Abstract
Subjective tinnitus is an auditory phantom perceptual disorder without an objective biomarker. Bothersome tinnitus in single-sided deafness (SSD) is particularly challenging to treat because the deaf ear can no longer be stimulated by acoustic means. We contrasted an SSD cohort with bothersome tinnitus (TIN; N = 15) against an SSD cohort with no or non-bothersome tinnitus (NO TIN; N = 15) using resting-state functional magnetic resonance imaging (fMRI). All study participants had normal hearing in one ear and severe or profound hearing loss in the other. We evaluated corticostriatal functional connectivity differences by placing seeds in the caudate nucleus and Heschl’s Gyrus (HG) of both hemispheres. The TIN cohort showed increased functional connectivity between the left caudate and left HG, and left and right HG and the left caudate. Within the TIN cohort, functional connectivity between the right caudate and cuneus was correlated with the Tinnitus Functional Index (TFI) relaxation subscale. And, functional connectivity between the right caudate and superior lateral occipital cortex, and the right caudate and anterior supramarginal gyrus were correlated with the TFI control subscale. These findings support a striatal gating model of tinnitus and suggest tinnitus biomarkers to monitor treatment response and to target specific brain areas for innovative neuromodulation therapies.
Similar content being viewed by others
Introduction
Tinnitus is a common auditory phantom perceptual disorder where conventional audiometric hearing loss profiles alone cannot help clinicians to distinguish between patients who merely experience tinnitus from those who are troubled by tinnitus1,2. The search for physiological substrates that account for tinnitus persistence and tinnitus severity has led investigators to evaluate the central nervous system (CNS) using a variety of techniques. Some documented CNS changes are synchronous hyperactivity3,4,5, tonotopic map cortical plasticity6,7,8, thalamocortical dysrhythmia9,10, and gamma band oscillations11,12,13.
Human physiological studies14,15, case reports16,17, and an early clinical trial18 focused on the caudate nucleus of the basal ganglia support a striatal gating model15 of tinnitus awareness. This model delineates modulators of tinnitus persistence and tinnitus severity, where corticostriatal connections between the striatum and auditory cortex act to gate auditory phantom representations to reach perceptual awareness and connections between the striatum and limbic structures act to modulate auditory phantom distress.
A striatal gating model of phantom percept awareness is complementary to other CNS hypotheses, including those that posit tinnitus is primarily an expectation mismatch within the auditory system19,20 or is driven by abnormal auditory-limbic interactions21,22,23. Neuroimaging studies in support of abnormal striatal connectivity as a potential biomarker of chronic tinnitus24,25 have been reported in cohorts with inhomogeneous hearing loss profiles. Those studies used post-hoc statistical techniques to address the possible confound of hearing loss levels on neural correlates of tinnitus. However, there is not yet a neuroimaging investigation that incorporates a specific hearing loss pattern in a cohort contrast study design that could isolate differential network connectivity findings to chronic tinnitus.
Patients with bothersome tinnitus in single-sided deafness (SSD) or unilateral severe to profound hearing loss and normal or nearly normal thresholds in the only hearing ear represent an exceptional opportunity to study tinnitus not confounded by hearing loss variations. Bothersome tinnitus in adult acquired SSD is expected to be localized to the deaf ear2,26, although this is not necessarily the case in congenital SSD27. Tinnitus localized to the deaf ear eliminates the treatment option of masking sound delivery to the defective sensory end organ, as it is unresponsive to acoustic stimulation. Moreover, acoustic therapies (amplification, masking, customized acoustic stimuli) directed to the better hearing ear are of minimal to no benefit28,29,30. Behavioral therapies (Tinnitus Retraining and Cognitive Behavioral) may be beneficial in modulating tinnitus distress, but without effective sound therapy to the deaf ear, have little to no effect on tinnitus loudness31,32. Neuromodulation of the auditory periphery by cochlear implantation of the deaf ear, an alternative method of auditory system stimulation, often reduces tinnitus severity similar to acoustic therapies in an ear with hearing loss33,34,35,36. However, this intervention requires surgical implantation of hardware to the skull and complicates future head magnetic resonance imaging (MRI) examinations.
