Abstract
Inflammatory bowel diseases (IBD) are chronic relapsing disorders that have a negative impact on quality of life. They can be highly disabling and have been associated with sleep disturbance. The aim of our study was to evaluate the sleep quality of a large cohort of IBD patients to identify possible associated cofactors. We prospectively recruited consecutive patients attending the IBD Unit of “Azienda Ospedaliera” of Padua from November 2018 to May 2019 and collected demographics and clinical characteristics. The patients completed the Pittsburgh Sleep Quality Index (PSQI), the IBD questionnaire (IBDQ), the IBD-Disability Index (IBD-DI) questionnaire, and the Hospital Anxiety and Depression Scale (9-HADS). A multivariate regression model was applied to assess independent risk factors of sleep disturbance among IBD-related variables, disability, quality of life, anxiety, and depression. We investigated the sleep quality of 166 patients with IBD, finding 67.5% of them suffering from sleep disturbance. In particular, low quality of life, presence of disability and extraintestinal manifestations were identified as independent risk factors of sleep disturbance. We discovered that all depressed patients were also affected by sleep disturbance, while we found no difference in sleep disturbance between patients with or without anxiety state. However, a positive correlation was reported between both anxiety and depression scores and PSQI score (Spearman correlation: r = 0.31 and r = 0.38 respectively). Our study showed that sleep quality is not directly associated with an active or inactive IBD state or with the ongoing treatment, but it is mostly correlated with the patients’ mood state, disability, and quality of life. Gastroenterologists and psychologists should join forces during clinical outpatients’ visits to evaluate emotional states for a better IBD management.
Similar content being viewed by others
Introduction
Inflammatory bowel disease (IBD) is a group of chronic relapsing disorders that includes, principally, Crohn’s disease (CD) and ulcerative colitis (UC) and is characterized by a progressive course and potential development of complications, including extra-intestinal manifestations (EIMs)1.
Indeed, both diseases are associated with a marked reduction of health-related quality of life, severe fatigue, and work impairment2,3, as well as with depression and anxiety4. In particular, changes in mood and mental state are linked to both development and disease worsening in IBD patients5,6,7,8,9,10. Furthermore, sleep, considered as an environmental factor, has been investigated as a possible marker of subclinical inflammation6,11. An unsatisfactory sleep quality is commonly reported in the general population, due to several environmental and psychological factors. Alerting factors include older age, female gender, lower socio-economic status, marital status different from being married12, anxiety, and lack of optimism13,14. Patients with chronic medical disorders report an even worse quality of sleep, experiencing fewer hours and less restorative sleep when compared to healthy individuals5. In particular, some evidence suggests that sleep disorder is one of the main concerns of patients suffering from chronic intestinal disease, which can seriously impact health and work productivity in the long term15,16. Furthermore, it has been shown that sleep disturbance can worsen the subjective symptoms of the disorder, leading to more frequent relapses17.
Thus, a poor sleep quality is associated with troubles and distress in patients with IBD18, resulting in an impaired quality of life19. However, only a few studies6,20,21 have analyzed the risk factors associated with sleep disturbance in IBD patients. The aim of the present study was to evaluate sleep disturbance in IBD patients and identify its risk factors, including disease-related variables and mood states.
Results
Demographics and clinical characteristics
A total of 166 subjects completed the Inflammatory Bowel Disease Questionnaire (IBDQ) for quality of life assessment, a global score ≤170 is expression of low QoL; the IBD-Disability Index (IBD-DI) questionnaires for disability assessment, and a global score ≤3.5 is expression of greater disability, and the Pittsburgh Sleep Quality Index (PSQI) for sleep quality assessment. In particular, the latter is a self-reported survey that evaluates characteristics such as sleep duration, efficiency, and disturbances. A global score ≥5 reveals poor sleep quality.
Table 1 shows demographics and baseline characteristics of our population. The mean age was 44.39 years (±13.92; range 18–80). In particular, 82 patients (49.40%) were aged more than 45 years. Sex distribution was 79 females (47.59%) and 87 males (52.41%). In 134 patients (80.7%) disease was considered clinically in remission, although 47% of all population had calprotectin levels higher than 250. Forty-five (27.11%) patients were affected by at least one EIM associated to IBD with only 16 of them having an active disease at the time of the study.
