Abstract
Recent studies have shown that predatory mites used as biocontrol agents can be loaded with entomopathogenic fungal conidia to increase infection rates in pest populations. Under laboratory conditions, we determined the capacity of two phytoseiid mites, Amblyseius swirskii and Neoseiulus cucumeris to deliver the entomopathogenic fungus Beauveria bassiana to their prey, Frankliniella occidentalis. Predatory mites were loaded with conidia and released on plants that had been previously infested with first instar prey clustered on a bean leaf. We examined each plant section to characterize the spatial distribution of each interacting organism. Our results showed that A. swirskii delivered high numbers of conidia to thrips infested leaves, thereby increasing the proportion of thrips that came into contact with the fungus. The effect was larger when thrips infestation occurred on young leaves than on old leaves. Neoseiulus cucumeris delivered less conidia to the thrips infested leaves. These patterns result from differences in foraging activity between predatory mite species. Amblyseius swirskii stayed longer on plants, especially within thrips colonies, and had a stronger suppressing effect on thrips than N. cucumeris. Our study suggests that loading certain predatory mite species with fungal conidia can increase their capacity to suppress thrips populations by combining predation and dispersing pathogens.
Similar content being viewed by others
Introduction
Pathogens have evolved several ways to disperse and increase the probability of encountering their host. A pathogen can be transferred directly from an infected individual to an uninfected individual, indirectly when the host encounters the free-living infectious stage of the pathogen in the environment, or via a vector1,2. The rate of disease transmission within a host population is strongly influenced by the spatial distribution, temporal activity pattern and foraging behaviour of interacting species (i.e. pathogens, uninfected hosts, infected hosts, vectors)3,4,5.
A growing number of studies has shown that arthropods can act as dispersal agents and transmit pathogens passively to potential hosts without becoming themselves infected6,7,8,9. For example, in the soil environment, collembolans can facilitate fungal dispersion by carrying conidia attached to their bodies or located in their guts10,11. In honeybees, phoretic Varroa mites have been identified as common vectors of viruses and fungi causing mortality and colony collapse12,13. Arthropod vectors therefore have the potential to shape direct and indirect interactions between a microorganism and its host and consequently influence their population dynamics, as well as the structure and stability of communities8. Although such interactions should be common in nature, the role of arthropod dispersal agents in pathogen epidemiology remains poorly understood.
From an applied perspective, insect pollinators and arthropod biological control agents can be used for dispersing pathogens to agricultural pests14, weeds15 and antagonists to plant diseases16. For example, in addition to pollinate greenhouse tomato and sweet pepper, bumble bees have the capacity to co-disseminating two fungi Beauveria bassiana Balsamo Vuillemin (Ascomycota: Hypocreales) and Clonostachys rosea (Link: Fries) Schroers, Samuels, Seifert, and Gams (Ascomycota: Hypocreales) for control of insect pests (greenhouse whitefly and tarnished plant bug) and grey mould, respectively17. Similarly, some species of commercially mass-produced predatory mites have shown potential for dispersing entomopathogenic fungi to insect pests. Under laboratory conditions, two phytoseiid species, Neoseiulus cucumeris Oudemans (Acari: Phytoseiidae) and Amblyseius swirskii Athias-Henriot (Acari: Phytoseiidae) facilitated the dissemination of B. bassiana conidia to their prey, the Asian citrus psyllid Diaphorina citri Kuwayama (Homoptera: Psyllidae), a major pest of citrus18. Such findings stimulated research on techniques to load arthropod dispersal agents with optimal doses of infective fungal conidia before releasing them in the crop where they can disseminate the pathogen to the target pests19,20,21.
While the role of host and non-host arthropods in facilitating entomopathogenic fungi dispersal in the environment has been identified1,14, the underlying ecological and behavioural mechanisms still need to be examined. Arthropods can mediate the rate at which a disease is horizontally transmitted to susceptible hosts through either direct physical contact (e.g. during a predation or a parasitism attempt by natural enemies) or indirectly by releasing infective propagules (fungal spores) in the habitat. In such cases, when there is a close association between the dispersal agent and the host susceptible to the pathogen, the encounter between interacting species is not a random event. For example, the capacity of a predator to disperse fungal conidia to its prey will primarily depend on how conidia are dislodged from the cuticle (either by grooming or walking) and its foraging behaviour (e.g. habitat location, area-restricted searching behaviour, numerical response) that contribute to increasing the spatial co-occurrence with the prey22.
This study aimed at investigating the capacity of two species of predatory mites commonly used as biological control agents in dispersing conidia of an entomopathogenic fungus to their prey. We predicted that foraging predatory mites artificially loaded with conidia will move close to their prey, thereby increasing the spatial co-occurrence between the fungus and the prey and the fungal infection rate. Under laboratory conditions, we examined the (i) spatial distribution of conidia on plant parts when unloaded from predatory mite bodies, as well as the proportion of conidia delivered to the prey oviposition leaf, (ii) predation rates and (iii) proportion of prey bearing conidia on their body. These data provide valuable insights into mechanisms involved in dispersing fungal conidia when transported by an arthropod predator that is not harmed by the fungi. They will also inform the biological control community of researchers and practitioners about the potential of predators to induce fungal epizootics in pest populations.
