Abstract
Titanium dioxide represents one of the most widely studied transition metal oxides due to its high chemical stability, non-toxicity, abundance, electron transport capability in many classes of optoelectronic devices and excellent photocatalytic properties. Nevertheless, the wide bang gap of pristine oxide reduces its electron transport ability and photocatalytic activity. Doping with halides and other elements has been proven an efficient defect engineering strategy in order to reduce the band gap and maximize the photocatalytic activity. In the present study, we apply Density Functional Theory to investigate the influence of fluorine and chlorine doping on the electronic properties of TiO2. Furthermore, we present a complete investigation of spin polarized density functional theory of the (001) surface doped with F and Cl in order to elaborate changes in the electronic structure and compare them with the bulk TiO2.
Similar content being viewed by others
Introduction
Transition metal oxides such as titanium dioxide (TiO2) are considered as an extremely important class of materials due to their intense catalytic activity, superior chemical stability, long life cycle and high electrical conductivity1,2,3,4,5,6,7,8,9,10. Particularly, anatase TiO2 has been extensively studied because of its abundance and non-toxicity, high photocatalytic activity and responseto visible light arising from high its absorption coefficient and reflectivity1,2,3,4,5,6,7,8,9,10. Moreover, it represents the work-horse photoanode material in dye-sensitized solar cells (DSSCs)11,12,13,14,15,16, whereas recently has been widely applied as the device electron transport material in both organic-inorganic halide perovskite (PSC) and organic solar (OSC) cells17,18,19,20,21,22,23,24,25. Nevertheless, TiO2 exhibits a wide band gap of around 3.2 eV26,27 that limits its absorption in the visible and, especially, in the near infrared (NIR) region7. A common way to reduce the band gap of TiO2 is through doping with appropriate elements which has also significant impact on its electronic structure. A vast variety of literature reports previously demonstrated that by doping TiO2 with nitrogen (N), halogens such as fluorine (F) and chlorine (Cl), or several transition metal ions such as zinc (Zn) or nickel (Ni), significant changes in its electronic structure are observed28,29,30,31,32,33,34. Particularly, the formation of mid gap states resulting in a band gap reduction was evident. For instance, when titanium dioxide is doped with Ni the band gap decreases to 2.57 eV,33 whereas when it is doped with Cl mid gap states are formed and the band gap decreases to 3 eV31. Progress on the photocatalytic performance and heterogeneous catalysis of TiO2 and other oxides such as ZnO has also been accomplished by the introduction of oxygen vacancies35,36 combined with metallic doping.
Focusing on other defect related projects regarding the TiO2 and its application to photocatalysis, it is seen that TiO2 has a fast recombination of the conduction band electrons and valence band holes and as a result it is not a satisfactory photocatalytic for organic degradation. In order to solve that problem as well as to reduce the large band gap, it is seen in the literature that many doped models of TiO2 can have improved photocatalysis. For example, N doped TiO237 and Nb doped TiO238 is reported to have better photocatalytic properties than pure TiO2.
Although a profound band gap reduction can be beneficial to the material’s photocatalytic activity as it results in higher absorption of visible light, it might create mid gap states that usually act as charge traps hence having a negative impact on the performance of organic and perovskite solar cells utilizing TiO2 exclusively as electron transport/extraction material39. In those cases the photocatalytic ability of TiO2 should be suppressed as it degrades its interface with organic/perovskite semiconductor. The formation of mid gap states upon doping of TiO2 is therefore undesired in OSCs and PSCs. In the present study, fluorine and chlorine doping of the bulk and surface TiO2 is studied via Density Functional Theory (DFT) in order to examine the electrical structure before and after doping with F and Cl and investigate the potential improvement in the photocatalytic activity of the TiO2. Moreover we investigated many different defect sites for the F and Cl in the bulk system and we also calculated for the first time the interstitial sites and the changes in electrical properties of the (001) TiO2 surface after the F and Cl doping. Total density of states (DOS) and partial DOS (PDOS) of the energetically minimum sites of the defects are considered in order for the electrical structure changes to be fully understood. A band gap reduction is evident in both cases. Moreover, the formation of mid gap states in all cases is also predicted. Such states are highly beneficial for the photocatalytic applications of TiO2 though they can be detrimental for OSCs and PSCs performance as they constitute trap sites for the photogenerated charge carriers thus significantly reducing the device photocurrent.
