Abstract
The mitotic serine/threonine kinase aurora kinase-A (AURKA) has been identified as carcinogenic in hepatocellular carcinoma (HCC). AURKAPS1, a long non-coding RNA (lncRNA), is the pseudo-gene of AURKA, which play important roles in the cancer. Its underlying functions and mechanisms in liver cancer progression remain largely unknown. The mRNA expression of AURKAPS1 in HCC tumor tissues was significantly higher, which is associated with tumor size and TNM stage. The high expression of AURKAPS1 promotes cell movement, migration and invasion. AURKAPS1 can increases the protein expression of RAC1, promotes the activation of ERK, and enhance the formation of membrane ruffles by binding with miR-182, miR-155 and miR-142 competively. Thus, AURKAPS1 could be a useful marker, and the combination of AURKAPS1/miRNAs (miR-142, miR-155 and miR-182) may be a new theoretical basis for the treatment of HCC.
Similar content being viewed by others
Introduction
Hepatocellular carcinoma (HCC) is a lethal cancer with increasing frequency in the world in the recently decades. More than 700,000 new cases happened, and approximately 600,000 people die of liver cancer every year1,2,3. Despite medical technology development and progress that have led to great achievements in the comprehensive treatment of liver cancer, liver cancer recurrence and metastasis result in prognoses that are not optimistic4,5,6. Therefore, it is significant to study the underlying mechanisms of HCC progression to provide new information for improving HCC treatments.
Long noncoding RNAs (lncRNAs) are a kind of the most important noncoding RNAs which has more than 200 nucleotides in length and don’t have the ability to code proteins7,8,9. LncRNAs were reported to paly emportant roles in lots of diseases spatially and temporally, especially in cancers, which indicating specific functions for lncRNAs10,11,12,13,14. Additionally, lncRNAs can modulate the functions of target microRNAs (miRNAs) by acting as a competitive endogenous RNA (ceRNA)15.
AURKAPS1 is a long noncoding RNA, also the pseudogene of AURKA, which is located in the intron region of RAB3 GTPase activating non-catalytic protein subunit 2 (RAB3GAP2) on chromosome 1. The expression and functions of AURKAPS1 in tumours have not been reported. LncRNAs can function as a ceRNA or as a molecular sponge to modulate the expression and biological functions of miRNA, for example, via regulating the post-transcriptional proceed. Thus, an important crosstalk may exist between lncRNA and miRNA. However, whether AURKAPS1 affects tumourigenesis by regulating miRNAs remains unclear.
In this project, we found the expression of AURKAPS1 was significantly higher in HCC tissues and cell lines, also AURKAPS1 potentiates the invasion and metastasis of HCC cells by regulating miRNAs, so it may become a potential target for the treatment of HCC in the future.
Results
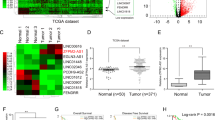
AURKAPS1 is the pseudogene of AURKA and is located in the intron region of RAB3GAP2 on chromosome 1 (Fig. 1A). Nevertheless, the expression and function of AURKAPS1 in tumours have not been reported. Through sequence comparison, we found that compared with the AURKA gene, the AURKAPS1 gene lacks a 359–560 coding region sequence, has a 25 bp difference in its 3′ extremity, and has some nucleotide mutations and losses (Fig. 1B). Furthermore, AURKAPS1 expression in 124 cases liver cancer tissues was detected by quantitative real-time PCR (qRT-PCR), and the results showed that AURKAPS1 expression was significantly higher in HCC tissues than in adjacent normal liver tissues (Fig. 1C). In addition, the expression level of AURKAPS1 was positively correlated with tumour size and TNM stage (Fig. 1D,E), but not with sex, age, history of hepatitis, or lymph node metastasis (Table 1), suggesting that AURKAPS1 may be associated with tumour invasion and metastasis.
