Abstract
Epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) are standard therapy for EGFR-mutant non-small cell lung cancer (NSCLC). Preclinically, HER3 ligand heregulin induces resistance to EGFR-TKIs, whereas the pan-human EGFR family inhibitor afatinib remains effective. Here, we examined whether soluble heregulin levels have clinical implications for EGFR-mutant NSCLC treated with EGFR-TKIs. Soluble heregulin was immunologically measured in plasma from EGFR-mutant NSCLC patients. Cutoff values were determined by 1-year PFS ROC curve. The relationship between soluble heregulin and PFS following EGFR-TKI therapy was analyzed by Cox proportional hazards model. Seventy-three patients were enrolled: 44 were treated with 1st-generation and 29 with 2nd-generation EGFR-TKIs. Soluble heregulin levels varied (range: 274–7,138 pg/mL, median: 739 pg/mL). Among patients treated with 1st-generation EGFR-TKIs, those with high heregulin (n = 20, >800 pg/mL) had a tendency for shorter PFS than those with low heregulin (n = 24, <800 pg/mL), with median PFS of 322 and 671 days, respectively. Cox proportional hazards model also indicated a trend toward resistance against 1st-generation EGFR-TKIs (HR: 1.825, 95% CI: 0.865–4.318) but not against 2nd-generation EGFR-TKIs. Soluble heregulin potentially correlates with resistance to EGFR-TKIs but not 2nd-generation EGFR-TKIs in patients with EGFR-mutant NSCLC.
Similar content being viewed by others
Introduction
Epidermal growth factor receptor (EGFR) is a critical molecular target of anti-cancer therapy in non-small cell lung cancer (NSCLC)1,2. Previous clinical trials have demonstrated that EGFR tyrosine kinase inhibitors (EGFR-TKIs), such as gefitinib and erlotinib, dramatically improve the survival of patients with NSCLC harboring EGFR-activating mutations3,4,5. EGFR genomic mutations alter its protein structure, at the site where ATP preferentially binds to its intracellular kinase domain, leading to spontaneous EGFR activation6,7. However, some tumors are refractory to EGFR-TKI therapy despite harboring EGFR-activating mutations. Even though tumors respond to EGFR-TKI therapy, they eventually become resistant to it. Several underlying mechanisms of resistance to EGFR inhibitors have already been identified8. In particular, the T790M secondary EGFR mutation was detected in approximately 50% of the patients with NSCLC harboring EGFR-activating mutations with acquired resistance to EGFR-TKIs9. Furthermore, other resistant mechanisms, including MET amplification, human EGFR 2 (HER2) amplification, and hepatocyte growth factor overexpression, have also been reported in NSCLC10,11,12. Intriguingly, MET aberrant expression leads to activation of HER3 and its downstream pathway, suggesting that HER3 plays a key role in EGFR-TKI resistance10. Based on these findings, it is imperative that novel treatment strategies are clinically investigated to overcome EGFR-TKI resistance.
Second- or third-generation EGFR-TKIs demonstrate superior clinical efficacy for EGFR-TKI-naïve patients compared to that of 1st-generation EGFR-TKI13,14,15. 2nd-generation EGFR-TKIs, including afatinib and dacomitinib, can irreversibly bind to EGFR and other HER family tyrosine kinases and thus are referred to as pan-HER inhibitors16,17. In randomized clinical trials, second-generation EGFR-TKIs were shown to significantly improve progression-free survival (PFS) as well as overall survival compared to first-generation EGFR-TKIs in patients with advanced NSCLC harboring EGFR-activating mutations13,14. However, second-generation EGFR-TKIs were not able to overcome EGFR T790M-induced resistance to EGFR-TKIs18. In fact, like those treated with first-generation EGFR-TKIs, the secondary EGFR T790M mutation is present in approximately 50% of patients treated with second-generation EGFR-TKIs19. In contrast to first- and second-generation EGFR-TKIs, third-generation EGFR-TKIs, such as osimertinib, have been shown to exhibit enhanced efficacy against NSCLC with the EGFR T790M mutation20,21. Furthermore, osimertinib significantly improves PFS in EGFR-TKI-naïve patients with NSCLC harboring EGFR-activating mutations compared to first-generation EGFR-TKIs15. However, despite pharmacodynamic improvements in EGFR-TKIs, a subset of patients with NSCLC harboring EGFR-activating mutations continue to exhibit resistance to EGFR-TKIs.
