Abstract
Intracranial stents have expanded endovascular therapy options for intracranial aneurysms. The braided Accero stent is available for clinical use since May 2015. To date, no clinical reports on the stent are available. Purpose of this study was the evaluation of the safety and efficacy of the Accero stent in stent-assisted coiling. All patients, in whom implantation of the stent was performed, were included. Primary endpoints were good clinical outcome (mRS ≤ 2) and aneurysm occlusion grades 1 and 2 (Raymond Roy Occlusion Classification). Secondary endpoints were procedural and device-related complications with permanent disability or death, complications in the course, and the recanalization rate. Between September 2015 and August 2018, thirty-four aneurysms were treated with stent-assisted coiling using the Accero. Sixteen aneurysms were untreated, four of these were ruptured. Mild neurological complications occurred in 2/34 (5.9%) treatments. Two stent occlusions occurred during follow-up. No patient had a poor procedure- or device-related outcome. After an average of 15 months of follow-up, 28/30 aneurysms were completely or near-completely occluded. The Accero stent proved to be safe and effective in the treatment of broad-based intracranial aneurysms. The complication rate and the rate of successful aneurysm occlusions are similar to those of other stents.
Similar content being viewed by others
Introduction
Stent-assisted coiling (SAC) is a long-established therapy option for intracranial aneurysms1,2. With this method it is possible to endovascularly treat even complex and broad-necked aneurysms, as stents form a scaffold and thus prevent protrusion or dislocation of coils into the parent artery. Over the years, a variety of stents with different properties have been developed. These include laser-cut stents in open-cell or closed-cell design, as well as braided stents3,4,5. The requirements for all stents are the same: advancement in the catheter and deployment must be easy, they must provide sufficient coverage of the aneurysm neck and be flexible enough to maintain their shape and ensure complete wall apposition even in tortuous vascular anatomy. But above all, usage of the device must be safe and lead to good clinical results with regard to aneurysm occlusion. We present the first results of SAC with a new, braided stent (Accero, Acandis, Pforzheim, Germany).
Materials and Methods
The data was extracted from a database of our institution that was created for all neurovascular interventions within the framework of a quality control. From this database, the prospectively collected data on all patients, in whom an Accero stent was implanted or intended to be implanted, were extracted and retrospectively analyzed. STROBE guidelines were applied6. The study was conducted according to the guidelines and with the approval of the Local Ethics Committee of the University of Magdeburg in compliance with national legislation and the Code of Ethical Principles for Medical Research Involving Human Subjects of the World Medical Association (Declaration of Helsinki). The Local Review Board stated that the retrospective use of the clinical and imaging data does not require separate informed consent.
Device information
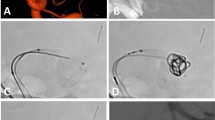
The Accero stent is a commercially available, self-expandable braided stent that is approved for the treatment of intracranial aneurysms. The device is characterized by an entire contour visibility due to platinum-nitinol composite wires (Fig. 1). Moreover, the Accero Stent is provided with the BlueXide surface finishing, that aims to optimize hemocompatibility and facilitates stent delivery by using a corrosion-protective surface, ensuring an extremely low nickel ion release7. Furthermore, high oxygen and nitrogen intensities of the protective titanium oxide/oxynitride film will work to reduce platelet adhesion. Available diameters range from 2.5 mm to 4.5 mm with lengths up to 25 mm. The Accero stent can be delivered through 0.0165–0.0170” microcatheters. Re-sheathing is still possible when 95% of its length are released.
High-resolution volume-of-interest (VOI) imaging of two implanted Accero stents. (A) Thick-slab maximum intensity projection after multiplanar reconstruction after deployment of the stent in the left PICA. The stent has opened completely and shows a complete wall-apposition. The metal artifacts caused by the previous coiling do not affect the assessability of the stent lumen. (B) Incomplete opening at the distal end of the stent (white arrow). Note the intended bulging of the stent into the aneurysmal lumen (white arrowhead), which, however, substantially increased by trying to reach the distal end of the stent with a balloon catheter. However, the bulging was not sufficient to cover the ostium sufficiently for coiling, so that a second stent was implanted.
