Abstract
Multicellular organisms repair injured epithelium by evolutionarily conserved biological processes including activation of c-Jun N-terminal kinase (JNK) signaling. Here, we show in Drosophila imaginal epithelium that physical injury leads to the emergence of dying cells, which are extruded from the wounded tissue by JNK-induced Slit-Roundabout2 (Robo2) repulsive signaling. Reducing Slit-Robo2 signaling in the wounded tissue suppresses extrusion of dying cells and generates aberrant cells with highly upregulated growth factors Wingless (Wg) and Decapentaplegic (Dpp). The inappropriately elevated Wg and Dpp impairs wound repair, as halving one of these growth factor genes cancelled wound healing defects caused by Slit-Robo2 downregulation. Our data suggest that JNK-mediated Slit-Robo2 signaling contributes to epithelial wound repair by promoting extrusion of dying cells from the wounded tissue, which facilitates transient and appropriate induction of growth factors for proper wound healing.
Similar content being viewed by others
Introduction
Wound repair is an evolutionarily conserved process that maintains tissue homeostasis upon injury1,2,3. It has been reported that JNK signaling acts as an essential regulator of wound repair in Drosophila epithelial tissue4,5,6,7, planarians body8,9, and zebrafish tail fin10. Genetic studies in Drosophila have shown that JNK signaling contributes to (1) actin remodeling to close wound edges6,11, (2) reconstruction of lost tissue parts by activating growth promoters such as Yorkie (Yki, a YAP homolog)12,13, Wg (a Wnt homolog)14, Dpp (a TGF-β/BMP family member)15 and Myc14, (3) facilitating cell reprograming via reducing the activity of polycomb-dependent silencing16, and (4) induction of developmental delay by upregulating Drosophila insulin-like peptide 8 (Dilp8) to prolong the developmental period for recovery17. Particularly, JNK-dependent induction of Wg promotes regenerative growth of Drosophila wing imaginal discs after genetic ablation of the tissue14. In addition, JNK-mediated upregulation of Wg and Dpp plays critical roles in compensatory proliferation of imaginal cells after induction of massive cell death15,18,19. JNK signaling also induces apoptosis20,21, which is required for regeneration of planarian body9 or wound repair in Drosophila epithelial tissue22,23,24. Together, JNK regulates multiple steps of repair process from beginning to end.
Dying cells emerged in the epithelial tissue are extruded basally or apically by a coordinated mechanism25. For instance, overcrowding of cells within a limited space triggers extrusion of living or dying cells from Madin-Darby canine kidney (MDCK) epithelial monolayer26, developing zebrafish tail fin26, and Drosophila notum27. In Drosophila embryonic development, extrusion of apoptotic cells from amnioserosa promotes dorsal closure28,29, the process that shares common JNK-dependent events with epithelial wound repair, which include actin remodeling, cell migration, and epithelial zipping30,31. Similarly, JNK-dependent cell extrusion is required for tumor-suppressive cell competition, the process in which oncogenic polarity-deficient cells such as scribble (scrib) or discs large (dlg) mutant cells are actively eliminated from epithelia when surrounded by wild-type cells32,33,34,35,36,37. Importantly, extrusion of polarity-deficient cells by cell competition is driven by JNK-mediated activation of Slit-Robo2 axonal repulsive signaling that downregulates E-cadherin, as the ligand Slit, its receptor Robo2, and the downstream effector Enabled (Ena)/Vasp are all induced by JNK signaling35. During Drosophila embryonic development, the N-terminus of Slit produced from midline glial cells binds to the immunoglobulin (Ig) motif of Robo2 expressed in commissural axons, thereby regulating midline crossing of commissural axons via cell-cell repulsion38,39,40,41,42,43 and the system is well conserved throughout evolution44. Interestingly, it has been shown that extrusion of dying cells by Semaphorin-PlexinA axonal repulsive signaling is required for wound repair in Drosophila and zebrafish epithelia45, although the role of cell extrusion in wound repair and the upstream trigger for cell-extrusion signaling remain unknown.
Here, we found in Drosophila epithelium that physical injury induces JNK activation, which promotes extrusion of dying cells via Slit-Robo2 signaling. The Slit-Robo2-mediated cell extrusion facilitates epithelial wound repair by preventing excessive expression of growth factors Wg and Dpp upon injury.
