Abstract
Human cytomegalovirus (CMV) is the leading non-genetic cause of fetal malformation in developed countries. CMV placental infection is a pre-requisite for materno-fetal transmission of virus, and fetal infection. We investigated the roles of the viral pentameric complex gH/gL/pUL128-pUL131A, and cellular platelet-derived growth factor receptor-α (PDGFRα) for CMV infection in first trimester extravillous-derived (SGHPL-4) and villous-derived (HTR-8/SVneo) trophoblast cells. Infection with four CMV clinical and laboratory strains (Merlin, TB40E, Towne, AD169), and Merlin deletion mutants of UL128-, UL130-, and UL131A-genes, showed a cell type-dependent requirement of the viral pentameric complex for infection of trophoblast cells. The viral pentameric complex was essential for infection of villous trophoblasts, but non-essential for extravillous trophoblasts. Blocking of PDGFRα in extravillous trophoblasts, which naturally express PDGFRα, inhibited entry of pentameric complex-deficient CMV strains, but not the entry of pentameric positive CMV strains. Transient expression of PDGFRα in villous trophoblasts, which are naturally deficient in PDGFRα, promoted the entry of CMV strains lacking gH/gL/pUL128-pUL131A, but had no effect on entry of pentameric positive CMV strains. These results suggest PDGFRα is an important cell receptor for entry of CMV mutant strains lacking gH/gL/pUL128-pUL131A complexes in some placental cells, suggesting these entry pathways could be potential antiviral targets.
Similar content being viewed by others
Introduction
Human cytomegalovirus (CMV) is the leading viral cause of congenital infection in developed countries, with a global incidence of 0.3–0.7% of all live births1,2. Congenital CMV infection may result in fetal death3,4 or permanent neurodevelopmental sequelae including sensorineural hearing loss and mental disability5. The risk of congenital CMV is highest in pregnant women who acquire primary CMV during pregnancy, with 32% chance of transmission to the fetus1,6. Reactivation of latent virus or re-infection with a new strain of CMV can also lead to congenital CMV transmission, but at a much lower transmission rate of 1.4%1. CMV transmission can occur throughout pregnancy, although congenital infection during the first trimester of pregnancy presents the greatest risk to the developing fetus in terms of disease severity and long-term sequelae3,7.
The exact mechanism by which CMV transmission occurs in utero is unknown. However, evidence suggests CMV infection of the placenta is a critical step in materno-fetal transmission of the virus8,9. CMV infection of placental trophoblasts has been demonstrated in placental tissue from congenitally infected infants using immunohistochemical and in situ PCR techniques10,11. CMV-DNA, CMV-transcripts and CMV-proteins from immediate-early (IE), early (E), and late times have been detected in all placental cell types of naturally-infected, early and late gestation placentae12,13. Productive CMV replication has also been demonstrated in ex vivo villous explant models, where CMV infection of villous cytotrophoblasts precedes infection of other placental cell types14,15. These observations indicate trophoblast cells are important sites for placental CMV infection and most likely transmission of the virus to the fetus.
In addition to placental explant models, studies have utilised primary cytotrophoblasts16, syncytiotrophoblast-like cells16,17,18,19, and trophoblast-derived cell lines19,20,21 to investigate CMV infection in vitro. Laboratory strains of CMV such as AD169 and Towne have been used most frequently for in vitro studies16,17,18,19,20,21,22. These CMV strains are known to have significant alterations in their genomes due to extensive propagation in cell culture23,24,25. The AD169, Towne and other CMV strains passaged extensively in fibroblasts have frame-shift mutations in one of three genes (UL128, UL130 and UL131A) of the UL128 locus24,26. Intact UL128 protein (pUL128), pUL130, and pUL131A can independently assemble onto glycoprotein H (gH)/gL heterodimers to form the gH/gL/pUL128-131A pentameric complex that is critical for entry into epithelial cells, endothelial cells, monocytes, and dendritic cells27,28,29,30,31. Mutations within the UL128 locus inhibit formation of the pentameric complex in AD169 and Towne strains and render these viruses incapable of infecting epithelial cells, endothelial cells, monocytes and dendritic cells.
The CMV gH/gL proteins also assemble into complexes with gO to form gH/gL/gO trimers, which is a necessary pre-requisite for virus entry into fibroblasts30,32,33,34,35. Some studies show gO promotes the incorporation of gH/gL into the virion, and gH/gL, but not gH/gL/gO, mediates CMV entry into fibroblasts36,37. However, further investigation from the same group has revealed all strains of CMV contain different compositions of gH/gL/gO trimers and gH/gL/pUL128-131A pentamers, and these envelope complexes are necessary for virus entry into host cells38. Interference studies through overexpression of gH/gL/pUL128-131A or gH/gL/gO in susceptible cells suggest these glycoprotein complexes bind to cell type-specific receptors, leading to resistance to CMV entry35,39. In addition to these envelope glycoprotein complexes, CMV gB homodimers are also essential for CMV entry into host cells via fusion with the plasma membrane40,41,42. A recent study has demonstrated gB, but not gH/gL/pUL128-pUL131A complex is required for CMV infection of placental trophoblast progenitor cells43. Since the human placenta is comprised of diverse trophoblast populations, including trophoblast progenitor cells, syncytiotrophoblasts, cell column cytotrophoblasts (proximal and distal), and extravillous trophoblasts, investigating the significance of viral pentameric complex on infection of these trophoblast cell types is of critical importance, with such studies of relevance for pathogenesis and vaccine research44.
The platelet-derived growth factor receptor-α (PDGFRα), epidermal growth factor receptor (EGFR), integrins, annexin II, dendritic cell-specific ICAM-grabbing nonintegrin, neurophilin-2 and OR14I1 have been proposed as CMV receptors for infection of different cell types including fibroblasts, epithelial cells, endothelial cells, lymphocytes, and dendritic cells45,46,47,48,49,50,51,52,53. Several of these receptors (PDGFRα, EGFR, integrin β1/αVβ3, annexin II) interact with gB, gH, or gO and this has been shown to facilitate viral entry and activation of cellular signalling pathways45,46,48,54,55,56. In placental trophoblasts, co-expression of EGFR and integrins (αVβ3 and α1β1) has been associated with increased susceptibility to CMV infection and viral replication57. Although the roles of PDGFRα in CMV cell entry have been inconsistent between studies of different cell types and virus strains, there is evidence for PDGFRα promoting entry of AD169 and UL131A-deleted CMV TR strains into epithelial and endothelial cells58. This suggests PDGFRα may be necessary for cellular entry of CMV strains lacking the pentameric gH/gL/pUL128-131A complex. Recent studies have shown virion gH/gL/gO binds to PDGFRα on the surface of fibroblasts and either directly or indirectly recruits gB to this complex to facilitate virus cell59,60 entry, with the N terminus of gO contributing to interactions with PDGFRα56.
