lysed with sonicator. The soluble and insoluble fractions were separated by centrifugation at 10,000 rpm for 30 min at 4 °C and analyzed by sodium dodecy1 polyacrylamide gel electrophoresis (SDS-PAGE). The recombinant pN11R and pG31P were treated with Prescission Protease (GE, Piscataway, NJ, U.S.A.) at 4 °C for 16 h and purified through GSTrapTM FF (GE, Piscataway, NJ, U.S.A.) to remove glutathione S-transferase (GST)-tag.
Western blot analysis
The pN11R and pG31P were subjected to 15% SDS-PAGE and transferred to 0.22 μm polyvinylidene fluoride membrane (PVDF). The membrane was blocked with 5% skim milk (Sigma, St. Louis, MO, U.S.A.), incubated in mouse anti-human CXCL8 antibody (CSB-PA08327A0Rb, CUSABIO, Wuhan, China) and HRP-conjugated goat-anti-rabbit IgG (CUSABIO, Wuhan, China), and visualized by ECL Western Blotting Substrate (Thermo Fisher, Waltham, MA, U.S.A.).
Neutrophil chemotaxis assay
The under-agarose chemotactic model was performed as described previously51. Briefly, 3 mL 1.2% agarose solution included 50% 1 × HBSS with Ca2+ and Mg2+, 50% RPMI 1640 culture medium, and 20% heat-inactivated FBS was put in the 6-well culture plate. Once the agarose solidified, 3 wells in a straight line, 3.5 mm in diameter and 2.8 mm apart, were generated on the gel. Neutrophils were isolated from peripheral blood of healthy pigs using Porcine Neutrophil Isolation Kit (Solarbio, Beijing, China) and added into the two side wells (10 μL/107cells/well). The porcine CXCL8 (pCXCL8), pN11R, pG31P, and cell culture medium were added into the middle well (10 μL/1 μg/well) respectively. The gels were incubated at 37 °C/5% CO2 for 6 h, and the migration distance of neutrophil was measured under microscope (Leica, Wetzlar, Germany).
Animal procedures
18–20 g female BALB/c mice (7-week-old), which purchased from Laboratory Animal Center of Huazhong Agricultural University (Wuhan, China), were randomly divided into 5 groups, each group of 9 mice. The mice were anesthetized by diethyl ether and held on a plate titled 45°. Mice in the control group were intranasal inoculated with 20 μL normal saline. Mice in the groups 1–4 were first intranasally inoculated with 10 μL (10 μg/μL) lipopolysaccharide (LPS) to induce pneumonia. Then, groups 1–4 were intranasal inoculated with 10 μL (500 μg/kg) pCXCL8, 10 μL (500 μg/kg) pN11R, 10 μL (500 μg/kg) pG31P, and 10 μL normal saline respectively. After 24 h of inoculation, all mice were anesthetized with sodium pentobarbital (100 μl, 75 mg/ml). 3 mice in each group were collected lung for histopathological examination, 3 mice in each group were collected lung for molecular biological detection, and 3 mice in each group were collected bronchiolar alveolar lavage (BAL) for neutrophil count.
Lung histopathology
Mice lung tissues was fixed in 10% formalin, embedded in paraffin, sectioned into 4 μm thickness, and stained with hematoxylin-eosin (H.E.). The histopathological changes were observed and recorded under a microscope (NiKon, Tokyo, Japan).
Bronchiolar alveolar lavage collection
The lung of mice was lavaged three times with 1 mL normal saline. Lavage fluid was mixed with red blood cell lysis buffer and centrifuged for 10 min at 1,750 g. The pellet cells were resuspended in the 1 mL normal saline. 10 μL cell suspension was adhered to glass slides and stained using H.E. Meanwhile, 10 μL cell suspension was added to the cell-count boards to count the number of neutrophils.
Myeloperoxidase (MPO) assay
About 100 mg frozen lung was homogenized with 1 mL saline, and centrifuged at 12,000 rpm for 10 minutes at 4 °C. The supernatant was used for MPO activity analysis according to the instruction of Mouse Myeloperoxidase ELISA Kit (Solarbio, Beijing, China).