Heregulin is a ligand for HER3 and HER4 and is aberrantly overexpressed in cancer cells, including NSCLC or cancer-associated fibroblast cells22,23,24. Heregulin alters the conformational structure of its binding receptors and may activate HER3, HER4, and its coupling partner HER2 in cancer cells in an autocrine or paracrine manner25,26. Previously, preclinical studies have suggested that heregulin may cause resistance to first-generation EGFR-TKIs such as erlotinib in NSCLC patients harboring EGFR-activating mutations, as heregulin promotes HER2-HER3 coupling and activates anti-apoptotic HER2-HER3-Akt bypass signaling27. In contrast to 1st-generation EGFR-TKIs, 2nd-generation EGFR-TKIs such as afatinib or dacomitinib, unique pan-HER family inhibitors, have been shown preclinically to overcome heregulin-mediated resistance28. Heregulin expression varies in patients with NSCLC harboring EGFR-activating mutations, although its clinical implications are unclear, especially in terms of EGFR-TKI therapeutic efficacy28.
In the current study, we aimed to exploratively examine whether the soluble heregulin (sHRG) level in plasma has clinical implications for EGFR-TKI efficacy in patients with NSCLC harboring EGFR-activating mutations. First- and second-generation of EGFR-TKIs were assessed to determine their efficacy in patients with high heregulin expression.
Results
Patient characteristics and cutoff values for sHRG
A total of 73 patients with NSCLC harboring EGFR-activating mutations were enrolled in this study. All patients had been treated with EGFR-TKIs between February 2015 and July 2018. Of those patients, 44 patients received first-generation EGFR-TKIs (gefitinib or erlotinib), and 29 patients received second-generation EGFR-TKIs (afatinib or dacomitinib). Plasma samples had been collected prior to EGFR-TKI therapy in all 73 patients and was used for measuring sHRG protein levels. The sHRG distribution in these patients is shown in Fig. 1A. sHRG levels varied, ranging from 274 pg/mL (the lower limit of detection) to 7,138 pg/mL, with a median concentration of 739 pg/mL. Patients treated with first- or second-generation EGFR-TKIs were grouped together for analysis. The sHRG levels did not significantly differ between those groups (Fig. 1B). Baseline characteristics for all patients, as well as for the 1-generation EGFR-TKI population and the 2nd-generation EGFR-TKI population are shown in Table 1. Characteristics were similar between these two subpopulations, although those receiving second-generation EGFR-TKI therapy more frequently included smokers and patients with minor EGFR mutations, such as exon 20 insertion and exon 18 point mutations G719S, G719A, and G719C. Those characteristics did not significantly correlate with sHRG levels (Supplemental Fig. 1). The median PFS for the 1st-generation EGFR-TKI population and 2nd-generation EGFR-TKI population were 446 and 393 days, respectively, and a survival curve for each population is shown in Supplemental Fig. 2.
(A) Soluble heregulin expression in patients with NSCLC with EGFR-activating mutations. Soluble heregulin was measured in plasma obtained from patients prior to EGFR-TKI treatment by quantitative sandwich immune assay (n = 76). X-axis, individual patients; y-axis, plasma heregulin concentration, pg/mL. (B) Boxplot shows soluble heregulin expression for patients on 1st and 2nd generation EGFR-TKI. The Mann-Whitney test was used to compare differences between patients on 1st and 2nd generation EGFR-TKI.