Results
Number and proportion of B. bassiana conidia delivered by predatory mites
The number of CFUs recovered from the entire plant significantly differed among treatments (generalized linear model with negative binomial distribution, treatment χ2 = 36.75, p < 0.001, Fig. 1). Both A. swirskii (multiple comparisons with ‘glht’ function, Tukey method, z = 6.45, p < 0.001) and N. cucumeris (z = 5.37, p < 0.001) contributed to increase total CFUs on plants compared to control. Both predatory mites delivered the same quantity of CFUs to the plant (z = 1.09, p = 0.519). One plant from the control treatment was excluded from the analysis because extremely high number of conidia (~19,800) landed on a single leaf; this outlier was more than three times of absolute deviation above the median23.
Number of B. bassiana colony-forming units (CFUs) recovered on a plant 48 hours after the beginning of the experiment on plants without (control) and with predatory mites, N. cucumeris or A. swirskii. Different letters indicate a significant treatment effect (p < 0.05 generalized linear model with negative binomial distribution, multiple comparisons with ‘glht’ function, Tukey method). Dots identify outliers as defined by ggplot, i.e. values exceeding 1.5 interquartile range.
There was an interaction between the thrips oviposition leaf and treatment (generalized linear model with negative binomial distribution, interaction χ2 = 31.47, p < 0.001; Fig. 2). Simple effects (the effect of each independent variable within each level of the other independent variable) were examined. For A. swirskii, this effect was greater when thrips eggs were laid on the young leaf than on the old leaf (generalized linear model with negative binomial distribution, χ2 = 5.29, z = 2.32, p = 0.020). Amblyseius swirskii increased the number of CFUs recovered from the thrips oviposition leaf compared to control, but N. cucumeris did not (Kruskal-Wallis test, when thrips eggs were laid on old leaf: treatment simple effect χ2 = 19.81, p < 0.001; Kruskal-Wallis test, when thrips eggs were laid on young leaf: treatment simple effect χ2 = 18.55, p < 0.001).
Number of B. bassiana colony-forming units (CFUs) recovered from the thrips oviposition leaf (young vs. old) 48 hours after the beginning of the experiment on plants without (control) and with predatory mites, N. cucumeris or A. swirskii. Thrips oviposition leaf refers to the leaf where thrips females were caged for 24 hours to lay eggs prior to treatments. Dots identify outliers as defined by ggplot, i.e. values exceeding 1.5 interquartile range. Different capital and lower case letters indicate significant treatment effect for young and old leaf, respectively (p < 0.05, Kruskal-Wallis test with multiple comparisons). The asterisk indicates a significant difference (0.05 < p < 0.01) between thrips oviposition leaf: n.s. = not significant (Kruskal-Wallis test within treatment ‘control’ and treatment ‘cucumeris’, generalized linear model with negative binomial distribution within treatment ‘swirskii’).
At the beginning of the experiment, it was not possible to load the two predatory mite species with a similar number of conidia. Therefore, the proportion of B. bassiana delivered to the thrips oviposition leaf by the two predatory mite species was evaluated. The proportion of CFUs recovered from the thrips oviposition leaf varied among treatments (generalized linear model, χ2 = 23.00 p < 0.001; Fig. 3) with A. swirskii increasing the proportion of B. bassiana on the thrips oviposition leaf compared to control (generalized linear model, followed by multiple comparisons with ‘glht’ function, Tukey method, z = 4.14, p < 0.001), but not N. cucumeris (z = 0.06, p = 0.998). Amblyseius swirskii delivered a significantly higher proportion of B. bassiana to the thrips oviposition leaf than N. cucumeris (z = 4.15, p < 0.001).
Proportion of B. bassiana colony-forming units (CFUs) recovered from the thrips oviposition leaf 48 hours after the beginning of the experiment on plants without (control) and with predatory mites, N. cucumeris or A. swirskii. Different letters indicate significant differences between treatments (p < 0.05, generalized linear model with normal distribution, followed by multiple comparisons with ‘glht’ function, Tukey method). Dots identify outliers as defined by ggplot, i.e. values exceeding 1.5 interquartile range.
Proportion of thrips contacting B. bassiana delivered by predatory mites
The proportion of thrips coming into contact with B. bassiana was significantly affected by both treatment and thrips oviposition leaf (generalized linear model with normal distribution, treatment χ2 = 22.37, p < 0.001; oviposition leaf χ2 = 10.78, p = 0.001; Fig. 4), as well as by their interaction (χ2 = 8.00, p = 0.018). For A. swirskii, this effect was much greater when thrips laid eggs on the young leaf rather than the old leaf (generalized linear model with normal distribution, followed by multiple comparisons with ‘glht’ function, Tukey method, z = 3.03, p = 0.002).
Proportion of thrips bearing B. bassiana 48 hours after the release of B. bassiana on plants without (control) and with predatory mites, N. cucumeris or A. swirskii. Thrips oviposition leaf refers to the leaf where thrips females were caged for 24 hours to lay eggs prior to treatments, old: leaf No. 5, young: leaf No. 2. Different capital letters indicate significant treatment simple effect for the young leaf (p < 0.05, generalized linear model with normal distribution, followed by multiple comparisons with ‘glht’ function, Tukey method) while different lower case letters indicate significant treatment simple effect for the old leaf (p < 0.01, generalized linear model with normal distribution, followed by multiple comparisons with ‘glht’ function, Tukey method). Differences between thrips oviposition leaves within a treatment are shown above bars: n.s. = not significant (p > 0.05), **0.001 < p < 0.01 (generalized linear model). Dots identify outliers as defined by ggplot, i.e. values exceeding 1.5 interquartile range.