Cytokine bioassay
The total RNA extraction and cDNA synthesis were described above. The levels of TNF-α, CXCL8, IL-1β were detected by Real-Time PCR with the primers listed in Table 1. The cycling parameters were 95 °C for 10 min; 40 cycles at 94 °C for 30 sec; 60 °C for 1 min.
Statistical analysis
All data was analyzed using GraphPad Prism (San Diego, CA, U.S.A). Graph bar represent means +/− S.E.M of three replicate experiments performed in triplicate (n = 3). The significance of variability among different groups was determined by unpaired t-test, P values < 0.05 was considered to be statistically significant, and P values < 0.01 was considered to be extremely significant.
References
Opriessnig, T., Gimenez-Lirola, L. G. & Halbur, P. G. Polymicrobial respiratory disease in pigs. Anim. Health Res. Rev. 12, 133–148 (2011).
Choi, Y. K., Goyal, S. M. & Joo, H. S. Retrospective analysis of etiologic agents associated with respiratory diseases in pigs. Can. Vet. J. 44, 735–737 (2003).
Palzer, A., Ritzmann, M., Wolf, G. & Heinritzi, K. Associations between pathogens in healthy pigs and pigs with pneumonia. Vet. Rec. 162, 267–271 (2008).
Reeves, P. T. Drug residues. Handb. Expe. Pharmacol. 199, 265–290 (2010).
Villarino, N. et al. An acute reversible experimental model of pneumonia in pigs: time-related histological and clinical signs changes. J. Vet. Pharmacol. Ther. 36, 241–247 (2013).
Kolaczkowska, E. & Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 13, 159–175 (2013).
de Oliveira, S. et al. Cxcl8 (IL-8) mediates neutrophil recruitment and behavior in the zebrafish inflammatory response. J. Immunol. 190, 4349–4359 (2013).
Yoshimura, T., Matsushima, K., Oppenheim, J. J. & Leonard, E. J. Neutrophil chemotactic factor produced by lipopolysaccharide (LPS)-stimulated human blood mononuclear leukocytes: partial characterization and separation from interleukin 1 (IL 1). J. Immunol. 139, 788–793 (1987).
John, M. et al. Expression and release of interleukin-8 by human airway smooth muscle cells: inhibition by Th-2 cytokines and corticosteroids. Am. J. Respir. Cell Mol. Biol. 18, 84–90 (1998).
Ribeiro, F. P. et al. mRNA expression and release of interleukin-8 induced by serum amyloid A in neutrophils and monocytes. Mediat. Inflamm. 12, 173–178 (2003).
Nakamura, H., Yoshimura, K., Jaffe, H. A. & Crystal, R. G. Interleukin-8 gene expression in human bronchial epithelial cells. J. Biol. Chem. 266, 19611–19617 (1991).
Linevsky, J. K. et al. IL-8 release and neutrophil activation by Clostridium difficile toxin-exposed human monocytes. Am. J. Physiol. 273, G1333–1340 (1997).
Horuk, R. Chemokine receptors. Cytokine Growth Factor. Rev. 12, 313–335 (2001).
D’Ambrosio, D., Panina-Bordignon, P. & Sinigaglia, F. Chemokine receptors in inflammation: an overview. J. Immunol. Methods 273, 3–13 (2003).
Akdis, M. et al. Interleukins (from IL-1 to IL-38), interferons, transforming growth factor beta, and TNF-alpha: Receptors, functions, and roles in diseases. J. Allergy Clin. Immunol. 138, 984–1010 (2016).
Bizzarri, C. et al. ELR+ CXC chemokines and their receptors (CXC chemokine receptor 1 and CXC chemokine receptor 2) as new therapeutic targets. Pharmacol. Ther. 112, 139–149 (2006).
White, J. R. et al. Identification of a potent, selective non-peptide CXCR2 antagonist that inhibits interleukin-8-induced neutrophil migration. J. Biol. Chem. 273, 10095–10098 (1998).
Matera, M. G., Calzetta, L., Segreti, A. & Cazzola, M. Emerging drugs for chronic obstructive pulmonary disease. Expert. Opin. Emerg. Drugs 17, 61–82 (2012).
