Abstract
Understanding mechanisms of thermal sensitivity is key to predict responses of marine organisms to changing temperatures. Sustaining heart function is critical for complex organisms to oxygenate tissues, particularly under temperature stress. Yet, specific mechanisms that define thermal sensitivity of cardiac function remain unclear. Here we investigated whole animal metabolism, cardiac performance and mitochondrial function in response to elevated temperatures for temperate, subtropical and tropical spiny lobster species. While oxygen demands increased with rising temperatures, heart function became limited or declined in all three species of lobsters. The decline in cardiac performance coincided with decreases in mitochondrial efficiency through increasing mitochondrial proton leakage, which predicts impaired compensation of ATP production. Species differences were marked by shifts in mitochondrial function, with the least thermal scope apparent for tropical lobsters. We conclude that acute temperature stress of spiny lobsters, irrespective of their climatic origin, is marked by declining cellular energetic function of the heart, contributing to an increasing loss of whole animal performance. Better understanding of physiological thermal stress cascades will help to improve forecasts of how changing environmental temperatures affect the fitness of these ecologically and commercially important species.
Similar content being viewed by others
Introduction
Temperature is one of the major driving forces shaping survival and physiological performance of marine organsims1. Particularly in shallow coastal and intertidal waters, organisms require to cope with frequent changes of temperatures across space and time. Outcomes of acute or chronic thermal stress can be significant, not only to individuals but whole populations, leading to large scale re-distribution, decline or even collapse of local populations2,3,4. Lobsters are ecological key players, both as predators and prey5 and crucial to sustain ecosystem resilience by e.g. buffering destructive species shift6. They are one of the most highly valued marine food sources7,8 and promising candidates for an emerging global aquaculture industry9,10. However, lobsters are particularly exposed to temperature changes, due to their inability to regulate body temperature (ectothermic) and the distribution of numerous species in shallow coastal waters, where temperatures vary naturally during tidal cycles, strong winds or across thermoclines by more than 10 °C11,12. For some species, thermal limits have been exceeded in some areas13,14,15,16, which causes concern given that ocean are predicted to warm on average by 2.7 °C by the end of this century (from 1990, RCP8.517). An increasing frequency, intensity and duration of heat waves and localised above average warming trends18,19, will likely exacerbate long term trends, and may pose acute risks to local populations with small thermal safety margins16,20. Understanding when and why lobsters reach thermal limits will improve mechanistic models to predict the wellbeing of future populations21,22,23, as well as inform protective management actions to e.g. identify and preserve thermal refugia24,25,26.
The mechanisms underlying temperature limitation of marine ectotherms remain debated, as these may vary, depending on a species physiology, life stage (e.g. larvae vs adult), physiological state (e.g. age, disease, growth, reproduction), and on intensity and exposure (acute or chronic) of temperature stress27. Oxygen limitation has been proposed as primary factor in limiting thermal tolerance in aquatic ectotherms28,29. Accordingly, marine ectotherms increasingly struggle to sustain adequate tissue oxygen levels when temperatures increase, due to an inability of the ventilatory and cardiovascular system to match the steady increase of cellular oxygen demands30. This concept remains controversial with support for and against e.g.31,32,33,34. One reason may be the lack of detailed understanding if and how different components of the oxygen delivery system may trigger a cascade of thermal failure27,35.
The mechanisms of thermal limitation are still poorly understood for crustaceans. An impaired response to increase of ventilation with rising temperature, and a coincident declining arterial oxygenation have been suggested to be significant factors that combine to limit oxygen supply at high temperatures33. In contrast, the role of cardiac performance in thermal limitation was proposed to be minor for most crustaceans33. However, this interpretation is limited to heart rate measures as proxies of performance36,37,38,39. Indeed, other cardiac performance measures cease to rise and compensate for rising temperatures, or even decrease. These include cardiac stroke volume12,40,41,42, cardiac output12,43, or cardiac contraction force12. While this may be linked to decreased arterial oxygen supplies to cardiac tissues, crustacean hearts - unlike most teleost hearts44 - are less likely to become oxygen depleted as their hearts receive oxygenated blood directly from the gills. Further, control by cardio-regulatory nerves and neurohormones arising from other parts of the central nervous system are also unlikely to drive cardiac temperature dependence, given that similar thermal sensitivities of hearts are apparent in vivo and ex vivo12. This indicates that some intrinsic factors mediate the temperature dependence of cardiac performance. The cardiac ganglion is the prime candidate for control of the crustacean neurogenic heart45, however, alternative intrinsic mechanisms that define thermal sensitivity have not been well considered. Mitochondria present one such target within cardiac tissues and are well known to respond to rising temperatures through a decline in oxidative phosphorylation efficiency through impaired inner mitochondrial membrane integrity46,47,48,49.
To date the relation between the thermal sensitivity of mitochondrial function and cardiac performance has been poorly assessed in crustaceans. So far it has only been tested by comparison of two species of portunid crab, but not for other crustaceans of diverse climatic origins49. It is also unclear whether patterns of thermal sensitivity remain consistent across closely related species of lobsters that have differing thermal histories (i.e. thermal adaptation) or un-linear change occurs, as the case for distinct populations of European green crabs50.
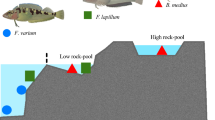
We compared three spiny lobsters species from different thermal habitats, (1) the cold-temperate to temperate Jasus edwardsii, (2) the subtropical Sagmariasus verreauxi and (3) the tropical Panulirus ornatus (Fig. 1). We then tested (1) how whole animal metabolism and cardiac performance respond to acute increases in temperature, (2) if cardiac thermal sensitivity mirrors cardiac mitochondrial function and (3) if there are universal and species-specific patterns that dictate thermal sensitivity or cardiac performance.
Combined whole animal respirometry and heart rate logging was undertaken, followed by measurements of cardiac mitochondrial energy production. This revealed a species-independent divergence between increasing oxygen demand and limited or declining cardiac performance with increasing temperatures. This paralleled decreasing cardiac mitochondrial stability of respiratory complex I and mitochondrial membrane stability, where the latter resulted in decreased energetic efficiency in terms of work done to maintain a membrane potential, and ATP production. The tropical spiny lobster showed the greatest response in mitochondrial function with increasing temperature, and the least margin between optimal and critical cellular temperatures.
Results
Thermal divergence between oxygen demand and cardiac performance
Simultaneous measurements of whole animal oxygen consumption and heart rate revealed a growing gap between increasing demand for oxygen and plateauing or declining cardiac performance with acutely rising temperatures (Fig. 2). While oxygen consumption increased in all three species with increasing temperature (Fig. 2a, 14–24 °C for J. edwardsii, 20–28 °C for S. verreauxi, 27–35 °C for P. ornatus), heart rate did not increase significantly in J. edwardsii and P. ornatus, except for S. verreauxi (Fig. 2b). Pseudo cardiac output did not increase significantly with increasing temperature in any of the three species and declined significantly in P. ornatus beyond 33 °C (Fig. 2c). More clearly, heart rate and pseudo cardiac output changed at much lower rates than oxygen consumption relative to their initial starting temperature (Fig. 2a–c, refer to percentage values), with a maximal gap between relative change of oxygen consumption (82% ± CI 54%) and pseudo cardiac output (−29% ± CI 10%) in P. ornatus (Fig. 2a,c). Ratios of pseudo cardiac output to oxygen consumption rate highlighted a general decrease of pseudo cardiac output relative to the total oxygen utilised by the animal with increasing temperature (Linear regression F(1,107) = 87.15, P < 0.01, R2adj. = 0.44, Fig. 3).
(a) Change of total oxygen consumption, (b) heart rate and (c) pseudo cardiac output (expressed in arbitrary units AU) with increasing temperatures for three spiny lobster species - cold-temperate J. edwardsii (blue), subtropical S. verreauxi (green) and tropical P. ornatus (red). Data presented as means ±95% CI, n = 6–8. Open circles indicate significant difference relative to the lowest temperature for each species. Percentage values indicate relative change between starting and final temperature. Coloured tick marks at bottom x-axes indicate optimal temperatures for growth corresponding to each species (20.6 °C for J. edwardsii100, 21.5 °C for S. verreauxi34 and 28 °C for P. ornatus82).
Pseudo cardiac output decreases linearly relative to spiny lobster oxygen consumption with increasing temperatures (y = −0.10 × +4.34). A linear regression model was applied to the pooled data set, as a more complex model with species as co-factor did not significantly improve the linear regression model (ANOVA (F(4, 107) = 0.60, P = 0.67).