Platelet derived growth factor (PDGF) consists of a family of homodimeric and heterodimeric forms of A- and B-chains and is generally described as a mitogen for fibroblasts, smooth muscle cells, and other cell types61. The PDGF ligands and their receptors, PDGFRα and PDGFRβ, are also expressed in different subpopulations of term placental extravillous trophoblasts, and reported to function as a growth factor in the development of cytotrophoblasts62. Therefore, we hypothesise that PDGFRα may be differentially expressed in diverse trophoblast cell types, and potentially influence the cellular entry of CMV into these cell types. In this research, we investigated the importance of viral gH/gL/pUL128-pUL131A complexes and cellular PDGFRα in CMV infection of two distinct trophoblast cell lines, extravillous-derived SGHPL-4 and villous derived HTR-8/SVneo cells. We demonstrated that CMV uses different entry pathways, involving at least the viral pentameric complex and cellular PDGFRα receptor for entry into placental trophoblasts.
Materials and Methods
Cells
Simian virus 40-transformed first trimester primary extravillous-derived trophoblasts (SGHPL-4 cells) were kindly provided by Guy Whitley (St. George’s University of London, Cranmer Terrace, London)63. These cells were cultured in F10 Ham’s medium (Life Technologies) containing 10% Fetal Bovine Serum (FBS) and 1% Penicillin Streptomycin Glutamine (PSG). Simian virus 40-transformed first trimester primary villous-derived trophoblasts (HTR-8/SVneo) were kindly provided by Charles Graham (The Department of Anatomy and Cell Biology, Queen’s University, Kingston) and Amanda Highet (School of Paediatrics and Reproductive Health, University of Adelaide)64. HTR-8/SVneo cells were maintained in RPMI-1640 Medium (Life Technologies) supplemented with 10% FBS and PSG. Cells were characterised by expression of trophoblast markers cytokeratin, human chorionic gonadotrophin, human placental lactogen, as well as in vitro invasiveness63,64,65. Retinal pigment epithelial (RPE-1) cells were kindly provided by Barry Slobedman (University of Sydney) and cultured in DMEM/F12 + GlutaMAX Medium (Life Technologies) containing 10% FBS and PSG. MRC-5 fibroblasts (ECACC, UK) were maintained in Modified Minimum Essential Medium (Life Technologies) containing 10% FBS and PSG.
Viruses
A bacterial artificial chromosome (BAC) recombinant of CMV clinical strain Merlin, clone pAL1120 containing wild-type UL128 locus was kindly provided by Richard Stanton (Cardiff University)66. For reconstitution of Merlin, Merlin-BAC DNA was extracted from bacterial culture using Nucleobond BAC 100 extraction kit (Macherey-Nagel), then transfected into MRC-5 fibroblasts using Lipofectamine 2000 (Life Technologies). The reconstituted Merlin was then further propagated in RPE-1 epithelial cells to minimise mutation.
The UL128-, UL130-, and UL131A-deletion mutants of Merlin (Merlin ∆UL128, Merlin ∆UL130, Merlin ∆UL131A) were constructed by recombination in Escherichia coli SW10267. Briefly, primers with 3′ homology to the LacZ/Ampicillin/sacB cassette (~20 bp) and 5′ homology to sequences flanking the target viral gene (~80 bp) were used to amplify pAL1141 DNA67 and generate DNA fragments containing selectable markers with short regions of CMV homology. The sequences of primers used are listed in Table 1. Methylated template DNA was removed from PCR reactions by DpnI digestion (New England Biolabs), followed by gel purification using Wizard gel purification system (Promega). Purified PCR amplicons were electroporated into E. coli SW102 and colonies containing BAC with selectable markers were selected using ampicillin, X-gal and IPTG. In the second round of recombination, oligonucleotides with 50 bp homology to the left and right of the deleted gene were electroporated into E. coli SW102 resulting from the first round of recombination. Colonies containing correct oligonucleotide insertions were selected on media containing sucrose, X-gal and IPTG. Deletion of the target gene was confirmed by restriction digestion analysis and DNA sequencing of the gene region.
CMV TB40E-BAC4 was provided by Christian Sinzger (University Hospital Ulm, Germany) and reconstituted as described above. Merlin ∆UL128, Merlin ∆UL130, Merlin ∆UL131A, and TB40E were further propagated in MRC-5 fibroblasts for two to four passages following reconstitution. CMV laboratory strains, AD169 (ATCC, USA) and Towne (provided by Barry Slobedman, University of Sydney), were also propagated in MRC-5 cells. Cell-free supernatant of virus cultures were stored at −80 °C and standard plaque assay in MRC-5 cells was performed to measure the virus titre (pfu/cell) of all virus strains.
PDGFRα expression vectors
Expression plasmids containing complete coding regions of human PDGFRα receptor were constructed by cDNA synthesis and PCR amplification of PDGFRα-specific sequences from RNA extracts of MRC-5 fibroblasts, followed by cloning into pcDNA3.1/V5-HisB plasmid (provided by Caroline Ford, University of New South Wales). Briefly, total RNA was extracted from MRC-5 cells using the RNAqueous kit (Ambion), and then cDNA was synthesized using iScript cDNA synthesis kit (BioRad), and PCR-amplified using primers containing PDGFRα-specific and restriction-site specific sequences (Table 1). PCR amplification was followed by restriction digestion (BamH1 and Xho1 restriction enzymes; New England Biolabs) and ligation of the DNA encoding PDGFRα receptor into pcDNA3.1/V5-HisB. The vector containing PDGFRα-specific cDNA sequence was transformed in E. coli JM109 (Promega), and a colony containing the desired insert was subjected to overnight culture in LB medium, followed by plasmid DNA extraction using PureLink HiPure plasmid midiprep kit (Life Technologies). In addition, commercially available expression plasmid pCMV-SPORT6 containing human PDGFRα cDNA (accession number: BC063414) was also obtained (Thermo Scientific).
The PDGFRα expression plasmids with deleted coding regions for extracellular domains 2–3 or 4–5, were constructed using the phosphorothioate method for the generation of deletion mutants from a plasmid DNA, as described previously68. The sequences of partially phosphorothioate-modified primers used in the inverse PCR are listed in Table 1. The deletion of targeted coding regions for extracellular domains 2–3 or 4–5 was confirmed by DNA sequencing.
CMV infection assays
The SGHPL-4, HTR-8/SVneo, and MRC-5 cells seeded in 8-well chamber culture slides (BD Biosciences) were grown to 80–90% confluence, and then inoculated with cell-free CMV (Merlin wild type, Merlin ∆UL128, Merlin ∆UL130, Merlin ∆UL131A, TB40E wild type, AD169, or Towne) at a multiplicity of infection of 1 pfu/cell. Cells were incubated at 37 °C with 5% CO2 for 3 h, then washed once with 1 x PBS to remove the virus inoculum and incubated in appropriate growth medium (F-10 Ham’s, RPMI-1640 or Minimum Essential Medium, with 2% (v/v) FBS). For antibody inhibition assays, cells were pre-incubated with 20 μg/ml goat anti-PDGFRα antibody (AF-307-NA, R&D systems) or normal goat serum (Sigma-Aldrich) at 37 °C for 1 h, and then inoculated with different strains of CMV (Merlin wild type, Merlin ∆UL128, Merlin ∆UL130, Merlin ∆UL131A, TB40E wild type, AD169, or Towne). After 3 h inoculation, cells were washed once with PBS and incubated in fresh media containing the same concentration of PDGFRα antibody or goat serum control. For ligand competition assays, cells were pre-incubated with 20 ng/ml PDGF-AA ligand (R&D systems) at 4 °C for 1 h, and then inoculated with CMV strains at 37 °C with 5% CO2 for 3 h. After initial inoculation, cells were washed once with PBS and incubated in media containing the same concentration of PDGF-AA ligand. At 2 dpi, cells were fixed and stained for CMV immediate-early IE1p72 and early pUL44 (IE/E) antigens using immunofluorescence.