(A) Gene structure and localization of AURKAPS1. (B) Sequence comparison of AURKA and AURKAPS1. (C) Relative expression of AURKAPS1 in 124 pairs of HCC tissues (p = 0.0012)). (D) Relative expression of AURKAPS1 in different tumour sizes(p = 0.0241). (E) Relative expression of AURKAPS1 in different TNM stages(p = 0.0227). AURKAPS1 expression was examined by qRT-PCR. P < 0.05 was considered to indicate a statistically significant difference. The means ± SD are shown. Statistical analysis was conducted using Student’s t-test.
We consctruct AURKAPS1 Lentivirus vector using Ubi-MCS-SV40-EGFP-IRES-puromycin by Genechem Co. Adopt psPAX, pMD2.G entivirus packaging system to pack entivirus, then construct the AURKAPS1 overexpression stable cell lines, and identify them by RT-PCR (Fig. 2A–C). We evaluated cancer cell migration and invasion through Transwell assays. The migration and invasion of HepG2 and BEL-7402 cells were also significantly higher in the AURKAPS1-overexpression group than in the control group (Fig. 2D–G). These results indicated that AURKAPS1 may act as an oncogene to promote the migration and invasion of HCC cells.
(A) Enzyme digestion identification of lentivirus plasmids. (B) Detection of cell activity after infection with lentivirus at different doses. (C) The expression of AURKAPS1 in stably transformed cells was detected byRT- PCR. (D) Effect of AURKAPS1 overexpression on HepG2 cell migration and invasion. (E) Effect of AURKAPS1 overexpression on BEL-7402 cell migration and invasion. (F) Effects of AURKAPS1 transfection with lentivirus on AURKA. The data are presented as the mean ± SD. *P < 0.05. Scale bars, 20 µm.
When AURKAPS1 was overpressed in HepG2 and BEL-7402 cell lines, in order to prove whether the expression of AURKA was affected, we tested the expression of AURKA in both cell lines at 24 and 48h after transfection by western blot, and found there were no change for AURKA protein after the overexpression of AURKAPS1 (Fig. 2F).
Invasion and metastasis are the cruces of malignant tumour recurrence. We simulated liver cancer metastasis by injecting HCC cells into the tail veins of mice. This metastasis tumour burden assay in the liver showed that the number and diameter of tumour nodules were significantly fewer and smaller in the control group than in the AURKAPS1 overexpression group (Fig. 3). Taken together, these in vivo results demonstrated that AURKAPS1 plays a crucial role in liver cancer cell migration and invasion.
Through DIANA software analysis, we found that AURKAPS1 had ten miRNA binding sites (miR-134, miR-302, miR-636, miR-192, miR-182, miR-640, miR-1912, miR-767, miR-452 and miR-218). Functional bioinformatics prediction was then performed on the potential target genes of these miRNAs. By functional cross analysis, we identified six hub target genes (NM _000601 HGF, NM _000875 IGF1R, NM _002745 MAPK1, NM _006908 RAC1, NM _005359 Smad4, and NM _003392 Wnt5A). These results also suggest that AURKAPS1 might be involved in regulating the expression of these six genes as a ceRNA. To further verify the above hypothesis, we selected RAC1 and MAPK1, which are associated with tumour invasion and metastasis, as study subjects. Western blot analysis showed that AURKAPS1 overexpression could upregulate the RAC1 protein, but not MAPK1 (Fig. 4A). Because RAC1 can activate the MAPK1 pathway, we used phosphorylated MAPK1 antibodies to detect its active form. The results suggest that AURKAPS1 overexpression promoted the activation of MAPK1 (Fig. 4B).
We constructed RAC1 3′UTR and AURKAPS1 reporter gene plasmids to confirm which miRNA regulates RAC1 and AURKAPS1. In this study, five potential miRNA binding sites of AURKAPS1 (miR-134, miR-182, miR-192, miR-218 and miR-636) predicted by DIANA software and three underlying binding sites of the RAC1 3′UTR (miR-142, miR-155 and miR-194) predicted by TargetScan were selected for analysis (the above three binding sites were also present in AURKAPS1 as analysed by RNA22). The luciferase reporter gene assay indicated that miR-142, miR-155, miR-182 and miR-194 could suppress the reporter gene activity of RAC1 when the RAC1 3′UTR was cotransfected with the selected potential miRNAs (Fig. 5B–D,F), while miR-134, miR-192, miR-218 and miR-636 had no significant differences (Fig. 5A,E,G,H).