Results and Discussion
Bulk anatase TiO2
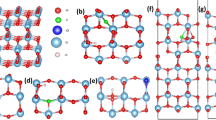
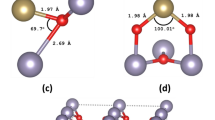
There are three polymorphs of TiO2 (rutile, anatase and brookite) with the anatase being the prevalent choice for photovoltaic applications as it has superior photocatalytic properties40. The crystal structure for the anatase is tetragonal with space group I4/amd and its experimental structural parameters calculated from neutron diffraction are a = 3.782 Å, b = 3.782 Å and c = 9.502 Å41. Our theoretically calculated lattice parameters of the anatase are a = 3.804 Å, b = 3.804 Å and c = 9.729 Å which also agree with other theoretical results42,43. The percentage of our dopants was 1 dopant atom per 109 atoms of TiO2 (0.91% doping). In the surface system we calculated 1 dopant atom per 96 atoms of TiO2 (1.04% additional doping). As regards the density of F and Cl atoms in the bulk systems, we calculated that for the F interstitial we have a density of 7.863 1020 cm−3, for the F substitutional we have a density of 7.872∙1020 cm−3 and lastly for the Cl interstitial and Cl substitutional we have a density of 7.825∙1020 cm−3 and 7.829 1020 cm−3 respectively. On the other hand, for the surfaces, we calculated a density of 4.451∙1020 cm−3 for the F interstitial and 4.423∙1020 cm−3 for the Cl interstitial. According to our DFT calculations, the F atom is stable either as an oxygen substitutional defect or an interstitial defect in the bulk TiO2 system. We found that in the case of interstitial, the fluorine atom is located at a distance of 1.983 Å from the nearest oxygen atom (see Figs. 1a and 2a) in agreement with previous studies33. Examining thoroughly the various defect formations, we concluded that the minimum energy configuration is when fluorine occupies an oxygen site displacing the oxygen to an interstitial site, as seen in Figs. 1b and 2b. Moreover, the simple substitution of an O atom with F has also been examined (Figs. 1c, 2c). Concerning Cl atom doping of bulk TiO2, we show that the Cl can either substitute an oxygen atom or relax in a substitutional position, at a distance of 2.15 Å from the nearest oxygen atom (Figs. 1d,e and 2d,e).
(a) The minimum energy structure of fluorine interstitial doped anatase TiO2, (b) The minimum energy structure of the F doped bulk TiO2 where F occupies an oxygen site, (c) The minimum energy structure of the substitutional F on the bulk anatase TiO2, (d) The minimum energy structure of the chlorine substitutional doped bulkanatase TiO2, (e) The minimum energy structure of the chlorine interstitial doped bulk anatase TiO2.
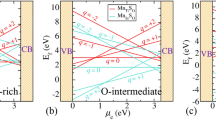
For each supercell, we calculated the DOS (Fig. 3(a)–(f)) and, in Fig. 3a, the total DOS of the pure TiO2 is shown as a reference. Our calculations were performed with DFT + U model with the Hubbard-U parameter equal to 8.2 eV33,34. We calculated the band gap at 3.14 eV, in agreement with previous theoretical studies33,34,44, which show a band gap narrowing from 3 to 3.16 eV and close to the experimental value of 3.2 eV.
(a) DOS of the undoped bulk anatase TiO2, (b) DOS of interstitially F - doped TiO2 (c) DOS of F:TiO2 with F substituting an Oxygen site and displacing it to an interstitial site. (d) DOS of the F:TiO2 when F is a substitution to O, (e) The Density of States graph of the Cl-Doped TiO2 with Cl as an interstitial, (f) The Density of States graph of the Cl:TiO2 when Cl is a substitution to O.