Transient transfection assays
HTR-8/SVneo trophoblasts grown in 8-well chamber culture slides (BD Biosciences) were transfected with 0.32 μg of various PDGFRα expression constructs (PDGFRα WT, PDGFRα truncated, ∆D2&3, or ∆D4&5) using Lipofectamine 2000 (Life Technologies). At day 2 post-transfection, cells were infected with Merlin ∆UL128 mutant, and then analysed for PDGFRα and CMV IE/E expressions at 2 dpi. For western blotting, HTR-8/SVneo trophoblasts grown in 6-well culture plates were transfected with 4 μg of PDGFRα expression constructs, and cells were harvested at day 2 post-transfection for protein extraction and analysis.
Indirect immunofluorescence analysis
Immunofluorescence staining was performed as previously described69. CMV infection and/or PDGFRα expression was detected using mouse anti-HCMV immediate early (IE1p72) and early (pUL44) antibody cocktail (IE/E; clones DDG9 and CCH2; Dako)/goat anti-PDGFRα antibody (AF-307-NA, R&D systems) and Alexa Fluor 594 donkey anti-mouse/Alexa Fluor 488 donkey anti-goat secondary antibody (Life Technologies). Nuclei were counterstained with ProLong Gold Antifade Reagent containing DAPI (Life Technologies). Immunofluorescence images were taken at room temperature (21 °C) using a Nikon DS camera unit attached to a Nikon Eclipse E400 microscope with Y-FL Epi Fluorescence emission unit. Infectivity of CMV variants were calculated as percentage of IE/E-positive nuclei in randomly selected fields under 200 × magnification (n = 15 from three separate experiments).
Membrane protein extraction, SDS-PAGE and western blotting
The SGHPL-4, HTR-8/SVneo, MRC-5 and RPE-1 cells seeded in 25 cm2 culture flasks were grown to approximately 90% confluence. Membrane protein extraction was performed using the “ProteoExtract native Membrane Protein Extraction Kit (M-PEK, Calbiochem, UK)”, with slight modifications to manufacturer’s protocol. Briefly, the cell monolayer was washed twice with 2 ml ice cold M-PEK wash buffer (Calbiochem), and incubated in 2 ml of M-PEK extraction buffer I containing protease inhibitor for 10 min at 4 °C under gentle agitation. Following 10 min incubation, the supernatant containing soluble proteins was discarded. The cell monolayer was then removed gently using a cell scraper, and cells were further incubated in 300 µl of M-PEK extraction buffer II containing protease inhibitor for 30 min at 4 °C, with gentle agitation on rotary mixer. Insoluble material was removed by centrifugation at 16,000 g, 4 °C for 15 min. The supernatant containing membrane fraction enriched in integral membrane and membrane-associated proteins was collected, and boiled in 2 × laemmli buffer for 10 min.
Western blotting was performed as previously described69 using goat anti-PDGFRα (R&D systems) or rabbit anti-caveolin-1 (Abcam) primary antibodies and HRP-conjugated anti-goat (R&D systems) or donkey anti-rabbit (Thermo Scientific) secondary antibodies. Protein bands were visualised by chemiluminescence using Image Lab 4.1 software (Bio-Rad) and densitometry of immunostaining was performed using ImageJ software.
Statistical analysis
The statistical significance of differences in infectivity was determined using the two-tailed Mann-Whitney U test analysis, with a P-value of less than 0.001 considered highly significant, and a P-value of less than 0.05 considered statistically significant. Analysis was performed using SPSS version 19.0 (SPSS Inc, Chicago).
Results
CMV gH/gL/pUL128-pUL131A complex is essential for infection of villous HTR-8/SVneo, but non-essential for infection of extravillous SGHPL-4 trophoblasts
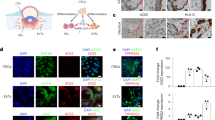
Inoculation of MRC-5 fibroblasts with CMV strains Merlin, Merlin ∆UL128, Merlin ∆UL130, Merlin ∆UL131A, TB40E wild type, AD169, and Towne (serving as a control for infectivity) resulted in similar levels of infection between the strains. Approximately, 70% of fibroblasts expressed CMV immediate-early/early (IE/E) antigens at 2 days post-infection (dpi) (Fig. 1A). Only the wild-type strains of Merlin and TB40E were able to infect HTR-8/SVneo trophoblast cells (Fig. 1A,B). CMV strains unable to assemble the gH/gL/pUL128-pUL131A complex (Merlin ∆UL128, Merlin ∆UL130, Merlin ∆UL131A, AD169, Towne), did not infect HTR-8/SVneo cells. In contrast, wild-type Merlin and TB40E, as well as CMV mutants lacking the pentameric complex were able to infect SGHPL-4 trophoblast cells (Fig. 1A,C). However, infection with wild-type Merlin in SGHPL-4 trophoblasts was significantly more efficient than the Merlin UL128-UL131A-deletion mutant counterparts (21.3% versus 15.8% IE/E-positive cells, P = 0.042). These data indicate that the gH/gL/pUL128-pUL131A complex is essential for infection of villous HTR-8/SVneo trophoblasts, and the pentameric complex contributes to efficient infection of extravillous SGHPL-4 trophoblasts, although it is not essential for infection of these cells.
Infection efficiencies of various CMV strains in extravillous SGHPL-4 and villous HTR-8/SVneo trophoblasts. (A) HTR-8/SVneo trophoblasts, SGHPL-4 trophoblasts, and MRC-5 fibroblasts (control) infected with indicated CMV strains at moi of 1 pfu/cell. Viral IE/E antigens (red); Cell nuclei (blue). Scale bars represent 100 μm. Infection efficiencies of CMV strains in HTR-8/SVneo (B) and SGHPL-4 (C) cells are presented as percentage of viral IE/E-positive nuclei in randomly selected fields (n = 15 from three independent experiments). Mean values and standard error of the mean are shown. *P < 0.05; ***P < 0.001.
PDGFRα receptor is differentially expressed in extravillous SGHPL-4 and villous HTR-8/SVneo trophoblasts
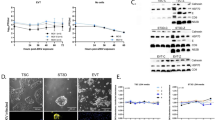
Since different trophoblast subpopulations may differentially express cell surface receptors, we next analysed the expression of PDGFRα, a receptor for entry into fibroblast and glioma cells. Western blot analysis of membrane fractions demonstrated high level expression of PDGFRα in MRC-5 fibroblasts, whilst extravillous SGHPL-4 cells exhibited low level PDGFRα expression on their membranes (Fig. 2A). In contrast, PDGFRα was not detected in the membrane lysates of RPE-1 or villous HTR-8/SVneo cells (Fig. 2A). Densitometric analyses were performed next to semi-quantify the expression levels of PDGFRα relative to caveolin-1 in the different cell lines. PDGFRα/caveolin-1 expression ratio was significantly higher in SGHPL-4 cells, compared with HTR-8/SVneo trophoblasts (P < 0.001) (Fig. 2B).
