Abstract
The acute phase response (APR) is a systemic first-line defense against challenges including infection, trauma, stress, and neoplasia. Alteration of acute phase protein (APP) levels in plasma is the most important change during acute phase response. C-reactive protein (CRP), which increases dramatically during inflammation onset, is an indicator of inflammation. To monitor the process of APR, we generated human CRP promoter-driven luciferase transgenic (hCRP-Luc) mice to quantify the hCRP promoter activation in vivo. The naïve female hCRP-Luc mice express low basal levels of liver bioluminescence, but the naïve male hCRP-Luc mice do not. Thus, female hCRP-Luc mice are suitable for monitoring the process of APR. The liver bioluminescence of female hCRP-Luc mice can be induced by several toll-like receptor (TLR) ligands. The expression of liver bioluminescence was highly sensitive to endotoxin stimulation in a dose-dependent manner. On-off-on bioluminescence response was noted in female hCRP-Luc mice upon two endotoxin stimulations one month apart. The LPS-induced bioluminescence of the female hCRP-Luc mice was IL-6-mediated and associated with APP alpha-1-acid glycoprotein expression. In conclusion, the female hCRP-Luc mouse is a non-invasive, sensitive and reusable reporter tool for APR.
Similar content being viewed by others
Introduction
Acute phase response (APR) is a core function of the innate immune system to combat numerous immediate challenges, including infection, trauma, burns, neoplasia, or even strenuous exercise, heatstroke, labor, psychological stress, and psychiatric illness1,2. One of the most important changes during the APR is the alteration of acute phase protein (APP) levels in plasma2. Acute phase proteins are classified as positive APP (those induced by more than 25%) and negative APP (those repressed by more than 25%) in plasma during APR2,3. Positive APPs participate in and regulate several innate immunological responses, including the complement system, coagulation system, cytokine antagonists, and opsonization2,4. In clinical application, positive APPs are often used as indicators of inflammation. One of the most widely applied APPs in medical and biological research is C-reactive protein (CRP). The major role of CRP is to opsonize pathogens and dead cells to facilitate phagocytosis5, and it triggers the classical complement cascade6,7.
In humans, CRP expression increases rapidly within 6–8 hours during infection or inflammatory response and returns to the basal level quickly after inflammation is diminished2,8. CRP concentration in peripheral blood may increase 10,000 fold upon acute phase stimulus in humans9, making it an excellent biomarker for such conditions10,11. CRP is predominately synthesized by hepatocytes in response to pro-inflammatory cytokines, complements, and hormones12,13,14,15. Pro-inflammatory cytokines secreted by macrophages and monocytes, including IL-6, IL-1β, and TNFα, mediate CRP activation16,17. Among these cytokines, IL-6 is the major regulator of APP synthesis in adult human hepatocytes14,16,17,18,19. Both siltuximab (a chimeric anti-IL-6 antibody) and tocilizumab (a humanized anti-IL-6R antibody) can downregulate CRP production20,21. Other cytokines have been shown to have inconsistent effects in different reports. IL-1β was found to induce CRP production in several human hepatoma cell lines19,22,23. However, neither IL-1β- nor TNFα-stimulation induced CRP production in cultured human primary hepatocytes14. Moshage and his colleagues demonstrated that IL-1β-induced CRP production in human hepatocytes was mediated by IL-624. Collectively, these findings indicate that the inflammatory response leading to CRP expression is an IL-6 dependent process.
CRP expression can also be affected by other non-inflammatory factors. Orchiectomy decreases circulating CRP concentrations without changing serum IL-1β and IL-6 concentrations in humans25, indicating that testicular hormones might also regulate CRP production. Indeed, testosterone could induce human CRP gene expression in hCRP transgenic mice15.
Unlike that in humans, CRP in mice is only a modest APP. The mouse serum CRP level is 5–9 mg/L at baseline and peaks at 17 mg/L after LPS injection26, indicating that the regulatory mechanisms in response to inflammatory stimulus of the two species are different. This species-specific pattern of CRP gene expression hinders the usage of CRP as a marker of inflammation in mice. Human CRP-expressing mice with the regulatory elements of the human gene have been generated using a cosmid-based strategy. hCRP expression in these mice is highly inducible and tissue specific under inflammatory stimulus12,27. These animals have been used for studying the mechanisms of APR and the biological functions of hCRP protein.
In this study, we generated a human CRP transgenic reporter mouse, using a bacterial artificial chromosome (BAC)-based approach to include regions of the human CRP promoter linked with luciferase cDNA sequences. In vivo imaging showed that this transgenic mouse exhibited a rapid, strong, and on-off-on bioluminescence signal upon several pattern recognition receptor (PRR) ligand stimulations, presumably via an IL-6 dependent pathway. This transgenic mouse has the advantage of reporting CRP gene instead of protein activation in vivo and may be used as a tool to monitor APR.