For subsequent analysis of the 1st-generation EGFR-TKI population, 1-year PFS ROC curve analysis was performed for determining the cutoff values for classifying patients into sHRG-high and sHRG-low groups. The optimal cutoff value was located between 787 and 884 pg/mL; therefore a cutoff value of 800 pg/mL was selected, and 25 patients were classified into the sHRG-low group, with 22 patients classified into the sHRG-high group (Supplemental Fig. 3).
PFS in the 1st-generation EGFR-TKI population
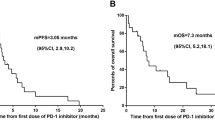
For the 1st-generation EGFR-TKI population, treatment efficacy as measured by PFS was shorter in the sHRG-high subgroup than in the sHRG-low subgroup (Fig. 2A). The median PFS of the sHRG-high and sHRG-low subgroups were 322 days and 671 days, respectively [hazard ratio (HR): 1.825; 95% CI: 0.865–4.318; log-rank test p-value = 0.1137]. Furthermore, Cox proportional hazards analysis for PFS showed that the sHRG-high subgroup tended to exhibit resistance to EGFR-TKI treatment, after correcting for several factors including age, performance status, type of EGFR mutation, and smoking (HR: 1.911; 95% CI: 0.837–4.360; p-value = 0.124, Fig. 2B).
Kaplan–Meier curves of progression-free survival in the 1st-generation EGF-TKI population. (A) Kaplan–Meier survival curve was drawn for patients classified as sHRG-high (n = 20) and sHRG-low (n = 24). (B) Cox proportional hazards model adjusted by factors including smoking, type of EGFR mutation, performance status, age, and heregulin expression.
PFS in the second-generation EGFR-TKI population
Subsequently, we examined whether resistance to second-generation EGFR-TKIs was similarly related to sHRG levels, as observed in the first-generation EGFR-TKI population. Twenty-nine patients were classified into sHRG-low (n = 17) and sHRG-high subgroups (n = 12) using the same cutoff value of 800 pg/mL as determined for the 1st-generation EGFR-TKI population. In contrast to the results for the first-generation EGFR-TKI population, the efficacy of second-generation EGFR-TKIs was more durable in the sHRG-high subgroup than in the sHRG-low subgroup (Fig. 3A). The median PFS of the sHRG-high and sHRG-low subgroups were 535 days and 228 days, respectively (HR: 0.5978; 95% CI: 0.262–1.298; log-rank test p-value = 0.2019). However, it should be noted that patients with minor EGFR mutations were frequently included in the sHRG-low subgroup. Cox proportional hazards regression analysis for PFS indicated that in the sHRG-high group, there was no obvious correlation between sHRG expression and EGFR-TKI resistance, after correcting for several factors including age, type of EGFR mutation, and smoking (HR: 0.879; 95% CI: 0.325–2.376; p-value = 0.799, Fig. 3B).
Kaplan–Meier curves of progression-free survival in 2nd-generation EGF-TKI population. (A) Kaplan-Meier survival curve was drawn for patients classified as sHRG-high (n = 12) and sHRG-low (n = 17). (B) Cox proportional hazards model adjusted by factors including smoking, type of EGFR mutation, age, and heregulin expression.
Discussion
In this study, we observed the potential implications of heregulin expression in EGFR-TKI–treated NSCLC patients who harbored EGFR-activating mutations. The efficacy of 1st-generation EGFR-TKIs was less durable in patients with high sHRG plasma levels than in patients with low sHRG plasma levels. Furthermore, Cox regression analysis showed that this tendency was maintained after adjusting for multiple influential factors such as PS, smoking history, and age29. This study generated a new hypothesis, which states that soluble heregulin levels might be associated with the limited efficacy of EGFR-TKIs in NSCLC patients who harbor EGFR-activating mutations.
