Abstract
B7-H6, a member of the B7 family molecules, participates in the clearance of tumor cells by binding to NKp30 on NK cells. B7-H6 expression level in esophageal squamous cell carcinoma (ESCC) and the clinical value remain unknown. The goal of this study was to determine the expression of B7-H6 in ESCC and further explore its clinical significance. We retrospectively collected the clinical data of 145 patients diagnosed with ESCC between January 2007 and December 2008. The expression of B7-H6 of the pathological tissue samples was detected by immunohistochemistry. The chi-square (χ2) test was used to analyse the relationships of B7-H6 and clinicopathological characteristics. Survival and hazard functions were estimated using the Kaplan-Meier method, and survival between groups was compared using the two-sided log-rank test. The Cox proportional hazards regression model was used to adjust for the risk factors related to overall survival (OS). 133/145 (91.72%) of the ESCC tissue samples exhibited B7-H6 expression. The expression level of B7-H6 was correlated with T stage (P = 0.036) and lymphatic metastasis status (P = 0.044). High B7-H6 expression (P = 0.003) was associated with a significantly worse OS than low B7-H6 expression. Multivariate Cox proportional hazards regression analysis demonstrated that tumour size (P = 0.021), B7-H6 expression (P = 0.025) and lymphatic metastasis status (P = 0.049) were independent prognostic factors of OS for ESCC. Collectively, our findings suggest that B7-H6 is widely expressed in ESCC samples. And B7-H6 may represent a predictor of poor prognosis for ESCC.
Similar content being viewed by others
Introduction
Esophageal cancer is one of the most common malignant tumours in the world, ranking as the 6th most common cause of cancer-related death and the 8th most common cancer in the world, with an increasing incidence1. Esophageal squamous cell carcinoma (ESCC) accounts for about 90% of esophageal cancer cases, which always in advanced stage when first diagnosed, with a low 5-year overall survival (OS) rate of about15~25%1. Recently, with the rapid development of surgery, radiotherapy, chemotherapy, biological therapy and other comprehensive treatment methods, the prognosis of ESCC is still poor. Therefore, in-depth studies of the molecular mechanism underlying the occurrence and development of ESCC and the search for new molecular targets for diagnosis and prognostic monitoring have important clinical application value.
Tumour immune escape is an important molecular mechanism in the processes of tumourigenesis, invasion and metastasis. Tumour cells are usually unable to provide effective antigen signals, or the body has a defective immune response and is immunosuppressed, Further, they escape the surveillance and monitoring of the immune effector cells2. In this process, costimulatory molecules and their regulatory networks play an important role. Costimulatory molecules are mainly divided into two superfamilies, B7/CD28 and TNF/TNFR3. Recently, new discovered members of the B7 family of costimulatory molecules, such as B7-H1, B7-H3, B7-H4 and B7-H6, are widely expressed in many human tumour tissue types and can participate in the negative regulation of the T/natural killer (NK) cell-mediated antitumour immune response, thus attracting wide attention3,4. Studies have revealed that the expression of PD-L1 (B7-H1) and B7-H3 in esophageal cancer were associated with prognosis5,6,7. Ling Wang et al. reported B7-H3 and B7-H4 were widely expressed in ESCC. The high expression level of B7-H3 and B7-H4 were related to TNM stages and lymph node metastasis. Patients with both high levels of B7-H3 and B7-H4 had the poorest prognosis8. Additionally, Lijie Chen et al. demonstrated that B7-H4 expression was associated with ESCC progression and survival by reducing tumor immunosurveillance9.
B7-H6 is a recently discovered member in the B7 family10,11. It is a type I transmembrane protein that shows similar structure to B7-H1 and B7-H3 proteins12. The extracelluar region of B7-H6 consists of an IgV-like domain and an IgC-like domain. Gordon Joyce et al. have verified that the extracellular region of NKp30 interacts with the extracellular region of B7-H6 by directly and selectively binding, which used residue mutation strategy13. Studies have shown that B7-H6 can be activated by binding with the activation receptor NKp30 on the surface of NK cells and that B7-H6 promotes TNF- and IFN-mediated killing of tumour cells by NK cells, which is one of the important mechanisms of NK cell-mediated antitumour immunity12. B7-H6 expression in mRNA level was found in human primary lymphoma, leukemia, ovarian cancer, brain tumour, breast cancers, renal cell carcinoma, and various sarcomas11. The B7-H6 expression level was significantly upregulated in tumour tissue samples compared with the normal tissue, which was closely related to the clinicopathological characteristics and prognosis of patients14,15,16,17. However, until now, the clinical significance of B7-H6 expression in ESCC has not been reported. In this study, we investigated B7-H6 expression in ESCC tissue samples and explored the clinical implications.