Kaur, M. & Singh, D. Neutrophil chemotaxis caused by chronic obstructive pulmonary disease alveolar macrophages: the role of CXCL8 and the receptors CXCR1/CXCR2. J. Pharmacol. Exp. Ther. 347, 173–180 (2013).
Todd, C. M. et al. The effects of a CXCR1/CXCR2 antagonist on neutrophil migration in mild atopic asthmatic subjects. Pulm. Pharmacol. Ther. 41, 34–39 (2016).
Pawlick, R. L. et al. Reparixin, a CXCR1/2 Inhibitor in Islet Allotransplantation. Islets 8, 115–124 (2016).
Dwyer, M. P. et al. Discovery of 2-hydroxy-N,N-dimethyl-3-{2-[[(R)-1-(5- methylfuran-2-yl)propyl]amino]-3,4-dioxocyclobut-1-enylamino}benzamide (SCH 527123): a potent, orally bioavailable CXCR2/CXCR1 receptor antagonist. J. Med. Chem. 49, 7603–7606 (2006).
Gonsiorek, W. et al. Pharmacological characterization of Sch527123, a potent allosteric CXCR1/CXCR2 antagonist. J. Pharmacol. Exp. Ther. 322, 477–485 (2007).
Zarbock, A., Allegretti, M. & Ley, K. Therapeutic inhibition of CXCR2 by Reparixin attenuates acute lung injury in mice. Br. J. Pharmacol. 155, 357–364 (2008).
Nair, P. et al. Safety and efficacy of a CXCR2 antagonist in patients with severe asthma and sputum neutrophils: a randomized, placebo-controlled clinical trial. Clin. Exp. Allergy 42, 1097–1103 (2012).
Gordon, J. R. et al. Amelioration of pathology by ELR-CXC chemokine antagonism in a swine model of airway endotoxin exposure. J. Agromedicine 14, 235–241 (2009).
Gordon, J. R. et al. The combined CXCR1/CXCR2 antagonist CXCL8(3-74)K11R/G31P blocks neutrophil infiltration, pyrexia, and pulmonary vascular pathology in endotoxemic animals. J. Leukoc. Biol. 78, 1265–1272 (2005).
Cao, Q., Li, B., Wang, X., Sun, K. & Guo, Y. Therapeutic inhibition of CXC chemokine receptor 2 by SB225002 attenuates LPS-induced acute lung injury in mice. Arch. Med. Sci. 14, 635–644 (2018).
Petry, D. B. et al. Differential immunity in pigs with high and low responses to porcine reproductive and respiratory syndrome virus infection. J. Anim. Sci. 85, 2075–2092 (2007).
Chen, Y. et al. Haemophilus parasuis infection activates the NF-kappaB pathway in PK-15 cells through IkappaB degradation. Vet. Microbiol. 160, 259–263 (2012).
Chang, H. W. et al. Immunopathological effects of porcine circovirus type 2 (PCV2) on swine alveolar macrophages by in vitro inoculation. Vet. Immunol. Immunopathol. 110, 207–219 (2006).
Carioto, L. Pathologic Basis of Veterinary Disease, 5th edition. (Elsevier LTD, Oxford, 2014).
Wang, J. Neutrophils in tissue injury and repair. Cell Tissue Res. 371, 531–539 (2018).
Russo, R. C., Garcia, C. C., Teixeira, M. M. & Amaral, F. A. The CXCL8/IL-8 chemokine family and its receptors in inflammatory diseases. Expert. Rev. Clin. Immunol. 10, 593–619 (2014).
Lerner, C. A., Lei, W., Sundar, I. K. & Rahman, I. Genetic Ablation of CXCR2 Protects against Cigarette Smoke-Induced Lung Inflammation and Injury. Front. Pharmacol. 7, 391 (2016).
Sue, R. D. et al. CXCR2 is critical to hyperoxia-induced lung injury. J. Immunol. 172, 3860–3868 (2004).
Lee, Y. K., Hippe-Sanwald, S., Lee, S. C., Hohenberg, H. & Hwang, B. K. Distribution of the interleukin-8 receptors, CXCR1 and CXCR2, in inflamed gut tissue. J. Pathol. 192, 533–539 (2015).