Decline of cardiac performance
Subsequent mitochondrial assays on cardiac tissues of the experimental lobsters revealed declining efficiency of aerobic energy release by cardiac mitochondria in all three lobsters with increasing temperatures (Fig. 4). Independent measurements of mitochondrial leak respiration (i.e. state III respiration using oligomycin) and membrane potential (i.e. fluorescence of membrane selective TMRM) showed that proton leakage across the inner mitochondrial membrane into the matrix space likely increases with increasing temperature (Figs. 4a,b, 5a,b). Estimates of mitochondrial production of the main energy carrier ATP followed an optimum temperature pattern, with predicted ATP production peaking at 25 °C for J. edwardsii, 30 °C for S. verreauxi, and 30 °C for P. ornatus (Figs. 4c, 5a–c). Below peak temperatures for ATP production, mitochondrial proton leak and ATP production increased at similar levels, but then diverged beyond optimal temperatures, with proton leak increasing further and ATP production plateauing or rapidly declining (Fig. 5a–c). As a result, mitochondrial capacity to produce ATP likely decreases precipitously above optimal temperature (i.e. intact fraction of oxidative phosphorylation, Fig. 4d).
Cardiac tissue change of (a) leak respiration (following addition of complex III inhibitor oligomycin), (b) mitochondrial membrane potential, (c) predicted ATP synthesis rates and (d) fraction of intact mitochondrial ATP production (i.e. oxidative phosphorylation (state 3 respiration - leak respiration)/state 3 respiration) with increasing temperatures in comparison between cold-temperate J. edwardsii (blue), subtropical S. verreauxi (green) and tropical P. ornatus (red). Data presented as means ±95% CI, n = 6–8. Open circles indicate significant difference relative to the lowest temperature for each species. Coloured tick marks at bottom x-axes indicate optimal temperatures for growth corresponding to each species (20.6 °C for J. edwardsii100, 21.5 °C for S. verreauxi34 and 28 °C for P. ornatus82).
Relative change (%) of spiny lobster cardiac leak respiration (dotted line), ATP production (solid line) and membrane potential (dashed line) in comparison between (a) J. edwardsii (blue), (b) S. verreauxi (green) and (c) P. ornatus (red). Data presented as means ±95% CI, n = 6–8. Open circles indicate significant difference relative to the lowest temperature for each species. Coloured bars indicate margins between optimal temperature for growth and temperatures, at which leak respiration and ATP production start misaligning. Image courtesy of Craig Mostyn Group and Seafood New Zealand.
Species trends and climate origin
Thermal sensitivity of spiny lobster from different geographic and climate origins revealed similar patterns of cardiac performance, yet also species-specific characteristics. All three species displayed declining pseudo cardiac output relative to whole-animal oxygen consumption (Fig. 3). This trend was similar among species and suggests similar responses regardless of climatic/thermal habitat origin (i.e. linear regression models for each single species did not perform better than linear regression for the pooled data set, ANOVA, F(4,103) = 0.59, P = 0.67, Fig. 3). Similarly, mitochondrial leak respiration increased significantly with temperature (Linear regression, F(1,81) = 155.89, P < 0.01, R2adj. = 0.65) and this pattern was indistinguishable among species (i.e. linear model comparison pooled vs. species data, ANOVA, F(4,77) = 1.49, P = 0.21, Fig. 4). Membrane potential also decreased similarly in all three species (Linear regression with temperature as co-factor, F(5,74) = 10.52, P < 0.01, R2adj. = 0.38), however, there was a significant temperature offset between J. edwardsii and S. verreauxi (P = 0.01).
Differences among species were most pronounced for the tropical rock lobster, P. ornatus. While predicted ATP production was similar among all lobsters at 25 °C, the rates of P. ornatus tripled at 30 °C, exceeding the plateauing rates observed for J. edwardsii and S. verreauxi by 1.8–2.0 times. Accordingly, unlike for J. edwardsii and S. verreauxi, oxidative phosphorylation in P. ornatus remained intact up to 30 °C, and then decreased due to a steep decline of predicted ATP production (Fig. 4c,d). Notably, P. ornatus showed the smallest margins between optimal temperatures for growth and temperatures at which relative changes of cardiac leak respiration and predicted ATP production started to diverge (2 °C in P. ornatus versus 4.4 °C for J. edwardsii and 8.5 °C for S. verreauxi, Fig. 5). Lastly, the contribution of mitochondrial complex II to ATP production was 2–3 times higher in P. ornatus compared to J. edwardsii and S. verreauxi respectively (Fig. 6).
The ratio of mitochondrial complex II respiration (succinate + ADP) to complex I respiration (complex I substrates + ADP) increases from temperate J. edwardsii, subtropical S. verreauxi, to tropical P. ornatus. Letters indicate significant differences between species (Chi square = 23.3, P < 0.01, df = 2). Data were pooled for each species, due to lack of significant differences among experimental temperatures.
Discussions
Our data demonstrated a key role of declining cardiac mitochondrial function in defining thermal sensitivity of spiny lobsters. Successive increases in mitochondrial proton leak alongside limited compensation in predicted ATP production rates indicates an increasing mismatch of cardiac performance and whole animal oxygen consumption, irrespective of whether lobsters originated from cool-temperate, subtropical or warm-tropical waters.
Cardiac performance in Australian spiny lobsters increasingly diverges with increasing whole-animal oxygen consumption when temperatures rise acutely, indicating a role of cardiac limitation in thermal tolerance. This was supported by declining pseudo cardiac output relative to individual oxygen demand, irrespective of species or their climate origin (Fig. 3). By contrast, a continuous increase of heart rate but declining ventilation capacity prior to critical temperatures has been reported for several crustacean species, and it was suggested that cardiac performance is of minor importance in limiting thermal tolerance as opposed to branchial oxygen uptake33. However, many studies solely used heart rate as performance proxy, without including cardiac stroke volume to estimate overall cardiac output36,37,38,39. In fact, in many crustaceans, cardiac stroke volume plateaus or decreases with increasing temperature, inversely to heart rate, thus reducing or offsetting a thermal increase of cardiac output12,40,41,42,43,51. Wooden et al.12 explained this discrepancy by an independent control of heart rate and the heart beat strength in response to temperature. Heart rate is directly paced by the cardiac ganglion motorneurons12,45 and may also affect stroke volume indirectly, by defining the available time period for the heart to relax and contract to reach its full pump volume52. However, cardiac contraction strength and dependent stroke volume are uncoupled from heart rate in American lobsters, indicating that the overall cardiac output, results from the thermal sensitivities of two independent mechanisms12. Consequently, despite increasing heart rate, overall cardiac output and thus circulatory oxygen supply may indeed become limiting in crustaceans prior to critical temperatures.
The observed lower thermal increase of heart rates relative to oxygen consumption rates in all three species (Fig. 2b), further indicates divergence between cardiac performance and oxygen demand at higher temperatures, but also indicates some oxygen buffering mechanism, or increased dependence on anaerobic capacities. Intuitively, the relative change of heart rates is expected to align with oxygen consumption to match blood oxygen supply with increasing oxygen demands. However, thermal sensitivities of heart rates are highly variable among species or life stage, either being less40, similar36,37 or greater in response to relative changes in oxygen consumption rate51. A lower thermal increase of heart rate - as observed in this study - may lead to an oxygen deficit if there is no compensation. This could be achieved by increasing stroke volume, which however, plateaued or declined in all three spiny lobsters, indicating the presence of other compensatory mechanisms, or limitations on the heart. First, unlike primitive crustaceans, decapod crustaceans can directly control the flow of haemolymph to different parts of their body via seven arteries exiting the heart, each controlled by cardio-arterial valves53,54. Dungeness crabs redistribute their haemolymph flow when temperatures increase41 and this is more pronounced during hypoxia, and may act to preserve oxygen for critical organs such as ventilatory muscles of scaphognathites55. Spiny lobster too may selectively regulate cardiac haemolymph efflux to organs when temperatures rise, to lower systemic oxygen demand and consequently cardiac pumping, resulting in a lower thermal increase of heart rates. This may also explain selective organ damage, such as of crustacean´s locomotor muscle, which suffer from reduced mitochondrial membrane potential and aerobic fibre area56, following severe temperature or hypoxic stress. Second, the blood pigment haemocyanin has been shown to support blood oxygen supply by increased unloading at higher temperatures, thus decreasing demands for cardiac pumping40,43,57. Lastly, physiological structures differ between species and life stage and may alter cardiac oxygen supply. For instance, millimetre-sized crustaceans or larvae are able to supply peripheral tissues by diffusive oxygen uptake directly via the exoskeleton, thus decreasing the dependence on convective oxygen transport, particularly when ambient-tissue oxygen gradients are steep58,59,60. Overall, the combination of dampening of elevation of the heart rate with rising temperatures, and a stable or decreasing pseudo cardiac output indicate increasing cardiac limitation at higher temperatures in spiny lobsters, where buffering mechanism (e.g. selective haemolymph distribution, haemocyanin oxygen reserve, cutaneous respiration) may sustain circulatory oxygen supply for short durations.