Expression levels of PDGFRα receptor in HTR-8/SVneo and SGHPL-4 trophoblasts. (A) Membrane lysates of RPE-1 (control), MRC-5 (control), HTR-8/SVneo, and SGHPL-4 cells were subject to Western blot analysis using PDGFRα and caveolin-1 antibodies. Full-length blots are presented in Supplementary Fig. S1. (B) Densitometric analyses of PDGFRα expression levels relative to caveolin-1 in different cell lines. PDGFRα/Caveolin-1 values from three independent experiments are presented as mean ± standard error of the mean. ***P < 0.001.
PDGFRα contributes to the entry of CMV into extravillous SGHPL-4 trophoblasts in strains lacking the pentameric complex more than strains with intact pentameric complexes
In order to determine whether PDGFRα is involved in CMV infection of trophoblast cells, CMV entry assays were performed in the presence of PDGFRα-specific antibody or recombinant PDGF-AA ligand. Blocking with PDGFRα neutralising antibody did not reduce entry of wild-type CMV strains Merlin and TB40E into SGHPL-4 or HTR-8/SVneo trophoblasts (Fig. 3A). However, PDGFRα neutralisation with specific antibody in SGHPL-4 cells blocked the entry of CMV strains lacking gH/gL/pUL128-pUL131A complex, including Merlin ∆UL128, Merlin ∆UL130, Merlin ∆UL131A, AD169, and Towne (Fig. 3A). SGHPL-4 trophoblasts were also treated with PDGFRβ-specific antibody (served as antibody control) to determine the specificity of neutralisation effects observed with PDGFRα antibody. Neutralisation with PDGFRβ antibody did not block the entry of wild-type Merlin or Merlin ∆UL128 strains, whilst the treatment with PDGFRα specifically blocked the entry of Merlin ∆UL128 into SGHPL-4 trophoblast cells (data not shown). In addition, SGHPL-4 and HTR-8/SVneo cells were treated with 20 ng/ml dose of PDGF-AA ligand, and infection efficiencies of CMV strains were analysed. There was no evidence for inhibition of wild type Merlin and TB40E entry into SGHPL-4 or HTR-8/SVneo trophoblasts upon treatment with PDGF-AA (Fig. 3B). Similar to the PDGFRα neutralisation experiments, treatment with PDGF-AA reduced the entry of gH/gL/pUL128-pUL131A-deficient CMV strains into SGHPL-4 trophoblasts (Fig. 3B). However, the levels of inhibition on entry of CMV mutants were less pronounced with PDGF-AA ligand competition compared to receptor neutralisation using PDGFRα-specific antibody.
Effects of PDGFRα neutralising antibody and PDGF-AA ligand on infection of HTR-8/SVneo and SGHPL-4 trophoblasts. HTR-8/SVneo and SGHPL-4 trophoblasts treated with PDGFRα-specific antibody (A) or PDGF-AA ligand (B) were infected with indicated CMV strains, and then CMV IE/E antigens were detected using immunofluorescence. Relative infection is calculated by comparing the IE/E-positive cells in antibody-/ligand-treated samples versus no treatment controls (results from three separate experiments). Mean values and standard error of the mean are shown. ***P < 0.001.
Transient expression of PDGFRα promotes the entry of CMV UL128-UL131A mutants into villous HTR-8/SVneo trophoblasts
The effect of PDGFRα on viral entry into placental trophoblasts was also investigated using transient expression of PDGFRα in villous HTR-8/SVneo trophoblasts, which are known to be deficient for the receptor. HTR-8/SVneo trophoblasts were transfected with a wild-type PDGFRα expression plasmid (PDGFRα WT)70 or with a commercially obtained PDGFRα expression plasmid lacking the second tyrosine kinase segment (PDGFRα truncated; Fig. 4A). This truncated form of PDGFRα retains important autophosphorylation sites at tyrosine residues Y572, Y574, Y720, Y742, and Y754, whilst lacking Y988 and Y1018. HTR-8/SVneo trophoblasts were also transfected with wild-type PDGFRα expression plasmid where coding regions for extracellular domains 2–3 or 4–5 were deleted (PDGFRα mutants, ΔD2-3 and ΔD4-5).
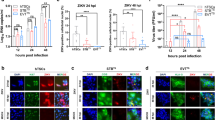
Transient expression of PDGFRα receptors in HTR-8/SVneo trophoblasts. (A) Schematic diagram of PDGFRα cDNA clones, representing wild type (PDGFRα WT), C-terminal truncated version (PDGFRα truncated), and extracellular domain deletion mutants (ΔD2-3, ΔD4-5). Extracellular domains (D1-5), transmembrane region, and tyrosine kinase segments (S1-2) encoded by the cDNA clones are illustrated in the diagram. (B) Western blot analysis of membrane fractions from HTR-8/SVneo trophoblasts transiently expressing PDGFRα receptors (PDGFRα WT, ΔD2-3, ΔD4-5 and PDGFRα truncated). Full-length blots are presented in Supplementary Fig. S2. (C) HTR-8/SVneo trophoblasts transiently expressing PDGFRα were infected with Merlin ∆UL128 mutant, and analysed for cellular PDGFRα and viral IE/E expressions using indirect immunofluorescence. Results show percentage of infected cells as mean ± standard deviation (n = 3). (D) Representative images of immunofluorescence staining with inset images illustrating enlargement of the representative areas. Scale bars represent 100 μm.
Western blot analysis using polyclonal antibody raised against extracellular domains 1–5 of PDGFRα, detected PDGFRα WT (~170 kDa), PDGFRα truncated (~130 kDa), PDGFRα ΔD2-3 (~155 kDa), and PDGFRα ΔD4-5 (~160 kDa) proteins in the membrane lysates of HTR-8/SVneo cells transiently expressing respective receptors (Fig. 4B). This is consistent with predicted molecular weight of these PDGFRα receptor proteins, and also showed that the deletion of D2-3 or D4-5 did not affect cell surface expression of these mutated PDGFRα receptors. In addition to western blot analysis, immunofluorescence staining was also performed to examine transient expression of different PDGFRα receptors in HTR-8/SVneo trophoblasts. A semi-quantitative comparison of PDGFRα expression by immunofluorescence showed more efficient expression of PDGFRα WT (27 ± 2%) and PDGFRα truncated (25 ± 3%), compared with PDGFRα ΔD2-3 (17 ± 2%) and PDGFRα ΔD4-5 (15 ± 1%) mutant receptors (mean ± SD).