Relative RAC1 3′UTR luciferase activity in HepG2 cells treated with miRNAs. (A–H) Relative luciferase activity of the RAC1 3′UTR in HepG2 cells lines transfected with miR-134/miR-142/miR-155/miR-182/miR-192/miR-194/miR-218/miR-636. (I) Reporter gene vector map and construction. (J) Identification of AURKAPS1 and RAC1 3′utr reporter recombinant plasmids. Luciferase activity of the RAC13′UTR was examined by luciferase reporter gene assay. *P < 0.05, **P < 0.01. The means ± SD are shown. Statistical analysis was conducted using Student’s t-test.
Subsequently, the luciferase reporter gene assay was used to analyse whether the 4 screened miRNAs that regulated RAC1 could simultaneously bind to AURKAPS1. We found that miR-142, miR-155 and miR-182 could also significantly inhibit the luciferase activity of AURKAPS1 (Fig. 6A–C), while miR-194 had no significant effect on the luciferase activity of AURKAPS1 (Fig. 6D).
Relative AURKAPS1 luciferase activity in HepG2 cells treated with miRNAs. (A–D) Relative luciferase activity of AURKAPS1 in HepG2 cells lines transfected with miR-142./miR-155/miR-182./miR-194. The luciferase activity of AURKAPS1 was examined by luciferase reporter gene assay. *P < 0.05, **P < 0.01. The means ± SD are shown. Statistical analysis was conducted using Student’s t-test.
As lncRNA can regulate the function of miRNA as a ceRNA, we conducted further reporter gene experiments to verify whether AURKAPS1 can be used as a competitive binding molecule to control the regulation of RAC1 by miRNA. The results revealed that the luciferase activity of RAC1 decreased when miR-142 (Fig. 7A), miR-155 (Fig. 7B) and miR-182 (Fig. 7C) were overexpressed, and this suppression was significantly weakened when AURKAPS1 was overexpressed at the same time. These data confirmed that AURPAPS1 could suppress the targeting effect of miR-142, miR-152 and miR-182 on RAC1 via binding to miRNAs, thus improving the luciferase activity of RAC1.
Relative RAC13′UTR luciferase activity in HepG2 cells treated with miRNAs/miRNAs+ AURKAPS1. (A) Relative luciferase activity of the RAC1 3′UTR in HepG2 cells lines transfected with miR-142/miR-142+ AURKAPS1. (B) Relative luciferase activity of the RAC1 3′UTR in HepG2 cells lines transfected with miR-155/miR-155+ AURKAPS1. (C) Relative luciferase activity of the RAC1 3′UTR in HepG2 cells lines transfected with miR-182/miR-182+ AURKAPS1. The RAC1 3′UTR luciferase activity was examined by luciferase reporter gene assay. *P < 0.05, **P < 0.01. The means ± SD are shown. Statistical analysis was conducted using Student’s t-test.
Western blot experiments were performed to further validate the competitive effect of AURKAPS1. RAC1 protein expression was significantly decreased when miR-142, miR-155 and miR-182 were overexpressed in the control cells. Moreover, the decrease in RAC1 levels was greater when these three miRNAs were cotransfected than when single miRNAs were transfected. The targeted inhibitory effect of these miRNAs on RAC1 was partially restored in AURKAPS1-overexpressing cells. This result also confirmed that AURKAPS1 could competitively inhibit the effects of miRNA downregulation (miR-142, miR-155 and miR-182) on RAC1 and promote ERK activation (Fig. 8A,B).
(A) Effect of AURKAPS1 overexpression in HepG2 cells on RAC1 and phosphorylated ERK1/2 downregulation by miR-142, miR-155 and miR-182. (B) Effect of AURKAPS1 overexpression in BEL-7402 cells on RAC1 and phosphorylated ERK1/2 downregulation by miR-142, miR-155 and miR-182. (C) Immunohistochemistry was employed to determine RAC1 expression in liver cancer tissues. Scale bars, 100 µm.