Endovascular procedure
All interventions were performed on a biplane angiography system (Artis Q, Siemens Healthineers, Forchheim, Germany) under general anesthesia by one of four neuroradiologists with many years of experience in neurovascular interventions. Written informed consent was obtained prior to the treatment in elective cases. Since the device is approved for stent-assisted coiling, only general information about the risks of stent-assisted coiling with braided stents was provided. The need for informed consent was waived in emergency cases, in such cases, the therapy strategy was determined on an interdisciplinary basis. Commercially available 6F or 8F femoral sheaths and guide catheters were used. In the case of elective treatments, patients were given 100 mg acetylsalicylic acid (ASA) and 75 mg clopidogrel daily for at least 5 days prior to the intervention, the efficacy of both was verified by light emission aggregometry and the Multiplate Analyzer (Roche Diagnostics, Rotkreuz, Switzerland). In addition, heparin adapted to weight was administered intravenously after groin puncture. In patients with subarachnoid hemorrhage, prior to stenting, 500 mg ASA were given intravenously and 180 mg ticagrelor via a nasogastric tube. Post-interventionally, ticagrelor was changed to clopidogrel and efficacy was determined as soon as possible. Clopidogrel was discontinued after 3 months, whereas ASA was given permanently.
The size of the stents was determined by measurements in the DSA working projections, for the choice of length also the local vascular anatomy was considered using 3D-rotational angiography (3D-RA). For the deployment of the stent, a neuroslider 17 (Acandis GmbH, Pforzheim, Germany) was used, which can be used for coiling as well. If Y-stenting was necessary, the second stent was laser-cut (Acclino flex, Acandis GmbH, Pforzheim, Germany) in all cases, which was placed through the meshes of the Accero. High-resolution volume-of-interest (VOI) imaging was used to assess wall apposition and opening at the ends in untreated aneurysms and in those, in which artifacts due to coils didn’t affect the assessment of the stent lumen. At the discretion of the interventionalist, incidental aneurysms were coiled in the same or a second session.
All relevant details of the intervention regarding the aneurysms (size, localization, neck width, aspect ratio, dome-to-neck ratio, prior treatment), the stents (number, size, deployment behavior, advancement or wall adherence), the coiling (jailing, crossing, staged, coils used) and the complications were documented and analyzed.
Follow-Up
Patients were examined for neurological or other symptoms before and immediately after the intervention as well as at discharge. In addition, the patients were evaluated clinically prior to each follow-up imaging.
According to the imaging follow-up protocol of our institution, a DSA is performed 6 and 18 months after stent implantation, usually in combination with a contrast-enhanced MR-angiography (CE-MRA). After that, only CE-MRA will be performed. In case of abnormal findings (for example suspected reperfusion, intimal hyperplasia or in-stent stenosis), the interval is shortened or re-treatment is performed.
Treatment results for the aneurysms were assessed immediately after the intervention and in the follow-up examinations using the Raymond-Roy occlusion classification (RROC)8.
End-points
Primary end-points were the rate of patients with a good clinical outcome (mRS ≤ 2) and the proportion of patients with complete/near complete aneurysm occlusion (Raymond Roy Occlusion Grades (RROG) 1 and 2, respectively) at the time of latest follow-up. Secondary end-points comprised of complications that were related either to the procedure or the device and caused disabling stroke/death immediately or during the course. Procedure-related complications included thromboembolic events, vasospasm requiring treatment, dissection and perforation, whereas for example detachment or deployment problems, incomplete opening and kinking were rated device-related.
Statistical analysis
Data were analyzed using IBM SPSS Statistics, Version 24 (Armonk, New York, USA). The two-sided T-test was applied to search for statistically significant differences between subgroups with respect to morphologic parameters. Statistical significance of differences in categorical data between the pretreated and the untreated subgroup was analyzed by a Fisher´s exact test with 2-sided p-values.