Results
Study population
Patient characteristics were shown in Table 1. The median age of the study group was 60 years (range from 34 to 88 years). 82 cases (56.6%) patients were diagnosed with grade I-II and 63 cases (43.4%) were grade III- IV, according to TNM staging. Of the 145 patients examined, 79 (54.5%) died before the last follow-up evaluation period.
B7-H6 expression in esophageal tissue
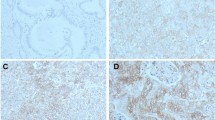
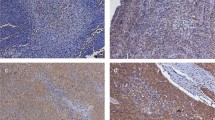
To detect the B7-H6 expression level in ESCC tissue, immunohistochemical analysis was used (Fig. 1). The approach showed that B7-H6 was present in 133/145 (91.72%) of the samples, which localized in the cytoplasm, while weak B7-H6 staining was found in normal esophageal tissue. The median value of the B7-H6 staining H-score was 40 (0–180).
Immunohistochemical staining (magnification: left, 5×; right, 100×). Results: B7-H6 expression (brown) in ESCC tissue samples and adjacent normal tissue samples. Negative B7-H6 expression (A); Low B7-H6 expression (B); High B7-H6 expression in ESCC tissue samples(C). Low B7-H6 expression in adjacent normal tissue samples(D).
The clinical significance of B7-H6 expression
The clinicopathological characteristics and B7-H6 expression of all patients were presented in Table 2. All patients were divided into two major subgroups according to the median B7-H6 intensity of staining of 40. This stratification revealed that B7-H6 expression was significantly correlated with T stage (P = 0.036) and lymphatic metastasis status (P = 0.044); however, B7-H6 expression did not correlate with other risk factors, including gender, age, tumour size, tumour location, differentiation grade, TNM stages, local recurrence status and metastasis status (P > 0.05).
Survival outcomes
With H-score = 90 as the cut-off value, the 145 patients were divided into two subgroups: low B7-H6 expression group (H-score ≤ 90) and high B7-H6 expression group (H-score > 90). The survival analysis using the log-rank test demonstrated that the patients with tumour size ≥ 3.0 cm (P = 0.001; Fig. 2A), with lymphatic metastasis (P < 0.001; Fig. 2B) and high B7-H6 expression (P = 0.003; Fig. 2C) expressed significantly worse survival, respectively. Univariate Cox regression analysis demonstrated that the clinical parameters tumour size, T stage, lymphatic metastasis, TNM stages and B7-H6 expression were significantly associated with survival. Multi-factors Cox regression analyses showed that tumour size (HR: 1.749, 95% CI: 1.089–2.807; P = 0.021), lymphatic metastasis status (HR: 2.157, 95% CI: 1.002–4.644; P = 0.049) and B7-H6 expression (HR: 1.751, 95% CI: 1.071–2.861; P = 0.025) were independent prognostic factors in ESCC, as shown in Table 3.
Discussion
The superfamily member B7/CD28 has been shown to play an important role in the immune response, and they are seen as effective markers in cancer diagnosis and treatment3,18. B7-H6, the new member of the B7 family, interacted with NK cell surface receptor NKp30 and played an obvious role in NK cell-mediated immune responses11. NK cells were important immune cells in the body. It was a core cell of the natural immune system and can kill tumour cells. NK-cells activation was regulated by some activation receptors or inhibition receptors on the cell surface10. The major activating receptors included NKG2D and the natural cytotoxicity receptors (NCRs) such as NKp46, NKp30, and NKp4419. NKp30 can promote NK cells to recognize and kill tumor cells, either alone or together with other stimulation receptors20,21,22. The HLA–B-associated transcript 3 (BAT3) and the pp65 proteins have been revealed to bind NKp30, but they don’t bind to ligands on the surface of tumour cell because pp65 was a cytomegalovirus tegument protein23 and BAT3 was a nuclear protein released after heat-shock treatment24. B7-H6 is a potent ligand for NKp30, and it doesn’t bind any other CD28 family members nor other NCRs12. B7-H6 expressed on tumour cells contacted NKp30 in a unique way that is the complementarity-determining region (CDR)-like loops of its V-like domain25. NK cells eliminate B7-H6-expressing tumour cells either directly via cytotoxicity or indirectly by cytokine secretion11. Eva Schlecke et al. illustrated that tumour cells impeded NK-mediated recognition by metalloprotease-mediated shedding of B7-H626. Soluble B7-H6 generated by ectodomain shedding is another form of B7-H611. Soluble B7-H6 had the ability to block the connection between anti-NKp30 mAbs and NKp30, thus inhibiting NKp30-mediated NK cell triggering26,27. Taken together, these data on B7-H6/ NKp30 interaction provided a theoretical basis for the development of novel cancer treatments.