At the time of the study, 98 (59.4%) patients were receiving therapy with biologic drugs (Infliximab, Adalimumab, Vedolizumab or Golimumab) and 29 (17.47%) with immunosuppressants (Azathioprine/AZA or Metotrexate). Subjects with a low IBDQ score (≤170) were 74 (44.58%); subjects with a low IBD-DI score (≤3.5) were 90 (54.22%) (Table 1).
Sleep, quality of life, and disability assessment
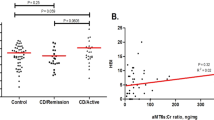
Patients who reported a pathological score of PSQI (≥5) were 112 (67.5%) (Table 1). In particular, we found that 64.9% (87/134) of patients in clinical remission and 78.1% (25/32) of patients with active disease (p = 0.15) had poor sleep quality. Considering each sleep component of PSQI, we found the highest sub-score in sleep disturbances (mean 1.49, SD 0.6) and the lowest one in habitual use of sleep medication (mean 0.33, SD 0.9) (Table 2). Sixty-two (55.4%) patients with bad sleep quality also had a poor quality of life, whereas 65.2% (73/112) had a low disability score. Considering each sub-score of both IBDQ and IBD-DI questionnaires, patients with poor sleep quality had statistically significant lower scores compared to patients reporting no sleep disturbance except for one IBD-DI sub-score: body structures (Supplementary Material, Tables S1 and S2).
Table 3 describes the factors that were significantly associated with the PSQI score. Based on the univariate analysis, we observed that patients who suffered from extra-intestinal manifestations, patients with pathologic IBD-DI score (≤3.5), with lower IBDQ score (≤170), with no occasional alcohol consumption and females were more likely to have a PSQI score ≥5 (Table 3). Age, type of disease (CD or UC), disease extent and phenotype, ongoing treatment, low hemoglobin levels, and elevated faecal calprotectin were instead not identified as risk factors for bad sleep quality (data not shown). We designed two different multivariate regression models including IBDQ and IBD-DI groups separately, together with the other significant risk factors found in the univariate analysis (presence of EIMs, alcohol consumption and sex), to respect the rules of multi-collinearity (Spearman correlation between IBD-DI and IBDQ scores were >0.80). Using the two models, only IBDQ, IBD-DI and EIMs were identified as independent risk factors of sleep disturbance. In particular subjects with low scores of IBD-DI and IBDQ were between three and four times more likely to have poor sleep quality (OR 3.6 95% CI 1.77–7.29 and OR 3.8, 95% CI 1.78–8.10, respectively). Moreover, in both models, patients with EIMs had a risk of sleep disturbance more than twice higher compared to patients without EIMs (Table 3).
As shown in Table 4, we observed a moderately strong negative correlation between PSQI score and IBDQ (r −0.50, p < 0.001) (Fig. 1), and between PSQI and IBD-DI (r −0.45, p < 0.001) (Fig. 2).
Sleep and mood state (depression and anxiety) assessment
A sub-group of patients (n = 74: 35 females, mean age 44.3 ± 13.23, 35 UC) also completed the HADS scale. Among these, 31 patients showed anxiety and 8 depressive mood. We found that all patients with depressive mood had sleep disturbance (100%) while it was present in 66.7% of those without depression (p = 0.05). In contrast, we did not find any statistically significant difference in sleep disturbance between patients with and without anxiety (p = 0.25) (Table 5). However, we observed a moderately positive correlation between PSQI and HADS score for both anxiety (r 0.31, p 0.006) and depression (r 0.38, p < 0.001) in the subset of patients.
Discussion
Main results
While it has been long recognized that IBD patients suffer from poor sleep quality, only a handful of studies6,20,21 have evaluated the prevalence and risk factors associated with sleep disturbance and severity in inflammatory bowel condition4,15. For this reason, we decided to investigate the sleep quality of 166 patients with IBD using validated questionnaires. We found that two thirds of patients with IBD suffered from sleep disturbance. Poor sleep quality was associated with low quality of life, greater disability and presence of EIMs whereas it did not appear to be associated to disease variables such as subtype, disease activity, or type of therapy taken. Absence of correlation with IBD-related characteristic can be due to a generalized state of discomfort. Furthermore, we showed that all our depressed patients were affected by sleep disturbance, while an anxiety state did not significantly impact on sleep quality. However, a positive correlation was found between PSQI score and both anxiety and depression scores.