Results
Between September 2015 and August 2018, 32 patients with 34 aneurysms received an Accero stent as part of a stent-assisted coiling (SAC). The mean age was 57,1 ± 13,1 years, and twenty-seven (84,4%) patients were female. Four (12,5%) patients had acute SAH with WFNS grades of 1 (n = 3) and 4 (n = 1), respectively. One aneurysm was symptomatic due to mass effect. Approximately half of the patients were retreated after coiling (n = 16) or clipping (n = 2). All aneurysms had a neck width of ≥4 mm and/or a dome-to-neck ratio of ≤2. Twenty-five aneurysms were smaller than 5 mm. For details of the aneurysm characteristics see Table 1. Aneurysm localizations were as follows: anterior communicating artery (n = 13), MCA-bifurcation (n = 7), basilar artery (n = 4), posterior communicating artery (n = 3), pericallosal artery (n = 2), posterior inferior cerebellar artery (PICA, n = 2), internal carotid artery (ICA, n = 1), carotid-t (ICA-T, n = 1) and posterior cerebral artery (n = 1).
Procedural results
SAC with a single Accero stent was planned in 25 aneurysms, and in 9 cases, primary Y-stenting was considered necessary. Deployment of the stent was successful in all 34 treatments. In one case (2,9%), proximal dislocation of the stent occurred by entangling of the pusher wire with the stent struts, which required placement of a second stent. No further rescue stents were required in this or any other patient. So in total, 35 Accero stents were placed in 34 aneurysms. Twenty-eight (82,4%) aneurysms were coiled in the same session, 24 (85,7%) of these with the jailing technique. Two of the patients with SAH had SAC with a single stent, two had Y-Stenting, all without new neurological deficits during the hospital stay.
Complete aneurysm occlusion (RROG 1) was achieved in 18 (52,9%) aneurysms, a neck remnant (RROG 2) was present in 10 (29,4%) aneurysms. Residual filling of the sac was observed in six (17,6%) aneurysms, including two patients, in whom the aneurysm couldn’t be entered for coiling after stenting, in both of which coiling of an anterior communicating artery aneurysm was tried in a second session 8 weeks after stenting. In one of these cases a distinct endothelial layer formed over that portion of the stent that covered the ostium, which prevented probing through the stent meshes. Since the aneurysm was significantly smaller after the stenting alone, it was decided to carry out follow-up examinations. In the second case, the angle between the stent axis and the aneurysm was unfavorable. The attending physician decided to terminate the intervention, although the aneurysm could have been entered from the contralateral side. The patient refused, as the size of the remaining aneurysm was also regredient. In only three of nine patients with Y-Stenting RROG 1 was achieved.
In 4 (11.8%) aneurysms, the distal end of the stent didn’t open completely. In two of these cases, this was not detected under fluoroscopy, but only by VOI imaging, which was performed after deployment. In one case, the stent was unintentionally deployed in an attempt to resheath it. This stent was released until just before the point of no return. In the fourth case, the incomplete opening was accepted, as the interventionalist felt that correction by balloon dilatation would be possible without much effort. However, in no case could the incomplete stent opening be corrected because the balloons could not be advanced to the distal end of the stent. After the 4th issue, VOI imaging was performed before final release in cases where the distal stent opening could not be clearly assessed by fluoroscopy.
Two procedure-related clinical complications occurred. One patient with Y-stenting for an MCA bifurcation aneurysm had multiple embolisms in the ACA- and MCA-territories with mild hemiparesis. She was one of the patients in whom the stent did not open completely distally.
Another patient with an Acom-aneurysm was coiled in a second session eight weeks after uncomplicated single stenting. During coiling a thrombus formed at the site where the stent didn’t cover the ostium. Despite instant medical treatment the patient developed a mild weakness of the foot. Both complications were not disabling (mRS 1 and 2 at discharge, respectively). No other procedural complications were observed.
Follow-Up
Follow-up examinations were performed in 30 (88.2%) aneurysms, with a mean duration of 444 (range 90–880) days. Reasons for missing follow-up were: death unrelated to the intervention (n = 2, accident and mesenteric infarction due to newly diagnosed atrial fibrillation, respectively), transfer to home country (n = 1) and refusal (n = 1).
In 21 (70,0%) patients, the aneurysm was completely occluded at the time of the last control. Seven patients improved from RROG 2 or 3 to complete occlusion (see Fig. 2). In two previously occluded aneurysms, a neck remnant developed during the course, and two aneurysms showed progressive filling (RROG 2 to 3), one of which needed re-treatment. Both aneurysms that could not be probed improved from RROG 3 to RROG 1 and 2, respectively. In total 93,3% of the aneurysms showed a complete/near complete occlusion (see Table 2). No change of the stent position was observed. Also, the configuration of the four stents that were distally incompletely open was unchanged.