Predatory mites and thrips remaining on the plant
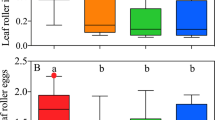
Forty-eight hours following predatory mites released, higher numbers of A. swirskii (8.94 ± 0.88, mean ± S.E.) were recovered from the plants than N. cucumeris (2.61 ± 0.50 S.E.) (generalized linear model with negative binomial distribution, treatment χ2 = 46.82, z = 6.49, p < 0.001). The numbers of thrips recovered on plants at the end of the experiment also varied between treatments (generalized linear model with negative binomial distribution, followed by multiple comparisons with ‘glht’ function, Tukey method, χ2 = 15.92, p < 0.001, Fig. 5). Amblyseius swirskii significantly reduced thrips number on plants (z = −3.91, p < 0.001; Fig. 5) compared to control, but not N. cucumeris (z = −0.85, p = 0.395).
Number of thrips recovered on plant 48 hours after the beginning of the experiment on plants without (control) and with predatory mites, N. cucumeris or A. swirskii. Different letters above bars indicate significant differences between treatments (p < 0.05, generalized linear model with negative binomial distribution, followed by multiple comparisons with ‘glht’ function, Tukey method). Dots identify outliers as defined by ggplot, i.e. values exceeding 1.5 interquartile range.
Discussion
Our results demonstrate that A. swirskii and N. cucumeris both have the capacity to disseminate B. bassiana conidia on plants when foraging. However, A. swirskii is more efficient than N. cucumeris as they delivered a higher proportion of conidia to thrips colonies.
There are mainly two ways in which conidia can be dislodged from the predatory mite body and be dispersed on the plant. They can either be actively groomed off by mites or rubbed off on the plant surface when predatory mites move along (Lin et al., unpublished data). Grooming, the use of legs to clean the body, has been observed in phytoseiid mites when they encounter potentially pathogenic fungi24,25. However, grooming is not efficient to remove all conidia from a mite, especially those located on the dorsal sections of their body. We further showed that A. swirskii and N. cucumeris mostly dislodged conidia from their body by walking on the plant surface. Indeed, the duration of walking is correlated to conidia removal for both species (Lin et al., unpublished data). Trichomes and other structures associated with the surface of bean leaves are likely to facilitate the dislodgement of conidia when mites are walking (Fig. 6B). Foraging predatory mites thus actively disperse B. bassiana conidia in the environment.
(A) Neoseiulus cucumeris bearing Beauveria bassiana conidia. (B) Amblyseius swirskii bearing Beauveria bassiana conidia, released on a bean leaf. The hair-like structures are dense bean trichomes. We observed and took photos of the specimens using a low temperature scan electron microscope (LT-SEM) with the same method described in Bolton, et al.61.
When mediated by predatory mites, transfer of conidia to thrips can either be a passive or an active process. Conidia are unloaded on plant surfaces and can subsequently passively attach to thrips cuticle when they forage on a contaminated substrate. Alternatively, conidia can be directly transferred from predatory mites to thrips during an unsuccessful predation event involving a physical contact between the two protagonists. Thrips are defensive prey that display counterattack behaviours. They can swing their abdomen to ‘slap’ predatory mites26 or secrete irritating anal fluid, which causes predatory mites to withdraw27. Moreover, the presence of predatory mites in the vicinity of thrips colonies can affect their behaviour28. Following detection of predators, thrips may switch state from stationary feeding to escaping, thereby increasing the probability of coming into contact with spores disseminated on plant surfaces14.
The observed differences between A. swirskii and N. cucumeris in their capacity to disseminate B. bassiana conidia to thrips colonies might arise from differences in predator foraging patterns. Amblyseius swirskii is classified as subtype III-b generalist predatory mite adapted to mostly living on glabrous leaves, whereas N. cucumeris is classified as subtype III-e generalist predatory mite from soil/litter habitats29. As a consequence, more A. swirskii individuals were recovered from the plant than N. cucumeris at the end of our experiment. Such a pattern was also observed on cucumber plants infested with thrips30. However, habitat preference cannot alone justify differences in spore dispersal patterns between the two predatory mite species since they both deliver similar numbers of conidia to the plant. Amblyseius swirskii, a more efficient predator30,31, seems to be better adapted to detect thrips colonies and subdue this type of prey than N. cucumeris, as shown by A. swirskii suppressing thrips more strongly than N. cucumeris in our experimental setup. The difference in proportions of conidia delivered to thrips patches by the two predatory mite species attests to the better capacity of A. swirskii to exploit thrips on bean plants. In another study system32, showed that the predatory mite Neoseiulus (Amblyseius) barkeri Hughes (Acarina: Phytoseiidae) did not increase B. bassiana transmission to thrips. Neosiulus barkeri is a less voracious thrips predator than N. cucumeris with a relatively low capture success when attacking first and second instar larvae of Thrips tabaci Lindeman (Thysanoptera: Thripidae)26. Furthermore, the level of foraging activity of N. barkeri is lower than that of N. cucumeris30. These results suggest that the foraging capacity of a predator and the strength of its interaction with a prey would be essential determinants of its potential efficiency as a dispersal agent of entomopathogens.
The rate at which B. bassiana is contacting its host is crucial in the context of biological control, not only because it is directly linked to the infection rate but also because the viability of conidia is very sensitive to environmental conditions such as UV and humidity33,34. Typically, entomopathogens are used like pesticides with single or multiple applications of large quantities of pathogens in crops. However, in some instances, aerial applications are not effective to reach target pests. For example, due to its thigmokinetic behaviour, Western flower thrips are often concealed in plant crevices and flower buds35. As a result, spraying fungal pathogens has little effect on thrips infection level36. In such circumstances, the capacity of predatory mites in delivering pathogens to thrips colonies could increase disease transmission. In our experiment, the thrips mortality after contacted with pathogens was not evaluated. Nevertheless, it was shown that the LD50 is relative low for technical grade powder of B. bassiana: approximately 50 conidia per 2nd instar F. occidentalis larva and only 5 per adult37.