Previous literature
We found a prevalence of sleep disturbances in IBD of 67.5%, which is higher than that described in other studies on the healthy population22. We did not find an association between the two major forms of IBD and PSQI pathologic score. This is in line with previous evidence that reports no difference in sleep quality disturbance between CD and UC patients (6.8 ± 3.4 versus 7.4 ± 3.1; p = 0.48)20. Conversely, we did not confirm the previous evidence that associates being female with more frequent PSQI pathologic score20. We only found a difference in sleep quality in the univariate analysis and it was not confirmed in the multivariate ones. This difference in sleep quality might be due to the gender differences in the prevalence of psychiatric morbidities, socio-cultural factors, and coping strategies23. We found that all subjects with depression had sleep disturbance and that a moderate positive correlation between anxiety and depression scores and PSQI score was present. These results are in line to those described in the general population24,25,26. For this reason, psychological factors can be associated with a bad sleep quality in IBD patients, more than with the disease per se.
Notably, IBD patients could suffer from sleep problems linked to medication side effects. For this reason, several studies regarding sleep and fatigue have previously taken into consideration the ongoing therapy. In 2018, Lee et al. assessed sleep disturbance in IBD patients receiving immunosuppressant and biologic drugs27. None of these therapies turned out to be predictive of disturbed sleep. This is in line with our findings highlighting that sleep quality is not associated with the current treatment. Sobolewska et al. investigated sleep quality in a small cohort of IBD patients. They found that poor sleep was assessed in 78% of clinically-active patients (40/51) and in 35% of patients considered in remission (5/14) (p = 0.002; OR 6.5, 95% CI 1.8–23.6)20. Conversely and analogous to our findings, Ranjbaran et al. used survey data to demonstrate that poor sleep quality and poor quality of life are associated also in patients which are clinically considered in remission21. Similar results were also found by Bar-Gil Shitrit et al.28. Furthermore, a previous study reported that poor sleep was more common in IBD patients with high C-Reactive Protein levels than in those with normal values29, while we did not find an association between quality of sleep and faecal calprotectin. However, only this latter is considered a specific inflammation marker for IBD30. Therefore, we did not find an association either with clinical or with biochemical signs of disease activity. Finally, certain EIMs, such as peripheral arthritis or erythema nodosum, are frequently associated with active intestinal inflammation while other EIMs, such as uveitis or ankylosing spondylitis, usually occur independently of intestinal inflammatory activity31. We found an independent association between sleep disturbance and presence of EIMs but not between sleep disturbance and active disease. This result can be justified by the fact that in our population only 35 patients with EIM had an active disease at the time of the study. We have recently published a study which reports the presence of EIMs as an independent risk factor of poor QOL and presence of disability32.
Strengths and limitations
Our study has multiple strengths. First, it is based on a large population referring to the same IBD Unit, thus different environmental factors as well as different diseases approaches did not influence our results. Moreover, we applied state-of-the-art methods to assess all the variables investigated, while the questionnaires were all administered by one interviewer in order to ensure that all patients understand the questions. Finally, to the best of our knowledge, this is the first study aimed at assessing sleep disturbance associated with disability and mood disorders in IBD patients.
We have also identified a number of limitations. First, the results of our investigations are based on self-reported measures and lack an objective measurement of sleep (i.e. polysomnography). However, it is relevant to note that, in the past, Keefer et al. have demonstrated a good correlation between polysomnography measure and subjective sleep perception investigated with PSQI questionnaire21,33. Second, our results were not associated with objective evidence of disease (endoscopy), however we used faecal calprotectin level which has been considered a good alternative to endoscopy evaluation34. Third, a cross-sectional protocol provides limited evidence concerning changes over time, which would be better assessed by a future longitudinal study. Moreover, considering that IBD subjects often have overlapping IBS35, another limitation is the lack of assessment of IBS in our study population. Finally, we only started administering the HADS questionnaire after the study had started, meaning that it was only completed by a subset of patients. However, in the absence of a selection bias (all consecutive patients have also completed this questionnaire since February 2019), we believe that this sub-group is still representative of the entire population.
Conclusions
We demonstrated that a poor quality of life in IBD patients is strongly related to poor quality of life, disability, and mood state. In IBD management not only disease progression, but also sleep and disability should be investigated during clinical outpatients’ visits. This would also allow for a novel multidisciplinary approach based on collaborative efforts between gastroenterologists and psychologists with the aim to improve sleep and quality of life in general, and to reduce disability and the restriction and limitations that sleep deprivation can induce in daily life.