Initial treatment result and follow-up after SAC of an aneurysm at the pericallosal artery with the jailing technique. (A) Working projection with microcatheters immediately proximal to the aneurysm. The aneurysm develops from the apex of a sharp curve. A little further distally again sharp bend of the vessel. (B) Result immediately after the intervention. Still little inflow into the aneurysm sac (RROG 3, white arrows). Note the slightly different vascular anatomy after stenting. (C) In the control-DSA after 6 months complete aneurysm occlusion (RROG 1). Note the discreet intimal hyperplasia in the region of the aneurysm ostium (white arrowhead).
Table 1: Comparison of occlusion rates immediately after treatment (initial results) with those after the last imaging (follow-up results) of the whole patient population. The numbers below the main diagonal correspond to improvements. There were no significant differences between untreated and pretreated patients (p = 0.472).
In one patient (3.3%), dual platelet inhibition was discontinued at day 29 after Y-stenting in an outside hospital due to pseudoaneurysm surgery at the puncture site, but without consulting the attending neurointerventionalist. This resulted in an acute occlusion of the Accero stent in the right inferior M2-branch. It was the same patient, who experienced embolism during the intervention. The new event again caused a slight hemiparesis (mRS 2 at discharge). The symptoms improved over time, but didn’t resolve completely. In another patient, asymptomatic occlusion of the A2-segment following SAC of an Acom-aneurysm was detected in the first follow-up DSA. In this patient, the stent also hadn’t opened completely distally. Mild intimal hyperplasia occurred in 6 (20,0%) patients, three of them had Y-Stenting. No in-stent-stenosis was observed. In the patient who had mild foot palsy after coiling, the symptoms completely receded.
Discussion
The introduction of specially designed self-expandable stents for intracranial use has significantly expanded the spectrum of endovascular aneurysm therapy. The first report on such a stent, the Neuroform, was published in 2002, others followed shortly thereafter1,9,10.
A variety of stents with different properties have been developed since then, all with different advantages and disadvantages. Open-cell stents have the disadvantage that the cell size increases significantly at the outer curvature of tortuous vessels, which can lead to coil prolapse11,12,13. In addition, re-sheathing is not possible, so it frequently has to be removed in case of dislocation during deployment14,15. On the other hand, laser-cut stents with closed-cell design can ovalize or even kink in tight curves16. Migration has also been observed17.
Braided stents such as the Accero, which was investigated in this study, should be superior to the laser-cut stents in these regards. They are more flexible and adapt well to the vascular anatomy without kinking. Indeed, no intervention in our series had to be terminated because of technical complications, in all cases an Accero could be placed as intended. In one case, a second stent had to deployed due to detachment problems, though. Also, the wall apposition was complete even in tight curves, no flattening or kinking was observed. However, the stent didn’t open completely distally in 4 cases (11,8%). Incomplete opening not only at the ends has also been observed with other stents, though less frequently as in this study. Usually this can be corrected and in most cases doesn’t cause any clinical symptoms18,19,20. However, one of these patients developed persistent clinical symptoms, but whether these resulted from the incomplete opening is questionable. The embolisms that occurred intra-procedurally did not only affect the vascular territory downstream of the stent, and the stent occlusion after 4 weeks in the same patient happened after discontinuation of the platelet inhibitors. But as another patient with an incomplete opening had an asymptomatic occlusion, at least the later complication must be considered stent-related. In both of these patients, the difference between vessel diameters proximal and distal was each greater than 1 millimeter, and in all four cases the distal vessel diameters were approximately 1.5 millimeters, which is smaller than in most other patients. It may be possible that a small vessel diameter favors the incomplete stent opening. However, by performing a VOI imaging before final release whenever the complete deployment of the stent could not be clearly assessed, this technical complication could be avoided after the fourth case. Overall, the rate of technical difficulties is similar to the 6–11% that are reported for other braided stents20,21,22. With 5, 9% minor neurological intra-procedural complications, one of them with complete remission, and no device- or procedure-related disabling strokes or deaths our results are in line with those of other groups, which also reported no or only low rates of serious complications, regardless of the stent used19,23,24,25.