Results
Male sex hormone modulated hCRP expression in hCRP-Luc mice
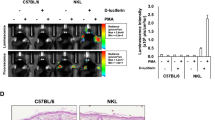
The luciferase cDNA was introduced into the BAC construct containing the human CRP promoter as described in Methods (Fig. 1a). During our initial characterization of the hCRP-Luc mice, we observed that male mice exhibited a bioluminescence basal level higher than that of their female counterparts. We thus further characterized the differences in LPS-induced bioluminescence in male and female hCRP-Luc mice. Female hCRP-Luc mice showed a relatively low basal level (3.39 × 105 photons/sec) with increased bioluminescence signals upon LPS stimulation (100 ng/mouse; 5.81 × 107 photons/sec; Fig. 1b, p = 0.005). The male mice had a high intrinsic luciferase activity (9.81 × 108 photons/sec), which was already higher than that of LPS-stimulated female mice, and did not increase upon LPS stimulation (6.13 × 108 photons/sec). Previous reports indicated that testosterone can modulate hCRP expression independent of IL-613,15,25. We therefore evaluated the effect of castration on the bioluminescence intensity of male hCRP-Luc mice. As shown in Fig. 1c, normal male hCRP-Luc mice showed high bioluminescence signals (5.78 × 108 photons/sec), whereas castrated male hCRP-Luc mice had a 98% reduction in bioluminescence (1.63 × 107 photons/sec; p = 0.0302). Serum IL-6 levels between the castrated and normal male mice were not significantly different (Fig. 1d). The high basal level of bioluminescence signals in male hCRP-Luc mice was thus likely regulated by an IL-6-independent mechanism. This result confirmed our speculation, and female hCRP-Luc mice were chosen for the following experiments.
Male sex hormone modulated hCRP expression in hCRP-Luc mice. (a) Construction of human CRP-luciferase reporter. Recombination cassette containing the IRES-Luciferase-2x poly A and a 50-bp targeting homologous sequence was introduced into the BAC clone RP11-158E22 by Red/ET-mediated homologous recombination in XL-1 blue electroporation competent cells. The targeting homologous site was designed to replace the sequence between the exon 2 leader sequence and those sequences just behind the stop codon of human CRP with IRES-Luciferase-2x polyA. The end product was microinjected into mouse embryos at pronuclear stages. (b) hCRP-Luc mice were intraperitoneally injected with sterile PBS or LPS (100 ng/mouse). Mice were subjected to liver bioluminescence analysis by IVIS at 22 h post LPS injection. Data are means ± SEM (LPS-injected, male group, n = 5; PBS-injected, male group, n = 5; LPS-injected, female group, n = 8; PBS-injected, female group, n = 4). (c) Male hCRP-Luc mice (n = 5) were castrated and rested for 2 weeks, and the bioluminescence was compared with age-matched normal male hCRP-Luc mice by AmiHT (n = 7). Data are means ± SEM of two replicated experiments. (d) Serum IL-6 levels of the castrated (n = 5) and normal mice (n = 7) were determined by immunoassay.
Multiple TLR ligands induced liver bioluminescence in female hCRP-Luc mice with dose-dependent responses
We next tested the sensitivity of bioluminescence induction of female hCRP-Luc mice in response to different TLR ligands (Fig. 2). We found that 2 ng/mouse of LPS induction resulted in an approximately 32.6-fold increase in bioluminescence intensity (5.75 × 107 photons/sec) as compared to the basal level (1.76 × 106 photons/sec). Ten-fold and 100-fold increases of the initial dosages resulted in additional 5.4-fold (3.11 × 108 photons/sec) and 16.1 fold (9.23 × 108 photons/sec) induction of the signal increase of the bioluminescence signal, respectively. Zymosan at the predetermined dosage of 2 μg/mouse resulted in a 4.6-fold increase of bioluminescence signal as compared to the basal level (8.11 × 106 photons/sec). Increasing the dosages 10- and 100-fold resulted in further 55-fold (9.72 × 107 photons/sec) and 897-fold (1.58 × 109 photons/sec) increases in bioluminescence signal, respectively. Unlike LPS or zymosan, poly(I:C) at the predetermined dosage of 0.1 μg/mouse did not increase the bioluminescence signal (1.54 × 106 photons/sec), and a 10-fold increase of the dosage resulted in a 19.4-fold increase in bioluminescence signal (3.42 × 107 photons/sec). In addition, a 100-fold increase of the initial dosage resulted in a 78.4-fold increase in bioluminescence signal (1.38 × 108 photons/sec). These results not only confirmed the responses of the female hCRP-Luc mice to different TLR ligands but also showed that these mice could respond to the ligands in a dose-dependent manner.
Titration of TLR-induced bioluminescence in female hCRP-Luc mice. Female hCRP-Luc mice were intraperitoneally injected with increasing doses of LPS (2, 20, 200 ng/mouse), poly(I:C) (0.1, 1, 10 μg/mouse), or zymosan (2, 20, 200 μg/mouse) and the bioluminescence of these mice was analyzed by AmiHT at 22 h post injection. Data are means ± SEM (n = 3–5) of two repeated experiments.