The goal of this study was to identify candidate biomarkers to monitor tinnitus treatment response and targets for brain-based neuromodulation approaches. We evaluated basal ganglia and cortical connectivity patterns by contrasting an SSD cohort with bothersome tinnitus (TIN) against an SSD cohort no or non-bothersome tinnitus (NO TIN). We used resting-state functional magnetic resonance imaging (fMRI) to define whole-brain connectivity patterns of the caudate nucleus and auditory cortex. We report on connectivity differences between TIN and NO TIN cohorts, and voxelwise connectivity strength correlations with subscale scores of the validated Tinnitus Functional Index (TFI)37 within the TIN cohort.
Results
Demographics and audiometrics
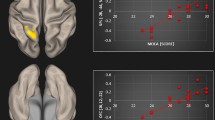
Data on TFI score, age, sex, deafness duration, diagnosis of vestibular schwannoma, and deaf ear laterality for SSD TIN and SSD NO TIN cohorts are shown in Table 1. Descriptive statistics, using mean (standard error) and ratio conventions were computed for each cohort. The two cohorts differed only in TFI score (t-test, p < 0.001), an expected result of the study design; all other comparisons were not significantly different. Pure tone audiometric thresholds for low, middle, and high frequency bands of the normal and deaf ears were not significantly different for the two cohorts (Fig. 1). The mean (standard error) of the three frequency bands were as follows: Low (normal ear): TIN = 9.1 (2.3), NO TIN = 10.3 (1.5); Low (deaf ear): TIN = 78.5 (3.3), NO TIN = 89.5 (5.2). Middle (normal ear): TIN = 11.0 (2.2), NO TIN = 11.0 (1.8); Middle (deaf ear): TIN = 86.3 (5.3), NO TIN = 98.6 (2.3). High (normal ear): TIN = 15.9 (2.5), NO TIN = 15.8 (3.1); High (deaf ear): TIN = 91.8 (3.0), NO TIN = 97.7 (2.3). When thresholds exceeded the limits of the audiometer, the limit of the equipment (100–110 dB) was used for analysis.
Audiometric threshold Tukey boxplots of low frequency (average of 250 and 500 Hz), middle frequency (average of 1000 and 2000 Hz) and high frequency (average of 4000, 6000 and 8000 Hz) bands of normal (circle) and deaf (square) ears for both cohorts show no significant differences across all bands. TIN – bothersome tinnitus. NO TIN - no or non-bothersome tinnitus (NO TIN).
Bothersome vs non-bothersome tinnitus
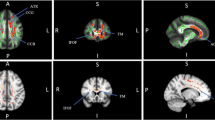
Resting-state functional connectivity maps were reconstructed for TIN and NO TIN cohorts for the four seed regions (Heschl’s gyrus (HG), and caudate, bilaterally). Functional connectivity seeded from the left caudate was significant with the right caudate, and with multiple regions of pre-frontal cortex (PFC) in both TIN and NO TIN cohorts (Fig. 2A,B). The TIN cohort exhibited increased functional connectivity between the left caudate and a region of the left HG/insula, and between the left caudate and the right supplementary motor area (Fig. 2C). There were no differences between the two cohorts in functional connectivity for the other three seed regions (right caudate, right HG, and left HG). Table 2 lists the centroid coordinates of regions with increased functional connectivity referenced to ROI seeds, in compliance with reporting standards for neuroimaging studies.
Functional connectivity of the left caudate in both cohorts. (A) TIN within-group connectivity. (B) NO TIN within-group connectivity. (C) TIN > NO TIN connectivity differences. The TIN cohort exhibits increased functional connectivity from the left caudate to left HG (corticostriatal) and right supplementary motor area. The color bar represents the t-statistic of differences in functional connectivity within (A,B) or between (C) cohorts. Positive values (red colors) indicate increased connectivity and negative values (blue colors) indicate decreased connectivity. HG – Heschl’s gyrus.
TFI subscale correlation with functional connectivity
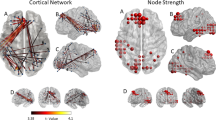
Within the TIN cohort, voxelwise correlations with TFI subscales showed increased connectivity between the right caudate and cuneus for the relaxation subscale, where increased connectivity was correlated with higher interference with the ability to relax (Fig. 3). Voxelwise correlations analysis also showed increased connectivity between the right caudate and superior lateral occipital cortex and the right caudate and anterior supramarginal gyrus for the control subscale, where increased connectivity was correlated with the sense of reduced control over the tinnitus percept (Fig. 3). No other TFI subscale scores showed statistically significant correlations with right caudate connectivity. Table 3 lists centroid coordinates of brain areas with increased connectivity to the right caudate for the relaxation and control TFI subscales.