Our findings about the relative potential of A. swirskii and N. cucumeris in dispersing B. bassiana conidia to thrips are consistent with the conclusion drawn by Zhang et al.18 who studied a similar biological system on the tropical shrub, Murraya paniculata (L.) Jack (Rutaceae), infested by the Asian citrus psyllid, Diaphorina citri, in the laboratory. Higher mortality in D. citri populations was achieved when B. bassiana was delivered by A. swirskii rather than by N. cucumeris, and compared to B. bassiana being sprayed evenly onto plants. We can thus conclude that under our experimental conditions, A. swirskii is a better biological control agent because it reduced thrips more strongly and transmitted conidia to a larger number of thrips escaping from predation. However, N. cucumeris could show good potential both as a predator and an entomopathogen dispersal agent when used in a different crop-pest association. For example, in greenhouses from temperate regions, it has been shown that N. cucumeris showed similar performance as A. swirskii as a thrips biocontrol agent under simulated winter conditions38.
Finally, how can we apply such a system in a biological control program? Growers periodically release predatory mites and spray B. bassiana onto crops to control thrips. The strategy we proposed does not require two separate applications, but solely a premix of B. bassiana conidia (technical grade powder) into commercially available predatory mite package (if approved by regulatory agencies)20. The predatory mites would likely increase disease transmission rate to concealed pests. The overall quality of a predatory mite species as a pathogen dispersal agent would depend on its capacity to be loaded with conidia, its capacity to resist pathogenic infection and, as shown by the present study, its foraging activity. Predatory mites should be closely associated to the target pest and have the ability to search for, locate and engage in interactions with the pest on the plant, so they can disperse spores on the plant like little pebbles strewn about by Tom Thumb39.
Methods
The study system
The biological system under study consisted of the entomopathogenic fungus Beauveria bassiana, two species of predatory mites Amblyseius swirskii and Neoseiulus cucumeris as potential fungal dispersal agents and the western flower thrips Frankliniella occidentalis Pergande (Thysanoptera: Thripidae) as a resource for both the fungus and the predators. These species share similar habitats (i.e. plants supporting thrips populations) and can coexist in commercial greenhouses applying biological control programs. In a previous study, we showed that B. bassiana strain ANT-03 is virulent to thrips (all stages, except first instar larva), slightly virulent to N. cucumeris and avirulent to A. swirskii20. This system thus perfectly fits the profile of a suitable pathogen, vector and host association, in which the pathogen is virulent against host and benign towards the vector40.
Beauveria bassiana is a generalist entomopathogenic fungus that exploits more than 200 species from most insect orders, with some isolates showing a high degree of specificity41,42. Conidia are responsible for infection and natural dispersal by air movement because of their small size (1–3 μm)43, by contact with infected hosts or via a dispersal agent1,14,19. Conidia adhere to the host cuticle, germinate, penetrate in the host by enzymatic and mechanical processes and next reproduce by exploiting host hemolymph and various host tissues44,45,46. Once host nutrients are depleted, the fungus breaches the cuticle from inside out and sporulates in large numbers45. Commercial strains of B. bassiana are used for the control of arthropod pests in biological control programs. They are typically sprayed over the crops like pesticides and the probability of contact with the host depends on the spatial distribution of the pests36,42.
The two phytoseiid species are generalist predators that actively search for prey29. Foraging phytoseiid mites typically respond to chemical cues emitted by plants when attacked by herbivores and move towards infested areas47. They are both commercialized and successfully released on vegetable and ornamental crops to control insect pests, including thrips29. They both mostly attacked first instar thrips larvae because larger prey successfully counterattack predatory mites27. Small and large thrips larvae live together in colonies on plant parts and larger larvae can protect their younger siblings from predation48.
Frankliniella occidentalis is a cosmopolitan and highly polyphagous insect that feeds on almost every plant parts, from leaves to flower and pods49,50,51, it can also vector a number of plant virus52. Their eggs are laid in plant tissues and then go through three stages (two larval and one prepupal stages) before pupation52. Frankliniella occidentalis can hide in concealed parts of plants where pesticides cannot reach them, and they rapidly develop resistance to chemicals52,53.
Arthropod colonies and fungal inoculum
A colony of N. cucumeris, provided by Anatis Bioprotection Inc., was maintained on a factitious prey Aleuroglyphus ovatus Toupeau (Acari: Acaridae) while A. swirskii, purchased from BioBest Canada, was reared on a diet mixture containing Carpoglyphus lactis L. (Acari: Carpoglyphidae) and cherry pollen (Firman Pollen Co., Yakima, WA). Frankliniella occidentalis was obtained from a lab colony in Anatis Bioprotection Inc. and reared on California red kidney bean plants Phaseolus vulgaris L. (Fabaceae), with cherry pollen supplied ad libitum on a weekly basis. All colonies were maintained at 25 °C, 60–70% relative humidity and under a 14 L: 10D light cycle.
Beauveria bassiana strain ANT-03 has been registered in North America for greenhouse thrips control. We used the technical grade powder produced by Anatis Bioprotection Inc. containing 5 × 1010 conidia g−1 for all experiments.