LPS-induced bioluminescence and AGP acute phase protein expression was IL-6-dependent
IL-6 is one of the most important cytokines mediating CRP induction18,19. A previous study using a human CRP transgenic model also showed that IL-6 is in part responsible for hCRP induction in mice27. To determine the involvement of IL-6 in our female hCRP-Luc mice, we blocked IL-6 signaling on the bioluminescence signal. We first evaluated whether IL-6 injection alone could trigger bioluminescence signal. IL-6 injection, similar to LPS, induced strong bioluminescence in the female hCRP-Luc mice (basal level: 3.02 × 105 photons/sec; LPS: 1.81 × 108 photons/sec, p = 0.001), and IL-6 (1.10 × 109 photons/sec, p = 0.0012; Fig. 3a). Co-injection of anti-IL-6 antibody with LPS also resulted in an increase of bioluminescence signal from the basal level (4.72 × 107 photons/sec and 5.83 × 105 photons/sec, respectively; Fig. 3b). However, the magnitude was significantly lower as compared to mice injected with LPS only (3.10 × 108 photons/sec, p = 0.0415) or with LPS and isotype matched antibody (2.03 × 108 photons/sec). This indicated that LPS-induced bioluminescence was partially mediated by IL-6 and that other IL-6-independent pathways may exist in the female hCRP-Luc mice. We further analyzed whether AGP, a known IL-6-regulated APP, was also induced in this model28. Serum AGP levels were upregulated in female hCRP-Luc mice by LPS (961 pg/mL, p = 0.0006) and IL-6 (703 pg/mL) (Fig. 3c). Anti-IL-6 mAb treatment significantly repressed the LPS-induced serum AGP levels (294 pg/mL, p = 0.0012). Because IL-6 plays a central role in LPS-induced CRP and AGP, we also determined serum IL-6 levels (Fig. 3d). As expected, LPS induced serum IL-6 production (17.1 pg/mL, p = 0.005) and anti-IL-6 mAb treatment reduced the LPS-induced IL-6 levels to the basal level (4.32 pg/mL, p = 0.002). Significant changes was not detected for other APPs (e.g. adipsin, A2M, SAP) and TNF in sera, suggesting that they were not involved in the LPS induced acute phase response in the female hCRP-Luc mice (Figure S1).
LPS-induced bioluminescence and AGP acute phase protein expression in female hCRP-Luc mice was mediated by IL-6. (a) Female hCRP-Luc mice were intraperitoneally injected with sterile PBS, LPS (100 ng/mouse), or IL-6 (1 μg/mouse). The liver bioluminescence was determined by IVIS at 22 h post LPS injection. Data are means ± SEM (n = 4–5) of two repeated experiments. (b) Female hCRP-Luc mice were intraperitoneally injected with sterile PBS, LPS (100 ng/mouse), co-injected with LPS (100 ng/mouse) and anti-IL-6 neutralized mAb (200 μg/mouse), or co-injected with LPS (100 ng/mouse) and isotype-matched irrelevant mAb (200 μg/mouse). The liver bioluminescence was determined by AmiHT at 22 h post LPS injection. Data are means ± SEM (n = 5–7) of two repeated experiments. (c) Serum samples were collected from mice described in (a) for determination of alpha-1-acid glycoprotein (AGP) and IL-6. Data are means ± SD (n = 4–5).
Human CRP-Luc mouse was a highly sensitive and reusable APR reporter
To evaluate the sensitivity of the female hCRP-Luc mouse, we compared its detection limit to limulus amebocyte lysate (LAL) assay, the current standard pyrogen test. LAL diluted standards (0.0256–256 EU) were injected intraperitoneally to female hCRP-Luc mice, and in vivo luciferase assays were performed at 22 h after injection. The results showed a dose-dependent induction with a good correlation (R2 = 0.9967; Fig. 4a). Notably, the bioluminescence of the lowest dose of the LAL standards (0.0256 EU/mouse) was still significantly higher than that of the control, indicating the female hCRP-Luc mice exerted a potential low detection limit. The same animals of this study were re-stimulated with LAL standards one month after the first treatment (Fig. 4b). The repeated stimulation also showed good correlation of dose-dependent luciferase activity (R2 = 0.9896). To determine the clearance time of luciferase, female hCRP-Luc mice were stimulated with LPS, and luciferase responses were determined 1, 3 and 7 days after stimulation (Fig. 4c). In female hCRP-Luc mice, strong bioluminescence (5.27 × 107 photons/sec) was induced at day 1 post stimulation and decreased to near the baseline level at day 7 (4.97 × 105 photons/sec). This indicated that the bioluminescence of female hCRP-Luc mice can return to the baseline after stimulant removal and suggested that repeated stimulation is possible.
High sensitivity and re-inducible APR reporter of female hCRP-Luc mice. Serial dilutions of LAL standard samples in endotoxin-free saline were intraperitoneally injected into female hCRP-Luc mice (200 μL/mouse) twice one month apart. The liver bioluminescence was determined by IVIS at 22 h after each injection. (a) Bioluminescence activity after the first injection. (b) Bioluminescence activity after the second injection. Data are means ± SD (n = 3–5). (c) Female hCRP-Luc mice were intraperitoneally injected with LPS (100 ng/mouse). The liver bioluminescence was determined before (day 0) or at day 1, 3 and 7 post injection. Data are means ± SD (n = 5).