Results and Discussion
Slit-Robo2 signaling acts downstream of JNK in wound repair
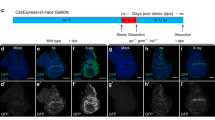
To dissect the mechanism of epithelial wound repair in Drosophila, we physically injured the wing imaginal disc, the larval epithelial primordia of adult wing. The right wing disc was injured with a tungsten needle by aseptic in situ wounding in living larvae without further damaging the animal (hereafter denoted as “wounded” disc), with the left wing disc remained undamaged as an internal control (hereafter denoted as “intact” disc) (Supplementary Fig. 1). Wounded wing discs were repaired during animal development and form essentially normal adult wings (Fig. 1a,a’, quantified in Fig. 1g). Blocking JNK signaling by knocking down Drosophila JNK basket (bsk) significantly impaired wound repair (Fig. 1b,b’, quantified in Fig. 1g), indicating that JNK signaling is essential for wound repair as reported previously4,5,6,7. In analyzing downstream effectors of JNK signaling, we found that Slit-Robo2 signaling, the cell-extrusion signaling activated by JNK during tumor-suppressive cell competition35, is required for wound repair. Downregulation of Slit or Robo2 by heterozygous deletion of these genes or by RNAi expression in the wing pouch significantly impaired wound repair (Fig. 1c’–f’, quantified in Fig. 1g) without affecting wing development in intact discs (Fig. 1c–f). Consistently, JNK activity and slit-lacZ expression were elevated around the wound at 6hrs after wounding, as visualized by the anti-Mmp146 and slit-lacZ reporter (Fig. 1h,i). The upregulations of Mmp1 and slit-lacZ expression were significantly suppressed by bsk-RNAi (Fig. 1j–k”, quantified in Supplementary Fig. 2). In addition, blocking Slit-Robo2 signaling did not exacerbate repair defect caused by bsk-RNAi (Supplementary Fig. 3a,b, quantified in Supplementary Fig. 3c), suggesting that JNK and Slit-Robo2 participate in wound healing process in the same pathway. These data suggest that Slit-Robo2 signaling acts downstream of JNK in wound repair. The wound-repair defect caused by blocking JNK was severer than blocking Slit-Robo2 (Fig. 1g), likely because JNK has multiple functions in wound repair.
Slit-Robo2 signaling acts downstream of JNK in wound repair. (a–f’) Control intact (a–f) and wounded (a’–f’) adult wings in each genotype taken from the same individuals. All pictures were taken at the same magnification. Scale bar, 500 µm. (g) Boxplot with dots representing wound repair (%) (see Methods) in each genotype (wild-type (n = 136), bsk-RNAi (n = 131), slit2/+ (n = 139), slit-RNAi (n = 113), robo22/+ (n = 154), and robo2-RNAi (n = 189)). Mann-Whitney U-test; *p < 0.05, **p < 0.01, and ***p < 0.001. (h–k”’) Wing discs of wild-type (h–i”’) and nub > bsk-RNAi (j–k”’) flies were dissected at 6hrs after wounding. Wing pouches were labeled with GFP using the nub-gal4 driver (green). JNK activity (magenta), slit-lacZ expression (white), and nuclei (cyan) were detected by anti-Mmp1, anti-β-gal (for slit-lacZ), and DAPI, respectively. Yellow dashed lines and arrowheads indicate locations of wounds. Red arrowheads indicate ectopic slit-lacZ expressions. Asterisks indicate the position of endogenous slit-lacZ expression. Scale bars, 50 µm. See Supplementary Information for detailed genotypes.
Slit-Robo2 signaling promotes extrusion of dying cells from the wounded tissue
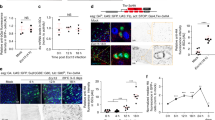
Our finding that Slit-Robo2 signaling plays a role in epithelial wound repair suggests that JNK-mediated cell extrusion is required for this process. We thus analyzed spatial locations of dying cells in the wounded tissue by immunostaining for the cleaved form of the effector caspase Dcp1 (c-Dcp1). In wild-type background, the number of dying cells in the wing pouch significantly increased at 6hrs after wounding (Supplementary Fig. 4a,b, quantified in Supplementary Fig. 4i). Importantly, the number of dying cells within the disc was 4-fold higher at the earlier time point (Fig. 2g, 3hrs, quantified in Fig. 2j, compare to Fig. 2b, 6hrs, quantified in Fig. 2e), suggesting that dying cells are extruded from the tissue over time. Supporting this notion, the analysis of extruding/extruded dying cells in the wounded discs by classifying their locations into three classes (“in disc”, “apically extruding”, and “basally extruding”; Fig. 2a) revealed that the ratio of dying cells within the disc over basally/apically extruding cells was significantly reduced over time (compare Fig. 2k (3 hrs, wild-type), Fig. 2f (6 hrs, wild-type), and Supplementary Fig. 5e,l (9 hrs, wild-type)). Crucially, heterozygous deletion of slit or robo2 gene significantly increased the number and ratio of dying cells remained in the wounded disc at 6hrs (Fig. 2c,d, quantified in Fig. 2e,f) and 9hrs (Supplementary Fig. 5f,g, quantified in Supplementary Fig. 5l) after wounding, while the tendency was not observed at 3hrs after wounding likely because extrusion has not proceeded sufficiently at this time point even in the wild-type tissue (Supplementary Fig. 5a,b, quantified in Supplementary Fig. 5j).