Prey patch establishment on a plant
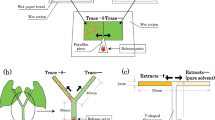
To test the capacity of predatory mites to deliver B. bassiana to thrips, we first established a spatial structure combining plant parts infested or not by thrips. To standardize the structure of a plant, we first trimmed bean plants (approximately 20 cm in height) to two sets of trifoliate (Fig. 7). To create a clumped distribution of thrips larvae on the plant, we enclosed 25 ovipositing female thrips for 24 hours in a clip cage on a single leaf. The clip cage was designed by F. Longpré, London Research and Development Center, Agriculture and AgriFood Canada, and made using a 3D printer. During the oviposition period, female thrips were assumed to have fed and left olfactory cues on the leaf that can further be used by predatory mites to locate the prey patch54,55. To avoid potential experimental bias related to leaf age or position, half of the plants had thrips on leaflet 2, the middle leaflet of the old trifoliate, while the second set of plants had thrips on leaflet 5, the middle leaflet of the young trifoliate (Fig. 7). Following oviposition, the clip cage and female thrips were removed from the plant. Four days later, when most eggs had developed into first instar larvae, the suitable prey stage for predatory mites, we released predatory mites loaded with B. bassiana on the plant.
Schematic drawing of the bean plant structure after being trimmed (left). Plant parts (leaflet and stem) are each identified by a number (right). An Eppendorf tube containing predatory mites and fungal conidia to be released was attached in position 8. An example of the spatial distribution of larval thrips is illustrated using yellow oval spots - in this case, thrips are mostly clumped on the oviposition leaflet 5. Drawn by Gongyu Lin with the software Adobe Illustrator.
Releasing predatory mites loaded with B. bassiana conidia
Adult female predatory mites of various ages were exposed to B. bassiana conidia in the commercial rearing substrate (2.5 × 109 conidia g−1 substrate) for two hours to obtain maximum conidia load on their body (Fig. 6)20. In a modified Eppendorf tube, we put 25 predatory mites with 0.2 g of B. bassiana contaminated rearing substrate20. The tube was attached on the stem, at equal distance to the base of the petiole of the two trifoliates (Fig. 7). To control for dispersal of B. bassiana conidia by air and potential mechanical disturbance during experimental manipulations, a tube containing 0.2 g of B. bassiana contaminated rearing substrate was attached to the plant (Control treatment). Each plant was isolated in a paper cylinder and the inner walls and the bottom of the paper cylinder were coated with rings of Tanglefoot® glue to prevent conidia and predatory mites dispersal between experimental units. For each set of plants (leaf 2 vs. leaf 5), there were three treatments: control, B. bassiana dispersed by N. cucumeris and B. bassiana dispersed by A. swirskii. The experiment was repeated nine times (temporal blocks, n = 9) with two plants per treatment in each block at 25 °C, 60–70% relative humidity and under a 14 L: 10D light cycle. The two blocks where no thrips left on plants were excluded from the analyses of proportion of thrips bearing B. bassiana because this parameter cannot be estimated in absence of thrips.
Recovery of predators and prey
Forty-eight hours after the release of phytoseiid mites, plants were carefully examined to establish the number and spatial distribution of surviving predators and prey. Each of the nine plant parts (Fig. 7) were collected and placed in a 2 oz black solo cup with lid. The cup was filled with carbon dioxide from SodaStream® to stop movement of thrips and predatory mites for the ease of handling and to avoid fungal cross-contamination between individuals. The number of mites and thrips found alive on each plant part was recorded. Thrips mortality was assumed to result from the presence of predators since B. bassiana conidia cannot germinate and invade thrips tissues within a 48 h period20,56.
Recovery of B. bassiana conidia from prey and plant parts
To detect the presence or absence of B. bassiana on living thrips that remained on plants until the end of the experiment, thrips were individually picked with a sterilized toothpick or clean fine brush (sterilized with 75% ethanol and rinsed with 0.1% Tween-80 between samples) and placed in a small Petri dish (Ø 35 mm) containing 2.5 ml of an oatmeal selective media for B. bassiana57. Petri dishes were examined 10 days later when colony-forming units (CFUs) can be visualized. The proportion of thrips bearing conidia was calculated.
To assess the number of conidia on each plant part following arthropod removal, leaves and stems were cut into small pieces (<2 cm in width or length) and put back into the solo cup. Conidia were washed off by adding 5 ml of 0.1% Tween-80 into each solo cup and the cups were put on a rotary shaker for 2 hours at a speed of 125 rpm58. Next, one aliquot of a 0.5 ml suspension was transferred onto the selective media for B. bassiana57 and CFUs were counted 9 days later. For each plant, we noted the sum of CFUs delivered to the entire plant and, more specifically, the quantity and the proportion of CFUs on the leaf where thrips females laid their eggs.