Transient expression of PDGFRα WT or PDGFRα truncated in HTR-8/SVneo cells promoted the entry of CMV mutants lacking gH/gL/pUL128-pUL131A complex (Merlin ∆UL128, Merlin ∆UL130, Merlin ∆UL131A) (Fig. 4C,D), consistent with the carboxy-terminal half of the receptor not playing an important role in entry of CMV mutants lacking the pentameric complex. There was no significant increase in entry of wild type CMV strains containing functional pentameric complex, upon transient expression of PDGFRα WT or PDGFRα truncated receptors (data not shown). In contrast, transient expression of PDGFRα mutants (ΔD2-3 or ΔD4-5) did not promote the entry of CMV mutants lacking gH/gL/pUL128-pUL131A complex (Fig. 4C,D). The lower transfection efficiencies of ΔD2-3 and ΔD4-5 constructs are unlikely to affect the determination of infection efficiencies due to the almost complete lack of infection in these transfected cells (0.1 ± 0.05% of cells infected). These results indicate extracellular domains 2-5 of PDGFRα are required for infection of placental trophoblasts with CMV strains unable to assemble the pentameric complex.
Discussion
The ability of CMV to infect diverse cell types of normal placenta, and move across from mother to fetus, is an important factor in transplacental transmission of this DNA virus, and may be important for other vertically transmitted viruses. The mechanism by which CMV infects placental trophoblasts is less well understood than understanding of CMV entry pathways into epithelial, endothelial and fibroblast cells. Using two trophoblast cell lines, extravillous SGHPL-4 cells and villous HTR-8/SVneo cells, we demonstrated that CMV entry into trophoblast cells involves the viral pentameric complex (gH/gL/pUL128-pUL131A) and cellular PDGFRα.
Broad cellular tropism of clinical CMV strains in vivo is facilitated by the presence of various receptor binding proteins mediating entry into different cell types. One essential virion component expressed in clinical CMV strains, is the pentameric complex of gH/gL/pUL128-pUL131A. Wild type CMV strains including Merlin, TB40E, and TR have been shown to express substantial amounts and differential ratios of gH/gL/pUL128-pUL131A pentameric complexes in the virion envelope28,38,71. This pentameric protein complex is necessary for entry into epithelial cells, endothelial cells, monocytes, and dendritic cells27,28,29,31,72,73. Laboratory strains of CMV such as AD169 and Towne cannot assemble gH/gL/pUL128-pUL131A due to mutations within the UL128-UL131A genes23,29. These laboratory-adapted CMV strains have lost the ability to infect epithelial cells, endothelial cells, monocytes, and dendritic cells in vitro, but maintain the ability to infect diploid fibroblasts.
The present study demonstrated the gH/gL/pUL128-pUL131A complex is essential for infection of villous HTR-8/SVneo trophoblasts, whilst it is non-essential for infection of extravillous SGHPL-4 trophoblasts since CMV strains lacking the pentameric complexes were able to infect SGHPL-4 cells (Fig. 1). These results suggest different trophoblast cell types may differentially express cellular receptors specific for CMV infection in a pentameric complex (gH/gL/pUL128-pUL131A)-dependent and -independent manner. This hypothesis is compatible with inconsistencies of infection efficiencies observed in previous studies on infection of primary and other trophoblasts with AD169 and Towne strains16,17,18,19,20,21. Discrepant infectivities were also noted in studies comparing the efficiency of trophoblast cell infection by clinical and laboratory strains. In one study, no differences in infectivity were observed between three clinical CMV isolates, and laboratory strains AD169 and Towne17, whilst another showed a significantly more efficient infection of villous and cell column cytotrophoblasts with VR1814 clinical strain (pentameric complex intact wild type) compared to AD16974. Notably, using different CMV strains (VR1814, AD169, TB40E, TB40E ∆UL131A), and neutralising antibody against viral envelope gB or pUL130/pUL131A, Zydek and colleagues43 demonstrated that CMV gB, but not the viral pentameric complex is necessary for infection of placental trophoblast progenitor cells, which give rise to various trophoblast cell types.
The cellular growth factor receptor, EGFR was reported as a CMV receptor through its interaction with gB45 although subsequent studies have demonstrated that EGFR is not required for CMV entry into fibroblasts, epithelial cells or endothelial cells58,75. In other reports, integrins α2β1, α6β1, and αVβ3 were shown to promote CMV entry into cells through binding with disintegrin-like domain of gB49,55. Blocking with peptide containing gB disintegrin-like domain or antibodies raised against this peptide inhibited CMV infection in human fibroblasts, indicating the importance of gB in entry into these cells55. Accordingly, Maidji and colleagues57 investigated the expression of potential CMV receptors in various populations of cytotrophoblasts and reported distinct viral replication sites in the placenta that correlate with co-expression of EGFR and integrins α1β1, αVβ3, and αVβ6. Although the mechanistic roles of EGFR and certain integrin receptors in CMV entry is unclear, it is likely that co-expression of these receptors promote the engagement of virions to trophoblast cells, thereby triggering gB to fuse with plasma membrane for virus entry. In another study, Wille and colleagues demonstrated that gH/gL complex binds to cellular receptor(s) to trigger gB, and gB acts as the viral fusion protein rather than a receptor-binding protein to promote CMV entry into cells42.
Soroceanu et al. were the first to implicate PDGFRa as a receptor for entry of clinical and laboratory CMV strains into human fibroblasts and glioma cells46. However, a study by Vanarsdall and colleagues58 showed that PDGFRα promotes entry of CMV mutants lacking the pentameric complex into epithelial and endothelial cells, whilst it is not necessary for entry of CMV TR strain, a virus which is able to assemble the pentameric complex. Recent studies have shown a direct interaction between the gO subunit of gH/gL/gO and PDGFRα51 and, in conflict with Soroceanu et al.46., that PDGFRα activation is not necessary for CMV infection59. The significance of PDGFRα is particularly interesting since only a subpopulation (10–15%) of term placental cytotrophoblasts have been reported to express PDGFRα on the cell surface62. This variability in expression of PDGFRα is reflected in our study using continuous cell lines derived from different trophoblast cell types, with SGHPL-4 extravillous trophoblasts able to express PDGFRα at low levels, whilst HTR-8/SVneo villous trophoblasts do not express detectable levels of PDGFRα (Fig. 2A). CMV entry assays involving the blockage of PDGFRα in SGHPL-4 trophoblasts suggest PDGFRα is an important receptor for entry of CMV strains unable to assemble the pentameric complex (Fig. 3). The fact that PDGFRα antibody or PDGF-AA did not affect the entry of wild-type CMV strains suggests the pentameric complex provides an alternate pathway into trophoblast cells during the blockage of functional PDGFRα. Consistent with our study, Vanarsdall and colleagues58 demonstrated that PDGFRα antibodies or PDGF-AA ligand did not block wild-type CMV TR entry into fibroblasts, epithelial and endothelial cells.