Slit-Robo2 promotes extrusion of dying cells from the wounded tissue. (a) Diagram of the analysis for dying cell locations in the cross-section of the wing disc. See Methods for further details. (b–k) Images show xy and yz cross-sections of wounded wing discs of wild-type (b), slit2/+ (c), and robo22/+ (d) larvae dissected at 6hrs after wounding; xy and yz cross-sections of wild-type (g), nub > slit (h), and nub > robo2 (i) larvae dissected at 3hrs after wounding. Dying cells were detected by anti-c-Dcp1 staining (magenta) and wing pouches were marked with Venus using the nub-gal4 driver (cyan), and F-actin was visualized with Phalloidin (green). Yellow dashed lines indicate the positions of wounds. White dashed lines indicate the positions of yz cross-section shown in the right panel. The two-direction arrow indicates apical (A) and basal (B) sides of the disc. Asterisks in right panels indicate dying cells classified as “in disc” (white), “apically extruding” (yellow), and “basally extruding” (blue). Scale bars, 50 µm. Quantification of the number of dying cells classified as “in disc” at 6hrs after wounding in each genotype (e) (wild-type (n = 12), slit2/+ (n = 12), and robo22/+ (n = 12)); 3hrs after wounding in each genotype (j) (wild-type (n = 11), nub > slit (n = 12), and nub > robo2 (n = 12)). Welch’s T-test; mean ± s.d.; *p < 0.05 and **p < 0.01. (f,k) Quantification of the ratio of dying cells classified into 3 types (as shown in A) at 6hrs after wounding in each genotype (f) (wild-type (n = 12), slit2/+ (n = 12), and robo22/+ (n = 14)); 3hrs after wounding in each genotype (k) (wild-type (n = 17), nub > slit (n = 13), and nub > robo2 (n = 13)). Chi-squared test; ***p < 0.001; # the absolute value of adjusted residual >2.56. See Supplementary Information for detailed genotypes.
Conversely, overexpression of slit or robo2 in the wing pouch significantly decreased the number and ratio of dying cells in the disc at 3hrs (Fig. 2h,i, quantified in 2j, k) and 6hrs (Supplementary Fig. 5c,d, quantified in Supplementary Fig. 5k) after wounding, while the tendency was not observed at 9hrs after wounding likely because most cells have already been extruded by this time point even in the wild-type tissue (Supplementary Fig. 5h,i, quantified in Supplementary Fig. 5l). Together, these data indicate that JNK-induced Slit-Robo2 signaling promotes extrusion of dying cells from the injured tissue, which is essential for wound repair. Notably, blocking JNK signaling in the damaged wing pouch abolished apoptosis (as visualized by c-Dcp1 staining) but not necrosis (as visualized by Propidium Iodide (PI) staining) (Supplementary Fig. 4), indicating that apoptosis induction is also JNK-dependent as reported previously22,23,24.
Slit-Robo2-mediated extrusion of dying cells prevents excessive growth factor expressions in the wounded tissue
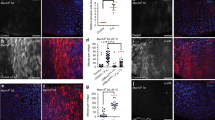
We next examined the role of Slit-Robo2-mediated extrusion of dying cells in wound repair. It has been shown that secreted growth factors Wg and Dpp are essential for regeneration of damaged epithelia in Drosophila14,15,18,19,47. Interestingly, we found that at 24hrs after wounding, aberrant dying cells with highly elevated Wg and Dpp expressions were emerged in slit2/+ or robo22/+ wounded wing discs (Fig. 3g,h,k,l) compared to wild-type background (Fig. 3c,d, quantified in Fig. 3m,n), while intact discs did not possess such aberrant cells (Fig. 3a,b,e,f,i,j, quantified in Fig. 3m,n). In addition, these aberrant cells frequently located nearby or within JNK-activated cells as visualized by TRE-DsRed reporter48 (Supplementary Fig. 6g (63.6%, n = 11), h (100%, n = 5), k (85.7%, n = 14), l (87.5%, n = 14)) compared to wild-type background (Supplementary Fig. 6c (30.8%, n = 13), d (22.2%, n = 9)). These data indicate that impaired Slit-Robo2 signaling in the wounded tissue results in the emergence of abnormal cells with excess production of Wg and Dpp, which may disturb the wound healing process. Indeed, downergulation of Wg or Dpp by halving the wg or dpp gene significantly suppressed the wound healing defects in slit2/+ or robo22/+ flies (Fig. 4b,c,e,f, quantified in Fig. 4g, and Supplementary Fig. 7b,c,e,f, h,i, quantified in Supplementary Fig. 7j), while heterozygosity for wg or dpp on its own did not affect wound repair (Fig. 4a,d, quantified in Fig. 4g, and Supplementary Fig. 7a,d,g, quantified in Supplementary Fig. 7j). In addition, halving the wg gene indeed suppressed the emergence of aberrant cells with excess Wg (Supplementary Fig. 8a–f, quantified in Supplementary Fig. 8g). These results suggest that the emergence of aberrant cells with excess growth factors is responsible for the repair defect. Together, our data indicate that JNK-induced Slit-Robo2 signaling contributes to wound repair by promoting extrusion of dying cells with excess growth factors (Fig. 5).