Methods
Patients
The present cross-sectional study was approved by the Ethics Committee of Padua (protocol number 4197/AO/17) in November 2018 and procedures applied were in conformity with a previous study we conducted32. We consecutively and prospectively recruited all IBD patients who visited the IBD Unit of the “Azienda Ospedaliera” of Padua from November 2018 to May 2019. Inclusion criteria were age ≥18 years and a confirmed diagnosis of UC or CD based on clinical, endoscopic and histological examinations according to international criteria36 from at least six months. The exclusion criteria were patients diagnosed with cognitive disorders or psychiatric illness, stomas, history of alcoholism, pregnancy, and patients who refused to sign the informed consent form.
All participants were informed about the nature, duration, and purpose of the study. The study protocol was performed accordingly to the ethical guidelines of the 1964 Declaration of Helsinki (6th revision, 2008) as reflected in a priori approval by the institution’s human research committee.
All patients enrolled were asked to complete the validated Italian versions of the Pittsburgh Sleep Quality Index (PSQI)37 and of the Inflammatory Bowel Disease Questionnaire (IBDQ)38 to respectively evaluate sleep disorder and quality of life. The IBD-Disability Index (IBD-DI) questionnaire, used as tool to assess disability, was administered by an interviewer since the questionnaire was not validated in Italian language1and was not designed as a self-reported questionnaire39. Furthermore, since February 2019 the patients have also been asked to complete the Hospital Anxiety and Depression Scale (HADS) to determine anxiety and depression. All questionnaires were dispensed with the support of one interviewer (CM) in order to ensure that all patients understand the questions.
Clinical IBD assessment
Outpatient medical records were used as source of demographics and clinical data (including duration, location phenotypes, and extension of intestinal disease according to Montreal classification40), while other information were taken in collaboration with the participants. For UC patients, the partial Mayo score was used to assess disease activity, while the Harvey Bradshaw Index was used for CD patients. Disease activity was also determined through faecal calprotectin measurement, as non-invasive marker34,41. Supplementary clinical data, like age at symptoms’ onset and at diagnosis, extra-intestinal manifestations associated to the IBD, history of IBD-related surgery, and current medical therapy were also collected.
Sleep disorders assessment
Patients were asked to complete the Pittsburgh Sleep Quality Index (PSQI) in Italian language to evaluate sleep quality and related disturbances37, considering events occurred over the previous month. The PSQI consists of 7 “component” scores, including subjective sleep quality, use of sleeping drug, and daytime disorders. Patients can answer “0” in case of no difficulty or “3” in case of severe difficulty for each component score. The sum of each component score produces a total score that ranges from 0 to 21. When it results ≥5, it is suggestive of significant bad sleep quality42.
Quality of life assessment
Patients completed the validated Italian version of the Inflammatory Bowel Disease Questionnaire (IBDQ) for general quality of life measurement38, often used as tool for treatment outcome measurement2,43,44,45. The IBDQ explores not only intestinal, but also emotional function (such as depression, anxiety, anger, and embarrassment), systemic symptoms (such as sleep disorders and fatigue) and social function. The sum of each component produces a total score representing poor or good quality of life (range 32–224). Scores higher than 170 were previously associated with patients with clinically inactive disease2.
Disability assessment
Patients fulfilled the IBD disability index (IBD-DI) that consists of 19 items organized in 28 parts. Health and disability sections are overall health, body functions (sleep/energy/fatigue, affect, body image, pain, diarrhea, body mass index, weight loss), activities and participation (regulating defecation, looking after one’s health, interpersonal activities, and work/education), body structures (blood in stool, arthralgia/arthritis), and environmental factors (exacerbating effect of medication, food, family, and professional health care)39.
The total score on the IBD disability represents maximum state of disability index or no disability (range −80–22). A previous study identified a cut-off of 3.5 as the differentiation point for IBD versus healthy controls1.