Within the TIN cohort, connectivity strength (regression t-score) between the caudate nucleus and other nonauditory structures is correlated with a specific TFI subscale score. (A) Right caudate nucleus to cuneus connectivity is significantly correlated with relaxation difficulty due to tinnitus. (B,C) Right caudate nucleus to superior lateral occipital cortex (sLOC) and to anterior supramarginal gyrus (aSMG) is correlated with sense of reduced control over tinnitus. Yellow indicates significantly increased connectivity. TFI – Tinnitus Functional Index.
Discussion
The key finding of this study is increased connectivity between the caudate nucleus and auditory cortex in the SSD cohort with chronic, bothersome tinnitus. All patients relied on monaural hearing with normal audiometric thresholds, thereby removing hearing loss variation as a possible confound and isolating abnormal striatal functional connectivity to chronic tinnitus. This independent replication of the critical finding in our prior studies, where tinnitus cohorts had variable hearing loss profiles, further supports a striatal gating model of tinnitus awareness where the limbic system may be driving tinnitus severity18. The striatum is believed to be normally restrictive, blocking out phantom percepts, but becomes dysfunctionally permissive in chronic tinnitus. Initial evidence in support of this model stems from results of acute direct stimulation of the striatum at the junction of the head and body subdivisions (area LC) during deep brain stimulation (DBS) surgery in movement disorders patients with chronic tinnitus, where auditory phantom loudness can be modulated14. In those without tinnitus, caudate DBS can trigger auditory phantom percepts15 and vascular infarction of the dorsal striatum results in enduring tinnitus loudness reduction16. Furthermore, chronic caudate DBS has been shown to significantly improve TFI in some patients with severe, chronic tinnitus18. Although the exact physiological mechanisms are not clear, alteration of excitation and inhibition balance either within the caudate nucleus or in its connections to auditory cortex may be modulating phantom percept gating permissiveness38,39. Findings from this study extend generalizability of results from human neurophysiological14,15,18 and resting-state fMRI24,25 studies in binaural patients.
Other tinnitus neuroimaging studies using EEG methodologies have yielded interesting insights that are relevant to our findings. Tinnitus severity response to Tinnitus Retraining Therapy40, as measured by the Tinnitus Handicap Inventory41 (THI) score, is positively correlated with pre-treatment activities of the left insula42. Increased functional connectivity between the caudate and the insula observed in this study suggests that the left insula may be an important structure within corticostriatal loops that link the auditory system with language networks and the limbic system43,44. Tinnitus awareness burden is negatively correlated with delta band activity of rostral and dorsal anterior cingulate cortices. Those areas are considered to be at the core of a descending noise cancellation system whose dysfunction may contribute to the percentage of daytime tinnitus awareness45. Increased striatal functional connectivity between the left caudate and the right supplementary motor area (SMA) observed in this study may possibly include neighboring dorso-rostral anterior cingulate cortices, thereby contributing to overall tinnitus distress.
The other key finding is significant relationships between right caudate connectivity with non-auditory brain regions and TFI subscale scores. Increased connectivity with the cuneus of the default mode network is correlated with increased difficulty to relax, indicating heightened introspection of the auditory phantom24. Increased connectivity with the superior lateral occipital cortex and anterior supramarginal gyrus is correlated with reduced sense of control over the phantom percept, regions that are part of task-positive visual and dorsal attention networks, suggesting enhanced attentional engagement for control of tinnitus46. Although the right caudate connectivity shows significant correlation with TFI subscale scores, it did not survive across cohort differences in functional connectivity, most likely due to the underlying small sample size. Nevertheless, the highest connectivity of the right caudate is with the left caudate (Fig. 2A), suggesting comparable functionality across the two caudate nuclei.