Discussion
In this study, we generated human CRP promoter-driven luciferase transgenic mice. Only female hCRP-Luc mice showed increased expression of liver bioluminescence signals with dose-dependent responses after TLR-ligand stimulations. Male hCRP-Luc mice showed high basal levels of bioluminescence signals, which could be reduced by castration. We also proved that the LPS-induced bioluminescence was IL-6-mediated and parallel to acute phase protein AGP expression. In addition, the female hCRP-Luc mice showed high sensitivity to LAL standard endotoxin stimulation (0.0256 EU/mouse) and good dose-dependent correlation with LAL endotoxin. The endotoxin-induced bioluminescence returned to the baseline value 7 days post-stimulation and could be induced repeatedly.
In our hCRP-Luc mice, the CRP coding sequence was replaced with a luciferase reporter gene, and APR was quantitated based on luminescence instead of CRP protein production. Two methods have been commonly used for APR evaluation: blood APP quantification and body temperature measurement. APP quantification requires blood sampling which may increase risks of minor APR induction at the puncture site29, and detection of CRP or SAP by immunoassays would increase variability in the evaluation of APR. Changes in body temperature, as being used in the rabbit pyrogen test, can be used as sign of early stage inflammation. Pharmaceutical products containing 13.81 EU/mL endotoxin can induce a body temperature rise of 0.5 °C in rabbit pyrogen tests30. However, the long-term restraining of animals not only raises animal welfare issues but also affects the results of the pyrogen test31. Moreover, it is hard to apply the same method to laboratory mice. Measuring APR with our female hCRP-luc mice not only eliminates all the aforementioned difficulties, the fact that the mice could be repeatedly induced by TLR ligands also make them suitable for detection of non-continuous APR stimulations.
Our female hCRP-Luc mice would be a good model for studying IL-6- or non-IL-6-regulated CRP induction. Several studies have indicated that IL-6 is the principle inducer of CRP gene expression in humans; IL-1β, TNF-α and complements synergize with IL-618,23,32,33,34. Previous reports have shown that IL-6 production in mice after LPS-stimulation is extremely poor compared to that in humans35, and IL-1 is a stronger pyrogen than IL-6 in mice36. However, one study demonstrated that IL-1β-induced CRP production in human hepatocytes was mediated by IL-624, so we focus on IL-6-stimulated CRP production in our study. We found that LPS could induce a low level of IL-6 production (<30 pg/mL in sera, Fig. 3d), and at this level, bioluminescence expression in female hCRP-Luc mice was observed. We attributed this hCRP-Luc expression to IL-6, for we found that blockage of IL-6 using anti-IL-6 antibody significantly reduced the LPS-induced bioluminescence of hCRP-Luc mice. On the other hand, blocking IL-6 did not fully eliminate either LPS-induced bioluminescence or AGP expression (Fig. 3b,c), indicating the possible involvement of non-IL-6-mediated regulation. These results are in agreement with previous findings of PRR-induced, multiple pathway-mediated APPs16,17,19.
CRP is a marker of inflammation in both male and female patients. Thus, a major limitation of our hCRP-Luc mice is that only female mice could be used for detection of APR. Median levels and 95 percentiles of plasma testosterone levels are 0.19–12.18 ng/mL in mature male mice, which is similar to the normal range in human males37. Our male hCRP-Luc mice showed high levels of autonomous bioluminescence signal, which could be reduced by 98% via castration. This finding is in line with a previous report that orchiectomy decreased circulating CRP concentrations without changing serum IL-1β and IL-6 concentrations in humans25. Therefore, it might indicate that testicular hormones are involved in the regulation of hCRP promoter driven bioluminescence. In addition, it has been reported that testosterone could induce human C-reactive protein gene expression in transgenic mice15. Testosterone is the major testicular hormone known for its anti-inflammatory effect38. For example, testosterone suppresses hepatic inflammation in an experimental acute cholangitis murine model39; testosterone also reduces TNFα and IL-1β pro-inflammatory cytokines13,40 and suppresses IL-6 production in murine bone marrow stromal cell lines41. Testosterone transduces its signal through binding to androgen receptors (AR). Interestingly, several studies have indicated that AR signaling might enhance inflammation under certain conditions. In macrophages, AR signaling enhances TNF-α expression42. It has also been reported that AR activation is associated with carcinogenesis in human hepatocellular carcinoma and pancreatic cancer with enhanced IL-6 signaling43,44,45. Thus, the AR signaling may enhance the IL-6-signaling strength in liver local inflammation, but not directly induced CRP expression in male mice.
