Abstract
Size and duration of the neuroplastic effects of tDCS depend on stimulation parameters, including stimulation duration and intensity of current. The impact of stimulation parameters on physiological effects is partially non-linear. To improve the utility of this intervention, it is critical to gather information about the impact of stimulation duration and intensity on neuroplasticity, while expanding the parameter space to improve efficacy. Anodal tDCS of 1–3 mA current intensity was applied for 15–30 minutes to study motor cortex plasticity. Sixteen healthy right-handed non-smoking volunteers participated in 10 sessions (intensity-duration pairs) of stimulation in a randomized cross-over design. Transcranial magnetic stimulation (TMS)-induced motor-evoked potentials (MEP) were recorded as outcome measures of tDCS effects until next evening after tDCS. All active stimulation conditions enhanced motor cortex excitability within the first 2 hours after stimulation. We observed no significant differences between the three stimulation intensities and durations on cortical excitability. A trend for larger cortical excitability enhancements was however observed for higher current intensities (1 vs 3 mA). These results add information about intensified tDCS protocols and suggest that the impact of anodal tDCS on neuroplasticity is relatively robust with respect to gradual alterations of stimulation intensity, and duration.
Similar content being viewed by others
Introduction
Neuroplasticity is the structural and functional modification of synaptic connections in response to internal or external stimuli. It is involved in cognitive and behavioural functions such as learning and memory formation in healthy organisms, but also in restitution of functions after brain lesions. In neurological and psychiatric diseases moreover, pathological alterations of neuroplasticity play a role. Thus, the exploration of mechanisms, and consequences of neuroplasticity is relevant. Modern intervention techniques have endowed us with the ability to induce neuroplasticity in the intact human brain non-invasively. These non-invasive brain stimulation (NIBS) tools, which include transcranial magnetic stimulation (TMS), and transcranial electric stimulation (tES) have enhanced our understanding of functional relationships between brain physiology and behaviour1. Transcranial direct current stimulation (tDCS) is one tES technique which has been shown to modulate cognitive functions in healthy humans2, and to alter psychiatric and neurological symptoms in patients via induction of plasticity3,4,5,6,7.
tDCS induces polarity-dependent neuroplastic changes in the brain, which can last for hours depending on the dosage of stimulation. In the primary motor cortex, an enhancement of cortical excitability is observed when the anode is placed over the target region with stimulation intensities for up to 2 mA, and stimulation durations for up to 20 min, and an excitability reduction with the cathode over the target region for a stimulation intensity of 1 mA and an electrode size of 35 cm2 8,9,10. Unlike other NIBS techniques, such as repetitive TMS, tDCS uses weak direct currents to induce gradual changes of the resting membrane potential of cortical neurons in a polarity dependent manner – anodal stimulation leads to a subthreshold depolarization whereas cathodal stimulation leads to hyperpolarization of neuronal compartments critical for the respective excitability, and neuronal activity alterations11,12.
In conventional tDCS, two rubber electrodes covered in a saline-soaked sponge are placed on the scalp – one over the target area, and the other over a remote region8. Direct current passes through the skull and cerebrospinal fluid before reaching the brain. Whereas the primary effects of tDCS are due to neuronal membrane polarization, after-effects are caused by a modification of synaptic strength related to long-term potentiation (LTP) and long-term depression (LTD). tDCS-induced plasticity depends on glutamatergic mechanisms. It is calcium-, N-methyl-D-aspartate (NMDA) receptor-13,14,15, and α-amino-3-hydroxy-5-methyl-4-isoxazoleprionic acid (AMPA) receptor-dependent16,17. A concomitant reduction of GABA might serve as a gating mechanism18,19. For calcium-dependent plasticity, the amount of calcium influx determines the type of plasticity observed20. Whereas relatively low calcium concentration results in LTD, a high amount of calcium results in LTP. Between these concentrations, a no man’s land, or transition zone does exist, which results in no plasticity21. Excessive calcium concentration results likewise in no plasticity due to counteracting mechanisms, which include activation of potassium channels22.
Within the parameter space of 0.2–1 mA current intensity, and 5–13 minutes duration, previous studies have shown stronger and longer after-effects induced by stronger and longer stimulation8,9. However, some non-linear relationships between stimulation parameters and excitability alterations have been reported for stronger and longer-lasting stimulation protocols23,24, which are compatible with the above-mentioned calcium hypothesis. These recent studies stress the relevance of a systematic titration of tDCS parameters, to identify protocols that are resulting in stable and unidirectional effects.