Abnormal brain regions identified by resting-state fMRI in this report may serve as biomarkers of tinnitus treatment response or serve as targets for brain-based neuromodulation approaches to mitigate troublesome tinnitus in SSD. For biomarker validation, auditory perceptual training may be adapted for clinical deployment to mitigate tinnitus47. Treatment response may be expressed as change in pre-treatment functional connectivity between the striatum and other brain regions, consistent with observations in the insula and anterior cingulate cortices42,48. For biomarker targeted brain-based neuromodulation, innovative treatments to disrupt increased corticostriatal functional connectivity49,50 may be considered. Techniques include caudate nucleus neuromodulation by direct electrical stimulation14,15, MR-guided high intensity focused ultrasound51, transcranial stimulation43,52 and gamma knife radiosurgery53,54.
There are several limitations to this study. Foam earplugs with a noise reduction rating of 32 dB help to mitigate MRI scanner noise, with a spectrum estimated to have a maximum frequency of 1.4 kHz and a peak amplitude of 131 dB over a 10 ms time window55. Nonetheless, tinnitus percepts could have been partially masked during data acquisition by noise delivered to the only hearing ear56,57. The sample size consists of 15 participants in each cohort that are well matched for gender, age, handedness, degree of hearing loss, and duration of deafness, but with heterogeneity in tinnitus laterality. The relatively small numbers in each cohort and inhomogeneous tinnitus lateralization may be contributory factors to our inability to observe increased right caudate connectivity with auditory cortex and left caudate connectivity with non-auditory brain regions that correlate with TFI subscales. The study design uses a conservative TFI cut-off ≥13 to partition the two cohorts, ensuring NO TIN cohort participants do not have bothersome tinnitus37. However, this procedure contributes to TFI score heterogeneity in both cohorts. A future study with larger numbers of participants controlled for tinnitus laterality, and absolute adherence to no tinnitus whatsoever for the NO TIN cohort and the lower bound score of TFI-defined intervals that categorize tinnitus severity58 for the TIN cohort would be ideal, subject to participant accrual success.
In conclusion, adults with bothersome tinnitus in acquired SSD exhibited increased functional connectivity between the caudate and auditory cortex, adding evidence to support a striatal gating model of tinnitus, where a dysfunctionally permissive caudate nucleus enables auditory phantoms to reach perceptual awareness. The strength of functional connectivity between the caudate and cuneus was correlated with higher interference with the ability to relax. And the strength of connectivity between the caudate and superior lateral occipital cortex, and the caudate and anterior supramarginal gyrus was correlated with the perception of reduced control over the tinnitus percept. Together, these findings suggest that corticostriatal functional connectivity changes in bothersome tinnitus in SSD may serve as biomarkers to monitor treatment response and suggest candidate targets to develop innovative brain-based neuromodulatory approaches to mitigate tinnitus severity.
Methods
Study participants
All 30 study participants had normal hearing in one ear and severe or profound hearing loss in the other. There were 15 TIN and 15 NO TIN participants, where a TFI score ≥13 was the cutoff for bothersome tinnitus or more than “not a problem” in accordance with severity categorization37,58. TIN participants had chronic (≥1 year), constant, non-pulsatile tinnitus (Table 1). Participants were recruited from Otolaryngology-Head and Neck Surgery and Audiology clinics affiliated with the University of California, San Francisco (UCSF) and a regional chapter of the Acoustic Neuroma Association. All participants completed the TFI instrument and a demographic questionnaire, and underwent standard clinical audiometry to measure pure tone thresholds. A TFI score of zero indicated no tinnitus whatsoever. All participants with tinnitus localized their auditory phantom to the deaf ear. Audiometric thresholds for low (250 and 500 Hz), middle (1000 and 2000 Hz) and high (4000, 6000 and 8000 Hz) frequency bands were averaged separately to assess hearing loss level for each ear. All participants gave written informed consent. The University of California San Francisco Institutional Review Board approved all study procedures (IRB# 13-10587) and experiments were conducted in accordance with the Declaration of Helsinki.
Tinnitus severity
The TFI total score and subscales scores were calculated for each participant, and subscale scores were normalized to 100 for subsequent correlation analyses with functional connectivity. The eight TFI subscales address intrusiveness of tinnitus, control over the phantom percept, cognitive interference, sleep disturbance, auditory difficulties, interference with relaxation, quality of life, and emotional distress37.