AURKAPS1 expression is significantly increased in HCC tissues and can promote RAC1 protein expression by competitively sponge miR-142, miR-155 and miR-182. We detected RAC1 protein expression in 100 pairs of liver cancer tissues through immunohistochemistry. The results indicated that RAC1 expression was also significantly higher in liver cancer tissues than in adjacent normal tissues (Fig. 8C).
Discussion
Recently, lots of studies focused on the functions and the regulatory mechanisms of lncRNAs16,17, people have revealed that lncRNAs took part in various human cancers progression18,19,20. However, the functions and mechanisms of AURKAPS1 in HCC have not been reported, and remains unknown.
In the present project, we found that AURKAPS1 acted as an oncogenic biomarker in HCC. AURKAPS1 can target to miR-142, miR-155 and miR-182, and there was a negative correlation between AURKAPS1 and miRNAs. AURKAPS1 served as a ceRNA to potentiate cancer progress partially due to its ability to suppress the expression of miR-142, miR-155 and miR-182. Hence, our results was significance to improve the theoretical basis for lncRNAs in cancer therapy.
Emerging evidence has proved that many kinds of lncRNAs are abnormally expressed in different cancers21. Because lncRNAs take part in the occurrence and progression of malignant tumor, lncRNAs could be used as diagnostic or prognostic markers and potential therapeutic targets. A previous study indicated that HULC was upregulated in hepatoma and promoted cell proliferation, migration and invasion10. We observed similar findings in this study, AURKAPS1 overexpression accelerated the malignant progression of hepatocellular carcinoma cells. Also, we explored RAC1 expression and found that AURKAPS1 overexpression significantly improved RAC1 levels in liver cancer cells. RAC1 activation and mutation are closely related to the onset of melanoma and non-small cell lung cancer22. RAC1 overexpression can activate the PAK or ERK pathway to enhance tumour growth and aggressiveness23. In addition, RAC1 was found to be strongly linked to F-actin assembly and implicated in epithelial-to-mesenchymal transition (EMT)24. However, whether RAC1 is also involved in the AURKAPS1-induced enhancement of liver cancer progression needs to be further studied.
To date, AURKAPS1 and its underlying regulatory mechanisms have remained largely unknown. The ceRNA hypothesis may explain the mechanism of the complex biological function of lncRNA in the pathogenesis of human cancer partly25,26,27,28,29,30, also provides a basic theory to predict the potential functions of novel lncRNAs. Interestingly, we found the underlying molecular mechanism of how AURKAPS1 participates in liver cancer progression, it functions as a ‘molecular sponge’ to regulate miRNAs. Many studies have demonstrated lncRNAs play a vital role in a variety of cell processes by acting as ceRNAs to regulate miRNAs8,15, such as lncRNA HULC and MEG331,32,33, which have been studied in several cancer research. In this study, we evaluated the effect of AURKAPS1 on liver cancer cells and discovered that AURKAPS1 took part in the development of pseudogene-miRNA-mRNA interaction networks and acted as an endogenous miRNA sponge, binding to miRNA and regulating its function. AURKAPS1 was confirmed to be a direct target gene for miR-142, miR-155 and miR-182, and RAC1 may be a potential regulatory target of AURKAPS1. Studies have indicated that miR-142/155/182 inhibits many cancers, such as lung cancer34,35, pancreatic cancer36,37,38, liver cancer39,40.
In conclusion, we found that the expression of AURKAPS1 and miRNAs (miR-142, miR-155 and miR-182) showed a significantly negative correlation in HCC cell lines. These three miRNAs remarkably downregulated AURKAPS1, while AURKAPS1 overexpression reversed the RAC1 reduction induced by miR-142, miR-155 and miR-182. Our results indicate that high AURKAPS1 expression promotes the tumourigenesis and progression of liver cancer through regulating miRNA expression. Thus, AURKAPS1 could be a useful marker, and the combination of AURKAPS1/miRNAs (miR-142, miR-155 and miR-182) may be a promising choice for the therapyof human liver cancer.