In recent years, immune checkpoint inhibitors that block cytotoxic T-lymphocyte antigen 4 (CTLA-4) and programmed cell death protein 1 (PD-1) have led to significant improvements in prognosis and have brought tumour immunotherapy into a new era28,29. Several clinical studies in esophageal cancer using PD-1 inhibitors, such as nivolumab or pembrolizumab, are in progress with recent promising results30,31,32. However, the relationship between PD-1/PD-L1 expression in esophageal cancer tissue and prognosis remains controversial. To date, no good biomarker has been found to guide treatment and prognosis. Abnormal expression of B7-H6 has been found in many cancers, revealing that B7-H6 expressed important clinical significance. Our study is the first to explore the connection between prognosis and clinical implications of B7-H6 expression in ESCC tissue. The results of IHC staining suggested that B7-H6 expression was present in most ESCC tissue samples, which was consistent with the findings of other studies14,17,33. We also found that the expression level of B7-H6 correlated with T stage and lymphatic metastasis status, which suggested that the expression of B7-H6 may be a marker to identify T stage and lymphatic metastasis status of ESCC. The result is similar to the findings of studies in gastric cancer, ovarian cancer, non-small cell lung cancer, astrocytoma, breast cancer and other cancers14,15,17,33,34,35. In addition, Cox regression model analysis and log-rank test demonstrated that the expression level of B7-H6 was an independent prognostic factor for ESCC. Patients with high B7-H6 expression had significantly worse survival than those with low B7-H6 expression, suggesting that high B7-H6 expression is a predictor of poor prognosis. This result is similar to data from other researchers15,34,35. Thus, B7-H6 might be a meaningful biomarker for predicting the OS of ESCC patients, and also serve as an independent prognostic index. In the past three years, some studies about the knockdown of B7-H6 expression in tumours have been carried out16,36,37 and implied that B7-H6 might be a meaningful target for cancer therapy. Therefore, it is believed that further studies on B7-H6 expression at the gene level and the knockdown of B7-H6 expression may also have certain clinical value in determining the prognosis of ESCC patients.
Still, our study has some limitations. Firstly, its retrospective nature, potential selection bias, and confounding bias, were unavoidable. Secondly, all tumour samples were all from patients of China, which may differ from other ethnics and region. Thirdly, it may be more meaningful to detect B7-H6 expression in the protein and gene level using western bolt, enzyme-linked immunosorbent assay (ELISA) or gene chip. And further validate the phenotype change via altering the expression of B7-H6 in ESCC is meaningful.
Material and Methods
Patient characteristics
We retrospectively collected clinical data of 145 patients with ESCC between January 2007 and December 2008 in Sun Yat-sen University Cancer Center (SYSUCC). These patients had all undergone surgery, pathologically diagnosed, and had not received chemotherapy or radiotherapy. Pathological tissue samples from the 145 patients were collected to detect the expression of B7-H6 by immunohistochemistry. In addition, 7 non-malignant esophageal tissue samples were collected and used as controls. The clinicopathological data of all patients were available and were used for statistical analysis. The study was approved by SYSUCC Ethics Committee, and informed consent was provided by all patients based on the Declaration of Helsinki.
Antibodies and major regents
Anti-B7-H6 antibody (ab121794) was purchased from Abcam (Cambridge, MA, USA; dilution 1/100), and horseradish peroxidase (HRP) secondary antibodies was purchased from Dako (Glostrup, Denmark). A DAB color kit was purchased from Beijing Zhongshan Jinqiao Biotechnology Co., Ltd. (Beijing, China; Cat. No. zli-9017).