Most importantly, our results demonstrated that thermal sensitivity of cardiac performance in all three species of spiny lobsters links to declining cellular cardiac function, due to an increase of leak respiration and a decrease in mitochondrial membrane potential when temperatures rise (Fig. 4). Therefore, aerobic ATP production by cardiac mitochondria becomes increasingly inefficient, as energy (i.e. the proton gradient) dissipates across the inner mitochondrial membrane back into the mitochondrial matrix, without being utilised for ATP production. We note that the estimated ATP production initially increases in parallel with leak respiration, and this should largely compensate for this loss of energy, although will incur increased energetic costs in terms of substrate oxidation and oxygen uptake. However, at 25 °C for J. edwardsii and 30 °C for S. verreauxi and P. ornatus, ATP production plateaus or declines (Figs. 4c, 5), while leak respiration continued to increase (Figs. 4a, 5). This likely marks a critical temperature beyond which cardiac mitochondrial energy production must deteriorate. Temperature dependent increases in proton leak and resulting loss of phosphorylation capacity is a frequently observed phenomena46,47,49,61,62 and is related to increases of membrane permeability63 and proton flux via mitochondrial membrane proteins such as uncoupling proteins (in mammals)48, dicarboxylate carriers64, the adenine nucleotide translocase65,66, or aspartate/glutamate antiporter67. Therefore, despite an increasing uptake of metabolic energy (e.g. carbohydrates) and oxygen, mitochondria may become increasingly unable to supply sufficient ATP to power cardiac muscle contraction in spiny lobsters. Although not measured in this study, cardiac contraction strength declines along with stroke volume in heat stressed American lobster heart12, suggesting that in part a declining energy supply by mitochondria to cardiac muscle fibres contributes to declining stroke volume as temperature increases. In addition, this finding could present an intrinsic cardiac mechanism that in part could contribute to thermal sensitivity of cardiac output, which is independent from the thermal sensitivity of the ganglion controlled heart rate.
The comparison of three species of spiny lobster from distinct climate origins revealed general trends of cardiac and cellular performance. All three species displayed decreasing energetic efficiency of cardiac mitochondria (i.e. increasing proton leak, Fig. 4a,b), indicating a functional link with the general decline of pseudo cardiac output relative to total oxygen demand (Fig. 3). Therefore, for spiny lobsters, loss of energetic efficiency poses an underlying thermal challenge, compromising cardiac performance under acute as well as chronic sub-lethal temperature stress. This may be particularly critical during periods of high metabolic demand such as locomotion, moulting, reproduction or digestion. Loss of energetic efficiency, may not be exclusive to hearts but likely applies to other aerobic tissues as well, causing a disproportional increase of overall energy demand and diversion of energy away from essential processes such as growth, reproduction or locomotion47,68.
Our data also highlighted marked differences among species. Unlike temperate J. edwardsii and subtropical S. verreauxi, tropical P. ornatus was able to sustain mitochondrial capacity up to 30 °C, by compensating the increase of proton leak via a sharp increase of ATP production (Figs. 4, 5). Consequently, increasing rates of mitochondrial ATP production, is a potential adaptive strategy to compensate for a temperature driven loss of cellular energy, as opposed to modulating proton leak itself. This is in line with findings for cold and warm adapted porcelain crabs, where differences in thermal sensitivity of cardiac cellular performance, were not due to changes in membrane fluidity but increased ATPase activity in the warm adapted species69. In contrast, fish can modulate proton leak directly in response to environmental temperature, by adjusting membrane lipid composition70 or uncoupling protein expression71,72. As a result, compensatory increase of ATP production in tropical spiny lobster may extend thermal limits of cellular energy production, but at a higher metabolic cost73, which could be a critical shortcoming in face of ongoing ocean warming.
Further, the steep decline of ATP production and mitochondrial function, beyond 30 °C in tropical P. ornatus, indicates a small margin to compensate for cellular proton leak (Fig. 4c). This decline occurred far closer to optimal temperatures for growth then for temperate J. edwardsii and subtropical S. verreauxi (Fig. 5). Consequently, tropical spiny lobster have a much decreased margin between optimal and acute critical cellular cardiac temperatures compared to spiny lobster adapted to colder climates. This is in agreement with other tropical adapted species that show narrower thermal tolerance limits than species from temperate latitudes, making them more vulnerable to temperature stress74. In addition, a minor drop of heart rate and oxygen consumption at 31 °C and a significant decline of pseudo cardiac output at 33 °C in P. ornatus (Fig. 2), indicate a direct response of cardiac performance to the parallel decline of ATP production beyond 30 °C (Fig. 4c). The recovery of heart rate and oxygen consumption at 35 °C (Fig. 2) suggest short term buffering in cardiac tissue such as by cellular ATP stores (arginine phosphate75) or anaerobic ATP production76. Sustaining cardiac function and thus blood circulation anaerobically for short periods, could be an intriguing strategy to prevent systemic oxygen collapse under acute heat stress. This interesting aspect remains to be tested in future studies together if similar patterns occur for J. edwardsii and S. verreauxi, beyond the temperatures tested in this study.
Finally, the larger contribution by mitochondrial complex II to mitochondrial energy turn-over in P. ornatus compared to J. edwardsii and S. verreauxi (Fig. 6), indicates a temperature dependent shift in mitochondrial ATP production and efficiency of ATP synthesis. Mitochondrial complex I and II are essential to convert substrate bound energy into a proton-motive force that drives ATP production. Temperature has shown to affect the balance between both complexes resulting from declining complex I activity such as in crabs49 or cuttlefish77. This may reflect differential thermal stabilities of different complexes or changes in substrate generating pathways49. Most often complex I appears to be the most susceptible to stress78. In addition, complex I and II may differ in their kinetic rates. While complex I electron transport chains translocate five protons per electron and complex II moves three, complex II may sustain greater rates of proton translocation79 and also provide greater stability for thermally challenged animals, but at the cost of less efficient substrate use. Although differential thermal optima of mitochondrial complexes may limit total ATP productivity – and therefore cardiac capacity to contract - at a particular temperature, it may also enable mitochondria to extend the upper thermal range at which mitochondria can operate, as it is the case for P. ornatus. A temperature dependent shift of complex I and II ratios thus suggests a cellular strategy to extend thermal tolerance of cardiac performance towards higher temperatures.
Overall, our data highlight that declines of cellular energetic function may contribute to whole animal performance decline beyond optimal temperatures. Although, likely not the only limiting mechanisms, it provides a mechanistic pathway to better understand thermal tolerance in spiny lobsters and their ecological response to acute or chronic warming. For example, critical temperatures range from 27 to 30 °C (based on aerobic scope) for S. verreauxi puerulus15, which aligns well with our findings of declining cardiac cellular function at (or below) 30 °C (Figs. 4, 5b) and their naturally observed thermal range of 12–28 °C80. If long term warming and acute thermal extremes enter those critical ranges as projected for the northern range of S. verreauxi15, populations may shift their distribution to cooler waters. This in fact, is strongly supported by an increasing abundance of S. verreauxi in temperate South-East Australian waters81. While tropical P. ornatus (Tcrit > 31 °C 82,83,) may re-distribute to cooler latitudes as well, temperate J. edwardsii, (local thermal range of 10.8–17.5 °C, Tcrit ~18–22 °C84,85) may face compression of its natural distribution range due to the lack of suitable poleward habitat (Fig. 1). In contrast, vertical distribution shifts to deeper cooler waters are unlikely if ecological necessities such as prey and suitable habitat become unavailable.
Some elements of this study limit interpretation and warrant further research. First, since animals needed to be preserved for mitochondrial experiments, we were unable to assess critical (lethal) temperatures. Future studies would need to address the exact sequence of failure of oxygen consumption, heart rate and cardiac output at critical temperatures and assess how this aligns with the patterns observed for mitochondrial function. Second, stroke volume was estimated based on peak height of the reflective infrared signal of the photoplethysmographs. Although this is a valid proxy, it only estimates relative changes rather than absolute stroke volume or selective haemolymph distribution, which could be obtained with a pulsed-Doppler flow meter55.
Future research may investigate how cellular efficiencies and critical temperature differ between different types of aerobic tissues combined with whole animal calorimetry to assess overall state of energy leak86. Furthermore, it is unclear whether acclimation due to seasonal variation or chronic temperature increase will modulate thermal sensitivity of proton leak or ATP production. It is further desirable to test whether oxygen supply from the gills compromises oxygenation of cardiac tissue in lobsters when temperatures increase and at which critical oxygen levels mitochondrial function declines.
Conclusion
This study assessed thermal sensitivity in spiny lobsters species to identify functional links between the cellular, organ and whole animal level that contribute to performance decline beyond optimal temperatures. We identified universal patterns between species of spiny lobsters irrespective of their distinct geographical and climatic origin, marked by 1) an increasing divergence between increasing overall oxygen demand but plateauing or declining cardiac performance with increasing temperatures and 2) a parallel decline of cardiac mitochondrial energy production due to increasing proton leak and limited compensation by ATP production. In contrast to temperate and subtropical lobsters, tropical spiny lobsters displayed smaller thermal margins and functional shifts in mitochondrial energy production. We conclude that thermal sensitivity of mitochondrial energy production can directly affect performance of critical organs such as hearts, and thus contribute to defining thermal tolerance in spiny lobsters. An appropriate understanding of the functional cascades driving physiological thermal stress will help to improve forecasts of spiny lobsters’ fitness in response to changing temperatures.