One other possibility to explain the significantly high basal expression level in male hCRP-luc mice is the differences in hCRP promoter activation by transcription factors in murine and human cells. Differences between human and mouse CRP gene cis-regulatory elements have been attributed to the different degree of CRP gene activation during APR between these two species. For example, human CRP promotor have both STAT3 and C/EBPβ binding site that can respond to IL-6 and IL-1 signalling while mouse CRP promotor has STAT3 but not C/EBPβ site46. The expression of hCRP in murine cells is dependent on the interaction between trans-acting mouse transcription factors and cis-acting human regulatory sequences. The presence of different trans-activating factors in mouse, especially those have differential expression pattern from human, might contribute to the different basal expression of hCRP in mice. For example, the expression level of HNF-1α, a trans-acting mouse transcription factor on hCRP promoter, is 2-fold higher in mouse liver than in human liver47. However, the extent of how each and every one of these human regulatory elements is regulated in mouse cells is relatively unknown. In addition, hCRP promoter activation in mouse liver may also be regulated by sex-specific mouse liver gene expression48. The CRP was the only annotated human gene in the 181-kb BAC transgene fragment we introduced. Our CRP-Luciferase transgene was dramatically larger than the one used in previous human CRP transgenic mice12,27. Therefore, our transgene was likely to include most of the cis-regulatory transcription elements that could regulate CRP transcription in humans32,49,50,51,52. In this regard, our hCRP-Luc mice provide a valuable model to study how gender specific genes regulate hCRP gene expression.
In summary, we generated a highly sensitive mouse that reported hCRP gene expression instead of serum protein measurement in vivo. The induction of liver bioluminescence in the animal was IL-6-mediated, highly sensitive to multiple TLR ligands, and feasible for repeated endotoxin stimulations. The female hCRP-Luc mice is a potential non-invasive reporter tool for monitoring APR.
Methods
Animals
An IRES-Luc cassette was inserted into the human BAC clone RP11-158E22 (BAC/PAC resources center, Children’s Hospital Oakland Research Institute) with human CRP gene (Fig. 1a). The IRES-Luc cassette contained the luciferase cDNA (from pGL3-basic plasmid, Promega) and the IRES element (from pLVX-IRES-tdTomato, Clontech Laboratories). In addition, the IRES-Luc sequences were followed by bovine growth hormone polyadenylation (BGH-pA) signal (from pcDNA3.1 plasmid, Invitrogen). The cassette was constructed and introduced into the human BAC clone RP11-158E22 using a Red/ET recombination kit (Gene Bridges). The transgenic BAC construct was isolated and linearized by Not I, and a 181-kb transgene fragment was purified by phenol-chloroform extraction for subsequent pronuclear injection in mouse embryos. The CRP-Luciferase transgene was located in the centre, with 64 kb of 5’ flanking sequence and 112 kb of 3’ flanking sequence. C57BL/6-Tg(hCRP-IRES-Luc) mice (hCRP-Luc mice) were generated by the Genetic Engineered Murine Model Service (GEMMS) and deposited in the Rodent Model Resource Center (RMRC13210, RMRC; http://www.nlac.org.tw/RMRC/). hCRP-Luc mice were bred and held at the National Laboratory Animal Center (NLAC), National Applied Research Laboratories (NARL). Hemizygous hCRP-Luc mice were bred with wildtype C57BL/6JNarl mice purchased from NLAC. The genomic DNA from hCRP-Luc offspring were collected and screened by PCR. Luc4637F (Forward, TCACGCAGGCAGTTCTATGAGG) and Luc5147R (Reverse, GAGGTGGACATCACTTACGCTGAG) primers were used to amplify a 510-bp fragment of the luciferase transgene. Mice at 8–16 weeks of age were used for experiments.
Approval
All animal procedures were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of National Laboratory Animal Center (NLAC; protocol numbers NLAC-107-M-005). Post approval monitoring (PAM) programs were implemented during the research. All methods were performed in accordance with the relevant guidelines and regulations.
In vivo induction of acute phase responses
Several TLR ligands and standard endotoxin were used in this study. Lipopolysaccharides (LPS) from Escherichia coli 0111:B4 were purchased from Sigma-Aldrich (L2630). LPS stock (2 mg/mL) was prepared with endotoxin-free saline (Taiwan Biotech). High-molecular weight polyinosinic-polycytidylic acid (poly(I:C)) was purchased from InvivoGen (tlrl-pic). Stock solution (1 mg/mL) of poly(I:C) in endotoxin-free saline was incubated for 10 min at 65–70 °C and cooled at room temperature for 1 h for proper annealing. Zymosan (Sigma-Aldrich Z4250), a polysaccharide connected by β-1,3-glycosidic linkages from Saccharomyces cerevisiae, was suspended in endotoxin-free saline (10 mg/mL). The zymosan suspension was boiled for 10 min at 100 °C and then kept on ice before use. All TLR ligand stock solutions were diluted to working concentrations (described in figure legends) with the same batch of endotoxin-free saline before injection.
To avoid endotoxin contamination during ligand preparation, poly(I:C) and zymosan were prepared using the same batch of endotoxin-free saline. The endotoxin concentration in poly(I:C) solution was <0.005 EU/mL as determined by Biolasco, Taiwan, using Endosafe Nexgen–PTS Test (PTS150, The Charles River Laboratory). The endotoxin level of zymosan could not be determined because zymosan itself could give false positive results.
E-Toxate Endotoxin standard was purchased from Sigma-Aldrich (E8029) and diluted serially with endotoxin-free saline. Recombinant murine IL-6 (1 μg/mouse, Catalog Number: 216-16, PeproTech) was injected intraperitoneally to induce bioluminescence of the female hCRP-Luc mice. For IL-6 neutralization test, anti-IL-6 monoclonal antibody (200 μg/mouse, Clone: MP5–20F3, Bio X cell) or isotype-matched irrelevant mAb (200 μg/mouse, Clone: HRPN, Bio X cell) was simultaneously co-injected with LPS via intraperitoneal route.