Defects in dying cell extrusion cause aberrant Wg and Dpp expression. (a–l”) xy cross-section images of intact and wounded wing discs of wild-type (a–d”), slit2/+ (e–h”), and robo22/+ (i–l”) larvae at 24hrs after wounding. Dying cells were detected with anti-c-Dcp1 antibody (magenta). Wg and Dpp expressions were detected using anti-Wg antibody and anti-β-gal antibody (for dpp-lacZ), respectively (green). Yellow arrowheads indicate representative dying cells expressing aberrantly high levels of Wg or Dpp. Asterisks indicate endogenous signals. Scale bars, 50 µm. (m,n) Boxplot with dots representing normalized intensity of anti-Wg in c-Dcp1-positive cells (see Methods) in each genotype (wild-type (intact: n = 12, wounded: n = 11), slit2/+ (intact: n = 16, wounded: n = 17), and robo22/+ (intact: n = 15, wounded: n = 13)). (m) Boxplot with dots representing normalized intensity of anti-β-gal antibody (for dpp-lacZ) in c-Dcp1-positive cells (see Methods) at 24hrs after wounding in each genotype (wild-type (intact: n = 9, wounded: n = 11), slit2/+ (intact: n = 5, wounded: n = 6), and robo22/+ (intact: n = 12, wounded: n = 10)). (n) Mann-Whitney U-test; *p < 0.05, **p < 0.01, ***p < 0.001; n.s.; not significant. See Supplementary Information for detailed genotypes.
wg and dpp mutants could rescue Slit-Robo2 defect. (a–f’) Control intact (a–f) and wounded (a’–f’) adult wings in each genotype taken from the same individuals after wounding. All pictures were taken at the same magnification. Scale bar, 500 µm. (g) Boxplot with dots representing wound repair (%) (see Methods for details) in each genotype (wild-type (n = 138), wg1/+ (n = 89), dppd6/+ (n = 64), slit2/+ (n = 139), slit2/+, wg1/+ (n = 105), slit2/+, dppd6/+ (n = 77), robo22/+ (n = 154), robo22/+, wg1/+ (n = 128), and robo22/+, dppd6/+ (n = 95)) Mann-Whitney U-test; *p < 0.05, **p < 0.01, ***p < 0.001; n.s.; not significant. See Supplementary Information for detailed genotypes.
Intriguingly, it has been reported that an axon guidance molecule PlexinA plays an important role in cell extrusion during epithelial wound repair in Drosophila and zebrafish45. In addition, Slit has been proposed to bind to PlexinA in mammals49, suggesting that multiple axon guidance signaling contribute to wound healing by promoting dying cell extrusion. Our findings suggest that dying cells remained in the tissue with excess growth factors need to be removed for proper wound healing, by promoting epithelial fusion and/or facilitating transient and appropriate production of growth factors.
Methods
Fly strains
Flies were cultured with standard food in plastic vials at 25 °C. 3rd instar wandering larvae were analyzed in all the experiments. Fly strains are used as follows: nub-gal4 (Bloomington Drosophila Stock Center [BDSC] #42699), rn-gal4GAL4-5 (BDSC #7405), UAS-CD8-PARP-Venus (gift from Yasushi Hiromi)50, UAS-bsk-RNAi (National Institute of Genetics [NIG] #5680R-2), slit2 (Drosophila Genomics and Genetic Resources [DGGR] #106948)51, UAS-slit-RNAi (BDSC #31468), robo22 (DGGR #106843)42, UAS-robo2-RNAi (BDSC #34589), slit05248 (slit-lacZ, BDSC #12189)52,53, UAS-slit (gift from Tom Kidd), UAS-robo2-HA (gift from Talia Volk), wg1 (BDSC #2978)54, wgSP-1 (BDSC #405)55,56, dppd6 (DGGR #106644)57, dpps11 (DGGR #106646)57, dpphr92 (DGGR #106649)58, TRE-DsRed (BDSC #59012)48, P{PZ}dpp10638 (dpp-lacZ, BDSC #12379)52.