Transient expression of PDGFRα (carboxy-terminal truncated version) in epithelial and endothelial cells has been shown to enhance the entry of CMV strains lacking the pentameric complex, as well as the entry of wild type CMV TR strain58. In this study, transient expression of PDGFRα wild type or carboxy-terminal truncated version of the receptor in HTR-8/SVneo trophoblasts equally permitted the entry of CMV strains lacking the pentameric complex (Fig. 4). These results indicate that the second tyrosine kinase segment of the PDGFRα receptor is not involved in entry of CMV strains unable to assemble the pentameric complex. However, there was no further increase in entry of CMV strains containing functional pentameric complex upon over-expression of PDGFRα in HTR-8/SVneo trophoblasts (data not shown). These results may indicate that CMV preferably utilises the pentameric complex for entry into villous HTR-8/SVneo trophoblasts. Alternatively, this could be due to Merlin virions containing significantly more gH/gL/pUL128-pUL131A than other envelope glycoprotein complexes38, thereby limiting CMV entry into trophoblast cells via different pathways. Interestingly, transient expression of mutant PDGFRα receptors (lacking extracellular domains 2–3 or domains 4–5) in HTR-8/SVneo trophoblasts did not permit the entry of pentameric-deficient CMV strains, indicating extracellular domains 2–5 of PDGFRα are involved in CMV entry into trophoblasts. These results are supported by recent findings demonstrating the extracellular domain 3 of PDGFRα contribute to wild type Merlin virus entry in PDGFRα-KO fibroblast cells60.
It has been demonstrated that ARPE-19 epithelial cells become resistant to infection with CMV TR strain upon transient expression of the pentameric gH/gL/pUL128-pUL131A complex39. This suggests the pentameric protein complex binds to cell-specific receptors that are required for entry into ARPE-19 cells, and therefore producing interference. However, co-expression of PDGFRα and gH/gL/pUL128-pUL131A complexes resulted in efficient entry of CMV TR into ARPE-19 cells, indicating expression of PDGFRα overcomes the interference produced by the pentameric complexes58. This evidence in combination with our observations that transient expression of PDGFRα in HTR-8/SVneo trophoblasts allowed the entry of CMV strains lacking the pentameric complex, suggests PDGFRα is involved in CMV entry pathway that is independent of gH/gL/pUL128-pUL131A-mediated entry. Using chemical inhibitors targeting various stages of endocytic entry, Vanarsdall and colleagues58 showed PDGFRα promotes the entry of CMV TR into epithelial cells via dynamin-dependent endocytosis of the virion, followed by pH-independent fusion with endosomal membranes, although this pathway is yet to be confirmed in trophoblast cells. On the other hand, Soroceanu and colleagues46 showed that PDGFRα directly interacts with gB for viral attachment/entry into host cells, suggesting a potent neutralising antibody against gB may be sufficient to block CMV entry via PDGFRα-mediated pathway.
The viral pentameric complex components, gH and pUL130, have been reported to induce high titre antibodies, with antiserum to these proteins have been demonstrated to effectively neutralise CMV entry into epithelial cells44,76, and are proposed as important targets for CMV vaccine. As PDGFRα provides an additional CMV entry pathway into trophoblasts, specific inhibitor(s) of this pathway, in combination with potent neutralising antibodies against viral pentameric complex and gB may provide a more effective prevention of CMV infection in placental trophoblast cells, and possibly other placental cell types. In a further study, chemically engineered sulfated glucans were also shown to have high antiviral activity against CMV at the stage of viral entry into human fibroblasts77, and may be promising candidates for drug development and antiviral strategies.
In summary, this study demonstrates CMV entry into placental trophoblast involves the viral pentameric complex of gH/gL/pUL128-pUL131A, and the cellular PDGFRα receptor. These findings suggest multiple therapeutic targets are required for prevention of CMV infection in diverse placental cell types, which may facilitate treatment strategies for prevention of CMV transmission during pregnancy.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Kenneson, A. & Cannon, M. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev. Med. Virol. 17, 253–276 (2007).
Dollard, S., Grosse, S. & Ross, D. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev. Med. Virol. 17, 355–363 (2007).
Stagno, S. et al. Primary cytomegalovirus infection in pregnancy. Incidence, transmission to fetus, and clinical outcome. JAMA 256, 1904–1908 (1986).
Iwasenko, J. et al. Human cytomegalovirus infection is detected frequently in stillbirths and is associated with fetal thrombotic vasculopathy. J. Infect. Dis. 203, 1526–1533 (2011).
Boppana, S., Pass, R., Britt, W., Stagno, S. & Alford, C. Symptomatic congenital cytomegalovirus infection: neonatal morbidity and mortality. Pediatr. Infect. Dis. J. 11, 93–99 (1992).
Stagno, S. et al. Primary Cytomegalovirus Infection in Pregnancy: Incidence, Transmission to Fetus, and Clinical Outcome. JAMA 256, 1904–1908, https://doi.org/10.1001/jama.1986.03380140074025 (1986).
Pass, R., Fowler, K., Boppana, S., Britt, W. & Stagno, S. Congenital cytomegalovirus infection following first trimester maternal infection: Symptoms at birth and outcome. J. Clin. Virol. 35, 216–220 (2006).
Mostoufi-zadeh, M., Driscoll, S., Biano, S. & Kundsin, R. Placental evidence of cytomegalovirus infection of the fetus and neonate. Arch. Pathol. Lab. Med. 108, 403–406 (1984).
Monif, G. & Dische, R. Viral placentitis in congenital cytomegalovirus infection. Am. J. Clin. Pathol. 58, 445–449 (1972).
Muhlemann, K., Miller, R. K., Metlay, L. & Menegus, M. A. Cytomegalovirus infection of the human placenta: an immunocytochemical study. Hum. Pathol. 23, 1234–1237 (1992).
Sinzger, C. et al. Cell types infected in human cytomegalovirus placentitis identified by immunohistochemical double staining. Virchows Arch. A Pathol. Anat. Histopathol. 423, 249–256 (1993).
Tamiolakis, D. et al. Human decidual cells activity in women with spontaneous abortions of probable CMV aetiology during the first trimester of gestation. An immunohistochemical study with CMV-associated antigen. Acta Medica (Hradec Kralove) 47, 195–199 (2004).
Trincado, D., Munro, S., Camaris, C. & Rawlinson, W. Highly sensitive detection and localization of maternally acquired human cytomegalovirus in placental tissue by in situ polymerase chain reaction. J. Infect. Dis. 192, 650–657 (2005).
Gabrielli, L. et al. Complete replication of human cytomegalovirus in explants of first trimester human placenta. J. Med. Virol. 64, 499–504 (2001).
Hamilton, S. et al. Human cytomegalovirus-induces cytokine changes in the placenta with implications for adverse pregnancy outcomes. PLoS ONE 7, e52899 (2012).
Chan, G., Hemmings, D., Yurochko, A. & Guilbert, L. Human cytomegalovirus-caused damage to placental trophoblasts mediated by immediate-early gene-induced tumor necrosis factor-alpha. Am. J. Pathol. 161, 1371–1381 (2002).
Halwachs-Baumann, G. et al. Human trophoblast cells are permissive to the complete replicative cycle of human cytomegalovirus. J. Virol. 72, 7598–7602 (1998).
Hemmings, D., Kilani, R., Nykiforuk, C., Preiksaitis, J. & Guilbert, L. Permissive cytomegalovirus infection of primary villous term and first trimester trophoblasts. J. Virol. 72, 4970–4979 (1998).
Halwachs-Baumann, G., Weihrauch, G., Gruber, H., Desoye, G. & Sinzger, C. hCMV induced IL-6 release in trophoblast and trophoblast like cells. J. Clin. Virol. 37, 91–97 (2006).