Bioluminescence analysis
The bioluminescence analyses of each mouse were performed at indicated times after TLR ligand injection. The mouse was injected intraperitoneally with 200 μl D-luciferin (15 mg/mL, Cat. L-123-250, GoldBio). After 10 minutes, the mouse was then anesthetized with isoflurane (3% vaporized in O2) and the bioluminescence signals were captured and analyzed using the In Vivo Imaging System (IVIS, IVIS 100, Caliper) or AmiHT (Spectral Instruments Imaging). Total flux of bioluminescence signals was analyzed from a fixed region-of-interest (ROI) over the abdomen using Living Image software (Caliper, for IVIS-collected images) or Aura image software (Caliper, for AmiHT-collected images).
Biomarker analysis
Peripheral blood samples were obtained from facial submandibular veins of mice using Golden Lancets (4 mm, Medipoint), and collected into a BD Microtainer Capillary Blood Collector (Cat. 365967). After centrifugation at 400 × g for 5 mins. Sera were harvested and frozen in a −20 °C freezer before analysis. Mouse APP concentration including serum amyloid protein/pentraxin-2 (SAP), adipsin, alpha-1 acid glycoprotein (AGP), and alpha-2 macroglobulin (A2M) were determined using a Multiplex kit (Millipore; MAP2MAG-76K-04) under standard procedures and analyzed by Luminex® 100 system. Mouse IL-6 and TNF were determined using the BD CBA flex set system (Cat. 558301 and 558299) and analyzed by FCAP Array software (BD Bioscience).
Statistics
Analyses of the bioluminescence, acute phase protein, and cytokine productions were performed using one-way ANOVA with post-hoc Tukey HSD (Honestly Significant Difference) test. The LAL standard endotoxin-induced, dose-dependent bioluminescence in female hCRP-Luc mice was analyzed by linear regression method. All statistics were calculated using GraphPad Prism version 6 for Windows. The results were statistically significant when p < 0.05.
References
Maes, M. et al. Acute phase proteins in schizophrenia, mania and major depression: modulation by psychotropic drugs. Psychiatry research 66, 1–11 (1997).
Gabay, C. & Kushner, I. Acute-phase proteins and other systemic responses to inflammation. The New England journal of medicine 340, 448–454, https://doi.org/10.1056/NEJM199902113400607 (1999).
Gruys, E., Toussaint, M. J., Niewold, T. A. & Koopmans, S. J. Acute phase reaction and acute phase proteins. Journal of Zhejiang University. Science. B 6, 1045–1056, https://doi.org/10.1631/jzus.2005.B1045 (2005).
Cray, C., Zaias, J. & Altman, N. H. Acute phase response in animals: a review. Comparative medicine 59, 517–526 (2009).
Marnell, L., Mold, C. & Du Clos, T. W. C-reactive protein: ligands, receptors and role in inflammation. Clinical immunology 117, 104–111, https://doi.org/10.1016/j.clim.2005.08.004 (2005).
Mold, C., Gewurz, H. & Du Clos, T. W. Regulation of complement activation by C-reactive protein. Immunopharmacology 42, 23–30 (1999).
Sjoberg, A. P., Trouw, L. A., McGrath, F. D., Hack, C. E. & Blom, A. M. Regulation of complement activation by C-reactive protein: targeting of the inhibitory activity of C4b-binding protein. Journal of immunology 176, 7612–7620 (2006).
Morley, J. J. & Kushner, I. Serum C-reactive protein levels in disease. Annals of the New York Academy of Sciences 389, 406–418 (1982).
Pepys, M. B. & Hirschfield, G. M. C-reactive protein: a critical update. J Clin Invest 111, 1805–1812, https://doi.org/10.1172/JCI18921 (2003).
Povoa, P. et al. C-reactive protein as a marker of infection in critically ill patients. Clin Microbiol Infect 11, 101–108, https://doi.org/10.1111/j.1469-0691.2004.01044.x (2005).
Povoa, P. C-reactive protein: a valuable marker of sepsis. Intensive Care Med 28, 235–243, https://doi.org/10.1007/s00134-002-1209-6 (2002).
Torzewski, M., Waqar, A. B. & Fan, J. Animal models of C-reactive protein. Mediators of inflammation 2014, 683598, https://doi.org/10.1155/2014/683598 (2014).
Corcoran, M. P. et al. Sex hormone modulation of proinflammatory cytokine and C-reactive protein expression in macrophages from older men and postmenopausal women. The Journal of endocrinology 206, 217–224, https://doi.org/10.1677/JOE-10-0057 (2010).
Castell, J. V. et al. Interleukin-6 is the major regulator of acute phase protein synthesis in adult human hepatocytes. FEBS letters 242, 237–239 (1989).
Szalai, A. J. et al. Testosterone and IL-6 requirements for human C-reactive protein gene expression in transgenic mice. Journal of immunology 160, 5294–5299 (1998).