Physical in situ wounding
3rd instar wandering larvae were randomly collected and anesthetized with ice-water for around 10 minutes. Then their wing discs (which were marked by fluorescent proteins GFP or Venus) were injured on ice with a sharpened 0.3 mm tungsten needle by performing aseptic in situ wounding (by pushing the wing pouch region using the needle) in living larvae without further damaging the animal. Wounding was performed under the fluorescence binocular microscope. After wounding, larvae were cultured in fresh food vials and kept at 25 °C again. Late 3rd instar larvae before wondering were wounded only when we analyzed wing discs 24 hrs after wounding. See Supplementary Fig. 1 for further information.
Histology
Larval tissues were fixed and stained using standard immunohistochemical methods with rabbit anti-cleaved-Dcp1 (1:100, Cell Signaling Technology), chicken anti-β-galactosidase (1:2500, abcam), mouse anti-Mmp1 (1:100, from cocktail of 3A6B4, 3B8D12 and 5H7B11, Developmental Studies Hybridoma Bank [DSHB]), mouse anti-Wingless (1:100, DSHB), anti-mouse Alexa 546, 647 (1:250, Molecular Probes), anti-rabbit Alexa 546, 647 (1:250, Molecular Probes), anti-chicken Alexa 647 (1:250, Molecular Probes), and were mounted with 49,6-diamidino-2-phenylindole (DAPI)-containing SlowFade Gold Antifade Reagent (Molecular Probes). For detecting necrotic cells, larval tissues were dissected in Schnieder’s Drosophila medium containing 5% fetal bovine serum (FBS) and were immediately moved into fresh medium containing Propidium Iodide (PI; 1:1000, Wako Pure Chemical Industries), then tissues were analyzed after three times washing with phosphate buffered saline (PBS). Images were taken with Leica TCS SP8 confocal microscopes with Leica Application Suite X ver. 2.0.1.14392 (Leica Microsystems).
Measurement of wing size
The right and left wings of adult flies were mounted on slide glasses. Leica binocular stereo microscope with LEICA FIRECAM ver. 3.4 (Leica Microsystems) was used to take pictures of the wings and whole bodies of adult flies. The wing size was automatically measured by Fiji ver. 2.0.0-rc-49/1.51k (https://imagej.net/Fiji). “Wing repair (%)” was defined as injured right wing area divided by intact left wing area (%), calculated by Microsoft Excel for Mac ver. 16.16.4 (Microsoft).
Analysis of dying cells
Apoptotic dying cells were detected by c-Dcp1 antibody. For the analysis of spatial locations of c-Dcp1-positive dying cells, the locations were classified into 3 classes: (1) “basally extruding”, as dying cells located at the basal tip of the disc proper, (2) “apically extruding”, as dying cells located at the apical tip of the disc proper, and (3) “in disc”, as dying cells located within the disc proper (see Fig. 2a for further information). Wing discs with wound that crosses center of the wing pouches were analyzed for special locations of c-Dcp1-positive cells. c-Dcp1-positive cells classified as “in disc” were manually counted using xz or yz cross-section images, and pouch areas were manually measured with Fiji and calculated with Microsoft Excel for Mac. For the analysis of total number of c-Dcp1-positive cells in the pouch, the number of c-Dcp1-positive cells in the wing pouch and the size of the wing pouch areas were automatically counted using Z-stacked images with Fiji and calculated with Microsoft Excel for Mac. For the analysis of necrotic dying cells detected by PI staining, the number of PI-positive cells in the wing pouch were automatically counted using single xy cross-section images with Fiji and calculated with Microsoft Excel for Mac.
Quantifications of signal intensities
To analyze expression levels of slit-lacZ or Mmp1, the signal intensity of anti-β-gal or anti-Mmp1 staining at the wounded area was measured and normalized with background intensity in the intact notum area. For the analysis of Wg or Dpp expression by anti-Wg or anti-β-gal antibody, the signal intensity in c-Dcp1-positive cells (other than endogenous Wg and Dpp) was measured and normalized with background intensity in the intact pouch area. All measurements were performed with Fiji and calculated with Microsoft Excel for Mac.