As we show in Fig. 3b, the F interstitial in the bulk TiO2 gives rise to a small peak inside the band gap at approximately 0.98 eV above the valence band (VB) and it also decreases the band gap to 3.04 eV. When an oxygen atom is replaced by a fluorine, the mid-gap impurity band is shifted towards the conduction band (Fig. 3d). Figure 4(a)–(d) represents the PDOS of doped and undoped bulk TiO2. In order to fully understand the emergence of the mid-gap peak in the case of the F interstitial, we calculated the partial DOS which is shown in Fig. 4a. Figure 5(a)–(f) examines in more detail the PDOS and the orbital contribution to the mid gap rise. There, one can see that the conduction band is mainly attributed to Ti while the valence band to O. In more detail,in Fig. 5a, it is shown that the main contributions to the valence and the conduction bands are attributed mainly to the O-2p and Ti-3d respectively. As far as the rise of mid-gap states (Fig. 5b) is concerned, we found that it is mainly attributed to the O-2p and F-2p orbitals. Concerning the minimum energy configuration with a fluorine replacing an oxygen which is displaced to an interstitial, two mid-gap levels emerge, one at 0.48 eV and one at 2.02 eV above the VB maximum. Moreover, it is seen that the band gap decreases by approximately 0.10 eV, reaching a value of 3.04 eV. To shed more light on these mid-gap peaks, a PDOS calculation was conducted again and presented in Fig. 4b. As one can see in Fig. 5d, the main contributions to these mid-gap levels come from O-2p and Ti-3d with and only a minimal contribution originates from fluorine, unlike the previous case of the F interstitial (Fig. 5b). A similar DFT research was conducted by Valentin and Pacchioni45 and they calculated that when F is inserted as a substitutional defect, the band gap is reduced to 3.08 eV which is also in a good agreement with the present results. Samsudin and Hamid46 have done an important experimental work on the band gap engineering of anion doped TiO2 and they calculated that the band gap of TiO2 is significantly decreased to 3.02 eV, which is again consistent with the present DFT work.
(a) The PDOS coming from the orbitals for the bulk F:TiO2, (b) The orbitals contribution to the mid gap rise of the bulk F:TiO2, (c) The PDOS from the orbitals of F:TiO2 when F occupies an oxygen position and displaces the oxygen to an interstitial site, (d) the orbitals contribution to the mid gap rise of F:TiO2 when F occupies an oxygen site and displaces oxygen to an interstitial, (e) The PDOS coming from the orbitals for the bulk Cl:TiO2, (f) The orbitals contribution to the mid gap rise of the bulk Cl:TiO2.
For the bulk Cl:TiO2, in the case of a Cl interstitial, the DOS (Fig. 3d) shows peaks at 0.19 eV and 1.38 eV above the conduction band minimum and, in addition, the band gap of the bulk TiO2 is significantly reduced to 2.89 eV. Looking at the PDOS of the bulk with Cl interstitial, we see that the valence and the conduction bands mainly consist of Ti-3d and O-2p and the mid-gap peak of O-2p and Cl-2p states. For the case of Cl substituting an O, one can see that a mid-gap peak appears again, at 1.77 eV, while the band gap is reduced to 2.84 eV (Figs. 1d and 2f). Focusing on the experimental work of Sun et al.47 it is seen that the band gap of the Cl:TiO2 reaches a value of 2.98 eV. This experimental work is also in agreement with our calculations, therefore it is suggested that band gap engineering of TiO2 can be used to a number of applications such as photocatalysis.
Surface of anatase TiO2
In order to develop better photocatalytic materials with visible light response and high activity, such as TiO2, more attention should be paid to surface doping with atoms or molecules. However, theoretical investigations of the effect of the surface doping on the surface electronic structure of TiO2 are at present scarce compared to the studies concerning the bulk system48. The aim of this section is to investigate the structural and electronic properties of the F- and Cl- doped TiO2 surface by using a DFT + U, in spin polarized calculations. We calculated the interatomic distances and angles, the electronic density of states of the undoped and doped TiO2 surface, as well as the changes of the band gap when the system is doped. For the simulation of the surfacewe used a slab model with a vacuum of 14 Å thickness vertical to the (001) plane and with periodic boundary conditions in the other directions. In our surface system, the top 4 layers were fully relaxed while the bottom 4 layers were kept fixed in order to simulate the bulk area. We chose this particular surface because it is a common choice in other studies concerning the absorption of different atoms and molecules, like CO249, but the interstitial-doping with fluorine or chlorine atoms has never been studied before. Instead, the (101) surface has mostly been considered for fluorine and chlorine doping50. However, the (001) TiO2 surface is considered one of the most highly energetic surfaces of the TiO2 and, as a result, it often plays the role of the active site in photocatalytic reactions51,52,53,54,55. Τo the best of our knowledge only Zhou p. et al.56 have studied the TiO2 (001) surface in the case of F doping as a substitution of an O atom. The undoped configuration is shown in Fig. 6a while the minimum energy structures for F and Cl doped TiO2 (001) surfaces are shown in Fig. 6b,c respectively.