Material and Methods
Animals
Study animals comprised three species of Australian spiny lobsters of distinct climatic origins. Cool-temperate southern rock lobster (Jasus edwardsii) were caught from the Taroona marine research reserve at Crayfish point, Tasmania, using baited lobster pots from 5–9th February 2018. F1 generation individuals of subtropical eastern rock lobster (Sagmariasus verreauxi) and tropical ornate lobster (Panulirus ornatus) were obtained from the Institute for Marine and Antarctic Studies (IMAS) onsite aquaculture facility in Taroona, Tasmania. Parental animals of S. verreauxi originated from wild caught post larvae at the southern extent of the distribution range at Bicheno, Tasmania in 1999, using a settlement collector, and P. ornatus from the Torres Strait regions of Queensland by diving.
The three species of spiny lobsters span the entire latitudinal coastline of Australia. Their distribution ranges from southern Victoria, around Tasmania and across South Australia into Western Australia as well as New Zealand waters for J. edwardsii, along the east Australian coastline between Brisbane and the North East coast of Tasmania, including the northern waters of New Zealand for S. verreauxi, and the northern Australian coastline from Brisbane to northern West Australia for P. ornatus (Fig. 1). All three species live at depths ranging from 5–200 metres at the Australian continental shelf 8. Natural temperature ranges are ~10.8–17.5 °C for J. edwardsii (Ion Pot, Tasmania87,88), ~12–28 °C for S. verreauxi80 and ~25–30 °C for P. ornatus89.
Jasus edwardsii were held at 13.3 °C (±SD 0.3 °C) outdoors, in a single coated glass-fibre tank (W × L × H in cm, 205 × 205 × 90), filled with 2.9 m3 untreated and aerated flow-through sea water, at a day/night cycle ranging from 11/13 to 14/10 hours. Due to aquaculture holding restrictions and maintenance of water temperatures, S. verreauxi and P. ornatus were held indoors, in two separate coated glass-fibre tanks (W × L × H in cm, 205 × 205 × 90) at 21.0 °C (±SD 0.1 °C) and 28.0 °C (±SD 0.2 °C) respectively, filled with 2.9 m3 filtered and ozone treated, flow-through sea water, at a 14 hour day/10 hour night cycle. Previous testing showed that water quality parameters such as nitrate, ammonia or heavy metals were below critical levels at flow through rates of 1500 L/h and therefore were not tested during holding. Tanks were equipped with off-ground artificial oyster mesh shelters and cleaned every second day to remove food scraps and sediments. Lobsters were acclimated to holding temperatures for at least three months prior to experimentation. All lobster were fed daily with live blue mussels or frozen squid. Weights of lobsters averaged 1030 g (95% CI 909–1153 g, n = 6) for J. edwardsii, 975 g (95% CI 889–1060 g, n = 7) for S. verreauxi, and 876 g (95% CI 803–948 g, n = 8) for P. ornatus.
Animal ethics were not required. Tropical lobsters were handled and prepared within onsite quarantine facilities according to local biosecurity restrictions and legislation (Tasmanian Government Special Authority, SA 18–75).
Respirometry
Whole animal oxygen consumption was measured using two intermittent flow respirometers90. Prior to respirometry, lobsters were collected from holding tanks with a scoop net and a wet towel covering their eyes to reduce transfer stress. Following the fixation of heart rate loggers, individuals were transferred into cylindrical 10 L respirometers (L × D in cm, 66 × 15), with tail first to prevent blocking and injury due to the lobster’s guarding posture. Lobsters could gain traction to oyster mesh added to the bottom of the respirometer, held in place with an open cut piece of plastic pipe (Fig. 7a)91. Chambers were sealed within two minutes after addition of lobsters. Oxygen concentration was measured continuously using a fibre optic two-channel oxygen meter (HQ40d, Hach, USA), with oxygen probes positioned into the external recirculation loop. Re-circulation pumps (Quite One® Pro 1200, Lifegard Aquatics, USA) provided continuous mixing of water within respirometers at a flow of 1200 L/min. Following a six-minute respiration cycle, flush pumps (Compact + 2000, Eheim, Germany) re- oxygenated chambers at a flow of ca. 1500 L/min for eight minutes, using a time controlled digital recycling timer (DRT-1, Sentinel, USA). The two adjacent respirometers were housed in a buffer tank (W × L × H in cm, 102 × 52 × 50), filled with 190 L filtered and ozonated flow-through sea water at a flow rate of 130–150 L/h. An air stone ensured homogenous mixing in the buffer tank and supply of aeration. The experimental setup was covered with black building foil to prevent visual disturbance and illuminated with an LED flood light set to yellow at low intensity. Lights were permanently turned on during experiments to reduce spontaneous activity of the nocturnal lobsters92. After each experiment, the respiration setup was cleaned and flushed with fresh water.
Water temperature was increased from ambient starting temperature using a 2000 Watt titanium heater (Istra Elements, Australia) modified with a programmable PID controller (SmartPID, Arzaman, Italy). Following an acclimation time within the respirometers of minimum 11 hours, temperatures were ramped from 14–23 °C for J. edwardsii, 20–28 °C for S. verreauxi, and 28–35 °C for P. ornatus at a heating rate of ca. 2.3 °C per hour. Respiration was measured for one hour at each temperature ramp. After completion of the final heating ramp, temperatures returned to initial acclimation temperature at about 3 °C per hour and lobsters were returned to the holding tank for at least 24 hours before further use for mitochondrial experiments, with the exception of four specimens of P. ornatus, which were used after five hours acclimation. Note that experimental temperature ranges were not chosen to cover the full natural thermal range of the respective species, but to test effects of elevated and close to critical temperatures on lobster metabolism and cardiac performance.
Respiration was calculated as in Svendsen et al.93. Oxygen saturation in respiration chambers decreased to a minimum of 87% (95% CI 85–89%) on average, and never fell below 75%.
Individual body volume of lobsters was accounted for in MO2 calculations and measured as the volume overflow in ml after adding lobsters to a water levelled container. Body density did not differ significantly between species (ANOVA, F(2, 18) = 2.01, P = 0.163) and averaged 1.33 g/ml (95% CI 1.25–1.41 g/ml). Background respiration was recorded before and after each experiment and accounted for 4.5% (95% CI 3.3–5.7 g/ml) on average relative to standard metabolic rate (no significant differences between species, ANOVA, F(2, 18) = 0.36, P = 0.701). Preliminary tests confirmed appropriate mixing of water in the chamber, the lack of leaks (i.e. dye test) and appropriate flush/respiration cycles.
Moulting can significantly increase standard and routine metabolic rates94. We accounted for this by measuring the Brix index95,96 of 100 µl haemolymph from each lobster, following centrifugation at 10.000 g for 3 min, using a refractometer (Hanna HI96801, Hanna Instruments, Australia). The Brix index did not show significant interaction with metabolic rates (ANOVA, F(1,14) = 1.178, P = 0.296), indicating that our metabolic data were not affected by the moulting stage of the experimental lobsters.
Heart rate
Heart rate of lobster was recorded simultaneously during oxygen consumption rate measurement using a self-assembled infrared photoplethysmographs (Fig. 7b) analogue to previous sensors used for decapod crustaceans36,97. This comprised four infrared LEDs (SFH 4544, 950 nm, T-1 3/4, 5 mm, Osram, Germany) arranged around one infrared phototransistor (SFH 313 FA-2/3 20°, 740–1080 nm, T-1 3/4, 5 mm, Osram, Germany) glued into a 3D printed ABS plastic casing (3D model in Supplementary File S). The electronics were water proofed with hot glue, the lid fixed with superglue and the plastic surface welded with acetone. The photoplethysmograph sensors was powered from a 5 VDC laptop USB port and connected to a 2-channel A/D recorder (PowerLab15T Model ML4818, AD Instruments, New Zealand) via a four-wire cable to record the analogue voltage signal from the phototransistor (Electrical circuit diagram in Supplementary Fig. S). A plastic cable gland in the lid of the respiration chamber secured and sealed the cable.
Prior to the addition to the respiration chambers, heart rate loggers were positioned dorsally above the heart and fixed with two cable strips. Dental wax between the logger and the lobster’s carapace reduced slipping and signal disturbance by water movements. Short test recordings during positioning and just prior to the beginning of the respiration experiment confirmed a viable heart rate signal. A wet towel covering the eyes and antennae of lobsters reduced handling stress during the attachment procedure.