This study could not confirm the statistical significance of the association between heregulin plasma levels and limitations in the efficacy of EGFR-TKIs. Moreover, the hazard ratio for PFS crossed 1.0 in the 1st-generation subgroup of EGFR-TKI patients. Our previous preclinical study suggested that heregulin expression causes EGFR-TKI resistance in EGFR-mutant NSCLC27. However, the degree of heregulin influence in clinical situations remains unknown. Thus, the optimal cutoff point for high heregulin expression levels could not be determined. For those reasons, we could not statistically determine appropriate sample sizes prior to this study. A subsequent study is warranted for validating our new hypothesis with statistically appropriate sample sizes in order to optimize EGFR-TKI therapy in patients with EGFR-mutant NSCLC.
Recently, the 3rd-generation EGFR-TKI osimertinib was shown to significantly improve PFS and overall survival rates in EGFR-TKI–naive patients compared to 1st generation EGFR-TKIs15,30. However, a preclinical study demonstrated that heregulin-expressing NSCLC cells are resistant to osimertinib (Supplement Fig. 4). Considering those results, the implications of heregulin expression should be investigated in osimertinib-treated patients with EGFR-mutant NSCLC.
This study is the first to report the clinical implications of heregulin expression in EGFR-TKI–treated NSCLC patients harboring EGFR-activating mutations; the observation indicates a prognostically unfavorable influence of heregulin. However, in the other cohort, we did not find any prognostic influence of heregulin in NSCLC (Supplemental Fig. 5). Thus, although this study had a limited sample size, we speculate that sHRG levels may predict resistance to EGFR-TKIs in this population. A previous preclinical study found that induced expression of the heregulin gene worsened the sensitivity to the EGFR-TKI erlotinib of an NSCLC cell line harboring an EGFR-activating mutation27. Although the current study did not evaluate heregulin expression levels in tumors, sHRG found in plasma may be potentially produced in tumors and may limit the efficacy of EGFR-TKIs. The current clinical observations have not yet been validated in other cohorts. However, similar to the findings of the current study, patients with advanced colorectal cancer with high plasma sHRG levels exhibited shorter PFS following anti-EGFR antibody therapy than those with low sHRG31. Considering these observations, sHRG expression may potentially be associated with resistance to EGFR-TKIs, regardless of cancer type, and confirmation of this relationship in an unbiased study is warranted.
In contrast to 1st-generation EGFR-TKIs, 2nd-generation EGFR-TKIs showed no obvious relationship between heregulin expression and EGFR-TKI resistance according to the Cox proportional hazards model. Although the sample size was small, this difference between the generations of EGFR-TKIs may be caused by differences in pharmacological action. Specifically, the anti-cancer effects of second-generation EGFR-TKIs on heregulin-expressing cancer cells by be sustained by pan-HER family inhibition. In fact, a preclinical study demonstrated that the second-generation EGFR-TKI afatinib uniquely decreased EGFR activation, as well as that of HER2, HER3, HER4, and their downstream Akt phosphorylation, in heregulin-expressing cancer cells, overcoming heregulin-mediated resistance28. Furthermore, second-generation EGFR-TKIs have demonstrated a superior survival benefit compared to that of first-generation EGFR-TKIs in randomized clinical trials, and the mechanism of this effect was considered to be an advantage in pan-HER family inhibition specific to second-generation EGFR-TKIs13,14. Although other mechanisms, such as a HER2 genomic amplification, may activate HER family members other than EGFR, heregulin may potentially play a critical role in HER2, 3, and 4 activation. Considering this, NSCLC patients with heregulin expression may be an optimal subpopulation for second-generation EGFR-TKI treatment.
The current study did not examine whether sHRG levels were correlated with tumor heregulin expression. However, we previously examined this relationship in another cohort of NSCLC patients but did not observe a significant correlation32. This may imply heterogeneous heregulin expression levels among tumors. Alternatively, the sHRG level in the plasma may be influenced by the tumor burden, such as the size of the tumor or the number of metastases. Moreover, the measurement of sHRG for evaluating the local heregulin expression in tumors may be technically limited, whereas the current results imply that sHRG levels may be advantageous for evaluating systemic heregulin expression and may by proxy reflect resistance to EGFR-TKIs.