MRI data acquisition
Imaging data were acquired using a 3-Tesla MRI scanner (Discovery MR750, GE Medical system, Waukesha, WI) to collect both high-resolution structural T1-weighted fast spoiled gradient echo brain volume images (120 axial slices, field of view = 512 × 512 mm, repetition time = 7,232 ms, echo time = 2.78 ms, in-plane voxel dimensions 0.5 × 0.5 mm, slice thickness = 1.5 mm) and to collect spontaneous fMRI data using a resting-state echo planar imaging (EPI) sequence (1.88 × 1.88 mm, 3.0 mm slice thickness, repetition time = 2000 ms, echo time = 28 ms, 100 repetitions). Foam earplugs with a 32 dB noise reduction rating were inserted into both ears during data acquisition.
Data preprocessing
Resting-state fMRI data were spatially preprocessed using a standardized pipeline implemented in the CONN toolbox. Imaging data preprocessing steps were as follows: functional realignment and unwarping, translation to center, outlier detection using Artifact Detection Tools (https://www.nitrc.org/projects/artifact_detect/), tissue segmentation, spatial normalization to the Montreal Neurological Institute (MNI) template, and spatial smoothing (using a 8 mm FWHM kernel). Prior to functional connectivity analyses, resting-state data were temporally filtered (0.008Hz-0.09 Hz bandpass) and denoised by applying a regression model using 12 realignment parameters and the global mean signal of the white matter.
Functional connectivity analyses and group statistics
All functional connectivity and group analyses were performed using the CONN toolbox. For resting-state functional connectivity, four seed regions (right/left Heschl’s gyrus (HG); right/left caudate) were anatomically defined using AAL labelled regions59 as implemented in the CONN toolbox. Correlation coefficients were computed across all voxels of these pre-defined regions of interest (ROIs) with the rest of the brain. Voxelwise regression analyses were performed only within the TIN cohort (n = 15) between functional connectivity and TFI subscale scores. Voxelwise analyses within and between groups (TIN versus NO TIN) were performed using parametric one-tailed t-tests for determining regions with increased and decreased connectivity separately for each of the four seeds. We report on within and across-group whole-brain analyses that survived a threshold of p < 0.05 following a false discovery rate based cluster-mass correction for multiple comparisons60.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request, subject to University of California applicable data release policies, rules, and regulations.
Change history
05 February 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41598-021-83101-7
References
Coles, R. R. Epidemiology of tinnitus: (1) prevalence. The Journal of laryngology and otology 9, 7–15 (1984).
Tsai, B. S., Sweetow, R. W. & Cheung, S. W. Audiometric asymmetry and tinnitus laterality. The Laryngoscope 122, 1148–1153, https://doi.org/10.1002/lary.23242 (2012).
Chen, G. D. & Jastreboff, P. J. Salicylate-induced abnormal activity in the inferior colliculus of rats. Hearing research 82, 158–178 (1995).
Kaltenbach, J. A. Summary of evidence pointing to a role of the dorsal cochlear nucleus in the etiology of tinnitus. Acta oto-laryngologica 20–26 (2006).
Norena, A. J. & Eggermont, J. J. Changes in spontaneous neural activity immediately after an acoustic trauma: implications for neural correlates of tinnitus. Hearing research 183, 137–153 (2003).
Komiya, H. & Eggermont, J. J. Spontaneous firing activity of cortical neurons in adult cats with reorganized tonotopic map following pure-tone trauma. Acta Otolaryngol 120, 750–756 (2000).
Roberts, L. E. et al. Ringing ears: the neuroscience of tinnitus. The Journal of neuroscience: the official journal of the Society for Neuroscience 30, 14972–14979, https://doi.org/10.1523/JNEUROSCI.4028-10.2010 (2010).
Syka, J. Plastic changes in the central auditory system after hearing loss, restoration of function, and during learning. Physiological reviews 82, 601–636, https://doi.org/10.1152/physrev.00002.2002 (2002).
Llinas, R. R., Ribary, U., Jeanmonod, D., Kronberg, E. & Mitra, P. P. Thalamocortical dysrhythmia: A neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proceedings of the National Academy of Sciences of the United States of America 96, 15222–15227 (1999).
Weisz, N. et al. The neural code of auditory phantom perception. The Journal of neuroscience: the official journal of the Society for Neuroscience 27, 1479–1484 (2007).
De Ridder, D. et al. Theta-gamma dysrhythmia and auditory phantom perception. Journal of neurosurgery 114, 912–921, https://doi.org/10.3171/2010.11.JNS10335 (2011).