Chou, D., Ma, Y., Zhang, J., McGrath, C. & Parry, S. Cytomegalovirus infection of trophoblast cells elicits an inflammatory response: A possible mechanism of placental dysfunction. Am. J. Obstet. Gynecol. 194, 535–541 (2006).
LaMarca, H., Sainz, J. B. & Morris, C. Permissive human cytomegalovirus infection of a first trimester extravillous cytotrophoblast cell line. Virol J 1 (2004).
Fisher, S., Genbacev, O., Maidji, E. & Pereira, L. Human cytomegalovirus infection of placental cytotrophoblasts in vitro and in utero: implications for transmission and pathogenesis. J. Virol. 74, 6808–6820 (2000).
Cha, T. et al. Human cytomegalovirus clinical isolates carry at least 19 genes not found in laboratory strains. J. Virol. 70, 78–83 (1996).
Murphy, E. et al. Coding potential of laboratory and clinical strains of human cytomegalovirus. Proc. Natl Acad. Sci. USA 100, 14976–14981 (2003).
Prichard, M., Penfold, M., Duke, G., Spaete, R. & Kemble, G. A review of genetic differences between limited and extensively passaged human cytomegalovirus strains. Rev. Med. Virol. 11, 191–200 (2001).
Akter, P. et al. Two novel spliced genes in human cytomegalovirus. J. Gen. Virol. 84, 1117–1122 (2003).
Ryckman, B., Jarvis, M., Drummond, D., Nelson, J. & Johnson, D. Human cytomegalovirus entry into epithelial and endothelial cells depends on genes UL128 to UL150 and occurs by endocytosis and low-pH fusion. J. Virol. 80, 710–722 (2006).
Ryckman, B. et al. Characterization of the human cytomegalovirus gH/gL/UL128-131 complex that mediates entry into epithelial and endothelial cells. J. Virol. 82, 60–70 (2008).
Hahn, G. et al. Human cytomegalovirus UL131-128 genes are indispensable for virus growth in endothelial cells and virus transfer to leukocytes. J. Virol. 78, 10023–10033 (2004).
Wang, D. & Shenk, T. Human cytomegalovirus virion protein complex required for epithelial and endothelial cell tropism. Proc. Natl Acad. Sci. USA 102, 18153–18158 (2005).
Straschewski, S. et al. Protein pUL128 of Human Cytomegalovirus Is Necessary for Monocyte Infection and Blocking of Migration. J. Virol. 85, 5150–5158 (2011).
Huber, M. & Compton, T. Characterization of a novel third member of the human cytomegalovirus glycoprotein H-glycoprotein L complex. J. Virol. 71, 5391–5398 (1997).
Huber, M. & Compton, T. The human cytomegalovirus UL74 gene encodes the third component of the glycoprotein H-glycoprotein L-containing envelope complex. J. Virol. 72, 8191–8197 (1998).
Kinzler, E., Theiler, R. & Compton, T. Expression and reconstitution of the gH/gL/gO complex of human cytomegalovirus. J. Clin. Virol. 25, S87–S95 (2002).
Vanarsdall, A., Chase, M. & Johnson, D. Human cytomegalovirus glycoprotein gO complexes with gH/gL, promoting interference with viral entry into human fibroblasts but not entry into epithelial cells. J. Virol. 85, 11638–11645 (2011).
Wille, P., Knoche, A., Nelson, J., Jarvis, M. & Johnson, D. A human cytomegalovirus gO-null mutant fails to incorporate gH/gL into the virion envelope and is unable to enter fibroblasts and epithelial and endothelial cells. J. Virol. 84, 2585–2596 (2010).
Ryckman, B., Chase, M. & Johnson, D. Human cytomegalovirus TR strain glycoprotein O acts as a chaperone promoting gH/gL incorporation into virions but is not present in virions. J. Virol. 84, 2597–2609 (2010).
Zhou, M., Yu, Q., Wechsler, A. & Ryckman, B. Comparative analysis of gO isoforms reveals that strains of human cytomegalovirus differ in the ratio of gH/gL/gO and gH/gL/UL128-131 in the virion envelope. J. Virol. 87, 9680–9690 (2013).
Ryckman, B., Chase, M. & Johnson, D. HCMV gH/gL/UL128-131 interferes with virus entry into epithelial cells: evidence for cell type-specific receptors. Proc. Natl Acad. Sci. USA 105, 14118–14123 (2008).
Britt, W. Neutralizing antibodies detect a disulfide-linked glycoprotein complex within the envelope of human cytomegalovirus. Virology 135, 369–378 (1984).
Navarro, D. et al. Glycoprotein B of human cytomegalovirus promotes virion penetration into cells, transmission of infection from cell to cell and fusion of infected cells. Virology 197, 143–158 (1993).
Wille, P., Wisner, T., Ryckman, B. & Johnson, D. Human cytomegalovirus (HCMV) glycoprotein B promotes virus entry in trans acting as the viral fusion protein rather than as a receptor-binding protein. MBio 4, e00332–00313 (2013).
Zydek, M. et al. HCMV infection of human trophoblast progenitor cells of the placenta is neutralized by a human monoclonal antibody to glycoprotein B and not by antibodies to the pentamer complex. Viruses 9, 1346–1364 (2014).
Freed, D. et al. Pentameric complex of viral glycoprotein H is the primary target for potent neutralization by a human cytomegalovirus vaccine. Proc. Natl Acad. Sci. USA 110, E4997–5005 (2013).
Wang, X., Huong, S., Chiu, M., Raab-Traub, N. & Huang, E. Epidermal growth factor receptor is a cellular receptor for human cytomegalovirus. Nature 424, 456–461 (2003).
Soroceanu, L., Akhavan, A. & Cobbs, C. Platelet-derived growth factor-α receptor activation is required for human cytomegalovirus infection. Nature 455, 391–395 (2008).
Halary, F. et al. Human cytomegalovirus binding to DC-SIGN is required for dendritic cell infection and target cell trans-infection. Immunity 17, 653–664 (2002).
Wang, X., Huang, D., Huong, S. & Huang, E. Integrin αvβ3 is a coreceptor for human cytomegalovirus infection. Nat. Med. 11, 515–521 (2005).
Feire, A., Koss, H. & Compton, T. Cellular integrins function as entry receptors for human cytomegalovirus via a highly conserved disintegrin-like domain. PNAS 101, 15470–15475 (2004).
Adlish, J., Lahijani, R. & Jeor, S. S. Identification of a putative cell receptor for human cytomegalovirus. Virology 176, 337–345 (1990).
Kabanova, A. et al. Platelet-derived growth factor-α receptor is the cellular receptor for human cytomegalovirus gHgLgO trimer. Nat. microbiology 1, 16082 (2016).
Martinez-Martin, N. et al. An unbiased screen for human cytomegalovirus identifies neuropilin-2 as a central viral receptor. Cell 174, 1158–1171. e1119 (2018).
Xiaofei, E. et al. OR14I1 is a receptor for the human cytomegalovirus pentameric complex and defines viral epithelial cell tropism. Proc. Natl Acad. Sci. 116, 7043–7052 (2019).