Anxiety and depression
Anxiety and depression were assessed using the Hospital Anxiety and Depression Scale (9- HADS) questionnaire first used by Zigmond and Snaith46 and validated in Italian in 199947. This instrument is a self-report questionnaire composed of 7 items to assess depression and 7 items for anxiety within the previous week. Each item score ranges from 0 to 3. The sum of each component score produces a total score for depression and a total score for anxiety (range 0–21). Higher global scores indicate worst symptoms. A score of ≥11 is considered a clinically significant disorder, whereas a score between 8 and 10 suggests a mild disorder46. In our population, we considered patients with a score ≥8 as having an anxiety and depression mood.
Statistical analysis
Categorical variables were expressed as frequency and continuous variables as mean ± Standard Deviation (SD) and median with 25–75th percentiles. Differences in categorical variables were calculated using Chi Square Test. We used univariate logistic regression models to assess whether demographical, IBD related variables, IBDQ, and IBD-DI scores were related to a pathological PSQI (score ≥5). Statistically significant variables in the univariate analyses were then included in a multivariate regression model to identify, using the backward elimination analysis, the independent risk factors of sleep disturbance. We used the Spearman’s rank correlations coefficient to evaluate the correlations between PSQI and the following variables: age, partial Mayo score, HBI, faecal calprotectin, hemoglobin, IBD-Q score, IBD-DI score, anxiety, and depression. A p value < 0.05 was considered statistically significant. STATA 11 software was used to perform statistical analysis.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Leong, R., Huang, T., Ko, Y., Jeon, A. & Chang, J. Prospective validation study of the International Classification of Functioning, Disability and Health score in Crohn’s disease and ulcerative colitis. Journal of Crohn’s and 1237–1245 (2014).
Irvine, E. J. et al. Quality of life: a valid and reliable measure of therapeutic efficacy in the treatment of inflammatory bowel disease. Canadian Crohn’s Relapse Prevention Trial Study Group. Gastroenterology 106, 287–96 (1994).
Lo, B., Julsgaard, M., Vester-Andersen, M. K., Vind, I. & Burisch, J. Disease activity, steroid use and extraintestinal manifestation are associated with increased disability in patients with inflammatory bowel disease using the inflammatory bowel disease disability index: a cross-sectional multicentre cohort study. European journal of gastroenterology & hepatology 1, https://doi.org/10.1097/MEG.0000000000001199 (2018).
Vedamurthy, A. & Ananthakrishnan, A. N. Influence of Environmental Factors in the Development and Outcomes of Inflammatory Bowel Disease. Gastroenterology & hepatology 15, 72–82 (2019).
Mikocka-Walus, A., Pittet, V., Rossel, J. B. & von Känel, R. Symptoms of Depression and Anxiety Are Independently Associated With Clinical Recurrence of Inflammatory Bowel Disease. Clinical Gastroenterology and Hepatology 14, 829–835.e1 (2016).
Ananthakrishnan, A. N., Long, M. D., Martin, C. F., Sandler, R. S. & Kappelman, M. D. Sleep disturbance and risk of active disease in patients with Crohn’s disease and ulcerative colitis. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association 11, 965–71 (2013).
Benhayon, D. et al. Characterization of relations among sleep, inflammation, and psychiatric dysfunction in depressed youth with Crohn disease. Journal of pediatric gastroenterology and nutrition 57, 335–42 (2013).
Bernstein, C. N. Psychological Stress and Depression: Risk Factors for IBD? In Digestive Diseases 34, 58–63 (S. Karger AG, 2016).
Navabi, S. et al. Influences and impact of Anxiety and Depression in the setting of inflammatory bowel disease. Inflammatory Bowel Diseases 24, 2303–2308 (2018).
Bhamre, R. et al. Psychiatric comorbidities in patients with inflammatory bowel disease. Indian Journal of Gastroenterology 37, 307–312 (2018).
Ali, T., Madhoun, M. F., Orr, W. C. & Rubin, D. T. Assessment of the Relationship Between Quality of Sleep and Disease Activity in Inflammatory Bowel Disease Patients. Inflammatory Bowel Diseases 19, 2440–2443 (2013).
van de Straat, V. & Bracke, P. How well does Europe sleep? A cross-national study of sleep problems in European older adults. International Journal of Public Health 60, 643–650 (2015).
Hinz, A. et al. Sleep quality in the general population: psychometric properties of the Pittsburgh Sleep Quality Index, derived from a German community sample of 9284 people. Sleep Medicine 30, 57–63 (2017).