Bode, J. G., Albrecht, U., Haussinger, D., Heinrich, P. C. & Schaper, F. Hepatic acute phase proteins–regulation by IL-6- and IL-1-type cytokines involving STAT3 and its crosstalk with NF-kappaB-dependent signaling. European journal of cell biology 91, 496–505, https://doi.org/10.1016/j.ejcb.2011.09.008 (2012).
Kushner, I. Regulation of the acute phase response by cytokines. Perspect Biol Med 36, 611–622 (1993).
Toniatti, C. et al. Regulation of the human C-reactive protein gene, a major marker of inflammation and cancer. Molecular biology & medicine 7, 199–212 (1990).
Ganter, U., Arcone, R., Toniatti, C., Morrone, G. & Ciliberto, G. Dual control of C-reactive protein gene expression by interleukin-1 and interleukin-6. The EMBO journal 8, 3773–3779 (1989).
Nishimoto, N. et al. Mechanisms and pathologic significances in increase in serum interleukin-6 (IL-6) and soluble IL-6 receptor after administration of an anti-IL-6 receptor antibody, tocilizumab, in patients with rheumatoid arthritis and Castleman disease. Blood 112, 3959–3964, https://doi.org/10.1182/blood-2008-05-155846 (2008).
van Rhee, F. et al. Siltuximab, a novel anti-interleukin-6 monoclonal antibody, for Castleman’s disease. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 28, 3701–3708, https://doi.org/10.1200/JCO.2009.27.2377 (2010).
Zhang, D., Jiang, S. L., Rzewnicki, D., Samols, D. & Kushner, I. The effect of interleukin-1 on C-reactive protein expression in Hep3B cells is exerted at the transcriptional level. Biochem J 310(Pt 1), 143–148 (1995).
Ganapathi, M. K., Rzewnicki, D., Samols, D., Jiang, S. L. & Kushner, I. Effect of combinations of cytokines and hormones on synthesis of serum amyloid A and C-reactive protein in Hep 3B cells. Journal of immunology 147, 1261–1265 (1991).
Moshage, H. J. et al. The effect of interleukin-1, interleukin-6 and its interrelationship on the synthesis of serum amyloid A and C-reactive protein in primary cultures of adult human hepatocytes. Biochemical and biophysical research communications 155, 112–117 (1988).
Kovacs, A. et al. Hormonal regulation of circulating C-reactive protein in men. Clin Chem 51, 911–913, https://doi.org/10.1373/clinchem.2004.046169 (2005).
Simons, J. P. et al. C-reactive protein is essential for innate resistance to pneumococcal infection. Immunology 142, 414–420, https://doi.org/10.1111/imm.12266 (2014).
Ciliberto, G., Arcone, R., Wagner, E. F. & Ruther, U. Inducible and tissue-specific expression of human C-reactive protein in transgenic mice. The EMBO journal 6, 4017–4022 (1987).
Daveau, M. et al. IL-6-induced changes in synthesis of alpha 1-acid glycoprotein in human hepatoma Hep3B cells are distinctively regulated by monoclonal antibodies directed against different epitopes of IL-6 receptor (gp80). European cytokine network 5, 601–608 (1994).
Heimann, M., Roth, D. R., Ledieu, D., Pfister, R. & Classen, W. Sublingual and submandibular blood collection in mice: a comparison of effects on body weight, food consumption and tissue damage. Lab Anim 44, 352–358, https://doi.org/10.1258/la.2010.010011 (2010).
Silveira, R. L., Andrade, S. S., Schmidt, C. A., Casali, R. G. & Dalmora, S. L. Comparative evaluation of pyrogens tests in pharmaceutical products. Braz J Microbiol 35, 48–53, https://doi.org/10.1590/S1517-83822004000100007 (2004).
Hartinger, J., Kulbs, D., Volkers, P. & Cussler, K. Suitability of temperature-sensitive transponders to measure body temperature during animal experiments required for regulatory tests. Altex 20, 65–70 (2003).
Volanakis, J. E. Human C-reactive protein: expression, structure, and function. Molecular immunology 38, 189–197, https://doi.org/10.1016/s0161-5890(01)00042-6 (2001).
Szalai, A. J., van Ginkel, F. W., Wang, Y., McGhee, J. R. & Volanakis, J. E. Complement-dependent acute-phase expression of C-reactive protein and serum amyloid P-component. Journal of immunology 165, 1030–1035 (2000).
Ivashchenko, Y. et al. Protein kinase C pathway is involved in transcriptional regulation of C-reactive protein synthesis in human hepatocytes. Arterioscler Thromb Vasc Biol 25, 186–192, https://doi.org/10.1161/01.ATV.0000150041.81963.68 (2005).
Warren, H. S. et al. Resilience to bacterial infection: difference between species could be due to proteins in serum. J Infect Dis 201, 223–232, https://doi.org/10.1086/649557 (2010).
Dinarello, C. A. et al. Interleukin-6 as an endogenous pyrogen: induction of prostaglandin E2 in brain but not in peripheral blood mononuclear cells. Brain Res 562, 199–206, https://doi.org/10.1016/0006-8993(91)90622-3 (1991).
Nelson, J. F., Latham, K. R. & Finch, C. E. Plasma testosterone levels in C57BL/6J male mice: effects of age and disease. Acta Endocrinol (Copenh) 80, 744–752, https://doi.org/10.1530/acta.0.0800744 (1975).