According to our DFT calculations, when fluorine is inserted in the (001) surface as an interstitial (Fig. 7a), it is located at a distance of 2.00 Å from the nearest oxygen while Cl (Fig. 7b) at 2.18 Å. Looking at the DOS for each supercell, in Fig. 8a it is seen that the F interstitial at the surface produces a small impurity band peak at approximately 0.35 eV above the VB. On the other hand, as it is seen in Fig. 8b, when the surface is doped with a Cl atom, two mid-gap peaks arise, which are observed at approx. 0.55 eV and 0.93 eV above the valence band. Concerning the band gap, when the TiO2 surface is doped with F, the band gap is predicted at a value of 2.24 eV whereas for the Cl–doped surface, the calculated band gap reaches a value of 2.31 eV. The band gap of the undoped TiO2 surface calculated at 2.37 eV(Fig. 8c) in agreement with other theoretical studies concerning (001) TiO2 surfaces57. Therefore, the insertion of F or Cl on the (001) surface, has a minor effect on the band gap of the TiO2 system.
Conclusions
DFT calculations were performed for fluorine and chlorine-doped anatase TiO2 bulk and surface structures in order to evaluate the effect this kind of doping on the band gap and the electronic structure of TiO2. Both substitutional and interstitial halogen defects were predicted. In all cases, occupied mid-gap states attributed to a hybridization of O-2p with halogen 1 s orbitals were also predicted. Although such states are beneficial for the oxide’s photocatalytic activity as they significantly reduce the optical band gap with respect to that of undoped TiO2, they might be detrimental for its application as electron transport material in other classes of photovoltaic devices such as organic and perovskite solar cells.
Methods
Computational methodology
We performed periodic DFT calculations using the CASTEP program58,59. The Perdew, Burke and Ernzerhof (PBE)60 generalized gradient approximation (GGA) functional was employed for the exchange and correlation interactions with ultrasoftpseudopotentials61. The cut-off Energy was chosen at 480 eV and a 3 × 3 × 1 Monkhorst-Pack (MP)62 k-points mesh while supercells of 108 atoms were adopted for the bulk system.The structure was optimized with the Broyden-Fletcher-Goldfarb-Shanno (BFGS) method. To consider the effects of electronlocalization, the DFT + U method was employed for spin-polarized calculations with on-site Coulomb repulsions of 8.2 eV for the 3d orbitals of Ti. Finally, for the DOS calculations, a 3 × 3 × 3 k-points mesh of was adopted while for the PDOS a 7 × 7 × 7. The efficacy of the present approach has been demonstrated in recent studies10,11. For the surface structures, a supercell consisting of 96 atoms was used, with an energy cut off of 480 eV and a MP k-point mesh of 2 × 2 × 1. Finally, for the density of states, we chose a 7 × 7 × 7 k-point mesh.
References
Fujishima, A. & Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 238, 5358 (1972).
Gratzel, M. Photoelectrochemical cells. Nature 414, 338–344 (2001).
Asahi, R., Morikawa, T., Ohwaki, T., Aoki, K. & Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 293, 269–271 (2001).
Khan, S. U. M., Al-Shahry, M. & Ingler, W. B. Efficient photochemical water splitting by a chemically modified n-TiO2. Science 297, 2243–2245 (2002).
Russo, S. P., Grey, I. E. & Wilson, N. C. Nitrogen/hydrogen codoping of anatase: A DFT study. J. Phys. Chem. C 112, 7653–7664 (2008).
Yang, H. G. et al. Anatase TiO2 single crystals with a large percentage of reactive facets. Nature 453, 638–641 (2008).
Gai, Y., Li, J., Li, S.-S., Xia, J.-B. & Wei, S.-H. Design of narrow-gap TiO2: A passivated codoping approach for enhanced photoelectrochemical activity. Phys. Rev. Lett. 102, 036402 (2009).
Dou, L. et al. Tandem Polymer Solar Cells Featuring a Spectrally Matched Low-Bandgap Polymer. Nat. Photonics 6, 180–185 (2012).
Sivula, K. & van de Krol, R. Semiconducting materials for photoelectrochemical energy conversion. Nat. Mater. Rev. 1, 15010 (2016).
Zhu, J. et al. Intrinsic defects and H doping in WO3. Sci. Rep. 7, 40882 (2017).