Materials and Methods
Sample preparation
All samples for the project were collected from patients who had undergone surgery and were diagnosed with liver cancer based on a pathological evaluation at the First Affiliated Hospital of Zhengzhou University. No other treatment related to HCC had been proceeded in the patients before their surgical treatment. All the specimens were immediately snap-frozen and preserved in liquid nitrogen then transfered to the −80 °C refrigerator until used in this study. All patients signed the informed consent form. This research was conducted by the Declaration of Helsinki. Human investigations were approved by the Institutional Review Board of the First Affiliated Hospital of Zhengzhou University. All animal procedures and experiments methods were carried out according to the Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use committee of the First Affiliated Hospital of Zhengzhou University.
Cell lines and culture
Human liver cancer cell lines HepG2 and BEL-7402 were purchased from the Shanghai Cell Bank of the Chinese Academy of Science (Shanghai, China). These cells were maintained in high-glucose Dulbecco’s modified Eagle’s medium (DMEM-H) (HyClone Co, USA) containing 10% FBS in a humidified atmosphere of 5% CO2 at 37 °C.
Cell transfection
The AURKAPS1 overexpression plasmid and the respective vector (negative control) were synthesized by GenePharma Co. (Shanghai, China). Cells were transiently transfected using Lipofectamine 2000 transfection reagent (Life Technologies Corp., Shanghai, China) according to the manufacturer’s protocol. The transfection efficiency was tested by qRT-PCR analysis.
RNA extraction and qRT-PCR
Total RNA was extracted from frozen liver samples and cells with TRIzol reagent (Invitrogen, Carlsbad, CA, USA). cDNA was synthesised from RNA using the 50 μl systerm with an RNA reverse transcription kit, diluted 1:10, and stored in −20 °C refrigerator (Takara Co., Dalian, China). Using One-Step SYBR Prime Script RT-PCR kit for qRT-PCR, and the endogenous control was GAPDH. The relative expression level (change fold) was calculated using the 2−ΔΔCt method. The formula was reffered to the ABI Prism 7300 sequence detection system protocol.
Cell migration and invasion experiments
In order to test cell migration, place 8-mm pore size culture inserts (Transwell; Corning Costar, USA) into 24-well culture plates, upper and the lower chambers were seperated. For the lower chamber, add DMEM containing 10% FBS. Place serum-free medium containing 5 × 104 cells in the upper chamber to examine migration and invasion. Then incubate at 37 °C for 48 h, the cells on the upper membrane surface were scraped off. The cells on the lower side of the membrane were fixed and then stained with 0.4% trypan blue dye. The number of cells that had migrated through the pores was quantified by counting 10 independent visual fields (magnification x20) under the microscope and then subjected to statistical analyses. Each experiment was performed at least 3 times.
Construction of luciferase reporter plasmid
Select pMIR-REPORTTM Luciferase as the reporter gene vector, and the vector information is discribed in Fig. 5I,J. The reporter vector was digested according to the following reaction system (Lentivirus vector plasmid 1 μg, SpeI incision enzyme 1 μL, MluI incision enzyme 1 μL, Rapid enzyme digestion buffer 3 μL, add Sterilization of water to 30 μL) to obtain the desired enzyme digestion vector.
PCR amplification of the target sequence
RNA sequences of RAC1 and AURKAPS1 were downloaded from the NCBI nucleic acid database, and then Primer Premier software was used to design reporter primers of RAC1 and AURKAPS1, respectively. Meanwhile, restriction enzyme sites were added according to sequence characteristics, and PCR primers were used to amplify RAC1’s 3′UTR (non-coding region of 3′ segment) and AURKAPS1, respectively.
RAC1 3′UTR upstream primer:(include SpeI enzyme loci):AGACTAGTATGTCTCAGCCCCTCGTTCTT; RAC1 3′UTR downstream primer(include Mul I enzyme loci):TTACGCGT TATGATTCAAGGATTTATTAAGTCATACAT; AURKAPS1upstream primer(include SpeI enzyme loci): AGACTAGT TCTCCAGTCACAAGCCAGTTCAG; AURKAPS1 downstream primer(include Mul I enzyme loci): TTACGCGTACTATATCTCTAGCAGCTGTCCACAGT.