Sedley, W. et al. Single-subject oscillatory gamma responses in tinnitus. Brain: a journal of neurology 135, 3089–3100, https://doi.org/10.1093/brain/aws220 (2012).
van der Loo, E. et al. Tinnitus intensity dependent gamma oscillations of the contralateral auditory cortex. PloS one 4, e7396 (2009).
Cheung, S. W. & Larson, P. S. Tinnitus modulation by deep brain stimulation in locus of caudate neurons (area LC). Neuroscience 169, 1768–1778, https://doi.org/10.1016/j.neuroscience.2010.06.007 (2010).
Larson, P. S. & Cheung, S. W. Deep brain stimulation in area LC controllably triggers auditory phantom percepts. Neurosurgery 70, 398–405, discussion 405–396, https://doi.org/10.1227/NEU.0b013e3182320ab5 (2012).
Larson, P. S. & Cheung, S. W. A stroke of silence: tinnitus suppression following placement of a deep brain stimulation electrode with infarction in area LC. Journal of neurosurgery 118, 192–194, https://doi.org/10.3171/2012.9.JNS12594 (2013).
Lowry, L. D., Eisenman, L. M. & Saunders, J. C. An absence of tinnitus. Otology & neurotology: official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology 25, 474–478 (2004).
Cheung, S. W. et al. Phase I trial of caudate deep brain stimulation for treatment-resistant tinnitus. Journal of neurosurgery, 1–10, https://doi.org/10.3171/2019.4.JNS19347 (2019).
Eggermont, J. J. & Roberts, L. E. The neuroscience of tinnitus. Trends in neurosciences 27, 676–682, https://doi.org/10.1016/j.tins.2004.08.010 (2004).
Roberts, L. E., Husain, F. T. & Eggermont, J. J. Role of attention in the generation and modulation of tinnitus. Neuroscience and biobehavioral reviews 37, 1754–1773, https://doi.org/10.1016/j.neubiorev.2013.07.007 (2013).
Chen, Y. C. et al. Tinnitus distress is linked to enhanced resting-state functional connectivity from the limbic system to the auditory cortex. Human brain mapping 38, 2384–2397, https://doi.org/10.1002/hbm.23525 (2017).
Leaver, A. M. et al. Dysregulation of limbic and auditory networks in tinnitus. Neuron 69, 33–43 (2011).
Seydell-Greenwald, A. et al. Functional MRI evidence for a role of ventral prefrontal cortex in tinnitus. Brain research 1485, 22–39, https://doi.org/10.1016/j.brainres.2012.08.052 (2012).
Hinkley, L. B., Mizuiri, D., Hong, O., Nagarajan, S. S. & Cheung, S. W. Increased striatal functional connectivity with auditory cortex in tinnitus. Frontiers in human neuroscience 9, 568, https://doi.org/10.3389/fnhum.2015.00568 (2015).
Perez, P. L. et al. Human caudate nucleus subdivisions in tinnitus modulation. Journal of neurosurgery, 1–7, https://doi.org/10.3171/2018.10.JNS181659 (2019).
Overdevest, J. B., Pross, S. E. & Cheung, S. W. Tinnitus following treatment for sporadic Acoustic neuroma. The Laryngoscope 126, 1639–1643, https://doi.org/10.1002/lary.25672 (2016).
Lee, S. Y. et al. No auditory experience, no tinnitus: Lessons from subjects with congenital- and acquired single-sided deafness. Hear Res 354, 9–15, https://doi.org/10.1016/j.heares.2017.08.002 (2017).
Arndt, S. et al. Comparison of pseudobinaural hearing to real binaural hearing rehabilitation after cochlear implantation in patients with unilateral deafness and tinnitus. Otology & neurotology: official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology 32, 39–47, https://doi.org/10.1097/MAO.0b013e3181fcf271 (2011).
Desmet, J. et al. Clinical need for a Baha trial in patients with single-sided sensorineural deafness. Analysis of a Baha database of 196 patients. European archives of oto-rhino-laryngology: official journal of the European Federation of Oto-Rhino-Laryngological Societies 269, 799–805, https://doi.org/10.1007/s00405-011-1733-5 (2012).
Faber, H. T. et al. Analysis of factors predicting the success of the bone conduction device headband trial in patients with single-sided deafness. Archives of otolaryngology–head & neck surgery 138, 1129–1135, https://doi.org/10.1001/jamaoto.2013.754 (2012).