The analogue raw signal from the photoplethysmographs (Fig. 7c) was analysed using LabChart 8 Pro (AD Instruments, New Zealand), applying the following settings to calculate. beats per minute: 500 mV voltage range, 400/s recording frequency, triangular smoothing with 151 samples, 5 Hz low pass filter and cyclic measurement rate with general sine shape and a minimum peak height of 0.18 SD. Peak height (in volts) was extracted as an indicator of cardiac stroke volume, assuming that decreasing fluid content (cardiac volume), increases signal intensity, due to reduced absorbance by water within the ventricle. A proxy for cardiac output was calculated as the peak height multiplied by heart beat rate (per minute) and denoted as pseudo cardiac output.
Mitochondrial function
For the analysis of cardiac mitochondrial function, the same animals used for respiration and heart rate experiments were used. Lobsters were removed from holding tank and anaesthetized by chilling them in 0 °C sea water. Once lobsters stopped responding to physical contact (~10–20 min), the carapace was opened dorsally by two longitudinal parallel cuts above the gill chamber and carefully cut off from connective tissue and muscle.
The heart was immediately dissected and immersed in 2 ml modified ice-cold relaxing buffer (in mmol/L: 2.77 CaK2EGTA, 7.23 K2EGTA, 5.77 Na2ATP, 6.56 MgCl2·6H2O, 20 taurine, 20 imidazole, 0.5 dithiothreitol, 50 MES, and 400 KCl, pH 7.1 at 0 °C, final osmotic concentration was 960 mOsm). The heart tissues were cut into small pieces and stored in relaxing buffer on ice until required and excess tissue snap frozen in liquid nitrogen and stored at −80 °C. Approximately 100 mg of tissue was accurately weighed into a micro-centrifuge tube with a 10 fold w/v volume of respiration media (in mmol/L: 0.5 EGTA, 3 MgCl2, 100 K-lactobionate, 20 taurine, 10 KH2PO4, 20 HEPES, 200 sucrose, 250 KCl and 1 g/L BSA, pH 7.24 at 20 °C; Gnaiger et al. (2000)), and then homogenized using a tissue homogenizer (Dremel, 985370–395, USA). Homogenate (totalling 10 mg of tissue for J. edwardsii and S. verreauxi and 5 mg tissue for P. ornatus) was added to each chamber of calibrated Oroboros O2K oxygraphs (Oroboros Instruments, Innsbruck, Austria). Tissue homogenates were used as this permitted 1) rapid analysis of tissues, 2) a measure of the respiratory capacity of the wet mass of heart tissue, 3) decreased optical disturbances that permeabilised fibres can produce, and 4) rapid uptake of the mitochondria membrane potential indicator (TMRM). Fresh homogenate was used for each experiment.
Respiration and membrane potential of cardiac tissue homogenate was measured simultaneously in two replicate 2 ml glass chambers, using two Oroboros O2K oxygraphs, each set at two different temperatures, which were specific for each species. Samples of J. edwardsii were assayed at 15, 20, 25 and 30 °C, S. verreauxi, at 20, 25, 30 and 35 °C and P. ornatus at 25, 30, 35 and 37.5 °C. Temperatures were alternated among oxygraphs to account for possible differences in sample holding times and oxygraphs. Mitochondrial membrane potential was measured using fluorimeters, attached to the transparent front of each glass chamber, each using green excitation LEDs (530 nm) with red (580 nm long-pass filters). The fluorescent dye Tetramethylrhodamine (TMRM, Thermofisher Scientific) was titrated into each chamber in two injections of 125 µmol/L (totalling 250 µmol/L TMRM). These were used to calibrate the TMRM signal, which was allowed to settle prior to addition of mitochondrial substrates. The mitochondrial substrates malate (2 mmol/L) and glutamate (10 mmol/L) were then added to elevate membrane potential and provide a measure of leak state respiration, which results from proton conductance (leakage) across the inner mitochondrial membrane. ADP (2.5 mmol/L) was then added to initiate oxidative phosphorylation supported by mitochondrial complex I. Oxidative phosphorylation commencement also partially depolarizes the mitochondrial membrane potential. Oxidative phosphorylation was further enhanced by addition of the amino acid substrate proline (10 mmol/L), and then succinate (10 mmol/L). Proline is oxidised by proline dehydrogenase and in vivo the ensuing products should also support mitochondrial complex I and complex II. Succinate is oxidized by mitochondrial complex II. The addition of proline and succinate therefore elevate oxygen flux JO2. Oligomycin was then added (2.5 µmol/L) to induce a maximal leak respiration state LEAKmax, which coincides with a high mitochondrial membrane potential that is higher than that with single substrates. Titrations (1–3 additions, dependent on temperature) of the un-coupler carbonyl cyanide m-chlorophenyl hydrazone (CCCP 0.5 µmol/L) was then added to measure the maximum capacity of the electron transport system (ETS) and to depolarize mitochondrial membranes. Unless stated, all chemicals were obtained from Sigma-Aldrich (St. Louis, MO, USA).
Simultaneous to mitochondrial membrane potential, mitochondrial respiration was measured in the same chambers, using high resolution polarographic oxygen sensors, as mass-specific oxygen flux [pmol O2/(sec mg wet weight)]. Oxygen flux JO2 was calculated in real time as the negative time derivative of the oxygen concentration using Oroboros DatLab Software V 7.1. (Oroboros Instruments, Innsbruck, Austria).
In addition, we estimated the total ATP formed in maximal oxidative phosphorylation through several assumptions. We assumed that the measured mitochondrial oxygen flux JO2, transfers to ten protons for complex I/NADH derived electrons and six protons for complex II derived electrons being pumped into the intermembrane space. We further assume that proline, which is FAD+ linked at proline dehydrogease (ProlDH, only), or FAD+ and NAD+ linked (ProlDH and complex I and II) is fully oxidized. This results in respective substrate ATP:Oxygen (P:O) ratios of approximately 2.5 and 1.5 for complex I and complex II respectively, and 1.5 and 2.15 for proline with incomplete or complete oxidation of the products of proline oxidation respectively. We assumed that there are eight subunits in the ATP synthase C-ring and one rotation forms three ATP, and that increases in oxygen flux JO2 resulting from substrate addition, were proportionate to the electron flow through each respiratory complex, and that there was no reallocation or restriction of the Q-pool. We note that if the proportion of electrons flowing from different complexes changes with substrate addition (i.e. due to the addition of FAD+ linked substrates after complex I linked substrates), we will overestimates ATP synthesis rates.
Mitochondrial membrane potentials were not calculated, or reported as relative fluorescence. This was because the mitochondrial volume within homogenates was unknown and relative fluorescence would not allow comparisons among species. Here we report TMRM data as the amount of probe imported or expelled from mitochondrial with respective polarization and depolarization, with units of nmol TMRM per mg of tissue. This was calculated from measuring the differences in signal before or after addition of specific substrates, oligomycin, and on uncoupling with CCCP. This permitted calculation of the amount of TMRM taken up in oxidative phosphorylation and at maximum leak respiration.
To incorporate the oxygen flux JO2 and TMRM data we considered that the amount of imported TMRM is dependent on oxygen flux JO2 in specific states, and this represents a form of work to maintain a membrane potential. Therefore, we converted the oxygen flux JO2 into Watts assuming that the reduction of oxygen to H2O equals 20 kJ/mol, and in this way we explored the amount of TMRM taken up per unit mitochondrial work (energy expended) in performed maximum oxidative phosphorylation. This represents the demands where maximal demands are placed on the oxidative phosphorylation system, and also in maximal leak respiration state LEAKmax, as this provides insights to the membrane integrity while under high load.
Data analysis
All data statistical data analysis was performed using R statistical software98 and RStudio99.
Temperature ramp data (Figs. 2–5) were analysed using repeated-measures ANOVA followed by Tukey Posthoc test with Bonferroni correction, to compare data between the starting temperature and data at successive temperature ramps. If data failed ANOVA assumptions for normality and homogeneity of variances, a non-parametric Kruskal-Wallis test was applied followed by Dunn test for multiple comparisons and “Benjamini-Hochberg” correction to decreases type I error (i.e. false positives). The relation between pseudo cardiac output and oxygen consumption (Fig. 3) was analysed, by comparing two linear regression models with and without species as co-factor. Complex II substrate use was compared between species on data pooled across temperatures (Fig. 6), using non-parametric Kruskal-Wallis test, followed by Dunn test for multiple comparisons with “Benjamini-Hochberg” correction.
Standard metabolic rate was calculated as the mean of the lowest 10% of values for the initial acclimation period, with outliers excluded below ±2 SD of the mean27. Routine metabolic rates for each temperature ramp were calculated as the average respiration of a ramping level each lasting for one hour.
Heart rate data were extracted specific to each respiration cycle used for metabolic rate calculations, excluding data outside this range (e.g. ramping periods, flush cycles). To reduce the impact of signal noise, due to animal movements, heart rate data were filtered choosing binned regions with low signal variance within each six minute respiration interval.