Statistical analysis
All experiments were repeated at least three times. Mann-Whitney non-parametric test was used for analyzing adult wing sizes and signal intensity, Welch’s T-test was used for analyzing the number of dying cells, and chi-squared test was used for analyzing spatial locations of dying cells. Error bars in all bar graphs indicate standard deviation (s.d.). n.s.; not significant indicates p ≧ 0.05 or the absolute value of adjusted residual ≦2.56. All bar graphs and stacked graphs were prepared with Microsoft Excel for Mac. All boxplot graphs include the data of all individuals as dots and were prepared with R ver. 3.2.3 (https://www.r-project.org).
References
Poss, K. D. Advances in understanding tissue regenerative capacity and mechanisms in animals. Nat. Rev. Genet. 11, 710–722 (2010).
Tanaka, E. M. & Reddien, P. W. The Cellular Basis for Animal Regeneration. Dev. Cell 21, 172–185 (2011).
Belacortu, Y. & Paricio, N. Drosophila as a model of wound healing and tissue regeneration in vertebrates. Developmental Dynamics 240, 2379–2404 (2011).
Rämet, M., Lanot, R., Zachary, D. & Manfruelli, P. JNK signaling pathway is required for efficient wound healing in Drosophila. Dev. Biol. 241, 145–56 (2002).
Galko, M. J. & Krasnow, M. A. Cellular and genetic analysis of wound healing in Drosophila larvae. PLoS Biol. 2, E239 (2004).
Bosch, M., Serras, F., Martín-Blanco, E. & Baguñà, J. JNK signaling pathway required for wound healing in regenerating Drosophila wing imaginal discs. Dev. Biol. 280, 73–86 (2005).
Hariharan, I. K. & Serras, F. Imaginal disc regeneration takes flight. Curr. Opin. Cell Biol. 48, 10–16 (2017).
Tasaki, J., Shibata, N., Sakurai, T., Agata, K. & Umesono, Y. Role of c-Jun N-terminal kinase activation in blastema formation during planarian regeneration. Dev. Growth Differ. 53, 389–400 (2011).
Almuedo-Castillo, M. et al. JNK Controls the Onset of Mitosis in Planarian Stem Cells and Triggers Apoptotic Cell Death Required for Regeneration and Remodeling. PLoS Genet. 10, e1004400 (2014).
Ishida, T., Nakajima, T., Kudo, A. & Kawakami, A. Phosphorylation of Junb family proteins by the Jun N-terminal kinase supports tissue regeneration in zebrafish. Dev. Biol. 340, 468–479 (2010).
Mattila, J., Omelyanchuk, L., Kyttälä, S., Turunen, H. & Nokkala, S. Role of Jun N-terminal Kinase (JNK) signaling in the wound healing and regeneration of a Drosophila melanogaster wing imaginal disc. Int. J. Dev. Biol. 49, 391–9 (2005).
Grusche, Fa, Degoutin, J. L., Richardson, H. E. & Harvey, K. F. The Salvador/Warts/Hippo pathway controls regenerative tissue growth in Drosophila melanogaster. Dev. Biol. 350, 255–66 (2011).
Tsai, C. R., Anderson, A. E., Burra, S., Jo, J. & Galko, M. J. Yorkie regulates epidermal wound healing in Drosophila larvae independently of cell proliferation and apoptosis. Dev. Biol. 427, 61–71 (2017).
Smith-Bolton, R. K., Worley, M. I., Kanda, H. & Hariharan, I. K. Regenerative Growth in Drosophila Imaginal Discs Is Regulated by Wingless and Myc. Dev. Cell 16, 797–809 (2009).
Perez-Garijo, A., Shlevkov, E. & Morata, G. The role of Dpp and Wg in compensatory proliferation and in the formation of hyperplastic overgrowths caused by apoptotic cells in the Drosophila wing disc. Development 136, 1169–1177 (2009).
Lee, N., Maurange, C., Ringrose, L. & Paro, R. Suppression of Polycomb group proteins by JNK signalling induces transdetermination in Drosophila imaginal discs. Nature 438, 234–237 (2005).
Katsuyama, T., Comoglio, F., Seimiya, M., Cabuy, E. & Paro, R. During Drosophila disc regeneration, JAK/STAT coordinates cell proliferation with Dilp8-mediated developmental delay. Proc. Natl. Acad. Sci. USA 112, E2327–36 (2015).
Perez-Garijo, A., Martín, F. A. & Morata, G. Caspase inhibition during apoptosis causes abnormal signalling and developmental aberrations in Drosophila. Development 131, 5591–5598 (2004).
Ryoo, H. D., Gorenc, T. & Steller, H. Apoptotic Cells Can Induce Compensatory Cell Proliferation through the JNK and the Wingless Signaling Pathways. Dev. Cell 7, 491–501 (2004).
Dhanasekaran, D. N. & Reddy, E. P. JNK signaling in apoptosis. Oncogene 27, 6245–6251 (2008).