Specchio, L. M. et al. Insomnia, quality of life and psychopathological features. Brain Research Bulletin 63, 385–391 (2004).
Qazi, T. & Farraye, F. A. Sleep and Inflammatory Bowel Disease: An Important Bi-Directional Relationship. Inflammatory Bowel Diseases 25, 843–852 (2019).
Ballou, S. et al. Sleep Disturbances Are Commonly Reported Among Patients Presenting to a Gastroenterology Clinic. Digestive Diseases and Sciences 1–9, https://doi.org/10.1007/s10620-018-5237-7 (2018).
Swanson, G. R., Burgess, H. J. & Keshavarzian, A. Sleep disturbances and inflammatory bowel disease: a potential trigger for disease flare? Expert review of clinical immunology 7, 29–36 (2011).
Drossman, D. A., Patrick, D. L., Mitchell, C. M., Zagami, E. A. & Appelbaum, M. I. Health-related quality of life in inflammatory bowel disease. Functional status and patient worries and concerns. Digestive diseases and sciences 34, 1379–86 (1989).
Hyphantis, T. N. et al. Psychological Distress, Somatization, and Defense Mechanisms Associated with Quality of Life in Inflammatory Bowel Disease Patients. Digestive Diseases and Sciences 55, 724–732 (2010).
Sobolewska-Wlodarczyk, A. et al. Sleep disturbance and disease activity in adult patients with inflammatory bowel diseases. Journal of physiology and pharmacology: an official journal of the Polish Physiological Society 69 (2018).
Ranjbaran, Z. et al. Impact of sleep disturbances in inflammatory bowel disease. Journal of Gastroenterology and Hepatology 22, 1748–1753 (2007).
Bliwise, D. L., King, A. C., Harris, R. B. & Haskell, W. L. Prevalence of self-reported poor sleep in a healthy population aged 50-65. Social science & medicine (1982) 34, 49–55 (1992).
Klink, M. E., Quan, S. F., Kaltenborn, W. T. & Lebowitz, M. D. Risk factors associated with complaints of insomnia in a general adult population. Influence of previous complaints of insomnia. Archives of internal medicine 152, 1634–7 (1992).
Zhai, L., Zhang, H. & Zhang, D. Sleep Duration And Depression Among Adults: A Meta-Analysis Of Prospective Studies. Depression and Anxiety 32, 664–670 (2015).
Moulton, C. D. et al. Depressive symptoms in inflammatory bowel disease: an extraintestinal manifestation of inflammation? Clinical and Experimental Immunology 197, 308–318 (2019).
Papadimitriou, G. N. & Linkowski, P. Sleep disturbance in anxiety disorders. International Review of Psychiatry 17, 229–236 (2005).
Lee, A. J. et al. Immunomodulator and Biologic Agent Effects on Sleep Quality in Patients With Inflammatory Bowel Disease. The Ochsner journal 18, 76–80 (2018).
Bar-Gil Shitrit, A. et al. Sleep Disturbances Can Be Prospectively Observed in Patients with an Inactive Inflammatory Bowel Disease. Digestive Diseases and Sciences 63, 2992–2997 (2018).
Wilson, R. G. et al. High C-Reactive Protein Is Associated with Poor Sleep Quality Independent of Nocturnal Symptoms in Patients with Inflammatory Bowel Disease. Digestive Diseases and Sciences 60, 2136–2143 (2015).
Moein, S., Qujeq, D., Vaghari Tabari, M., Kashifard, M. & Hajian-tilaki, K. Original Article Diagnostic accuracy of fecal calprotectin in assessing the severity of inflammatory bowel disease: From laboratory to clinic. Caspian J Intern Med 8, 178–182 (2017).
Vavricka, S. R. et al. Extraintestinal Manifestations of Inflammatory Bowel Disease. Inflammatory bowel diseases 21, 1982–92 (2015).
Marinelli, C. et al. Factors Influencing Disability and Quality of Life during Treatment: A Cross-Sectional Study on IBD Patients. Gastroenterology Research and Practice 2019 (2019).
Keefer, L., Stepanski, E. J., Ranjbaran, Z., Benson, L. M. & Keshavarzian, A. An initial report of sleep disturbance in inactive inflammatory bowel disease. Journal of clinical sleep medicine: JCSM: official publication of the American Academy of Sleep Medicine 2, 409–16 (2006).