Data availability
The datasets generated during the current study are available in the Figshare repository, doi 10.6084/m9.figshare.9162218.
References
Angilletta Jr., M. J. & Angilletta, M. J. Thermal adaptation: a theoretical and empirical synthesis. (Oxford University Press, 2009).
Frölicher, T. L. & Laufkötter, C. Emerging risks from marine heat waves. Nat. Commun. 9, 650, https://doi.org/10.1038/s41467-018-03163-6 (2018).
Garrabou, J. et al. Mass mortality in Northwestern Mediterranean rocky benthic communities: effects of the 2003 heat wave. Global Change Biol. 15, 1090–1103, https://doi.org/10.1111/j.1365-2486.2008.01823.x (2009).
Cavole, L. M. et al. Biological Impacts of the 2013–2015 Warm-Water Anomaly in the Northeast Pacific Winners, Losers, and the Future. Oceanography 29, 273–285, https://doi.org/10.5670/oceanog.2016.32 (2016).
Butler, M. J., Steneck, R. S. & Herrnkind, W. F. Juvenile and adult ecology. Lobsters: biology and management. Blackwell Scientific Press, Oxford, 263–309 (2006).
Ling, S. D., Johnson, C. R., Frusher, S. D. & Ridgway, K. R. Overfishing reduces resilience of kelp beds to climate-driven catastrophic phase shift. Proc. Natl. Acad. Sci. USA 106, 22341–22345, https://doi.org/10.1073/pnas.0907529106 (2009).
Phillips, B. F. Lobsters: biology, management, aquaculture and fisheries. (Wiley Online Library, 2013).
Holthuis, L. FAO species catalogue. v. 13: Marine lobsters of the world. An annotated and illustrated catalogue of species of interest to fisheries known to date. FAO Fisheries Synopsis (FAO). no. 125 (13) (1991).
Jeffs, A. Status and challenges for advancing lobster aquaculture. J. Mar. Biol. Assoc. India 52, 320–326 (2010).
Fitzgibbon, Q. P. & Battaglene, S. C. Effect of water temperature on the development and energetics of early, mid and late-stage phyllosoma larvae of spiny lobster Sagmariasus verreauxi. Aquaculture 344–349, 153–160, https://doi.org/10.1016/j.aquaculture.2012.03.008 (2012).
Manning, J. & Pelletier, E. Environmental monitors on lobster traps (eMOLT): long-term observations of New England’s bottom-water temperatures. J. Oper. Oceanogr. 2, 25–33, https://doi.org/10.1080/1755876X.2009.11020106 (2009).
Worden, M. K., Clark, C. M., Conaway, M. & Qadri, S. A. Temperature dependence of cardiac performance in the lobster Homarus americanus. J. Exp. Biol. 209, 1024–1034, https://doi.org/10.1242/jeb.02082 (2006).
Pearce, J. & Balcom, N. The 1999 Long Island Sound lobster mortality event: findings of the comprehensive research initiative. J. Shellfish Res. 24, 691–698, https://doi.org/10.2983/0730-8000(2005)24[691:TLISLM]2.0.CO;2 (2005).
Wahle, R. A., Dellinger, L., Olszewski, S. & Jekielek, P. American lobster nurseries of southern New England receding in the face of climate change. ICES J. Mar. Sci. 72, i69–i78, https://doi.org/10.1093/icesjms/fsv093 (2015).
Fitzgibbon, Q. P., Ruff, N., Tracey, S. R. & Battaglene, S. C. Thermal tolerance of the nektonic puerulus stage of spiny lobsters and implications of ocean warming. Mar. Ecol. Prog. Ser. 515, 173–186, https://doi.org/10.3354/meps10979 (2014).
Pearce, A. F. et al. The “marine heat wave” off Western Australia during the summer of 2010/11. (Western Australian Fisheries and Marine Research Laboratories, 2011).
Bopp, L. et al. Multiple stressors of ocean ecosystems in the 21st century: projections with CMIP5 models. Biogeosciences 10, 6225–6245, https://doi.org/10.5194/bg-10-6225-2013 (2013).
Caputi, N. et al. Management adaptation of invertebrate fisheries to an extreme marine heat wave event at a global warming hot spot. Ecol. Evol. 6, 3583–3593, https://doi.org/10.1002/ece3.2137 (2016).
Wernberg, T. et al. An extreme climatic event alters marine ecosystem structure in a global biodiversity hotspot. Nat. Clim. Change 3, 78, https://doi.org/10.1038/nclimate1627 (2012).
Bennett, S., Wernberg, T., Arackal Joy, B., de Bettignies, T. & Campbell, A. H. Central and rear-edge populations can be equally vulnerable to warming. Nat. Commun. 6, 10280, https://doi.org/10.1038/ncomms10280 (2015).
Deutsch, C., Ferrel, A., Seibel, B., Pörtner, H.-O. & Huey, R. B. Climate change tightens a metabolic constraint on marine habitats. Science 348, 1132–1135, https://doi.org/10.1126/science.aaa1605 (2015).
Kearney, M. & Porter, W. Mechanistic niche modelling: combining physiological and spatial data to predict species’ ranges. Ecol. Lett. 12, 334–350, https://doi.org/10.1111/j.1461-0248.2008.01277.x (2009).
Magozzi, S. & Calosi, P. Integrating metabolic performance, thermal tolerance, and plasticity enables for more accurate predictions on species vulnerability to acute and chronic effects of global warming. Global Change Biol 21, 181–194, https://doi.org/10.1111/gcb.12695 (2015).
Hitt, N. P., Snook, E. L. & Massie, D. L. Brook trout use of thermal refugia and foraging habitat influenced by brown trout. Can. J. Fish. Aquat. Sci. 74, 406–418, https://doi.org/10.1139/cjfas-2016-0255 (2016).
Morelli, T. L. et al. Managing Climate Change Refugia for Climate Adaptation. Plos One 11, e0159909, https://doi.org/10.1371/journal.pone.0159909 (2016).
Keppel, G. & Wardell-Johnson, G. W. Refugia: keys to climate change management. Global Change Biol 18, 2389–2391, https://doi.org/10.1111/j.1365-2486.2012.02729.x (2012).
Clark, T. D., Sandblom, E. & Jutfelt, F. Aerobic scope measurements of fishes in an era of climate change: respirometry, relevance and recommendations. J. Exp. Biol. 216, 2771–2782, https://doi.org/10.1242/jeb.084251 (2013).
Pörtner, H. O. & Knust, R. Climate change affects marine fishes through the oxygen limitation of thermal tolerance. Science 315, 95–97, https://doi.org/10.1126/science.1135471 (2007).
Pörtner, H. O. Oxygen-and capacity-limitation of thermal tolerance: a matrix for integrating climate-related stressor effects in marine ecosystems. J. Exp. Biol. 213, 881, https://doi.org/10.1242/jeb.037523 (2010).
Pörtner, H. O., Climate change. and temperature-dependent biogeography: oxygen limitation of thermal tolerance in animals. Naturwissenschaften 88, 137–146, https://doi.org/10.1007/s001140100216 (2001).
Jutfelt, F. et al. Oxygen- and capacity-limited thermal tolerance: blurring ecology and physiology. J. Exp. Biol. 221, jeb169615, https://doi.org/10.1242/jeb.169615 (2018).
Pörtner, H.-O., Bock, C. & Mark, F. C. Oxygen- and capacity-limited thermal tolerance: bridging ecology and physiology. J. Exp. Biol. 220, 2685–2696, https://doi.org/10.1242/jeb.134585 (2017).
Verberk, W. C. E. P. et al. Does oxygen limit thermal tolerance in arthropods? A critical review of current evidence. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 192, 64–78, https://doi.org/10.1016/j.cbpa.2015.10.020 (2016).
Fitzgibbon, Q. P., Simon, C. J., Smith, G. G., Carter, C. G. & Battaglene, S. C. Temperature dependent growth, feeding, nutritional condition and aerobic metabolism of juvenile spiny lobster, Sagmariasus verreauxi. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 207, 13–20, https://doi.org/10.1016/j.cbpa.2017.02.003 (2017).
Steinhausen, M. F., Sandblom, E., Eliason, E. J., Verhille, C. & Farrell, A. P. The effect of acute temperature increases on the cardiorespiratory performance of resting and swimming sockeye salmon Oncorhynchus nerka. J. Exp. Biol. 211, 3915–3926, https://doi.org/10.1242/jeb.019281 (2008).
Ern, R., Huong, D. T. T., Phuong, N. T., Wang, T. & Bayley, M. Oxygen delivery does not limit thermal tolerance in a tropical eurythermal crustacean. J. Exp. Biol. 217, 809–814, https://doi.org/10.1242/jeb.094169 (2014).
Ern, R., Phuong, N. T., Madsen, P. T., Wang, T. & Bayley, M. Some like it hot: Thermal tolerance and oxygen supply capacity in two eurythermal crustaceans. Scientific reports 5, https://doi.org/10.1038/srep10743 (2015).