In conclusion, results of the current study suggest the potential clinical implications of heregulin expression in EGFR-TKI treatment–naive NSCLC patients with EGFR-activating mutations. Specifically, sHRG levels potentially correlate with resistance to first-generation EGFR-TKIs, but not to second-generation EGFR-TKIs capable of pan-HER family inhibition.
Methods
Study design
This was a retrospective cohort study. Patients were eligible for enrollment in the study if they had histologically confirmed NSCLC with EGFR mutation; had undergone stage IIIB/IV, post-operative, or radiation therapy; had measurable disease (per Response Evaluation Criteria in Solid Tumors guidelines, version 1.1); and had been treated with an EGFR-TKI without prior EGFR-TKI therapy33. EGFR-TKI re-challenge therapy or secondary EGFR-TKI therapy was not eligible. The following EGFR-TKIs were included: gefitinib, erlotinib, afatinib, and dacomitinib. The primary objective was to assess the correlation between the heregulin level and PFS in patients treated with each generation of EGFR-TKIs. The protocol was approved by the institutional review boards of the participating institutions including the Institutional Review Board of Kindai University Faculty of Medicine, Kyushu University Institutional Review Board for Clinical Research, the Institutional Review Board of Kurume University, and the Institutional Review Board of the Kinki-Chuo Respiratory Medical Center. Subjects provided written informed consent, including consent to provide plasma samples for assessment of heregulin expression. All methods including sHRG measurement were performed in accordance with Ethical Guidelines for Medical and Health Research Involving Human Subjects.
Biomarker assay for sHRG
Plasma samples were obtained from participants prior to EGFR-TKI treatment. sHRG was measured using a validated quantitative sandwich immune assay using a commercially available kit (NRG1 beta 1 human ELISA Kit, Abcam, Cambridge, MA, USA) according to our modified method28. Specifically, a 96-well microplate coated with anti-NRG1-β1 capture antibody was incubated with samples and standards. The plate was washed, probed with anti-NRG1-β1 detection antibody, and labeled with a chromogen. Finally, the optical densities of samples and standards were determined using a spectrophotometric microplate reader at 450 nm. The sHRG concentration of each sample was determined based on standard curves.
Statistical analyses
PFS was defined as the duration from the initiation of EGFR-TKI therapy until tumor progression or death from any cause. Kaplan–Meier curves were generated for PFS and used to calculate the median and 95% CI of each treatment group. Two-sided p-values were determined by log-rank test, and hazard ratios (and 95% CIs) were determined by the Cox proportional hazards model, stratified by age, smoking history, clinical stage, type of EGFR mutation, and Eastern Cooperative Oncology Group performance status. Analyses were performed using SPSS (version 22, SPSS Inc., Chicago, IL, USA). Data were graphically displayed using GraphPad Prism v.5.0 for Windows (GraphPad Software, Inc., La Jolla, CA, USA).
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
References
Zhou, C. & Yao, L. D. Strategies to improve outcomes of patients with EGFR-mutant non–small cell lung cancer: review of the literature. J Thorac Oncol. 11, 174–186 (2016).
Mitsudomi, T., Kosaka, T. & Yatabe, Y. Biological and clinical implications of EGFR mutations in lung cancer. Int J Clin Oncol. 11, 190–198 (2006).
Maemondo, M. et al. North-East Japan Study Group. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 362, 2380–2388 (2010).
Mitsudomi, T. et al. West Japan Oncology Group. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 11, 121–128 (2010).
Mok, T. S. et al. Gefitinib or carboplatin–paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 361, 947–957 (2009).
Paez, J. G. et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 304, 1497–500 (2004).
Lynch, T. J. et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 350, 2129–2139 (2004).
Sequist, L. et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 3, 75ra26 (2011).
Kobayashi, S. et al. EGFR mutation and resistance of non-small cell lung cancer to gefitinib. N Engl J Med. 352, 786–792 (2005).