Put the reaction solution into the PCR amplification instrument and perform PCR amplification according to the procedure. After amplification, separate PCR reaction products by 1% agarose gel electrophoresis, and cut the target fragment under UV light, and use agarose gel recovery kit to recover the target fragment. The recovered products were digested by enzymes. At last, connect the target fragment and the carrier according to the reaction system (Recombinant plasmid 1 μl, SpeI incision enzyme 0.5 μl, MluI incision enzyme 0.5 μl, Rapid enzyme digestion buffer 1 μl, add Sterilization of water to 10 μL). Using 1% agarose gel electrophoresis to separate, and acquire the image under ultraviolet lamp.
Dual-luciferase repoerter gene assay
A biological information website was used to predict the promoter sequence of miRNA genes that may bind to AURKAPS1. After PCR amplification, the miRNA gene promoter sequence was inserted into a luciferase reporter gene vector to construct a luciferase reporter plasmid. Next, the miRNA promoter luciferase reporter plasmid and AURKAPS1 plasmid were cotransfected into HepG2 cells. Utilizing the Dual-Luciferase Reporter Assay System to exzamine the luciferase activity after 48–72 h to observe the changes in the fluorescence value.
Western blot
After extacting the total protein from the cell, prepare 10% SDS-page gel, reheat the diluted sample at 55 °C for 5min, then add 15 μl sample to each well, and 7 μl maker in the first well. After adding the running buffer, Electrophoresis was performed at 90 V constant pressure for 30 minutes and 110 V for 70 minutes. PVDF film of 8.5 cm × 5.5 cm size was cut and soaked with methanol for 1 min. Then transfer the film at a constant pressure of 100 V under the condition of ice bath for 1.5 h. Rinsed with methanol again with TBST 5 min × 4 times, and sealed with TBST containing 5% milk at room temperature for 1 h. Add the properly diluted primary antibody according to the antibody instructions and incubate at 4 °C overnight. Second day, appropriately diluted peroxidase-labeled secondary antibody was added, and incubated at room temperature for 1 h. The incubated protein membrane was washed with TBST buffer for 5 min × 4 times. Put the film into the tray of the ECL gel imager, drop the appropriate amount of ECL substrate onto the film, click the appropriate exposure time and collect the image.
Tumour xenografts
Six weeks old female nude BALB/7 mice were purchased from Beijing Weitong Lihua Laboratory Animal Co, Ltd. (Beijing, China). The mice were given free access to sterile food and water during the experimental period. All animal experiments using nude mice were performed strictly in accordance with a protocol approved by the Beijing Research Center of Laboratory Animals. The mice were divided to twogroups (n = 7 in each group). The first group is tumor invasion experiment group, the mice were injected 1 × 106/100 μL l HepG2 (over expressed AURKAPS1) cells with a 1 ml syringe through the tail vein; the second group is the control groupcontrol group cells (1 × 106/100 μl PBS) were injected 0.8 weeks after the transplantation, sacrifice all the mice to harvest the liver to detect the tumor metastasis.
Immunohistochemistry
A continuous section of the wax (3–4 μm thickness) mass with tumour tissue was selected for immunohistochemical staining. A sodium citrate buffer solution (0.01 M, pH 6.0) was heated in a pressure cooker and kept warm for antigen repair. After blocking with 5% normal goat serum and 0.2% Triton X-100 in PBS for 1 h at room temperature, the liver sections were incubated overnight at 4 °C with 1% normal goat serum and 0.2% Triton X-100 in PBS containing primary antibodies against RAC1 to visualize hepatoma cells (rabbit, 1:500; Abcam, USA). After washing, the sections were then incubated with the species-appropriate biotin-labelled secondary antibodies overnight at 4 °C or for 2 h at room temperature. The slides were then coated with glycerol and covered for microscopic analysis.
Statistical analysis
All the data are showed as the mean ± standard deviation (SD). All experimental results were statistically analysed with Student’s t-test or one-way analysis of variance (ANOVA). All statistical analyses were performed with Graphad prism 8.0 version. A value of P < 0.05 was considered to indicate a statistically significant difference.