Kim, S. H., Byun, J. Y., Yeo, S. G. & Park, M. S. Tinnitus Retraining Therapy in Unilateral Tinnitus Patients with Single Side Deafness. The journal of international advanced otology 12, 72–76, https://doi.org/10.5152/iao.2016.1949 (2016).
Martinez-Devesa, P., Perera, R., Theodoulou, M. & Waddell, A. Cognitive behavioural therapy for tinnitus. The Cochrane database of systematic reviews, CD005233, https://doi.org/10.1002/14651858.CD005233.pub3 (2010).
Arts, R. A. et al. Tinnitus Suppression by Intracochlear Electrical Stimulation in Single Sided Deafness - A Prospective Clinical Trial: Follow-Up. PloS one 11, e0153131, https://doi.org/10.1371/journal.pone.0153131 (2016).
Galvin, J. J. III. et al. Benefits of Cochlear Implantation for Single-Sided Deafness: Data From the House Clinic-University of Southern California-University of California, Los Angeles Clinical Trial. Ear and hearing, https://doi.org/10.1097/AUD.0000000000000671 (2018).
Holder, J. T., O’Connell, B., Hedley-Williams, A. & Wanna, G. Cochlear implantation for single-sided deafness and tinnitus suppression. American journal of otolaryngology 38, 226–229, https://doi.org/10.1016/j.amjoto.2017.01.020 (2017).
Mertens, G., Kleine Punte, A., De Ridder, D. & Van de Heyning, P. Tinnitus in a single-sided deaf ear reduces speech reception in the nontinnitus ear. Otology & neurotology: official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology 34, 662–666, https://doi.org/10.1097/MAO.0b013e31828779f0 (2013).
Meikle, M. B. et al. The tinnitus functional index: development of a new clinical measure for chronic, intrusive tinnitus. Ear and hearing 33, 153–176, https://doi.org/10.1097/AUD.0b013e31822f67c0 (2012).
Calabresi, P. et al. Synaptic transmission in the striatum: from plasticity to neurodegeneration. Progress in neurobiology 61, 231–265 (2000).
Goubard, V., Fino, E. & Venance, L. Contribution of astrocytic glutamate and GABA uptake to corticostriatal information processing. The Journal of physiology 589, 2301–2319, https://doi.org/10.1113/jphysiol.2010.203125 (2011).
Jastreboff, P. J. & Jastreboff, M. M. Tinnitus Retraining Therapy (TRT) as a method for treatment of tinnitus and hyperacusis patients. Journal of the American Academy of Audiology 11, 162–177 (2000).
Newman, C. W., Sandridge, S. A. & Jacobson, G. P. Psychometric adequacy of the Tinnitus Handicap Inventory (THI) for evaluating treatment outcome. Journal of the American Academy of Audiology 9, 153–160 (1998).
Kim, S. H. et al. Neural substrates predicting short-term improvement of tinnitus loudness and distress after modified tinnitus retraining therapy. Scientific reports 6, 29140, https://doi.org/10.1038/srep29140 (2016).
Biou, E. et al. Transcranial direct current stimulation in post-stroke aphasia rehabilitation: a systematic review. Annals of physical and rehabilitation medicine. https://doi.org/10.1016/j.rehab.2019.01.003 (2019).
Dionisio, S. et al. Connectivity of the human insula: A cortico-cortical evoked potential (CCEP) study. Cortex; a journal devoted to the study of the nervous system and behavior 120, 419–442, https://doi.org/10.1016/j.cortex.2019.05.019 (2019).
Song, J. J., Vanneste, S. & De Ridder, D. Dysfunctional noise cancelling of the rostral anterior cingulate cortex in tinnitus patients. PloS one 10, e0123538, https://doi.org/10.1371/journal.pone.0123538 (2015).
Yeo, B. T. et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of neurophysiology 106, 1125–1165, https://doi.org/10.1152/jn.00338.2011 (2011).
Hoare, D. J., Stacey, P. C. & Hall, D. A. The efficacy of auditory perceptual training for tinnitus: a systematic review. Annals of behavioral medicine: a publication of the Society of Behavioral Medicine 40, 313–324, https://doi.org/10.1007/s12160-010-9213-5 (2010).