Igaki, T. Correcting developmental errors by apoptosis: Lessons from Drosophila JNK signaling. Apoptosis 14, 1021–1028 (2009).
Ryoo, H. D. & Bergmann, A. The role of apoptosis-induced proliferation for regeneration and cancer. Cold Spring Harb. Perspect. Biol. 4, 1–18 (2012).
Shlevkov, E. & Morata, G. A dp53/JNK-dependant feedback amplification loop is essential for the apoptotic response to stress in Drosophila. Cell Death Differ. 19, 451–460 (2012).
Diaz-Garcia, S., Ahmed, S. & Baonza, A. Analysis of the function of apoptosis during imaginal wing disc regeneration in Drosophila melanogaster. PLoS One 11, e0165554 (2016).
Ohsawa, S., Vaughen, J. & Igaki, T. Cell Extrusion: A Stress-Responsive Force for Good or Evil in Epithelial Homeostasis. Dev. Cell 44, 284–296 (2018).
Eisenhoffer, G. T. et al. Crowding induces live cell extrusion to maintain homeostatic cell numbers in epithelia. Nature 484, 546–549 (2012).
Marinari, E. et al. Live-cell delamination counterbalances epithelial growth to limit tissue overcrowding. Nature 484, 542–545 (2012).
Toyama, Y., Xg, P., Ar, W., Dp, K. & Gs, E. Apoptotic force and tissue dynamics during Drosophila embryogenesis. Science. 321, 1683–1686 (2008).
Muliyil, S., Krishnakumar, P. & Narasimha, M. Spatial, temporal and molecular hierarchies in the link between death, delamination and dorsal closure. Development 138, 3043–3054 (2011).
Jacinto, A., Martinez-Arias, A. & Martin, P. Mechanisms of epithelial fusion and repair. Nat. Cell Biol. 3, E117–E123 (2001).
Hayes, P. & Solon, J. Drosophila dorsal closure: An orchestra of forces to zip shut the embryo. Mech. Dev. 144, 2–10 (2017).
Igaki, T., Pagliarini, R. A. & Xu, T. Loss of Cell Polarity Drives Tumor Growth and Invasion through JNK Activation in Drosophila. Curr. Biol. 16, 1139–1146 (2006).
Igaki, T., Pastor-Pareja, J. C., Aonuma, H., Miura, M. & Xu, T. Intrinsic tumor suppression and epithelial maintenance by endocytic activation of Eiger/TNF signaling in Drosophila. Dev. Cell 16, 458–65 (2009).
Ohsawa, S. et al. Elimination of oncogenic neighbors by JNK-mediated engulfment in Drosophila. Dev. Cell 20, 315–28 (2011).
Vaughen, J. & Igaki, T. Slit-Robo Repulsive Signaling Extrudes Tumorigenic Cells from Epithelia. Dev. Cell 39, 683–695 (2016).
Yamamoto, M., Ohsawa, S., Kunimasa, K. & Igaki, T. The ligand Sas and its receptor PTP10D drive tumour-suppressive cell competition. Nature 542, 246–250 (2017).
Katsukawa, M., Ohsawa, S., Zhang, L., Yan, Y. & Igaki, T. Serpin Facilitates Tumor-Suppressive Cell Competition by Blocking Toll-Mediated Yki Activation in Drosophila. Curr. Biol. 28, 1756–1767.e6 (2018).
Kidd, T., Bland, K. S. & Goodman, C. S. Slit is the midline repellent for the robo receptor in Drosophila. Cell 96, 785–794 (1999).
Kuan, H. W. et al. Biochemical purification of a mammalian slit protein as a positive regulator of sensory axon elongation and branching. Cell 96, 771–784 (1999).
Simpson, J. H., Bland, K. S., Fetter, R. D. & Goodman, C. S. Short-range and long-range guidance by Slit and its Robo receptors: A combinatorial code of Robo receptors controls lateral position. Cell 103, 1019–1032 (2000).
Simpson, J. H., Kidd, T., Bland, K. S. & Goodman, C. S. Short-range and long-range guidance by Slit and its robo receptors: Robo and Robo2 play distinct roles in midline guidance. Neuron 28, 753–766 (2000).
Rajagopalan, S., Vivancos, V., Nicolas, E. & Dickson, B. J. Selecting a longitudinal pathway: Robo receptors specify the lateral position of axons in the Drosophila CNS. Cell 103, 1033–1045 (2000).
Nguyen Ba-Charvet, K. T. et al. Diversity and specificity of actions of Slit2 proteolytic fragments in axon guidance. J. Neurosci. 21, 4281–4289 (2001).