Engelman, J. A. et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 316, 1039–1043 (2007).
Takezawa, K. et al. HER2 amplification: a potential mechanism of acquired resistance to EGFR inhibition in EGFR-mutant lung cancers that lack the second-site EGFRT790M mutation. Cancer Discov. 2, 922–933 (2012).
Yano, S. et al. Hepatocyte growth factor induces gefitinib resistance of lung adenocarcinoma with epidermal growth factor receptor-activating mutations. Cancer Res. 68, 9479–9487 (2008).
Park, K. et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol. 17, 577–589 (2016).
Wu, Y. L. et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol. 18, 1454–1466 (2017).
Soria, J. C. et al. FLAURA Investigators. Osimertinib in untreated EGFR-mutated advanced non–small-cell lung cancer. N Engl J Med. 378, 113–125 (2018).
Li, D. et al. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene. 27, 4702–4711 (2008).
Engelman, J. A. et al. PF00299804, an irreversible pan-ERBB inhibitor, is effective in lung cancer models with EGFR and ERBB2 mutations that are resistant to gefitinib. Cancer Res. 67, 11924–11932 (2007).
Katakami, N. et al. LUX-Lung 4: A Phase II trial of afatinib in patients with advanced non–small-cell lung cancer who progressed during prior treatment with erlotinib, gefitinib, or both. J Clin Oncol. 31, 3335–3341 (2013).
Tanaka, K. et al. Acquisition of the T790M resistance mutation during afatinib treatment in EGFR tyrosine kinase inhibitor–naïve patients with non–small cell lung cancer harboring EGFR mutations. Oncotarget. 8, 68123–68130 (2017).
Jänne, P. A. et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med. 372, 1689–1699 (2015).
Cross, D. A. et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. 4, 1046–1061 (2014).
Zhou, B. B. et al. Targeting ADAM-mediated ligand cleavage to inhibit HER3 and EGFR pathways in non-small cell lung cancer. Cancer Cell. 10, 39–50 (2006).
Mendella, J. et al. Clinical translation and validation of a predictive biomarker for patritumab, an anti-human epidermal growth factor receptor 3 (HER3) monoclonal antibody, in patients with advanced non-small cell lung cancer. EBioMedicine. 2, 264–271 (2015).
Capparelli, C., Rosenbaum, S., Berger, A. C. & Aplin, A. E. Fibroblast-derived neuregulin 1 promotes compensatory ErbB3 receptor signaling in mutant BRAF melanoma. J Biol Chem. 290, 24267–24277 (2015).
Kawakami, H. & Yonesaka, K. HER3 and its ligand, heregulin, as targets for cancer therapy. Recent Pat Anticancer Drug Discov. 11, 267–274 (2016).
Breuleux, M. Role of heregulin in human cancer. Cell Mol Life Sci. 64, 2358–2377 (2007).
Yonesaka, K. et al. Anti-HER3 monoclonal antibody patritumab sensitizes refractory non-small cell lung cancer to the epidermal growth factor receptor inhibitor erlotinib. Oncogene. 35, 878–886 (2016).
Yonesaka, K. et al. The pan-HER family tyrosine kinase inhibitor afatinib overcomes HER3 ligand heregulin-mediated resistance to EGFR inhibitors in non-small cell lung cancer. Oncotarget. 6, 33602–33611 (2015).
Zhang, Y. et al. Impact of smoking status on EGFR-TKI efficacy for advanced non–small-cell lung cancer in EGFR mutants: a meta-analysis. Clin Lung Cancer. 16, 144–151.e1 (2015).
Ramalingam, S. S. et al. Osimertinib vs comparator EGFR-TKI as first-line treatment for EGFRm advanced NSCLC (FLAURA): final overall survival analysis. Ann Oncol. 30, Supplement 5 (2019).