The Accero stent has a high metal coverage, it is specified by the manufacturer for a stent with the size of 3.5 × 20 mm with 15–19%, depending on the actual diameter. It is thus significantly larger than that of laser-cut stents and comparable to that given for other braided stents24,26.
This better coverage of the aneurysm ostium may also result in a greater change in the intra-aneurysmal flow that favors the aneurysmal occlusion in the course. At least in this study, the Raymond Roy occlusion grade improved in nine of the thirteen initially incompletely occluded aneurysms that were controlled, seven of which were completely occluded. Only in two cases (6,7%), there was a recanalization to the aneurysm sac, one required re-treatment. A positive effect of braided stents on the flow was noted by other authors as well13,27. On the other hand, better metal coverage might make probing of the aneurysm after stenting more difficult, in particular, when the stent is compressed over the ostium to enhance the effect on hemodynamics. In this study, this occurred in two cases. Although both aneurysms shrank in the course, with the use of braided stents, primarily a one-time procedure in jailing technique should be chosen in order to avoid the possibility that the stents can’t be crossed anymore and thus not be embolized properly.
No coil prolapse was observed in this study. This may also be explained by the high density of the stent struts and better retainment of coils. Thus, braided stents could reduce the complications that arises from coiling in the SAC.
Delayed complications after SAC may arise from interactions of the vessel wall with the implant. In this study, mild intimal hyperplasia was found in six patients (20.0%). This rate is higher than in most other publications already mentioned. However, the changes were only discreet and didn’t require any therapy. In addition, there have been no in-stent stenoses, whereas in the literature a frequency of up to 10% is reported28.
However, a therapy should not only be safe, but also efficient. In this study, SAC with the Accero stent resulted in complete/near complete occlusion in 93.3% of the cases, which is in line with the literature. Although an even higher rate of complete occlusion would be desirable, re-treatment of the mostly very small neck remnants would place patients at a disproportionate risk, given the very low risk of rupture of such remnants, especially of unruptured aneurysms29,30.
Limitations
The first limitation is that this study is a retrospective analysis. However, the data were collected prospectively, so that they are qualitatively better than in a purely retrospective survey. Second, this is a single center study with a relatively small number of cases. However, the main purpose of the study was to test the safety and efficacy of the Accero stent and in this regard doesn’t stand out from many other studies that reported the experience with a new device. The neuroradiologists who have implanted the Accero stent are all experienced in neurointerventions and especially in stenting for many years. It can therefore be assumed that a higher number of cases would not have produced a significantly different result in terms of complication and occlusion rates. To test the performance of the Accero over other available stents, however, requires a different study design with more patients. Finally, not all patients had a follow-up examination. Although this can hardly be avoided in the context of patient studies, the results can, of course, be influenced by this. In the vast majority of patients who had a follow-up examination, however, stable or better findings were observed.
Conclusion
Stent-assisted coiling with the new braided Accero stent appears to be safe and effective. The complication rates are low and the occlusion rates are comparable to those of other stents. Together with its compatibility with a 0.0165 “catheter regardless of the stent diameter, it is a good alternative for the treatment of intracranial aneurysms.
Data availability
Any additional unpublished data simply available upon request from the corresponding author.
References
Henkes, H. et al. Endovascular coil occlusion of intracranial aneurysms assisted by a novel self-expandable nitinol microstent (neuroform). Interv Neuroradiol 8, 107–119, https://doi.org/10.1177/159101990200800202 (2002).
Biondi, A. et al. Neuroform stent-assisted coil embolization of wide-neck intracranial aneurysms: strategies in stent deployment and midterm follow-up. Neurosurgery 61, 460–468; discussion 468–469, https://doi.org/10.1227/01.NEU.0000290890.62201.A9 (2007).
Higashida, R. T., Halbach, V. V., Dowd, C. F., Juravsky, L. & Meagher, S. Initial clinical experience with a new self-expanding nitinol stent for the treatment of intracranial cerebral aneurysms: the Cordis Enterprise stent. AJNR Am J Neuroradiol 26, 1751–1756 (2005).