Han, L. et al. Baseline Functional Connectivity Features of Neural Network Nodes Can Predict Improvement After Sound Therapy Through Adjusted Narrow Band Noise in Tinnitus Patients. Front Neurosci 13, 614, https://doi.org/10.3389/fnins.2019.00614 (2019).
Selemon, L. D. & Goldman-Rakic, P. S. Longitudinal topography and interdigitation of corticostriatal projections in the rhesus monkey. The Journal of neuroscience: the official journal of the Society for Neuroscience 5, 776–794 (1985).
Yeterian, E. H. & Pandya, D. N. Corticostriatal connections of the superior temporal region in rhesus monkeys. The Journal of comparative neurology 399, 384–402 (1998).
Lee, E. J., Fomenko, A. & Lozano, A. M. Magnetic Resonance-Guided Focused Ultrasound: Current Status and Future Perspectives in Thermal Ablation and Blood-Brain Barrier Opening. Journal of Korean Neurosurgical Society 62, 10–26, https://doi.org/10.3340/jkns.2018.0180 (2019).
Voigt, J., Carpenter, L. & Leuchter, A. A systematic literature review of the clinical efficacy of repetitive transcranial magnetic stimulation (rTMS) in non-treatment resistant patients with major depressive disorder. BMC psychiatry 19, 13, https://doi.org/10.1186/s12888-018-1989-z (2019).
Miguel, E. C. et al. Evolution of gamma knife capsulotomy for intractable obsessive-compulsive disorder. Molecular psychiatry, https://doi.org/10.1038/s41380-018-0054-0 (2018).
Roberts, D. G. & Pouratian, N. Stereotactic Radiosurgery for the Treatment of Chronic Intractable Pain: A Systematic Review. Operative neurosurgery 13, 543–551, https://doi.org/10.1093/ons/opx095 (2017).
Ravicz, M. E., Melcher, J. R. & Kiang, N. Y. Acoustic noise during functional magnetic resonance imaging. The Journal of the Acoustical Society of America 108, 1683–1696, https://doi.org/10.1121/1.1310190 (2000).
Lanting, C. P., De Kleine, E., Bartels, H. & Van Dijk, P. Functional imaging of unilateral tinnitus using fMRI. Acta Otolaryngol 128, 415–421, https://doi.org/10.1080/00016480701793743 (2008).
Melcher, J. R., Levine, R. A., Bergevin, C. & Norris, B. The auditory midbrain of people with tinnitus: abnormal sound-evoked activity revisited. Hearing research 257, 63–74, https://doi.org/10.1016/j.heares.2009.08.005 (2009).
Henry, J. A. et al. Tinnitus Functional Index—Development and Clinical Application. Audiology Today, 40–48 (2014).
Tzourio-Mazoyer, N. et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15, 273–289, https://doi.org/10.1006/nimg.2001.0978 (2002).
Chumbley, J. R. & Friston, K. J. False discovery rate revisited: FDR and topological inference using Gaussian random fields. NeuroImage 44, 62–70, https://doi.org/10.1016/j.neuroimage.2008.05.021 (2009).
Acknowledgements
This work was support by the Department of Defense (Grant Numbers W81XWH1310494 and W81XWH1810741), Coleman Memorial Fund, and Hong Kong Lounge II Research Fund, and Hearing Research, Inc.
Author information
Authors and Affiliations
Contributions
J.H.S. drafted the manuscript, and analyzed and interpreted data. Y.S. analyzed and interpreted data, and revised the manuscript. P.L.P., J.L.C. and S.E.P. interpreted data and revised the manuscript. A.M.F. and D.M. contributed to data acquisition and revision of the manuscript. L.B.H. and S.S.N. analyzed and interpreted data. S.W.C. drafted the manuscript, analyzed and interpreted data, and supervised the project. All authors approved the final copy of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Henderson-Sabes, J., Shang, Y., Perez, P.L. et al. Corticostriatal functional connectivity of bothersome tinnitus in single-sided deafness. Sci Rep 9, 19552 (2019). https://doi.org/10.1038/s41598-019-56127-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-56127-1
This article is cited by
-
Degree centrality and functional connections in presbycusis with and without cognitive impairments
Brain Imaging and Behavior (2022)
-
Resting-state Networks in Tinnitus
Clinical Neuroradiology (2022)
-
Cortical and subcortical gray matter changes in patients with chronic tinnitus sustaining after vestibular schwannoma surgery
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.