References
Forner, A., Llovet, J. M. & Bruix, J. Hepatocellular carcinoma. Lancet 379, 1245–1255 (2012).
Jemal, A. et al. Global cancer statistics. CA Cancer J Clin, 61, 69–90 (2011).
Poloz, Y. & Stambolic, V. Obesity and cancer, a case for insμLin signaling. Cell Death & Disease 6, e2037 (2015).
Marin, D. et al. CT Appearance of Hepatocellular Carcinoma after Locoregional Treatments: A Comprehensive Review. Gastroenterol Res Pract 2015, 1–10 (2015).
Li, Y. W. et al. Hepatocellular carcinoma and hepatitis B surface protein. World J Gastroenterol 22, 1943–1952 (2016).
Gu, S. et al. Alcohol, TLR4-TGF-beta antagonism, and liver cancer. Hepatol Int 8, 408–412 (2014).
Liz, J. & Esteller, M. lncRNAs and microRNAs with a role in cancer development. Biochim Biophys Acta 1859, 169–176 (2016).
Mercer, T. R., Dinger, M. E. & Mattick, J. S. Long non-coding RNAs: Insights into functions. Nat Rev Genet 10, 155–159 (2009).
Ørom, U. A. et al. Long noncoding RNAs with enhancer-like function in human cells. Cell 143, 46–58 (2010).
Wang, J. et al. CREB up-regulates long non-coding RNA, HULC expression through interaction with microRNA-372 in liver cancer. Nucleic Acids Res 38, 5366–538 (2010).
Zhao, X. et al. Gas5 exerts tumor-suppressive functions in human glioma cells by targeting miR-222. Mol Ther 23, 1899–1911 (2015).
Tsai, M. C. et al. Long noncoding RNA as modular scaffold of histone modification complexes. Science 329, 689–693 (2010).
Wang, K. C. & Chang, H. Y. Molecular mechanisms of long noncoding RNAs. Mol Cell 43, 904–914 (2011).
Yuan, J. et al. A long noncoding RNA activated by TGF-β promotes the invasion-metastasis cascade in hepato-cellular carcinoma. Cancer Cell 25, 666–681 (2014).
Qian, C. et al. Identification of functional lncRNAs in atrial fibrillation by integrative analysis of the lncRNA-mRNA network based on competing endogenous RNAs hypothesis. Journal of cellular physiology, 234, 11620–11630 (2019).
Geisler, S. et al. Decapping of long noncoding RNAs regulates inducible genes. Mol Cell 45, 279–291 (2012).
Huarte, M. et al. A large intergenic noncoding RNA induced by p53 mediates global gene repression in the p53 response. Cell 142, 409–419 (2010).
Barnhill, L. M. et al. High expression of CAI2, a 9p21-embedded long noncoding RNA, contributes to advanced-stage neuroblastoma. Cancer Res 74, 3753–3763 (2014).
Nie, F. et al. Long non-coding RNA MVIH indicates a poor prognosis for non-small cell lung cancer and promotes cell proliferation and invasion. Tumour Biol 35, 7587–7594 (2014).
Yang, F. et al. Repression of the long noncoding RNA-LET by histone deacetylase 3 contributes to hypoxia-mediated metastasis. Mol Cell 49, 1083–1096 (2013).
Gong, W. et al. Knockdown of NEAT1 restrained the malignant progression of glioma stem cells by activating microRNA let-7e. Oncotarget 19, 62208–62223 (2016).
Hodis, E. et al. A landscape of driver mutations in melanoma. Cell 150, 251–263 (2012).
Zhang, J. et al. Rich1 negatively regulates the epithelial cell cycle, proliferation and adhesion by CDC42/RAC1-PAK1-Erk1/2 pathway. Cellular signaling 27, 1703–1712 (2015).
Leng, R. et al. Rac1 expression in epithelial ovarian cancer: effect on cell EMT and clinical outcome. Medical oncology 32, 329 (2015).
Tay, Y., Rinn, J. & Pandolfi, P. P. The multilayered complexity of ceRNA crosstalk and competition. Nature 505, 344–352 (2014).