Immunohistochemistry
Paraffin-embedded tissue samples were cut into 5 μm sections and roasted for 30 minutes in a 60 °C constant-temperature box. The sections were dewaxed with xylene and rehydrated with different concentrations of ethanol. Blocking endogenous peroxidase activity with 3% hydrogen peroxide solution for 10 minutes, extracting antigen in sodium citrate buffer (0.01 mmol/L, pH 6.0) at 100 °C for 20 minutes. After being soaked in distilled water for 10 minutes, the sections were incubated with 10% foetal bovine serum to block nonspecific binding. Next, the sections were incubated with rabbit anti-human B7-H6 polyclonal antibody at 4 °C overnight, and then incubated with the HRP-conjugated goat anti-mouse/rabbit secondary antibody at room temperature for 60 minutes. The excess secondary antibody was removed by washing with TBS and developed with DAB colorant; the sections were stained with haematoxylin, dehydrated with an alcohol gradient, dried and sealed with neutral resin.
Evaluation of immunohistochemical (IHC) staining
The ESCC tissue samples were analyzed by two independent senior pathologists who did not know the clinical and pathological information of the patients. The B7-H6 immunohistochemical staining results were analyzed according to a previously described method9,38: H-score = (% non-stained tumour cells × 0) + (% weakly stained tumour cells × 1) + (% moderately stained tumour cells × 2) + (% strongly stained tumour cells × 3). The staining intensity was divided into four grades: “0” (non-stained), “1” (weakly stained), “2” (moderately stained), or “3” (strongly stained). The H-scores ranged from 0 to 300. The average results of two pathologists were taken for statistical analysis.
Statistical analyses
Overall survival (OS) was used to assess prognostic indicator. It is the time from surgery to patient death or last follow-up. The final follow-up date was December 24, 2018. The B7-H6 high and low expression groups were determined by receiver operating characteristic (ROC) curve analysis. The correlations between the B7-H6 expression level and different clinical parameters were analysed by the chi-square (χ2) test. Survival date were analysed using the Kaplan–Meier method. Cox regression model analysis were used to explore the effects of clinical variables and B7-H6 on survival. Statistical significance was defined at P < 0.05. All date was analysed by SPSS software (version 13.0; IBM Corp., Armonk, NY, USA).
Conclusion
Our present study indicated that B7-H6 was widely expressed in ESCC tissues and can serve as an independent prognostic marker for ESCC.
Data availability
The data supporting the conclusions is available in the repository [the Research Data Deposit public platform], [RDDA2019001075 in http://www.researchdata.org.cn].
References
Pennathur, A., Gibson, M. K., Jobe, B. A. & Luketich, J. D. Oesophageal carcinoma. Lancet 381, 400–412, https://doi.org/10.1016/S0140-6736(12)60643-6 (2013).
Schreiber, R. D., Old, L. J. & Smyth, M. J. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 331, 1565–1570, https://doi.org/10.1126/science.1203486 (2011).
Lu, B. et al. T-cell-mediated tumor immune surveillance and expression of B7 co-inhibitory molecules in cancers of the upper gastrointestinal tract. Immunologic research 50, 269–275, https://doi.org/10.1007/s12026-011-8227-9 (2011).
Zou, W. & Chen, L. Inhibitory B7-family molecules in the tumour microenvironment. Nature reviews. Immunology 8, 467–477, https://doi.org/10.1038/nri2326 (2008).
Leng, C. et al. Relationship between expression of PD-L1 and PD-L2 on esophageal squamous cell carcinoma and the antitumor effects of CD8(+) T cells. Oncology reports 35, 699–708, https://doi.org/10.3892/or.2015.4435 (2016).
Lim, S. H. et al. Changes in tumour expression of programmed death-ligand 1 after neoadjuvant concurrent chemoradiotherapy in patients with squamous oesophageal cancer. European journal of cancer 52, 1–9, https://doi.org/10.1016/j.ejca.2015.09.019 (2016).
Song, J. et al. Epidermal growth factor receptor and B7-H3 expression in esophageal squamous tissues correlate to patient prognosis. OncoTargets and therapy 9, 6257–6263, https://doi.org/10.2147/OTT.S111691 (2016).