Qiu, Y., Chen, W. & Yang, S. Double-layered photoanodes from variable-size anatase TiO2 nanospindles: a candidate for high-efficiency dye-sensitized solar cells. AngewandteChemie International Edition 49(21), 3675–3679 (2010).
Ullattil, S. G. et al. Sol-solvothermal processed ‘black TiO2 asphotoanode material in dye sensitized solar cells. Sol. Energy 155, 490–495 (2017).
Govindaraj, R., Santhosh, N., Pandian, M. S., Ramasamy, P. & Sumita, M. Fabrication of stable dye-sensitized solar cell with hydrothermally synthesized titanium dioxide nanorods as a photoanode material. J. Mater. Sci. Mater. Electron. 29, 3736–3743 (2018).
Sauvage, F. et al. Hierarchical, TiO2 photoanode for dye-sensitized solar cells. Nano Lett. 10(7), 2562–2567 (2010).
Zhu, K., Neale, N. R., Miedaner, A. & Frank, A. J. Enhanced charge-collection efficiencies and light scattering in dye-sensitized solar cells using oriented TiO2 nanotubes arrays. Nano Lett. 7(1), 69–74 (2007).
Mor, G. K., Shankar, K., Paulose, M., Varghese, O. K. & Grimes, C. A. Use of highly-ordered TiO2 nanotube arrays in dye-sensitized solar cells. Nano Let. 6(2), 215–218 (2006).
Wang, Z. et al. Enhanced performance of perovskite solar cells by ultraviolet-ozone treatment of mesoporous TiO2. Appl. Surf. Sci. 436, 596–602 (2018).
Zhang, D., Xie, F., Lin, P. & Choy, W. C. Al-TiO2 composite-modified single-layer graphene as an efficient transparent cathode for organic solar cells. ACS Nano 7(2), 1740–1747 (2013).
Seo, H. O. et al. Ultrathin TiO2 films on ZnO electron-collecting layers of inverted organic solar cell. J. Phys. Chem. C115(43), 21517–21520 (2011).
Zhang, D. et al. Plasmonic electrically functionalized TiO2 for high‐performance organic solar cells. Adv. Funct. Mater. 23(34), 4255–4261 (2013).
Lira-Cantu, M., Chafiq, A., Faissat, J., Gonzalez-Valls, I. & Yu, Y. Oxide/polymer interfaces for hybrid and organic solar cells: Anatase vs. Rutile TiO2. Sol. Energ. Mat. Sol. Cells 95(5), 1362–1374 (2011).
Lin, Z., Jiang, C., Zhu, C. & Zhang, J. Development of inverted organic solar cells with TiO2 interface layer by using low-temperature atomic layer deposition. ACS Appl. Mater. Interfaces 5(3), 713–718 (2013).
Green, M. A., Ho-Baillie, A. & Snaith, H. J. The emergence of perovskite solar cells. Nat. Photonics 8(7), 506–514 (2014).
Kim, H. S. & Park, N. G. Parameters affecting I–V hysteresis of CH3NH3PbI3perovskite solar cells: effects of perovskite crystal size and mesoporous TiO2 layer. J. Phys. ChemLett. 5(17), 2927–2934 (2014).
Pathak, S. K. et al. Performance and stability enhancement of dye-sensitized and perovskite solar cells by Al doping of TiO2. Adv. Funct. Mater. 24(38), 6046–6055 (2014).
Serpone, N. Is the Band Gap of Pristine TiO2 Narrowed by Anion- and Cation-Doping of Titanium Dioxide in Second-Generation Photocatalysts? J. Phys. Chem. B 110, 24287–24293 (2006).
Vasilopoulou, M. et al. Hydrogen and nitrogen codoping of anatase TiO2 for efficiency enhancement in organic solar cells. Sci. Rep. 7(1), 17839 (2017).
Wang, J. et al. Origin of photocatalytic activity of nitrogen-doped TiO2 nanobelts. J. Am. Chem. Soc. 131(34), 12290–12297 (2009).
Czoska, A. et al. The nature of defects in fluorine-doped TiO2. J. Phys. Chem. C112(24), 8951–8956 (2008).
Zhao, Y. et al. Zn-doped TiO2 nanoparticles with high photocatalytic activity synthesized by hydrogen–oxygen diffusion flame. Appl. Catal. B Environ. 79(3), 208–215 (2008).