Salmena, L. et al. A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell 146, 353–358 (2011).
Yang, J. et al. FOXO1 3 UTR functions as a ceRNA in repressing the metastases of breast cancer cells via regulating miRNA activity. FEBS Lett 588, 3218–3224 (2014).
Grote, P. et al. The tissue-specific lncRNA Fendrr is an essential regulator of heart and body wall development in the mouse. Dev. Cell 24, 206–214 (2013).
Franco-Zorrilla, J. M. et al. Target mimicry provides a new mechanism for regulation of microRNA activity. Nat. Genet. 39, 1033–1037 (2007).
Cazalla, D., Yario, T. & Steitz, J. A. Down-regulation of a host microRNA by a Herpesvirus saimiri noncoding RNA. Science 328, 1563–1566 (2010).
Sun, J. et al. Long non-coding RNAs: critical play; ers in hepatocellular carcinoma. Int J Mol Sci 15, 20434–20448 (2014).
Shariff, M. I. F. et al. Hepatocellular carcinoma: current trends in worldwide epidemiology, risk factors, diagnosis and therapeutics. Expert Rev Gastroenterol Hepatol 3, 353–367 (2009).
Du, Y. et al. Elevation of highly upregulated in liver cancer (HULC) by hepatitis B virus X protein promotes hepatoma cell proliferation via down-regulating p18. J Biol Chem 287, 26302–26311 (2012).
Liu, Y. et al. A genetic variant in long non-coding RNA HULC contributes to risk of HBV-related hepatocellular carcinoma in a Chinese population. PLoS One 7, e35145 (2012).
Lai, M. et al. Long non-coding RNA MALAT-1 overexpression predicts tumor recurrence of hepatocellular carcinoma after liver transplantation. Med Oncol 29, 1810–1816 (2012).
Yin, Q. et al. MicroRNA miR-155 inhibits bone morphogenetic protein (BMP) signaling and BMP-mediated Epstein-Barr virus reactivation. Journal of virology 84, 6318–27 (2010).
Wang, P. et al. Micro-RNA-155 is induced by K-Ras oncogenic signal and promotes ROS stress in pancreatic cancer. Oncotarget 6, 21148–21158 (2015).
Babar, I. A. et al. Inhibition of hypoxia-induced miR-155 radiosensitizes hypoxic lung cancer cells. Cancer biology & therapy 12, 908–914 (2011).
Wang, J. et al. MicroRN14A-182 downregulates metastasis suppressor 1 and contributes to metastasis of; hepatocellular carcinoma. BMC cancer 12, 227 (2012).
Abdelrahman, M. M. et al. Enhancing NK cell cytotoxicity by miR-182 in hepatocellular carcinoma. Human immunology 77, 667–673 (2016).
Acknowledgements
The project was supported by Foundation of cutting-edge technologies of Henan province (162300410114)/(201702067)/(SB201902003) and National Natural Science Foundation of China (NO. SBGJ2018002).
Author information
Authors and Affiliations
Contributions
Each author contributed to the design, performance and analysis of the project. Specifically, Professor Wenzhi Guo and Professor Jianhua Li. were responsible for experimental planning and data analyses. Shouhua Zheng, Zhen Deng, Pengfei Xu, and Wenping Xue finish the in vitro experiments. Professor Xinguang Qiu and Dr. Danhua Zhang analysed and discussed data with the rest of the authors. Jianhua Li supervised the study and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, J., Guo, W., Xue, W. et al. Long noncoding RNA AURKAPS1 potentiates malignant hepatocellular carcinoma progression by regulating miR-142, miR-155 and miR-182. Sci Rep 9, 19645 (2019). https://doi.org/10.1038/s41598-019-56036-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-56036-3
This article is cited by
-
Non-coding RNAs and epithelial mesenchymal transition in cancer: molecular mechanisms and clinical implications
Journal of Experimental & Clinical Cancer Research (2022)
-
Modulation of the TGF-β signaling pathway by long noncoding RNA in hepatocellular carcinoma
Biomarker Research (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.