Wang, L. et al. Roles of coinhibitory molecules B7-H3 and B7-H4 in esophageal squamous cell carcinoma. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine 37, 2961–2971, https://doi.org/10.1007/s13277-015-4132-5 (2016).
Chen, L. J. et al. B7-H4 expression associates with cancer progression and predicts patient’s survival in human esophageal squamous cell carcinoma. Cancer immunology, immunotherapy: CII 60, 1047–1055, https://doi.org/10.1007/s00262-011-1017-3 (2011).
Chen, Y., Mo, J., Jia, X. & He, Y. The B7 Family Member B7-H6: a New Bane of Tumor. Pathology oncology research: POR 24, 717–721, https://doi.org/10.1007/s12253-017-0357-5 (2018).
Ni, L. & Dong, C. New B7 Family Checkpoints in Human Cancers. Molecular cancer therapeutics 16, 1203–1211, https://doi.org/10.1158/1535-7163.MCT-16-0761 (2017).
Brandt, C. S. et al. The B7 family member B7-H6 is a tumor cell ligand for the activating natural killer cell receptor NKp30 in humans. The Journal of experimental medicine 206, 1495–1503, https://doi.org/10.1084/jem.20090681 (2009).
Joyce, M. G. et al. Crystal structure of human natural cytotoxicity receptor NKp30 and identification of its ligand binding site. Proceedings of the National Academy of Sciences of the United States of America 108, 6223–6228, https://doi.org/10.1073/pnas.1100622108 (2011).
Chen, X. J., Shen, J., Zhang, G. B. & Chen, W. C. B7-H6 protein expression has no prognostic significance in human gastric carcinoma. Pathology oncology research: POR 20, 203–207, https://doi.org/10.1007/s12253-013-9686-1 (2014).
Zhou, Y. et al. B7-H6 expression correlates with cancer progression and patient’s survival in human ovarian cancer. International journal of clinical and experimental pathology 8, 9428–9433 (2015).
Wu, F., Wang, J. & Ke, X. Knockdown of B7-H6 inhibits tumor progression and enhances chemosensitivity in B-cell non-Hodgkin lymphoma. International journal of oncology 48, 1561–1570, https://doi.org/10.3892/ijo.2016.3393 (2016).
Zhang, X., Zhang, G., Qin, Y., Bai, R. & Huang, J. B7-H6 expression in non-small cell lung cancers. International journal of clinical and experimental pathology 7, 6936–6942 (2014).
Seliger, B. & Quandt, D. The expression, function, and clinical relevance of B7 family members in cancer. Cancer immunology, immunotherapy: CII 61, 1327–1341, https://doi.org/10.1007/s00262-012-1293-6 (2012).
Kaifu, T., Escaliere, B., Gastinel, L. N., Vivier, E. & Baratin, M. B7-H6/NKp30 interaction: a mechanism of alerting NK cells against tumors. Cellular and molecular life sciences: CMLS 68, 3531–3539, https://doi.org/10.1007/s00018-011-0802-7 (2011).
Pende, D. et al. Major histocompatibility complex class I-related chain A and UL16-binding protein expression on tumor cell lines of different histotypes: analysis of tumor susceptibility to NKG2D-dependent natural killer cell cytotoxicity. Cancer research 62, 6178–6186 (2002).
Pende, D. et al. Identification and molecular characterization of NKp30, a novel triggering receptor involved in natural cytotoxicity mediated by human natural killer cells. The Journal of experimental medicine 190, 1505–1516, https://doi.org/10.1084/jem.190.10.1505 (1999).
Byrd, A., Hoffmann, S. C., Jarahian, M., Momburg, F. & Watzl, C. Expression analysis of the ligands for the Natural Killer cell receptors NKp30 and NKp44. PloS one 2, e1339, https://doi.org/10.1371/journal.pone.0001339 (2007).
Arnon, T. I. et al. Inhibition of the NKp30 activating receptor by pp65 of human cytomegalovirus. Nature immunology 6, 515–523, https://doi.org/10.1038/ni1190 (2005).
Pogge von Strandmann, E. et al. Human leukocyte antigen-B-associated transcript 3 is released from tumor cells and engages the NKp30 receptor on natural killer cells. Immunity 27, 965–974, https://doi.org/10.1016/j.immuni.2007.10.010 (2007).