The parameter space of tDCS however is large, with different parameters contributing to the direction and magnitude of after-effects. Intensity and duration of stimulation are two critical parameters. This notwithstanding, stimulation protocols have not systematically been further developed during the last years, with stimulation intensity and duration between 0.5–2 mA, and 10–30 minutes in most studies. Some study results suggest that increasing the current intensity does not necessarily lead to longer neuroplastic changes or treatment outcomes, or even convert the direction of after-effects23,24,25. Recently, Jamil and colleagues systematically evaluated the effect of four tDCS intensities - 0.5, 1, 1.5, and 2 mA – applied for the duration of 15 minutes, on motor cortical excitability, and found no significant differences in after-effects induced by these intensities for anodal tDCS26. Apart from the intensity of tDCS, stimulation duration also affects the amount of induced neuroplasticity9. When the duration of 1 mA anodal tDCS was doubled from 13 minutes to 26 minutes, stimulation did induce no excitability enhancement, but diminution, showing a dependence of directionality of tDCS-induced after-effects on the duration of stimulation24. This might be an effect of the above-mentioned calcium dynamics, where longer stimulation could lead to an overflow of calcium at the synapse, setting off a reversal of potentiation21. It remains to be established how different combinations of intensity and duration of stimulation affect cortical excitability, especially when current intensities and durations are increased beyond those commonly used. This is especially important because of non-linear dose-effect relations, as described above.
This study aims to expand and investigate systematically the parameter space of anodal tDCS with respect to current intensity and stimulation duration, to extend systematic information on optimally suited stimulation protocols for neuroplasticity induction based on the primary motor cortex model in healthy adults. Anodal tDCS was administered with three current intensities – 1, 2, and 3 mA, for three different durations – 15, 20, and 30 minutes. Cortical excitability changes were monitored with TMS-induced motor evoked potentials (MEP) as an index of neuroplasticity. It was hypothesized that anodal tDCS parameters might non-linearly correlate with stimulation after-effects. Previous studies have shown that anodal tDCS intensities up to 2 mA and durations up to 20 min result in excitability enhancements23,27, while longer stimulation led to a conversion of the polarity of after-effects24. We furthermore hypothesized that this reversal of excitability from enhancement to diminution with longer stimulation duration might also be observed for stronger intensities of stimulation, due to the calcium-dependency of induced plasticity.
Results
Data analysis was based on data collected from 16 participants. Each subject took part in 10 sessions of the experiment, with each session separated by at least one week (Fig. 1). For each session, 15 measurements of cortical excitability were made – one measurement before tDCS (baseline excitability), and 14 measurements after tDCS (after-effects) (Fig. 1).
Diagrammatic representation of the experimental procedure. Each participant took part in 10 sessions of the experiment, with a minimum one-week inter-session interval to prevent carry-over effects. A condition as defined here refers to Intensity-Duration pairs (eg. 1 mA–30 min). All conditions were randomized. Each experimental session started with the identification of the ‘hotspot’ of the ADM, and the TMS intensity which resulted in a MEP amplitude of approximately 1 mV (baseline TMS intensity) was determined. MEPs were then recorded as a baseline excitability measure, after which tDCS was applied. Immediately after tDCS, MEPs were again recorded with the same baseline TMS stimulus intensity every 5 min until 30 min, every 30 min until 2 hr, SE, NM, NN, and NE (modified from Jamil26, with permission of The Journal of Physiology, John Wiley and Sons).
Apart from slight tingling, burning sensations, and redness of the skin which are commonly reported in tDCS experiments28, all subjects tolerated the stimulation well. Side effects did not last more than 30 minutes after stimulation. Side effects did not differ between active and sham stimulation conditions. Blinding was not compromised; in no condition participants guessed the correct intensity more frequently than expected by chance level. The results of the analyses of side-effects and blinding data are presented in Tables 1–7 of the Supplementary Material.
Baseline MEPs as well as baseline TMS intensity were not significantly different between sessions (Table 1).
No significant impact of stimulation intensity and duration on the effects of tDCS
We investigated the influence of tDCS parameters – intensity, and duration - on cortical excitability. These analyses were conducted without the sham stimulation condition, which only included one intensity/duration combination.