Kis, B., Weber, W., Berlit, P. & Kuhne, D. Elective treatment of saccular and broad-necked intracranial aneurysms using a closed-cell nitinol stent (Leo). Neurosurgery 58, 443–450; discussion 443–450, https://doi.org/10.1227/01.NEU.0000197103.10364.0C (2006).
Klisch, J. et al. Stent-assisted coil embolization of posterior circulation aneurysms using solitaire ab: preliminary experience. Neurosurgery 65, 258–266; discussion 266, https://doi.org/10.1227/01.NEU.0000348295.44970.C8 (2009).
von Elm, E. et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg 12, 1495–1499, https://doi.org/10.1016/j.ijsu.2014.07.013 (2014).
Ley, D. et al. The Derivo Embolization Device, a Second-Generation Flow Diverter for the Treatment of Intracranial Aneurysms, Evaluated in an Elastase-Induced Aneurysm Model. Clin Neuroradiol 27, 335–343, https://doi.org/10.1007/s00062-015-0493-9 (2017).
Roy, D., Milot, G. & Raymond, J. Endovascular treatment of unruptured aneurysms. Stroke 32, 1998–2004 (2001).
Benitez, R. P., Silva, M. T., Klem, J., Veznedaroglu, E. & Rosenwasser, R. H. Endovascular occlusion of wide-necked aneurysms with a new intracranial microstent (Neuroform) and detachable coils. Neurosurgery 54, 1359–1367; discussion 1368 (2004).
Pumar, J. M. et al. Preliminary experience with Leo self-expanding stent for the treatment of intracranial aneurysms. AJNR Am J Neuroradiol 26, 2573–2577 (2005).
Benndorf, G., Claus, B., Strother, C. M., Chang, L. & Klucznik, R. P. Increased cell opening and prolapse of struts of a neuroform stent in curved vasculature: value of angiographic computed tomography: technical case report. Neurosurgery 58, ONS-E380; discussion ONS-E380, https://doi.org/10.1227/01.NEU.0000205287.06739.E1 (2006).
Ebrahimi, N., Claus, B., Lee, C. Y., Biondi, A. & Benndorf, G. Stent conformity in curved vascular models with simulated aneurysm necks using flat-panel CT: an in vitro study. AJNR Am J Neuroradiol 28, 823–829 (2007).
Fiorella, D. et al. Final results of the US humanitarian device exemption study of the low-profile visualized intraluminal support (LVIS) device. J Neurointerv Surg 8, 894–897, https://doi.org/10.1136/neurintsurg-2015-011937 (2016).
Broadbent, L. P., Moran, C. J., Cross, D. T. 3rd & Derdeyn, C. P. Management of neuroform stent dislodgement and misplacement. AJNR Am J Neuroradiol 24, 1819–1822 (2003).
King, B., Vaziri, S., Singla, A., Fargen, K. M. & Mocco, J. Clinical and angiographic outcomes after stent-assisted coiling of cerebral aneurysms with Enterprise and Neuroform stents: a comparative analysis of the literature. J Neurointerv Surg 7, 905–909, https://doi.org/10.1136/neurintsurg-2014-011457 (2015).
Chen, Y. A., Hussain, M., Zhang, J. Y., Zhang, K. P. & Pang, Q. Stent-assisted coiling of cerebral aneurysms using the Enterprise and the Solitaire devices. Neurol Res 36, 461–467, https://doi.org/10.1179/1743132814Y.0000000356 (2014).
Dashti, S. R. et al. Proximal migration and compaction of an Enterprise stent into a coiled basilar apex aneurysm: a posterior circulation phenomenon? J Neurointerv Surg 2, 356–358, https://doi.org/10.1136/jnis.2010.002444 (2010).
Gross, B. A. et al. A clinical comparison of Atlas and LVIS Jr stent-assisted aneurysm coiling. J Neurointerv Surg, https://doi.org/10.1136/neurintsurg-2018-014208 (2018).
Machi, P. et al. LEO Baby Stent Use following Balloon-Assisted Coiling: Single- and Dual-Stent Technique–Immediate and Midterm Results of 29 Consecutive Patients. AJNR Am J Neuroradiol 36, 2096–2103, https://doi.org/10.3174/ajnr.A4413 (2015).