Wang, L. et al. VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. The Journal of experimental medicine 208, 577–592, https://doi.org/10.1084/jem.20100619 (2011).
Schlecker, E. et al. Metalloprotease-mediated tumor cell shedding of B7-H6, the ligand of the natural killer cell-activating receptor NKp30. Cancer research 74, 3429–3440, https://doi.org/10.1158/0008-5472.CAN-13-3017 (2014).
Matta, J. et al. Induction of B7-H6, a ligand for the natural killer cell-activating receptor NKp30, in inflammatory conditions. Blood 122, 394–404, https://doi.org/10.1182/blood-2013-01-481705 (2013).
Redman, J. M., Gibney, G. T. & Atkins, M. B. Advances in immunotherapy for melanoma. BMC medicine 14, 20, https://doi.org/10.1186/s12916-016-0571-0 (2016).
Carlo, M. I., Voss, M. H. & Motzer, R. J. Checkpoint inhibitors and other novel immunotherapies for advanced renal cell carcinoma. Nature reviews. Urology 13, 420–431, https://doi.org/10.1038/nrurol.2016.103 (2016).
Kojima, T. & Doi, T. Immunotherapy for Esophageal Squamous Cell Carcinoma. Current oncology reports 19, 33, https://doi.org/10.1007/s11912-017-0590-9 (2017).
Kudo, T. et al. Nivolumab treatment for oesophageal squamous-cell carcinoma: an open-label, multicentre, phase 2 trial. The Lancet. Oncology 18, 631–639, https://doi.org/10.1016/S1470-2045(17)30181-X (2017).
Doi, T. et al. Safety and Antitumor Activity of the Anti-Programmed Death-1 Antibody Pembrolizumab in Patients With Advanced Esophageal Carcinoma. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 36, 61–67, https://doi.org/10.1200/JCO.2017.74.9846 (2018).
Guo, J. G. et al. Clinical significance of B7-H6 protein expression in astrocytoma. OncoTargets and therapy 9, 3291–3297, https://doi.org/10.2147/OTT.S103771 (2016).
Chen, L. et al. Expression of B7-H6 expression in human hepatocellular carcinoma and its clinical significance. Cancer cell international 18, 126, https://doi.org/10.1186/s12935-018-0627-7 (2018).
Sun, J. et al. Clinical significance of novel costimulatory molecule B7-H6 in human breast cancer. Oncology letters 14, 2405–2409, https://doi.org/10.3892/ol.2017.6417 (2017).
Che, F. et al. B7-H6 expression is induced by lipopolysaccharide and facilitates cancer invasion and metastasis in human gliomas. International immunopharmacology 59, 318–327, https://doi.org/10.1016/j.intimp.2018.03.020 (2018).
Jiang, T. et al. High expression of B7-H6 in human glioma tissues promotes tumor progression. Oncotarget 8, 37435–37447, https://doi.org/10.18632/oncotarget.16391 (2017).
Chen, L. et al. Immunochemical staining of MT2-MMP correlates positively to angiogenesis of human esophageal cancer. Anticancer research 30, 4363–4368 (2010).
Acknowledgements
The authors would like to thank the patients who participated in this study, and two independent senior pathologists for technical support. This study was supported by the Science and Technology Planning Project of the Guangdong Esophageal Cancer Research Institute (No. Q201607) and 2016 Youth Teacher Training Program of Sun Yat-sen University (No. 051703).
Author information
Authors and Affiliations
Contributions
S.Y. and X.C. conceived the original idea and designed the study. H.Z. and K.W. collected the date for the study, which were analysed by H.Z., J.D. and L.G. The date interpretation and manuscript drafting were performed by H.Z. and J.D. The manuscript was revised by X.W., L.G., X.C. and S.Y. All authors reviewed the manuscript and agreed to submit it.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhou, H., Dong, J., Guo, L. et al. The prognostic value of B7-H6 in esophageal squamous cell carcinoma. Sci Rep 9, 18122 (2019). https://doi.org/10.1038/s41598-019-54731-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-54731-9
This article is cited by
-
Establishment and validation of a prognostic risk classification for patients with stage T1-3N0M0 esophageal squamous cell carcinoma
Journal of Cardiothoracic Surgery (2023)
-
The possibilities of LOXL4 as a prognostic marker for carcinomas
Amino Acids (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.