The three-way repeated measures ANOVA conducted over all 15 time bins shows no significant main effect of Intensity (F(2,28) = 1.710, df = 2, η2p = 0.109, p = 0.199) and Duration (F(2,28) = 2.278, η2p = 0.140, p = 0.121), but a significant main effect of Time (F(3.962,55.475) = 4.614, η2p = 0.248, p = 0.003). A trend-wise effect was identified for the Intensity and Time interaction (F(28,392) = 1.471, η2p = 0.095, p = 0.060). There were however no significant interactions between Intensity and Duration (F(4,56) = 0.140, η2p = 0.010, p = 0.967), Duration and Time (F(28,392) = 0.791, η2p = 0.053, p = 0.770), and Intensity, Duration, and Time (F(56, 784) = 1.078, η2p = 0.072, p = 0.328) (Table 2).
In a second analysis, post-stimulation time was averaged (pooled) into three time bins: early (0–30 mins), late (60–120 mins), and very late (SE-NE) epochs. The ANOVA results show likewise no significant main effect of Intensity (F(2, 30) = 1.925, η2p = 0.114, p = 0.163) and Duration (F(2, 30) = 2.009, η2p = 0.118, p = 0.152) but a significant main effect of Epoch (F(3, 45) = 14.604, η2p = 0.493, p < 0.001). A trend-wise effect was present for the Intensity x Epoch interaction (F(6, 90) = 2.126, η2p = 0.124, p = 0.058), but no significant interactions between Intensity and Duration (F(4, 60) = 0.314, η2p = 0.020, p = 0.868), Duration and Epoch (F(6, 90) = 1.098, η2p = 0.068, p = 0.370), or Intensity, Duration, and Epoch (F(5.661, 84.908) = 0.832, df = 5.661, η2p = 0.053, p = 0.617) were revealed.
After-effects of tDCS on cortical excitability
In these analyses, we investigated the influence of active stimulation conditions on altered cortical excitability relative to sham. Stimulation conditions refer to intensity-duration combinations (Fig. 1).
The two-way repeated measures ANOVA shows significant main effects of Condition (F(9, 126) = 2.489, η2p = 0.151, p = 0.012) and Time (F(3.944, 55.210) = 4.330, η2p = 0.236, p = 0.004), but no significant interaction between Condition and Time (F(126, 1764) = 1.155, η2p = 0.076, p = 0.122) (see Table 2). Post-hoc comparisons of the various time points to baseline show that MEP amplitudes were enhanced or showed trend-wise increases in all, except for the sham condition within the first two-hour period after stimulation (Fig. 2A–C). Compared to their respective baselines, 1 mA–15 min resulted significant cortical excitability enhancements until 30 min (but not for the time points 5, 10, and 20 min), 1 mA–20 min resulted in excitability enhancements that lasted until same day evening (SE), whereas 1 mA–30 min elicited mostly trend-wise excitability enhancements, with significant increases at time points 20 and 25 min after tDCS. Comparisons with sham showed for the 1 mA–15 min stimulation condition significant excitability enhancements at time points 0, 15, 30, 90 min, and next noon (NN) after tDCS. The 1 mA–20 min condition enhanced cortical excitability until NN except at time points 25, 60, 120 min, and next morning (NM) after tDCS, while the 1 mA–30 min condition did not show any significant enhancements in cortical excitability when compared with sham except for the NN time point (Fig. 2A). For the 2 mA stimulation conditions, comparison with baseline showed that 2 mA–15 min did not significantly enhance cortical excitability post-tDCS except for time point 120 min, while 2 mA–20 min resulted excitability enhancements until NN except at the NM timepoint. The 2 mA–30 min condition resulted in delayed enhancements of excitability, with significant increases of MEP amplitudes at timepoints 20 to 60 min post-tDCS. Compared to the sham stimulation condition, 2 mA–15 min did not result in any significant enhancements of cortical excitability post-tDCS except at the NN timepoint, whereas the 2 mA–20 min condition induced an enhancement of MEP amplitudes until NN (except for the 25 min, 60 min, and NM time points). 2 mA–30 min only resulted in MEP enhancements significant at 20, 30, and 60 min post-stimulation when compared to sham (Fig. 2B). For the protocols including 3 mA stimulation intensity, compared to their respective baselines, all conditions induced an enhancement of cortical excitability within the first 2 hours post-tDCS (except at time points 5, and 20 min for 3 mA–30 min, 25 min for 3 mA–20 min, and 90 min for 3 mA–15 min). Likewise, comparisons with sham show that MEP amplitudes were significantly increased in the 3 mA–15 min condition until 90 minutes after stimulation except for time point 25 min. The after-effects of 3 mA–20 min however did last until the NN (except for the 25 min time point), and those of the 3 mA–30 min stimulation protocol lasted also until NN (but not for 5, 20, 25, 120 min, and NM time points), when compared with sham (Fig. 2C). Sham tDCS did not result in any excitability alteration, except a small but significant reduction of MEP amplitudes at the NN timepoint.