Yang, K., Dai, Y., Huang, B. & Whangbo, M. H. Density functional characterization of the band edges, the band gap states, and the preferred doping sites of halogen-doped TiO2. Chem. Mater. 20(20), 6528–6534 (2008).
Sharma, S. D. et al. Sol–gel-derived super-hydrophilic nickel doped TiO2 film as active photo-catalyst. Appl. Catal. A Gen. 314(1), 40–46 (2006).
Kordatos, A., Kelaidis, N. & Chroneos, A. Defect pair formation in fluorine and nitrogen codoped TiO2. J. Appl. Phys. 123(16), 161510 (2018).
Kelaidis, N., Kordatos, A., Christopoulos, S.-R. G. & Chroneos, A. A roadmap of strain in doped anatase TiO2. Sci. Rep. 8, 12790 (2018).
Pan, X., Yang, M. Q., Fu, X., Zhang, N. & Xu, Y. J. Defective TiO2 with oxygen vacancies: synthesis, properties and photocatalytic applications. Nanoscale 5, 3601–3614 (2013).
Pan, X., Yang, M.-Q. & Xu, Y.-J. Morphology control, defect engineering andphotoactivity tuning of ZnO crystals by grapheneoxide–a unique 2D macromolecular surfactant. Phys. Chem. Chem. Phys. 16, 5589–5599 (2014).
Babu, V. J., Nair, A. S., Peining, Z. & Ramakrishna, S. Synthesis and characterization of rice grains like Nitrogen-doped TiO2 nanostructures by electrospinning–photocatalysis. Mater.Lett. 65(19-20), 3064–3068 (2011).
Khan, S. et al. Defect engineering toward strong photocatalysis of Nb-doped anatase TiO2: Computational predictions and experimental verifications. Appl. Catal. B206, 520–530 (2017).
Lin, Y. et al. The electronic structure, optical absorption and photocatalytic water splitting of (Fe+ Ni)-codoped TiO2: A DFT+ U study. Int. J. Hydrogen Energy 42(8), 4966–4976 (2017).
Di Valentin, C. & Pacchioni, G. Trends in non-metal doping of anatase TiO2: B, C, N and F. Cat. today 206, 12–18 (2013).
Samsudin, E. M. & Hamid, S. B. A. Effect of band gap engineering in anionic-doped TiO2 photocatalyst. Appl. Surf. Sci. 391, 326–336 (2017).
Sun, H., Wang, S., Ang, H. M., Tadé, M. O. & Li, Q. Halogen element modified titanium dioxide for visible light photocatalysis. Chem. Eng. J. 162(2), 437–447 (2010).
Nagpal, P. & Klimov, V. I. Role of mid-gap states in charge transport and photoconductivity in semiconductor nanocrystal films. Nature Commun. 2, 486 (2011).
Luttrell, T. et al. Why is anatase a better photocatalyst than rutile?-Model studies on epitaxial TiO2 films. Sci. Rep. 4, 4043 (2014).
Burdett, J. K., Hughbanks, T., Miller, G. J., Richardson, J. W. Jr. & Smith, J. V. Structural-electronic relationships in inorganic solids: powder neutron diffraction studies of the rutile and anatase polymorphs of titanium dioxide at 15 and 295 K. J. Am. Chem. Soc. 109(12), 3639–3646 (1987).
Muscat, J., Swamy, V. & Harrison, N. M. First-principles calculations of the phase stability of TiO2. Phys. Rev. B65(22), 224112 (2002).
Tosoni, S., Lamiel-Garcia, O., Fernandez Hevia, D., Doña, J. M. & Illas, F. Electronic structure of F-doped bulk rutile, anatase, and brookite polymorphs of TiO2. J. Phys. Chem. C116(23), 12738–12746 (2012).
Araujo-Lopez, E., Varilla, L. A., Seriani, N. & Montoya, J. A. TiO2 anatase’s bulk and (001) surface, structural and electronic properties: a DFT study on the importance of Hubbard and van der Waals contributions. Surf. Sci. 653, 187–196 (2016).
Zhao, Z., Li, Z. & Zou, Z. First-Principles Calculations on Electronic Structures of N/V‐Doped and N‐V‐ DodopedAnatase TiO2 (101) Surfaces. Chem. Phys. Chem. 13(17), 3836–3847 (2012).