Statistical analyses
Our experimental design includes two categorical factors: treatment (3 levels: control, N. cucumeris and A. swirskii) and leaf where eggs were laid (2 levels: leaf 2 and leaf 5). When either factor was not identified as a significant predictor of the dependent variable following a log-likelihood test, it was eliminated from the initial statistical model to optimize the final model. The number of predatory mites remaining on the plants was analyzed using generalized linear models with negative binomial distribution and with species as a factor. The number of thrips remaining on the plants was analyzed with generalized linear models with negative binomial distribution and with treatment a factor. The proportion of thrips bearing B. bassiana was analyzed using generalized linear models with normal distribution with treatment and oviposition leaf as factors. The number of conidia delivered to the entire plant was analyzed with generalized linear models with negative binomial distribution and with treatment and oviposition leaf as factors. The number of conidia on the thrips oviposition leaf was analyzed with both generalized linear models with negative binomial distribution and Kruskal-Wallis tests, depending on whether the residuals were normally distributed or not, determined by Normal QQ-plot59. The proportion of conidia on the thrips oviposition leaf was analyzed with generalized linear models. Multiple comparisons were performed with the package ‘multcomp’ with ‘glht’ function and Tukey’s all-pair comparisons method. Kruskal-Wallis multiple comparison tests were performed to compare differences among means when residuals were not normally distributed. All the statistical analyses were carried out with R version 1.0.14360.
Data availability
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).
References
Fuxa, J. R. & Tanada, Y. Basic measurements and observations in Epizootiology In Epizootiology of insect diseases 3–21 (Wiley New York, (1987).
Woolhouse, M. E., Taylor, L. H. & Haydon, D. T. Population biology of multihost pathogens. Science 292, 1109–1112 (2001).
Grassly, N. C. & Fraser, C. Mathematical models of infectious disease transmission. Nat. Rev. Microbiol. 6, 477 (2008).
Sih, A., Spiegel, O., Godfrey, S., Leu, S. & Bull, C. M. Integrating social networks, animal personalities, movement ecology and parasites: a framework with examples from a lizard. Anim. Behav. 136, 195–205 (2018).
Dougherty, E. R., Seidel, D. P., Carlson, C. J., Spiegel, O. & Getz, W. M. Going through the motions: incorporating movement analyses into disease research. Ecol. Lett. 21, 588–604 (2018).
Hajek, A. & St. Leger, R. Interactions between fungal pathogens and insect hosts. Annu. Rev. Entomol. 39, 293–322 (1994).
Roy, H. E., Steinkraus, D. C., Eilenberg, J., Hajek, A. E. & Pell, J. K. Bizarre interactions and endgames: entomopathogenic fungi and their arthropod hosts. Annu. Rev. Entomol. 51, 331–357, https://doi.org/10.1146/annurev.ento.51.110104.150941 (2006).
Hofstetter, R. W. & Moser, J. C. The role of mites in insect-fungus associations. Annu. Rev. Entomol. 59, 537–557, https://doi.org/10.1146/annurev-ento-011613-162039 (2014).
Erban, T. et al. In-depth proteomic analysis of Varroa destructor: Detection of DWV-complex, ABPV, VdMLV and honeybee proteins in the mite. Sci. Rep. 5, 13907 (2015).
Dromph, K. M. Dispersal of entomopathogenic fungi by collembolans. Soil Biol. Biochem. 33, 2047–2051 (2001).
Dromph, K. M. Collembolans as vectors of entomopathogenic fungi. Pedobiologia 47, 245 (2003).
Shen, M., Yang, X., Cox-Foster, D. & Cui, L. The role of varroa mites in infections of Kashmir bee virus (KBV) and deformed wing virus (DWV) in honey bees. Virology 342, 141–149 (2005).
Di Prisco, G. et al. Varroa destructor is an effective vector of Israeli acute paralysis virus in the honeybee, Apis mellifera. J. Gen. Virol. 92, 151–155 (2011).
Baverstock, J., Roy, H. E. & Pell, J. K. Entomopathogenic fungi and insect behaviour: from unsuspecting hosts to targeted vectors In The Ecology of Fungal Entomopathogens 89–102 (Springer, (2010).
Martin, H. & Dale, M. Potential of Cactoblastis cactorum as a vector for fungi pathogenic to pricklypear, Opuntia inermis. Biol. Control 21, 258–263 (2001).
Yu, H. & Sutton, J. Effectiveness of bumblebees and honeybees for delivering inoculum of Gliocladium roseum to raspberry flowers to control Botrytis cinerea. Biol. Control 10, 113–122 (1997).
Kapongo, J., Shipp, L., Kevan, P. & Sutton, J. Co-vectoring of Beauveria bassiana and Clonostachys rosea by bumble bees (Bombus impatiens) for control of insect pests and suppression of grey mould in greenhouse tomato and sweet pepper. Biol. Control 46, 508–514 (2008).
Zhang, Y. et al. A novel use of predatory mites for dissemination of fungal pathogen for insect biocontrol: The case of Amblyseius swirskii and Neoseiulus cucumeris (Phytoseiidae) as vectors of Beauveria bassiana against Diaphorina citri (Psyllidae). Syst. Appl. Acarol. 20, 177–187 (2015).
Vega, F. E. et al. Identification of entomopathogenic fungi In Field manual of techniques in invertebrate pathology 153–177 (Springer, (2000).
Lin, G., Tanguay, A., Guertin, C., Todorova, S. & Brodeur, J. A new method for loading predatory mites with entomopathogenic fungi for biological control of their prey. Biol. Control 115, 105–111 (2017).
Al-Mazra’Awi, M. S., Kevan, P. G. & Shipp, L. Development of Beauveria bassiana dry formulation for vectoring by honey bees Apis mellifera (Hymenoptera: Apidae) to the flowers of crops for pest control. Biocontrol Sci. Technol. 17, 733–741 (2007).
Lima, S. L. Putting predators back into behavioral predator–prey interactions. Trends Ecol. Evol. 17, 70–75 (2002).
Leys, C., Ley, C., Klein, O., Bernard, P. & Licata, L. Detecting outliers: Do not use standard deviation around the mean, use absolute deviation around the median. J. Exp. Soc. Psychol. 49, 764–766 (2013).