Bianchi, V. E. Testosterone, myocardial function, and mortality. Heart Fail Rev 23, 773–788, https://doi.org/10.1007/s10741-018-9721-0 (2018).
Schwinge, D. et al. Testosterone suppresses hepatic inflammation by the downregulation of IL-17, CXCL-9, and CXCL-10 in a mouse model of experimental acute cholangitis. Journal of immunology 194, 2522–2530, https://doi.org/10.4049/jimmunol.1400076 (2015).
Malkin, C. J. et al. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J Clin Endocrinol Metab 89, 3313–3318, https://doi.org/10.1210/jc.2003-031069 (2004).
Bellido, T. et al. Regulation of interleukin-6, osteoclastogenesis, and bone mass by androgens. The role of the androgen receptor. J Clin Invest 95, 2886–2895, https://doi.org/10.1172/JCI117995 (1995).
Lai, J. J. et al. Monocyte/macrophage androgen receptor suppresses cutaneous wound healing in mice by enhancing local TNF-alpha expression. J Clin Invest 119, 3739–3751, https://doi.org/10.1172/JCI39335 (2009).
Okitsu, K. et al. Involvement of interleukin-6 and androgen receptor signaling in pancreatic cancer. Genes Cancer 1, 859–867, https://doi.org/10.1177/1947601910383417 (2010).
Kanda, T., Jiang, X. & Yokosuka, O. Androgen receptor signaling in hepatocellular carcinoma and pancreatic cancers. World J Gastroenterol 20, 9229–9236, https://doi.org/10.3748/wjg.v20.i28.9229 (2014).
Kanda, T. & Yokosuka, O. The androgen receptor as an emerging target in hepatocellular carcinoma. J Hepatocell Carcinoma 2, 91–99, https://doi.org/10.2147/JHC.S48956 (2015).
Ochrietor, J. D., Harrison, K. A., Zahedi, K. & Mortensen, R. F. Role of STAT3 and C/EBP in cytokine-dependent expression of the mouse serum amyloid P-component (SAP) and C-reactive protein (CRP) genes. Cytokine 12, 888–899, https://doi.org/10.1006/cyto.2000.0668 (2000).
Harries, L. W., Brown, J. E. & Gloyn, A. L. Species-specific differences in the expression of the HNF1A, HNF1B and HNF4A genes. PLoS One 4, e7855, https://doi.org/10.1371/journal.pone.0007855 (2009).
Conforto, T. L. & Waxman, D. J. Sex-specific mouse liver gene expression: genome-wide analysis of developmental changes from pre-pubertal period to young adulthood. Biol Sex Differ 3, 9, https://doi.org/10.1186/2042-6410-3-9 (2012).
Ruminy, P. et al. Gene transcription in hepatocytes during the acute phase of a systemic inflammation: from transcription factors to target genes. Inflamm Res 50, 383–390, https://doi.org/10.1007/PL00000260 (2001).
Nishikawa, T. et al. Transcriptional complex formation of c-Fos, STAT3, and hepatocyte NF-1 alpha is essential for cytokine-driven C-reactive protein gene expression. Journal of immunology 180, 3492–3501, https://doi.org/10.4049/jimmunol.180.5.3492 (2008).
Voleti, B., Hammond, D. J. Jr., Thirumalai, A. & Agrawal, A. Oct-1 acts as a transcriptional repressor on the C-reactive protein promoter. Molecular immunology 52, 242–248, https://doi.org/10.1016/j.molimm.2012.06.005 (2012).
Young, D. P., Kushner, I. & Samols, D. Binding of C/EBPbeta to the C-reactive protein (CRP) promoter in Hep3B cells is associated with transcription of CRP mRNA. Journal of immunology 181, 2420–2427, https://doi.org/10.4049/jimmunol.181.4.2420 (2008).
Acknowledgements
The authors thank Dr. Shu-Ching Chen, Dr. Yo-Ping Lai, (Department of Internal Medicine, National Taiwan University Hospital) and Dr. Chun-Keung Yu (National Laboratory Animal Center, National Applied Research Laboratories), for comments on the manuscript. The authors also thank the Contract Breeding and Research Division, NLAC, for assistance with mouse husbandry, mouse experiments, and biomarker analysis.
Author information
Authors and Affiliations
Contributions
Chun-Fang Huang and Shang-Yi Chiu designed the experiments and wrote the main manuscript. Hung-Wen Huang interpreted bioluminescence data. Chia-Chi Liao performed animal experiments. Bing-Ho Cheng performed biomarker analysis. Hsiu-Min Pan, Wei-Lun Huang, Hsiao-Hui Chang, and Si-Tse Jiang generated hCRP-Luc Tg mice. Yu-Chia Su supervised this project and finalized manuscript preparation. All authors contributed to scientific discussions and read and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huang, CF., Chiu, SY., Huang, HW. et al. A reporter mouse for non-invasive detection of toll-like receptor ligands induced acute phase responses. Sci Rep 9, 19065 (2019). https://doi.org/10.1038/s41598-019-55281-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-55281-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.