Jost, J. A., Podolski, S. M. & Frederich, M. Enhancing thermal tolerance by eliminating the pejus range: a comparative study with three decapod crustaceans. Mar. Ecol. Prog. Ser. 444, 263–274, https://doi.org/10.3354/meps09379 (2012).
Wittmann, A. C., Pörtner, H. O. & Sartoris, F. J. A role for oxygen delivery and extracellular magnesium in limiting cold tolerance of the sub-antarctic stone crab Paralomis granulosa? Physiol. Biochem. Zool. 85, 285–298, https://doi.org/10.1086/665328 (2012).
Giomi, F. & Pörtner, H.-O. A role for haemolymph oxygen capacity in heat tolerance of eurythermal crabs. Front. Physiol. 4, 110, https://doi.org/10.3389/fphys.2013.00110 (2013).
De Wachter, B. & McMahon, B. R. Temperature effects on heart performance and regional hemolymph flow in the crab Cancer magister. Comp. Biochem. Physiol. A Physiol. 114, 27–33, https://doi.org/10.1016/0300-9629(95)02084-5 (1996).
De Wachter, B. & Wilkens, J. L. Comparison of Temperature Effects on Heart Performance of the Dungeness Crab, Cancer magister, in vitro and in vivo. Biol. Bull. 190, 385–395, https://doi.org/10.2307/1543031 (1996).
Florey, E. & Kriebel, M. E. The effects of temperature, anoxia and sensory stimulation on the heart rate of unrestrained crabs. Comp. Biochem. Physiol. A Physiol. 48, 285–300, https://doi.org/10.1016/0300-9629(74)90709-9 (1974).
McGaw, I. J. The Decapod Crustacean Circulatory System: A Case That Is neither Open nor Closed. Microsc. Microanal. 11, 18–36, https://doi.org/10.1017/S1431927605050026 (2005).
Cooke, I. M. Reliable, responsive pacemaking and pattern generation with minimal cell numbers: the crustacean cardiac ganglion. Biol. Bull. 202, 108–136, https://doi.org/10.2307/1543649 (2002).
Pörtner, H. O., Hardewig, I. & Peck, L. S. Mitochondrial function and critical temperature in the Antarctic bivalve, Laternula elliptica. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 124, 179–189 (1999).
Hardewig, I., Pörtner, H. O. & Peck, L. S. Thermal sensitivity of mitochondrial function in the Antarctic Notothenioid Lepidonotothen nudifrons. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 169, 597–604 (1999).
Brand, M., Chien, L., Ainscow, E., Rolfe, D. & Porter, R. The causes and functions of mitochondrial proton leak. Biochim. Biophys. Acta 1187, 132 (1994).
Iftikar, F. I., MacDonald, J. & Hickey, A. J. R. Thermal limits of portunid crab heart mitochondria: Could more thermo-stable mitochondria advantage invasive species? J. Exp. Mar. Biol. Ecol. 395, 232–239, https://doi.org/10.1016/j.jembe.2010.09.005 (2010).
Tepolt, C. K. & Somero, G. N. Master of all trades: thermal acclimation and adaptation of cardiac function in a broadly distributed marine invasive species, the European green crab, Carcinus maenas. J. Exp. Biol. 217, 1129–1138, https://doi.org/10.1242/jeb.093849 (2014).
Zainal, K. A. Y., Taylor, A. C. & Atkinson, R. J. A. The effect of temperature and hypoxia on the respiratory physiology of the squat lobsters, Munida rugosa and Munida sarsi (Anomura, Galatheidae). Comp. Biochem. Physiol. A Physiol. 101, 557–567 (1992).
McMahon, B. R. & Burnett, L. E. The Crustacean Open Circulatory System: A Reexamination. Physiol. Zool. 63, 35–71, https://doi.org/10.1086/physzool.63.1.30158153 (1990).
Wilkens, J. L. The control of cardiac rhythmicity and of blood distribution in crustaceans. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 124, 531–538, https://doi.org/10.1016/S1095-6433(99)00146-4 (1999).
McGaw, I. J., Airriess, C. N. & McMahon, B. R. Patterns of haemolymph-flow variation in decapod crustaceans. Mar. Biol. 121, 53–60, https://doi.org/10.1007/bf00349473 (1994).
Airriess, C. & McMahon, B. Cardiovascular adaptations enhance tolerance of environmental hypoxia in the crab Cancer magister. J. Exp. Biol. 190, 23–41 (1994).
Geihs, M. A., Vargas, M. A. & Nery, L. E. M. Damage caused during hypoxia and reoxygenation in the locomotor muscle of the crab Neohelice granulata (Decapoda: Varunidae). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 172, 1–9, https://doi.org/10.1016/j.cbpa.2014.02.010 (2014).
Oellermann, M., Lieb, B., Portner, H.-O., Semmens, J. & Mark, F. Blue blood on ice: modulated blood oxygen transport facilitates cold compensation and eurythermy in an Antarctic octopod. Front. Zool. 12, 6, https://doi.org/10.1186/s12983-015-0097-x (2015).
Pirow, R., Wollinger, F. & Paul, R. J. The sites of respiratory gas exchange in the planktonic crustacean Daphnia magna: an in vivo study employing blood haemoglobin as an internal oxygen probe. J. Exp. Biol. 202, 3089–3099 (1999).
Spicer, J. I. & McMahon, B. R. Gill function in the amphipod Megalorchestia (Orchestoidea) californiana (Brandt, 1851) (Crustacea). Can. J. Zool. 72, 1155–1158, https://doi.org/10.1139/z94-154 (1994).
Pirow, R., Bäumer, C. & Paul, R. J. Crater landscape: two-dimensional oxygen gradients in the circulatory system of the microcrustacean Daphnia magna. J. Exp. Biol. 207, 4393–4405, https://doi.org/10.1242/jeb.01318 (2004).
Hilton, Z., Clements, K. D. & Hickey, A. J. R. Temperature sensitivity of cardiac mitochondria in intertidal and subtidal triplefin fishes. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 180, 979–990, https://doi.org/10.1007/s00360-010-0477-7 (2010).
Abele, D., Heise, K., Pörtner, H. O. & Puntarulo, S. Temperature-dependence of mitochondrial function and production of reactive oxygen species in the intertidal mud clam Mya arenaria. J. Exp. Biol. 205, 1831–1841 (2002).
Hazel, J. R. Thermal adaptation in biological membranes: is homeoviscous adaptation the explanation? Annu. Rev. Physiol. 57, 19–42 (1995).
Wieckowski, M. R. & Wojtczak, L. Involvement of the dicarboxylate carrier in the protonophoric action of long-chain fatty acids in mitochondria. Biochem. Biophys. Res. Commun. 232, 414–417, https://doi.org/10.1006/bbrc.1997.6298 (1997).
Cadenas, S. et al. AMP decreases the efficiency of skeletal-muscle mitochondria. Biochem. J. 351, 307–311 (2000).
Bertholet, A. M. et al. H+ transport is an integral function of the mitochondrial ADP/ATP carrier. Nature 571, 515–520, https://doi.org/10.1038/s41586-019-1400-3 (2019).
Samartsev, V. N. et al. Involvement of aspartate/glutamate antiporter in fatty acid-induced uncoupling of liver mitochondria. Biochim. Biophys. Acta 1319, 251–257, https://doi.org/10.1016/S0005-2728(96)00166-1 (1997).
Clarke, A. & Fraser, K. P. P. Why does metabolism scale with temperature? Funct. Ecol. 18, 243–251, https://doi.org/10.1111/j.0269-8463.2004.00841.x (2004).
Stillman, J. H. Causes and consequences of thermal tolerance limits in rocky intertidal porcelain crabs, genus Petrolisthes. Integr. Comp. Biol. 42, 790–796, https://doi.org/10.1093/icb/42.4.790 (2002).
Kraffe, E., Marty, Y. & Guderley, H. Changes in mitochondrial oxidative capacities during thermal acclimation of rainbow trout Oncorhynchus mykiss: roles of membrane proteins, phospholipids and their fatty acid compositions. J. Exp. Biol. 210, 149–165, https://doi.org/10.1242/jeb.02628 (2007).
Mark, F. C., Lucassen, M. & Pörtner, H. O. Thermal sensitivity of uncoupling protein expression in polar and temperate fish. Comp. Biochem. Phys. D. 1, 365–374, https://doi.org/10.1016/j.cbd.2006.08.004 (2006).
Jastroch, M., Buckingham, J. A., Helwig, M., Klingenspor, M. & Brand, M. D. Functional characterisation of UCP1 in the common carp: uncoupling activity in liver mitochondria and cold-induced expression in the brain. J. Comp. Physiol. B 177, 743–752, https://doi.org/10.1007/s00360-007-0171-6 (2007).