Cho, Y. D. et al. Coil embolization of intracranial saccular aneurysms using the Low-profile Visualized Intraluminal Support (LVIS) device. Neuroradiology 56, 543–551, https://doi.org/10.1007/s00234-014-1363-x (2014).
Mohlenbruch, M. et al. The LVIS Jr. microstent to assist coil embolization of wide-neck intracranial aneurysms: clinical study to assess safety and efficacy. Neuroradiology 56, 389–395, https://doi.org/10.1007/s00234-014-1345-z (2014).
Poncyljusz, W. et al. The LVIS/LVIS Jr. stents in the treatment of wide-neck intracranial aneurysms: multicentre registry. J Neurointerv Surg 7, 524–529, https://doi.org/10.1136/neurintsurg-2014-011229 (2015).
Behme, D. et al. Low-profile Visualized Intraluminal Support device (LVIS Jr) as a novel tool in the treatment of wide-necked intracranial aneurysms: initial experience in 32 cases. J Neurointerv Surg 7, 281–285, https://doi.org/10.1136/neurintsurg-2014-011157 (2015).
Wang, J. et al. Stent-assisted coiling of cerebral aneurysms: a single-center clinical and angiographic analysis. J Neurointerv Surg 10, 687–692, https://doi.org/10.1136/neurintsurg-2017-013272 (2018).
Gory, B. et al. Solitaire AB stent-assisted coiling of wide-necked intracranial aneurysms: mid-term results from the SOLARE Study. Neurosurgery 75, 215–219; discussion 219, https://doi.org/10.1227/NEU.0000000000000415 (2014).
Cho, S. H. et al. Bench-top Comparison of Physical Properties of 4 Commercially-Available Self-Expanding Intracranial Stents. Neurointervention 12, 31–39, https://doi.org/10.5469/neuroint.2017.12.1.31 (2017).
Cagnazzo, F. et al. Flow-Diversion Effect of LEO Stents: Aneurysm Occlusion and Flow Remodeling of Covered Side Branches and Perforators. AJNR Am J Neuroradiol 39, 2057–2063, https://doi.org/10.3174/ajnr.A5803 (2018).
Park, S. Y., Oh, J. S., Oh, H. J., Yoon, S. M. & Bae, H. G. Safety and Efficacy of Low-Profile, Self-Expandable Stents for Treatment of Intracranial Aneurysms: Initial and Midterm Results - A Systematic Review and Meta-Analysis. Interv Neurol 6, 170–182, https://doi.org/10.1159/000471890 (2017).
Munich, S. A. et al. Neck Remnants and the Risk of Aneurysm Rupture After Endovascular Treatment With Coiling or Stent-Assisted Coiling: Much Ado About Nothing? Neurosurgery. https://doi.org/10.1093/neuros/nyy056 (2018).
Ji, W. et al. Stent-assisted coiling of very small wide-necked intracranial aneurysms: Complications, anatomical results and clinical outcomes. Neurol Neurochir Pol 50, 410–417, https://doi.org/10.1016/j.pjnns.2016.07.004 (2016).
Lauric, A., Baharoglu, M. I. & Malek, A. M. Ruptured status discrimination performance of aspect ratio, height/width, and bottleneck factor is highly dependent on aneurysm sizing methodology. Neurosurgery 71, 38–45, https://doi.org/10.1227/NEU.0b013e3182503bf9 (2012).
Acknowledgements
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. The contents of this manuscript have not been published elsewhere. The manuscript is not currently under review by another journal. Also, the results were not presented in full or in part.
Author information
Authors and Affiliations
Contributions
Oliver Beuing has substantial contribution in the conception, data acquisition and analysis, drafting and interpretation of the work. Anja Lenz, Aneta Donitza and Mathias Becker have substantial contribution in data acquisition and/or critical revisal of the manuscript. Steffen Serowy has substantial contribution in data analysis, drafting and critical revisal of the manuscript. Martin Skalej has substantial contribution in data acquisition, conception of the work and critical revisal of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Beuing, O., Lenz, A., Donitza, A. et al. Stent-assisted coiling of broad-necked intracranial aneurysms with a new braided microstent (Accero): procedural results and long-term follow-up. Sci Rep 10, 412 (2020). https://doi.org/10.1038/s41598-019-57102-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-57102-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.