Yonesaka, K. et al. Activation of ERBB2 signaling causes resistance to the EGFR-directed therapeutic antibody cetuximab. Sci Transl Med. 3, 99ra86 (2011).
Yonesaka, K. et al. Circulating heregulin level is associated with the efficacy of patritumab combined with erlotinib in patients with non-small cell lung cancer. Lung Cancer. 105, 1–6 (2017).
Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 45, 228–247 (2009).
Acknowledgements
The authors are grateful to the staff at the investigator sites for their support of this study, and to the patients who participated in this study. This work was supported by a research fund from Boehringer Ingelheim. The funding source had no involvement in the preparation of the article, including the study design, analysis, or interpretation of data.
Author information
Authors and Affiliations
Contributions
K.Y., H.H., Y.C. and K.N. designed the study. K.Y., E.I., H.H., S.S., R.K., S.W., T.T., J.T., K.T., M.T., K.A., S.A. and I.O. collected clinical data. K.Y. and K.S. performed biomarker assay for sHRG. K.Y., E.I., K.N., I.O. and K.N. critically revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
Dr. Iwama reports personal fees from AstraZeneca. Atagi reports grants and personal fees from Boehringer Ingelheim, grants and personal fees from MSD, grants and personal fees from Chugai, grants and personal fees from AstraZeneca, grants and personal fees from Taiho, grants and personal fees from Ono, grants and personal fees from Pfizer, grants and personal fees from Bristol-Myers Squibb, personal fees from Hisamitsu. Azuma reports personal fees from Boehringer Ingelheim, personal fees from AstraZeneca. Hayashi reports grants and personal fees from Boehringer Ingelheim, grants and personal fees from AstraZeneca, grants from Chugai. Nishio reports grants from Boehringer Ingelheim. Takahama reports personal fees from Boehringer Ingelheim, personal fees from AstraZeneca. Takeda reports personal fees from Boehringer Ingelheim. Tanaka reports personal fees from Boehringer Ingelheim, personal fees from AstraZeneca, personal fees from Bristol-Myers Squibb, personal fees from MSD Oncology. Tanizaki reports personal fees from Boehringer Ingelheim, personal fees from AstraZeneca, personal fees from Taiho Pharmaceutical, personal fees from Bristol-Myers Squibb, personal fees from Eli Lilly, personal fees from MSD Oncology. Dr. Okamoto reports grants and personal fees from AstraZeneca, grants and personal fees from Taiho Pharmaceutical, grants and personal fees from Boehringer Ingelheim, grants and personal fees from Ono Pharmaceutical, grants and personal fees from MSD Oncology, grants and personal fees from Lilly, grants from Astellas Pharma, grants and personal fees from Bristol-Myers Squibb, grants from Novartis, grants and personal fees from Chugai Pharma, personal fees from Pfizer, grants from AbbVie. Yonesaka reports grants and personal fees from Boehringer Ingelheim. Nakagawa reports grants and personal fees from AstraZeneca, grants and personal fees from Taiho Pharmaceutical, grants and personal fees from Boehringer Ingelheim, grants and personal fees from Ono Pharmaceutical, grants and personal fees from MSD Oncology, grants and personal fees from Lilly, grants and personal fees from Astellas Pharma, grants and personal fees from Bristol-Myers Squibb, grants from Novartis, grants and personal fees from Chugai Pharma, personal fees from Pfizer, grants from AbbVie, grants and personal fees from Takeda Pharma, grants and personal fees from Daiichi-sankyo.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yonesaka, K., Iwama, E., Hayashi, H. et al. Heregulin expression and its clinical implication for patients with EGFR-mutant non-small cell lung cancer treated with EGFR-tyrosine kinase inhibitors. Sci Rep 9, 19501 (2019). https://doi.org/10.1038/s41598-019-55939-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-55939-5
This article is cited by
-
Osimertinib resistance prognostic gene signature: STRIP2 is associated with immune infiltration and tumor progression in lung adenocarcinoma
Journal of Cancer Research and Clinical Oncology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.