Schneberger, D. et al. CXCR1/CXCR2 antagonist CXCL8(3-74)K11R/G31P blocks lung inflammation in swine barn dust-instilled mice. Pulm. Pharmacol. Ther. 31, 55–62 (2015).
Ha, H., Debnath, B. & Neamati, N. Role of the CXCL8-CXCR1/2 Axis in Cancer and Inflammatory Diseases. Theranostics 7, 1543–1588 (2017).
Wei, J. et al. CXCR1/CXCR2 antagonism is effective in pulmonary defense against Klebsiella pneumoniae infection. Biomed. Res. IntI. 2013, 720975 (2013).
Li, F., Zhang, X. & Gordon, J. R. CXCL8((3-73))K11R/G31P antagonizes ligand binding to the neutrophil CXCR1 and CXCR2 receptors and cellular responses to CXCL8/IL-8. Biochem. Biophys. Res. Commun. 293, 939–944 (2002).
Hammond, M. E. et al. Receptor recognition and specificity of interleukin-8 is determined by residues that cluster near a surface-accessible hydrophobic pocket. J. Biol. Chem. 271, 8228–8235 (1996).
Cheng, H. T., Yu, H. Y., Gordon, J. R., Li, F. & Cheng, J. W. Effects of K11R and G31P Mutations on the Structure and Biological Activities of CXCL8: Solution Structure of Human CXCL8(3-72)K11R/G31P. Molecules 22, 1229–1241 (2017).
Zhao, X. et al. Blockade of neutrophil responses in aspiration pneumonia via ELR-CXC chemokine antagonism does not predispose to airway bacterial outgrowth. Pulm. Pharmacol. Ther. 23, 22–28 (2010).
Grommes, J. & Soehnlein, O. Contribution of neutrophils to acute lung injury. Mol. Med. 17, 293–307 (2011).
Gilowska, I. CXCL8 (interleukin 8)–the key inflammatory mediator in chronic obstructive pulmonary disease? Postepy. Hig. Med. Dosw. (Online) 68, 842–850 (2014).
Song, H., Li, G. W., Ye, J. & Qian, Y. S. Modulation of mouse neutrophil cytokine secretion by Klebsiella pneumoniae. Comp. Clin. Pathol. 13, 14–18 (2004).
Faccioli, L. H., Souza, G. E., Cunha, F. Q., Poole, S. & Ferreira, S. H. Recombinant interleukin-1 and tumor necrosis factor induce neutrophil migration “in vivo” by indirect mechanisms. Agents Actions 30, 344–349 (1990).
Li, F. & Gordon, J. R. Il-8((3-73))K11R is a high affinity agonist of the neutrophil CXCR1 and CXCR2. Biochem. Biophys. Res. Commun. 286, 595–600 (2001).
Morohashi, H. et al. Expression of both types of human interleukin-8 receptors on mature neutrophils, monocytes, and natural killer cells. J. Leukoc. Biol. 57, 180–187 (1995).
Xu, X. et al. Adenosine effectively restores endotoxin-induced inhibition of human neutrophil chemotaxis via A1 receptor-p38 pathway. Inflamm. Res. 66, 353–364 (2017).
Acknowledgements
This work is supported by Da Bei Nong Group Promoted Project for Young Scholar of HZAU (Grant No. 2017DBN004).
Author information
Authors and Affiliations
Contributions
X.W., Y.C.L. and L.T.L. conceived and performed the experiments. X.L.L. and Z.J. prepared the reagents and samples. X.W. analyzed the data and wrote the manuscript. C.Q.G., W.P.Z., G.F.C. and X.Y.H. performed the proof reading and gave the important suggestions. W.P.Z. as the corresponding author gave the guidance in the whole study. All authors have reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, X., Li, Y., Li, L. et al. Porcine CXCR1/2 antagonist CXCL8(3–72)G31P inhibits lung inflammation in LPS-challenged mice. Sci Rep 10, 1210 (2020). https://doi.org/10.1038/s41598-020-57737-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-57737-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.