Wu, S. et al. Effects of Beauveria bassiana on predation and behavior of the predatory mite Phytoseiulus persimilis. J. Invertebr. Pathol. 153, 51–56 (2018).
Wekesa, V., Moraes, G. d, Knapp, M. & Delalibera, I. Jr. Interactions of two natural enemies of Tetranychus evansi, the fungal pathogen Neozygites floridana (Zygomycetes: Entomophthorales) and the predatory mite, Phytoseiulus longipes (Acari: Phytoseiidae). Biol. Control 41, 408–414 (2007).
Bakker, F. & Sabelis, M. How larvae of Thrips tabaci reduce the attack success of phytoseiid predators. Entomol. Exp. Appl. 50, 47–51 (1989).
Bakker, F. & Sabelis Med., F. Landbouww. Rijksuniv. 51, 1041–1044 (Ghent University, 1986).
Jandricic, S., Schmidt, D., Bryant, G. & Frank, S. Non-consumptive predator effects on a primary greenhouse pest: Predatory mite harassment reduces western flower thrips abundance and plant damage. Biol. Control 95, 5–12 (2016).
McMurtry, J. A., De Moraes, G. J. & Sourassou, N. F. Revision of the lifestyles of phytoseiid mites (Acari: Phytoseiidae) and implications for biological control strategies. Syst. Appl. Acarol. 18, 297–320 (2013).
Messelink, G. J., Van Steenpaal, S. E. & Ramakers, P. M. Evaluation of phytoseiid predators for control of western flower thrips on greenhouse cucumber. BioControl 51, 753–768 (2006).
Calvo, F. J., Knapp, M., van Houten, Y. M., Hoogerbrugge, H. & Belda, J. E. Amblyseius swirskii: What made this predatory mite such a successful biocontrol agent? Exp. Appl. Acarol. 65, 419–433 (2015).
Wu, S., Gao, Y., Smagghe, G., Xu, X. & Lei, Z. Interactions between the entomopathogenic fungus Beauveria bassiana and the predatory mite Neoseiulus barkeri and biological control of their shared prey/host Frankliniella occidentalis. Biol. Control 98, 43–51 (2016).
Inglis, G. D., Goettel, M. & Johnson, D. Influence of ultraviolet light protectants on persistence of the entomopathogenic fungus, Beauveria bassiana. Biol. Control 5, 581–590 (1995).
Shipp, J., Zhang, Y., Hunt, D. W. A. & Ferguson, G. Influence of humidity and greenhouse microclimate on the efficacy of Beauveria bassiana (Balsamo) for control of greenhouse arthropod pests. Environ. Entomol. 32, 1154–1163 (2003).
Broadbent, A. B., Rhainds, M., Shipp, L., Murphy, G. & Wainman, L. Pupation behaviour of western flower thrips (Thysanoptera: Thripidae) on potted chrysanthemum. Can. Entomol. 135, 741–744 (2003).
Ugine, T. A., Wraight, S. P. & Sanderson, J. P. Effects of manipulating spray-application parameters on efficacy of the entomopathogenic fungus Beauveria bassiana against western flower thrips, Frankliniella occidentalis, infesting greenhouse impatiens crops. Biocontrol Sci. Technol. 17, 193–219 (2007).
Ugine, T. A., Wraight, S. P., Brownbridge, M. & Sanderson, J. P. Development of a novel bioassay for estimation of median lethal concentrations (LC50) and doses (LD50) of the entomopathogenic fungus Beauveria bassiana, against western flower thrips, Frankliniella occidentalis. J. Invertebr. Pathol. 89, 210–218, https://doi.org/10.1016/j.jip.2005.05.010 (2005).
Hewitt, L. C., Shipp, L., Buitenhuis, R. & Scott-Dupree, C. Seasonal climatic variations influence the efficacy of predatory mites used for control of western flower thrips in greenhouse ornamental crops. Exp. Appl. Acarol. 65, 435–450 (2015).
Perrault, C. The complete fairy tales of Charles Perralt. 83–84 (Houghton Mifflin Harcourt New York (1993).
Ewald, P. W. Vectors, vertical transmission, and the evolution of virulence In Evolution of infectious disease. 46–47 (Oxford University Press, (1994).
Uma Devi, K. et al. A study of host specificity in the entomopathogenic fungus Beauveria bassiana (Hypocreales, Clavicipitaceae). Biocontrol Sci. Technol. 18, 975–989 (2008).
Brodeur, J. Host specificity in biological control: insights from opportunistic pathogens. Evol. appl. 5, 470–480 (2012).
Shimazu, M., Sato, H. & Maehara, N. Density of the entomopathogenic fungus, Beauveria bassiana Vuillemin (Deuteromycotina: Hyphomycetes) in forest air and soil. Appl. Entomol. Zool. 37, 19–26 (2002).
Boomsma, J. J., Jensen, A. B., Meyling, N. V. & Eilenberg, J. Evolutionary interaction networks of insect pathogenic fungi. Annu. Rev. Entomol. 59, 467–485 (2014).
Valero-Jiménez, C. A., Wiegers, H., Zwaan, B. J., Koenraadt, C. J. & van Kan, J. A. Genes involved in virulence of the entomopathogenic fungus Beauveria bassiana. J. Invertebr. Pathol. 133, 41–49 (2016).