Pietropaolo, R. & Compton, T. Direct interaction between human cytomegalovirus glycoprotein B and cellular annexin II. J. Virol. 71, 9803–9807 (1997).
Feire, A., Roy, R., Manley, K. & Compton, T. The glycoprotein B disintegrin-like domain binds beta 1 integrin to mediate cytomegalovirus entry. J. Virol. 84, 10026–10037 (2010).
Stegmann, C., Rothemund, F., Laib Sampaio, K., Adler, B. & Sinzger, C. The N Terminus of Human Cytomegalovirus Glycoprotein O Is Important for Binding to the Cellular Receptor PDGFRalpha. Journal of virology 93, https://doi.org/10.1128/JVI.00138-19 (2019).
Maidji, E., Genbacev, O., Chang, H. & Pereira, L. Developmental regulation of human cytomegalovirus receptors in cytotrophoblasts correlates with distinct replication sites in the placenta. J. Virol. 81, 4701–4712 (2007).
Vanarsdall, A., Wisner, T., Lei, H., Kazlauskas, A. & Johnson, D. PDGF receptor-α does not promote HCMV entry into epithelial and endothelial cells but increased quantities stimulate entry by an abnormal pathway. PLoS Pathog. 8, e1002905 (2012).
Wu, Y. et al. Human cytomegalovirus glycoprotein complex gH/gL/gO uses PDGFR-alpha as a key for entry. PLoS Pathog. 13, e1006281, https://doi.org/10.1371/journal.ppat.1006281 (2017).
Wu, K., Oberstein, A., Wang, W. & Shenk, T. Role of PDGF receptor-alpha during human cytomegalovirus entry into fibroblasts. Proc. Natl Acad. Sci. U S Am. 115, E9889–E9898, https://doi.org/10.1073/pnas.1806305115 (2018).
Heldin, C. & Westermark, B. Role of platelet-derived growth factor in vivo. 2nd edn, 249–273 (Plenum, 1996).
Holmgren, L., Claesson-Welsh, L., Heldin, C. & Ohlsson, R. The expression of PDGR α- and β-receptors in subpopulations of PDGF-producing cells implicates autocrine stimulatory loops in the control of proliferation in cytotrophoblasts that have invaded the maternal endometrium. Growth Factors 6, 219–231 (1992).
Choy, M. & Manyonda, I. The phagocytic activity of human first trimester extravillous trophoblasts. Hum. Repro 13, 2941–2949 (1998).
Graham, C. et al. Establishment and characterization of first trimester human trophoblast cells with extended lifespan. Exp. Cell Res. 206, 204–211 (1993).
Zhou, Y. et al. Human cytotrophoblasts adopt a vascular phenotype as they differentiate. J. Clin. Invest. 99, 2139–2151 (1997).
Stanton, R. et al. Reconstruction of the complete human cytomegalovirus genome in a BAC reveals RL13 to be a potent inhibitor of replication. J. Clin. Invest. 120, 3191–3208 (2010).
Stanton, R., McSharry, B., Armstrong, M., Tomasec, P. & Wilkinson, G. Re-engineering adenovirus vector systems to enable high-throughput analyses of gene function. Biotechniques 45(659–662), 664–668 (2008).
Stoynova, L., Solόrzano, R. & Collins, E. Generation of large deletion mutants from plasmid DNA. Biotechniques 36, 402–406 (2004).
Hamilton, S. T. et al. Human cytomegalovirus utilises cellular dual-specificity tyrosine phosphorylation-regulated kinases during placental replication. Placenta 72-73, 10–19, https://doi.org/10.1016/j.placenta.2018.10.002 (2018).
Claesson-Welsh, L., Eriksson, A., Westermark, B. & Heldin, C. cDNA cloning and expression of the human A-type platelet-derived growth factor (PDGF) receptor establishes structural similarity to the B-type PDGF receptor. Proc. Natl Acad. Sci. USA 86, 4917–4921 (1989).
Schuessler, A., Sampaio, K., Straschewski, S. & Sinzger, C. Mutational mapping of pUL131A of human cytomegalovirus emphasizes its central role for endothelial cell tropism. J. Virol. 86, 504–512 (2012).
Wang, D. & Shenk, T. Human cytomegalovirus UL131 open reading frame is required for epithelial cell tropism. J. Virol. 79, 10330–10338 (2005).
Gerna, G. et al. Dendritic-cell infection by human cytomegalovirus is restricted to strains carrying functional UL131-128 genes and mediates efficient viral antigen presentation to CD8+ T cells. J. Gen. Virol. 86, 275–284 (2005).
Tabata, T. et al. Cytomegalovirus impairs cytotrophoblast-induced lymphangiogenesis and vascular remodeling in an in vivo human placentation model. Am. J. Pathol. 181, 1540–1559 (2012).
Isaacson, M., Feire, A. & Compton, T. Epidermal growth factor receptor is not required for human cytomegalovirus entry or signaling. J. Virol. 81, 6241–6247 (2007).
Saccoccio, F. et al. Peptides from cytomegalovirus UL130 and UL131 proteins induce high titer antibodies that block viral entry into mucosal epithelial cells. Vaccine 29, 2705–2711 (2011).
Ray, B. et al. Chemically engineered sulfated glucans from rice bran exert strong antiviral activity at the stage of viral entry. J. Nat. Prod. 76, 2180–2188 (2013).
Acknowledgements
The authors thank Dr. Brian McSharry (University of Sydney), Prof. Barry Slobedman (University of Sydney), and Dr. Richard Stanton (Cardiff University) for generously providing CMV Merlin, and guidance with construction of Merlin mutants. We thank Prof. Christian Sinzger (Universitätsklinikum Ulm) for providing CMV TB40E. We also thank Prof. Guy Whitley (St. George’s University of London) for providing SGHPL-4 trophoblasts, Prof. Charles Graham (Queen’s University, Kingston) and Dr. Amanda Highet (University of Adelaide) for providing HTR-8/SVneo trophoblasts, Dr. Caroline Ford (University of New South Wales) for providing pcDNA3.1/V5-HisB vector, and Prof. Manfred Marschall (Universität Erlangen-Nürnberg) for his helpful comments on the manuscript. This project was supported by grants from the Australian Centre for Perinatal Science, the Australian National Health and Medical Research Council (grant APP1127717-Hamilton), the Thrasher Research Fund Early Career Award (grant RG181876-Hamilton) and the Rebecca L. Cooper Medical Research Foundation (grant 10875).
Author information
Authors and Affiliations
Contributions
Z.N., G.M.S. and W.D.R. designed the research; Z.N. and S.T.H. processed the acquired data, Z.N., S.T.H. and W.J.V.Z. performed the data analysis; Z.N., S.T.H., W.J.V.Z., G.M.S. and W.D.R. wrote the paper and revised the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Naing, Z., Hamilton, S.T., van Zuylen, W.J. et al. Differential Expression of PDGF Receptor-α in Human Placental Trophoblasts Leads to Different Entry Pathways by Human Cytomegalovirus Strains. Sci Rep 10, 1082 (2020). https://doi.org/10.1038/s41598-020-57471-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-57471-3
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.