Lazzeri, M., Vittadini, A. & Selloni, A. Structure and energetics of stoichiometric TiO2 anatase surfaces. Phys. Rev. B 63(15), 155409 (2001).
Liu, S., Yu, J. & Jaroniec, M. Anatase TiO2 with dominant high-energy {001} facets: synthesis, properties, and applications. Chem. Mater. 23(18), 4085–4093 (2011).
Wang, X., He, H., Chen, Y., Zhao, J. & Zhang, X. Anatase TiO2 hollow microspheres with exposed {001} facets: Facile synthesis and enhanced photocatalysis. Appl. Surf. Sci. 258(15), 5863–5868 (2012).
Liu, M., Li, H., Zeng, Y. & Huang, T. Anatase TiO2 single crystals with dominant {001} facets: Facile fabrication from Ti powders and enhanced photocatalytical activity. Appl. Surf. Sci. 274, 117–123 (2013).
Gu, L., Wang, J., Zou, Z. & Han, X. Graphitic-C3N4-hybridized TiO2 nanosheets with reactive {001} facets to enhance the UV-and visible-light photocatalytic activity. J. Hazard. Mater. 268, 216–223 (2014).
Long, J. et al. 2014. Gold-plasmon enhanced solar-to-hydrogen conversion on the {001} facets of anatase TiO2 nanosheets. Energy Environ. Sci. 7(3), 973–977 (2014).
Zhou, P. et al. Vectorial doping-promoting charge transfer in anatase TiO2 {0 0 1} surface. Appl. Surf. Sci. 319, 167–172 (2014).
Li, H., Guo, Y. & Robertson, J. Calculation of TiO2 surface and subsurface oxygen vacancy by the screened exchange functional. J. Phys. Chem. C119(32), 18160–18166 (2015).
Payne, M. C., Teter, M. P., Allan, D. C., Arias, T. A. & Joannopoulos, J. D. CASTEP 4.2 Academic version, licensed under the UKCP-MSI Agreement. Rev. Mod. Phys. 64, 1045–1097 (1992).
Segall, M. D. et al. First-principles simulation: ideas, illustrations and the CASTEP code. J. Phys. Cond. Matter 14(11), 2717–2744 (2002).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77(18), 3865–3868 (1996).
Vanderbilt, D. Soft self-consistent pseudopotentials in a generalized eigenvalue formalism. Phys. Rev. B41(11), 7892–7895 (1990).
Monkhorst, H. J. & Pack, J. D. Special points for Brillouin-zone integrations. Phys. Rev. B13(12), 5188–5192 (1976).
Acknowledgements
P.P.F., M.V., D.D. and A.C. are grateful for LRF ICON funding from the Lloyd’s Register Foundation, a charitable foundation helping to protect life and property by supporting engineering-related education, public engagement and the application of research. N.K. and N.N.L. acknowledge support by the projects “Advanced Materials and Devices” (MIS 5002409) and “National Infrastructure in Nanotechnology, Advanced Materials and Micro - / Nanoelectronics” (MIS 5002772), funded by the Operational Programme “Competitiveness, Entrepreneurship and Innovation” (NSRF 2014–2020), co-financed by Greece and the European Union (European Regional Development Fund).
Author information
Authors and Affiliations
Contributions
P.P.F. and N.K. performed the calculations. P.P.F., N.K., M.V., D.D., N.N.L. and A.C. contributed to the interpretation of the results and the writing of the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Filippatos, PP., Kelaidis, N., Vasilopoulou, M. et al. Defect processes in F and Cl doped anatase TiO2. Sci Rep 9, 19970 (2019). https://doi.org/10.1038/s41598-019-55518-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-55518-8
This article is cited by
-
Dopant incorporation into TiO2 semiconductor materials for optical, electronic, and physical property enhancement: doping strategy and trend analysis
Journal of the Australian Ceramic Society (2024)
-
Preparation of hydrogen, fluorine and chlorine doped and co-doped titanium dioxide photocatalysts: a theoretical and experimental approach
Scientific Reports (2021)
-
Impact of boron and indium doping on the structural, electronic and optical properties of SnO2
Scientific Reports (2021)
-
Fabrication of tantalum oxyfluoride and oxynitride thin films via ammonolysis of sol–gel processed tetraethoxo (β-diketonato) tantalum (V) precursors for enhanced photocatalytic activity
Journal of Materials Science: Materials in Electronics (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.