Blockus, H. & Chédotal, A. Slit-robo signaling. Dev. 143, 3037–3044 (2016).
Yoo, S. K. et al. Plexins function in epithelial repair in both Drosophila and zebrafish. Nat. Commun. 7, 12282 (2016).
Uhlirova, M. & Bohmann, D. JNK- and Fos-regulated Mmp1 expression cooperates with Ras to induce invasive tumors in Drosophila. EMBO J. 25, 5294–5304 (2006).
Wells, B. S., Yoshida, E. & Johnston, L. A. Compensatory Proliferation in Drosophila Imaginal Discs Requires Dronc-Dependent p53 Activity. Curr. Biol. 16, 1606–1615 (2006).
Chatterjee, N. & Bohmann, D. A versatile φC31 based reporter system for measuring AP-1 and NRF2 signaling in Drosophila and in tissue culture. PLoS One 7, e34063 (2012).
Delloye-Bourgeois, C. et al. PlexinA1 is a new Slit receptor and mediates axon guidance function of Slit C-terminal fragments. Nat. Neurosci. 18, 36–45 (2015).
Williams, D. W., Kondo, S., Krzyzanowska, A., Hiromi, Y. & Truman, J. W. Local caspase activity directs engulfment of dendrites during pruning. Nat. Neurosci. 9, 1234–1236 (2006).
Nambu, J. R., Franks, R. G., Hu, S. & Crews, S. T. The single-minded gene of Drosophila is required for the expression of genes important for the development of CNS midline cells. Cell 63, 63–75 (1990).
Karpen, G. H. & Spradling, A. C. Analysis of subtelomeric heterochromatin in the Drosophila minichromosome Dp1187 by single P element insertional mutagenesis. Genetics 132, 737–753 (1992).
Tayler, T. D., Robichaux, M. B. & Garrity, P. A. Compartmentalization of visual centers in the Drosophila brain requires Slit and Robo proteins. Development 131, 5935–5945 (2004).
Sharma, R. P. Wingless a new mutant in Drosophila melanogaster. Drosophila Information Service 50, 134 (1973).
Lindsley, D. L. & Zimm, G. G. Genome Of Drosophila Melanogaster. Acad. Press. San Diego (1992).
Neumann, C. J. & Cohen, S. M. Sternopleural is a Regulatory Mutation of wingless With Both Dominant and Recessive Effects on Larval Development of Drosophila melanogaster. Genatics 142, 1147–1155 (1996).
St. Johnston, R. D. et al. Molecular organization of the glutathione reductase gene in Drosophila melanogaster. Genes Dev. 4, 1114–1127 (1990).
Wharton, K., Ray, R. P., Findley, S. D., Duncan, H. E. & Gelbart, W. M. Molecular lesions associated with alleles of decapentaplegic identify residues necessary for TGF-β/BMP cell signaling in Drosophila melanogaster. Genetics 142, 493–505 (1996).
Acknowledgements
We thank Igaki and Morata lab members for discussions; K. Baba, M. Tanaka, and M. Koijima for technical supports; B. Dickson, Y. Hiromi, T. Kidd, M. Miura, T. Volk, Bloomington Drosophila Stock Center (Indiana, USA), National Institute of Genetics Stock Center (Mishima, Japan), and Drosophila Genomics and Genetic Resources (Kyoto, Japan) for fly stocks. This work was supported in part by grants from the MEXT/JSPS KAKENHI (grant number 26114002, 15H05862, 16H02505, and 16K07378) to K.T., S.O., and T.I., the Nakajima Foundation to S.O., the Inoue Science Research Award to S.O., the Naito Foundation to T.I., the Takeda Science Foundation to S.O. and T.I., Japan Agency for Medical Research and Development (Project for Elucidating and Controlling Mechanisms of Aging and Longevity) to T.I., Inamori Fundation to S.O., Toray Science Foundation to S.O., Senri Life Science Foundation to S.O., JSPS Research Fellowships for Young Scientists to C.I., and JSPS Overseas Challenge Program for Young Researchers to C.I.
Author information
Authors and Affiliations
Contributions
C.I., S.O., K.T., M.Y., G.M. and T.I. designed experiments; C.I. performed all experiments; C.I., S.O., K.T., M.Y., G.M. and T.I. analyzed the data; C.I., S.O., K.T. and T.I. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Iida, C., Ohsawa, S., Taniguchi, K. et al. JNK-mediated Slit-Robo signaling facilitates epithelial wound repair by extruding dying cells. Sci Rep 9, 19549 (2019). https://doi.org/10.1038/s41598-019-56137-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-56137-z
This article is cited by
-
Epigenetic adaptations of the masticatory mucosa to periodontal inflammation
Clinical Epigenetics (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.