Miller, N. A., Paganini, A. W. & Stillman, J. H. Differential thermal tolerance and energetic trajectories during ontogeny in porcelain crabs, genus Petrolisthes. J. Therm. Biol. 38, 79–85, https://doi.org/10.1016/j.jtherbio.2012.11.005 (2013).
Sunday, J. M., Bates, A. E. & Dulvy, N. K. Global analysis of thermal tolerance and latitude in ectotherms. Proc. R. Soc. Biol. Sci. Ser. B 278, 1823–1830, https://doi.org/10.1098/rspb.2010.1295 (2011).
England, W. R. & Baldwin, J. Anaerobic energy metabolism in the tail musculature of the Australian yabby Cherax destructor (Crustacea, Decapoda, Parastacidae): Role of phosphagens and anaerobic glycolysis during escape behavior. Physiol. Zool. 56, 614–622, https://doi.org/10.1086/physzool.56.4.30155884 (1983).
Livingstone, D. R. Invertebrate and vertebrate pathways of anaerobic metabolism: evolutionary considerations. J. Geol. Soc. London 140, 27–37, https://doi.org/10.1144/gsjgs.140.1.0027 (1983).
Oellermann, M., Pörtner, H. O. & Mark, F. C. Mitochondrial dynamics underlying thermal plasticity of cuttlefish (Sepia officinalis) hearts. J. Exp. Biol. 215, 2992–3000, https://doi.org/10.1242/jeb.068163 (2012).
Sharma, L. K., Lu, J. & Bai, Y. Mitochondrial respiratory complex I: structure, function and implication in human diseases. Curr. Med. Chem. 16, 1266–1277, https://doi.org/10.2174/092986709787846578 (2009).
Jin, Q. & Bethke, C. M. Kinetics of electron transfer through the respiratory chain. Biophys. J. 83, 1797–1808, https://doi.org/10.1016/S0006-3495(02)73945-3 (2002).
Montgomery, S. S., Liggins, G. W., Craig, J. R. & McLeod, J. R. Growth of the spiny lobster Jasus verreauxi (Decapoda: Palinuridae) off the east coast of Australia. N. Z. J. Mar. Freshw. Res. 43, 113–123, https://doi.org/10.1080/00288330909509986 (2009).
Robinson, L. M. et al. Rapid assessment of an ocean warming hotspot reveals “high” confidence in potential species’ range extensions. Global Environ Chang 31, 28–37, https://doi.org/10.1016/j.gloenvcha.2014.12.003 (2015).
Jones, C. M. Temperature and salinity tolerances of the tropical spiny lobster, Panulirus ornatus. J. World Aquacult. Soc. 40, 744–752, https://doi.org/10.1111/j.1749-7345.2009.00294.x (2009).
Sachlikidis, N. G., Jones, C. M. & Seymour, J. E. The effect of temperature on the incubation of eggs of the tropical rock lobster Panulirus ornatus. Aquaculture 305, 79–83, https://doi.org/10.1016/j.aquaculture.2010.04.015 (2010).
Bermudes, M. & Ritar, A. J. The ontogeny of physiological response to temperature in early stage spiny lobster (Jasus edwardsii) larvae. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 138, 161–168, https://doi.org/10.1016/j.cbpb.2004.03.010 (2004).
Smith, G. G., Ritar, A. J., Thompson, P. A., Dunstan, G. A. & Brown, M. R. The effect of embryo incubation temperature on indicators of larval viability in Stage I phyllosoma of the spiny lobster, Jasus edwardsii. Aquaculture 209, 157–167, https://doi.org/10.1016/S0044-8486(01)00758-X (2002).
van Ginneken, V. & van den Thillart, G. Metabolic depression in fish measured by direct calorimetry: A review. Thermochem. Acta 483, 1–7, https://doi.org/10.1016/j.tca.2008.09.027 (2009).
Stobart, B., Mayfield, S., Mundy, C., Hobday, A. J. & Hartog, J. R. Comparison of in situ and satellite sea surface-temperature data from South Australia and Tasmania: how reliable are satellite data as a proxy for coastal temperatures in temperate southern Australia? Mar. Freshw. Res. 67, https://doi.org/10.1071/mf14340 (2016).
Oliver, E. C. J. et al. The unprecedented 2015/16 Tasman Sea marine heatwave. Nat. Commun. 8, 16101, https://doi.org/10.1038/ncomms16101 (2017).
Suppiah, R., Bathols, J., Collier, M., Kent, D. & O’Grady, J. (CSIRO report for the Torres Strait Regional Authority, 2010).
Jensen, M. A., Fitzgibbon, Q. P., Carter, C. G. & Adams, L. R. Effect of body mass and activity on the metabolic rate and ammonia-N excretion of the spiny lobster Sagmariasus verreauxi during ontogeny. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 166, 191–198, https://doi.org/10.1016/j.cbpa.2013.06.003 (2013).
Dall, W. Indices of nutritional state in the western rock lobster, Panulirus longipes (Milne Edwards). I. Blood and tissue constituents and water content. J. Exp. Mar. Biol. Ecol. 16, 167–180 (1974).
Briceño, F. A., Polymeropoulos, E. T., Fitzgibbon, Q. P., Dambacher, J. M. & Pecl, G. T. Changes in metabolic rate of spiny lobster under predation risk. Mar. Ecol. Prog. Ser. 598, 71–84, https://doi.org/10.3354/meps12644 (2018).
Svendsen, M. B. S., Bushnell, P. G. & Steffensen, J. F. Design and setup of intermittent-flow respirometry system for aquatic organisms. J. Fish Biol. 88, 26–50, https://doi.org/10.1111/jfb.12797 (2016).
Fitzgibbon, Q. P., Jeffs, A. G. & Battaglene, S. C. The Achilles heel for spiny lobsters: the energetics of the non‐feeding post‐larval stage. Fish. Fish. 15, 312–326, https://doi.org/10.1111/faf.12018 (2014).
Battison, A. L. Use of the Brix value with cuticle indices to describe haemolymph biochemistry parameters in Homarus americanus H. Milne Edwards, 1837 (Decapoda: Malacostraca: Nephropidae). J. Crustac. Biol. 38, 625–634, https://doi.org/10.1093/jcbiol/ruy048 (2018).
Berry, S. E., Simon, C. J., Foote, A. R., Jerry, D. R. & Wade, N. M. Evaluation of baseline haemolymph biochemistry, volume and total body energetics to determine an accurate condition index in the black tiger shrimp, Penaeus monodon. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 228, 1–9, https://doi.org/10.1016/j.cbpb.2018.10.003 (2019).
Depledge, M. H. Photoplethysmography - a non-invasive technique for monitoring heart beat and ventilation rate in decapod crustaceans. Comp. Biochem. Physiol. A Physiol. 77, 369–371, https://doi.org/10.1016/0300-9629(84)90075-6 (1984).
Team, R. C. R: A language and environment for statistical computing. R Foundation for statistical computing, Vienna (2019).
Team, R. RStudio: integrated development environment for R. Boston, MA (2016).
Thomas, C. W., Crear, B. J. & Hart, P. R. The effect of temperature on survival, growth, feeding and metabolic activity of the southern rock lobster, Jasus edwardsii. Aquaculture 185, 73–84, https://doi.org/10.1016/S0044-8486(99)00341-5 (2000).
Acknowledgements
We would like to thank all staff at IMAS Taroona for their technical and logistical assistance, particularly Larnie Linton, Ross Goldsmid, Karl van Drunen, Craig Thomas, Alan Beech, Matthew Allen and Lisette Robertson. We further thank Samantha Twiname and Gretta Pecl for their manifold support and two anonymous reviewers whose comments helped improve and clarify this manuscript. We acknowledge the palawa, traditional custodians of lutruwita who have extensive knowledge of nubeena. MO was supported by a German Research Foundation fellowship OE 658/1-1. Tropical and sub-tropical lobsters were provided through collaboration with the Australian Research Council Industrial Transformation Research HUB (project number IH120100032).
Author information
Authors and Affiliations
Contributions
M.O. and A.J.R.H. conceived and conducted the experiments and processed the raw data. M.O. analysed the data and drafted the manuscript. Q.F. and G.S. provided animals, lab infrastructure and logistics. A.J.R.H. provided equipment for mitochondrial assays. All authors reviewed the data and all drafts of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Oellermann, M., Hickey, A.J.R., Fitzgibbon, Q.P. et al. Thermal sensitivity links to cellular cardiac decline in three spiny lobsters. Sci Rep 10, 202 (2020). https://doi.org/10.1038/s41598-019-56794-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-56794-0
This article is cited by
-
Metabolic plasticity improves lobster’s resilience to ocean warming but not to climate-driven novel species interactions
Scientific Reports (2022)
-
Effect of dietary protein on energy metabolism including protein synthesis in the spiny lobster Sagmariasus verreauxi
Scientific Reports (2021)
-
Coping with stress in a warming Gulf: the postlarval American lobster’s cellular stress response under future warming scenarios
Cell Stress and Chaperones (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.