Askary, H., Benhamou, N. & Brodeur, J. Ultrastructural and cytochemical characterization of aphid invasion by the hyphomycete Verticillium lecanii. J. Invertebr. Pathol. 74, 1–13, https://doi.org/10.1006/jipa.1999.4857 (1999).
Dicke, M., Takabayashi, J., Posthumus, M. A., Schütte, C. & Krips, O. E. Plant-phytoseiid interactions mediated by herbivore-induced plant volatiles: variation in production of cues and in responses of predatory mites. Exp. Appl. Acarol. 22, 311–333 (1998).
de Bruijn, P. J., Sabelis, M. W. & Egas, M. Effects of kinship or familiarity? Small thrips larvae experience lower predation risk only in groups of mixed-size siblings. Behav. Ecol. Sociobiol. 68, 1029–1035, https://doi.org/10.1007/s00265-014-1715-x (2014).
Zhi, J., Fitch, G. K., Margolies, D. C. & Nechols, J. R. Apple pollen as a supplemental food for the western flower thrips, Frankliniella occidentalis: response of individuals and populations. Entomol. Exp. Appl. 117, 185–192 (2005).
Trichilo, P. J. & Leigh, T. F. Influence of resource quality on the reproductive fitness of flower thrips (Thysanoptera: Thripidae). Ann. Entomol. Soc. Am. 81, 64–70 (1988).
De Jager, C., Butôt, R., De Jong, T., Klinkhamer, P. & Van Der Meijden, E. Population growth and survival of western flower thrips Frankliniella occidentalis Pergande (Thysanoptera, Thripidae) on different chrysanthemum cultivars: two methods for measuring resistance. J. Appl. Entomol. 115, 519–525 (1993).
Reitz, S. R. Biology and ecology of the western flower thrips (Thysanoptera: Thripidae): The making of a pest. Fla. Entomol. 92, 7–13, https://doi.org/10.1653/024.092.0102 (2009).
Mouden, S., Sarmiento, K. F., Klinkhamer, P. G. & Leiss, K. A. Integrated pest management in western flower thrips: past, present and future. Pest Manage. Sci. 73, 813–822 (2017).
Peterson, B. S. The effect of host plant on the biological control of western flower thrips by the predatory mite, Amblyseius cucumeris (a PhD thesis), Simon Fraser University, (1990).
Teerling, C., Gillespie, D. & Borden, J. Utilization of western flower thrips alarm pheromone as a prey-finding kairomone by predators. Can. Entomol. 125, 431–437 (1993).
Wu, S. et al. An entomopathogenic strain of Beauveria bassiana against Frankliniella occidentalis with no detrimental effect on the predatory mite Neoseiulus barkeri: evidence from laboratory bioassay and scanning electron microscopic observation. PLoS One 9, e84732, https://doi.org/10.1371/journal.pone.0084732 (2014).
Chase, A., Osborne, L. & Ferguson, V. Selective isolation of the entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae from an artificial potting medium. Fla. Entomol. 69, 285–292 (1986).
Kapongo, J. P., Shipp, L., Kevan, P. & Broadbent, B. Optimal concentration of Beauveria bassiana vectored by bumble bees in relation to pest and bee mortality in greenhouse tomato and sweet pepper. BioControl 53, 797–812, https://doi.org/10.1007/s10526-007-9142-9 (2007).
Kozak, M. & Piepho, H. P. What’s normal anyway? Residual plots are more telling than significance tests when checking ANOVA assumptions. J. Agron. Crop. Sci. 204, 86–98 (2018).
R, C. T. R: A language and environment for statistical computing. (2013).
Bolton, S. J., Klompen, H., Bauchan, G. R. & Ochoa, R. A new genus and species of Nematalycidae (Acari: Endeostigmata). J. Nat. Hist. 48, 1359–1373 (2014).
Acknowledgements
We thank members from laboratories of Jacques Brodeur, Claude Guertin, Étienne Yergeau for assistance and fruitful discussions; Félix Longpré and Jean-Philippe Parent from Agriculture and Agri-Food Canada for generous support and technical assistance; Ronald Ochoa and Gary Bauchan from Electron and Confocal Microscope Unit, United States Department of Agriculture, Agricultural Research Service for helping me take LT-SEM mite photos together; and Arne Janssen and two anonymous reviewers for helpful comments and suggestions on a previous version of this manuscript. This study was supported by Bursary in a Practice Environment (BMP) Innovation Research Scholarship Program from Natural Sciences and Engineering Research Council of Canada, Fonds de Recherche Nature et Technologies du Québec and Anatis Bioprotection Inc.
Author information
Authors and Affiliations
Contributions
G.L. and J.B. conceived and designed the experiments. C.G. and S.T. participated in the experimental design and provided editorial advice. G.L. and S.D.P. performed the experiments. G.L. analyzed the data. G.L. and J.B. wrote the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lin, G., Guertin, C., Di Paolo, SA. et al. Phytoseiid predatory mites can disperse entomopathogenic fungi to prey patches. Sci Rep 9, 19435 (2019). https://doi.org/10.1038/s41598-019-55499-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-55499-8
This article is cited by
-
Auto-dissemination of Cordyceps fumosorosea amongst adult females of the two-spotted spider mite
Experimental and Applied Acarology (2023)
-
Feeding lifestyles of the Phytoseiidae revisited: searching for a factitious rearing host for Neoseiulus fallacis (Acari: Phytoseiidae)
BioControl (2020)
-
Microscopic analysis of the microbiota of three commercial Phytoseiidae species (Acari: Mesostigmata)
Experimental and Applied Acarology (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.