Falvey, J. D. et al. Disease activity assessment in IBD: Clinical indices and biomarkers fail to predict endoscopic remission. Inflammatory Bowel Diseases 21, 824–831 (2015).
Spiller, R. & Major, G. IBS and IBD-separate entities or on a spectrum? Nature Reviews Gastroenterology and Hepatology 13, 613–621 (2016).
Dignass, A. et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis Part 1: Definitions and diagnosis. Journal of Crohn’s and Colitis 6, 965–990 (2012).
Curcio, G. et al. Validity of the Italian Version of the Pittsburgh Sleep Quality Index (PSQI). Neurological Sciences 34, 511–519 (2013).
Ciccocioppo, R. et al. Validation of the Italian translation of the Inflammatory Bowel Disease Questionnaire. Digestive and Liver Disease 43, 535–541 (2011).
Peyrin-Biroulet, L. et al. Development of the first disability index for inflammatory bowel disease based on the international classification of functioning, disability and health. Gut 61, 241–247 (2012).
Satsangi, J., Silverberg, M. S., Vermeire, S. & Colombel, J. F. The Montreal classification of inflammatory bowel disease: Controversies, consensus, and implications. Gut 55, 749–753 (2006).
Schroeder, K. W., Tremaine, W. J. & Ilstrup, D. M. Coated Oral 5-Aminosalicylic Acid Therapy for Mildly to Moderately Active Ulcerative Colitis. New England Journal of Medicine 317, 1625–1629 (1987).
Buysse, D. J., Reynolds, C. F., Monk, T. H., Berman, S. R. & Kupfer, D. J. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry research 28, 193–213 (1989).
Love, J. R., Irvine, E. J. & Fedorak, R. N. Quality of life in inflammatory bowel disease. Journal of clinical gastroenterology 14, 15–9 (1992).
Guyatt, G. et al. A new measure of health status for clinical trials in inflammatory bowel disease. Gastroenterology 96, 804–10 (1989).
Mitchell, A. et al. Quality of life in patients with inflammatory bowel disease. Journal of clinical gastroenterology 10, 306–10 (1988).
Zigmond, A. S. & Snaith, R. P. The hospital anxiety and depression scale. Acta psychiatrica Scandinavica 67, 361–70 (1983).
Costantini, M. et al. Detecting psychological distress in cancer patients: validity of the Italian version of the Hospital Anxiety and Depression Scale. Supportive care in cancer: official journal of the Multinational Association of Supportive Care in Cancer 7, 121–7 (1999).
Acknowledgements
Medical writing support granted by Abbvie. The research did not receive specific funding but was performed as part of the employment of the authors. Study’s results have been partially published as abstracts during the 25th National Congress of Digestive Diseases/Digestive and Liver Disease (Roma, 27-30 Mar 2019), and during the 10th National Congress of the Italian Group for the study of Inflammatory Bowel Disease (IG-IBD) (Riccione, 28-30 Nov 2019).
Author information
Authors and Affiliations
Contributions
C.M. performed data collection and analysis, wrote the manuscript, and approved the final version. E.S. performed data analysis, wrote the manuscript, and approved the final version. I.M., G.L., T.G. performed data collection and approved the final version. R.D. wrote the manuscript and approved the final version. F.Z. performed data analysis, wrote the manuscript, and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Marinelli, C., Savarino, E.V., Marsilio, I. et al. Sleep disturbance in Inflammatory Bowel Disease: prevalence and risk factors – A cross-sectional study. Sci Rep 10, 507 (2020). https://doi.org/10.1038/s41598-020-57460-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-57460-6
This article is cited by
-
Impact of insomnia upon inflammatory digestive diseases and biomarkers: a two-sample mendelian randomization research on Europeans
BMC Gastroenterology (2024)
-
Relationship between disease activity index and sleep disorders in children with inflammatory bowel diseases
European Journal of Pediatrics (2023)
-
Burden of Fatigue Among Patients with Ulcerative Colitis and Crohn’s Disease: Results from a Global Survey of Patients and Gastroenterologists
Advances in Therapy (2023)
-
Sarcopenia, severe anxiety and increased C-reactive protein are associated with severe fatigue in patients with inflammatory bowel diseases
Scientific Reports (2021)
-
Inflammatory Bowel Disease and Sleep Disturbance: As Usual, Quality Matters
Digestive Diseases and Sciences (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.