Averaged MEPs post-stimulation for all intervention conditions and monitored time bins. MEPs were obtained before, immediately after tDCS at 5-minute intervals until 30 min after intervention and then every half an hour until 120 mins; same day evening (SE), next morning (NM), next noon (NN) and next evening (NE) for all three tDCS intensity-duration combinations. tDCS resulted in an enhancement of cortical excitability post-stimulation when compared to the respective baselines values, and sham. Sham tDCS did not alter cortical excitability with the exception of significant reduction of MEP amplitudes at NN. The three intensities and durations of stimulation show comparable effects. Comparison of all real tDCS conditions showed no significant differences between the respective induced cortical excitability alterations. Error bars represent standard error of the mean. Filled symbols represent a significant difference of MEP amplitudes compared to the respective baselines. Floating symbols represent significant differences between real and sham stimulation conditions (paired t test, two-tailed, p < 0.05).
Time points post-stimulation were pooled into an early (0–30 min), late (60–120 min), and very late (SE-NE) epochs to control for variability within the time bins.
For the respective pooled data, the ANOVA results indicate significant main effects of Condition (F(4.791, 71.868) = 2.686, η2p = 0.152, p = 0.030) and Epoch (F(3, 45) = 13.491, η2p = 0.474, p < 0.001), as well as a significant interaction between Condition and Epoch (F(27, 405) = 1.613, η2p = 0.097, p = 0.029). Post-hoc comparisons show that all real stimulation conditions resulted in a significant enhancement of cortical excitability when compared to baseline and sham within the first 30 minutes after stimulation (early epoch), except for the 2 mA–15 min stimulation condition (Fig. 3). For the late epochs (60–120 mins after stimulation), all conditions except 1 mA–30 min, 2 mA–15, and 2 mA–30 min showed a significant increase of cortical excitability compared to their respective baselines and sham (Fig. 3). Cortical excitability was significantly enhanced for only the 2 mA–20 min and 3 mA–20 min conditions in the very late epoch (same day evening to next day evening after stimulation) when compared to their respective baselines, but for all conditions when compared to sham except for the 1 mA–30 min, 2 mA–30 min and 3 mA–15 min conditions (Fig. 3).
Average MEPs post-stimulation for all intervention conditions and pooled time bins. Post hoc comparisons with sham tDCS indicate that all stimulation conditions resulted in significant cortical excitability enhancements within the first 30 minutes after stimulation (early epoch), except for the 2 mA–15 min condition, which did alter excitability only trend-wise. For the late epoch (60–120 min after stimulation), all conditions except 1 mA–30 mins, 2 mA–15 mins, and 2 mA–30 mins show a cortical excitability increase. Cortical excitability was also enhanced for all conditions in the very late epoch (same day evening to next day evening after stimulation), except for the 1 mA–30 min, 2 mA–30 min, and 3 mA–15 min conditions. Error bars represent standard error of the mean. Filled symbols in the graph represent a significant difference of MEP amplitudes compared to the respective baselines. The floating symbols indicate significant differences between real stimulation and sham conditions (paired t test, two-tailed, p < 0.05).
Discussion
We explored the parameter space of anodal tDCS in the primary motor cortex of healthy humans. When compared to sham, all stimulation conditions resulted in significant enhancement of MEP amplitudes following anodal tDCS. There were no significant differences between the stimulation intensities, or durations. We however observed a trend-wise increase of excitability enhancements from the lower to the higher current intensities. Participants tolerated the stimulation well, and side-effects did not significantly differ for the respective real stimulation intensities versus sham. Blinding was successful, as participants could not accurately guess the stimulation intensities they received.
With respect to stimulation intensity, this result confirms those of previous studies, which showed only minor or no differences of stimulation intensities between 0.5 and 2 mA23,26,29,30. The results of the present study extend the tDCS intensity range showing similar effects for 3 mA. In the current study however, a trend was observed in the intensity domain, with increased effects with higher current intensity. It might thus be that enhancing stimulation intensity further results in higher efficacy of anodal tDCS.
Previous studies with lower current intensities (≤1 mA) and shorter durations (≤13 minutes) have reported an intensity- and duration-dependent effect of anodal tDCS, where increasing the respective parameters resulted in a linear dose-response relationship8,9. The relationship between current intensity and induced after-effects of tDCS is however partially non-linear for higher intensities23 for cathodal tDCS. Specifically, for anodal tDCS, increasing the current intensity from 1 to 2 mA did not result in a corresponding increase of MEP amplitudes26,29. Changes in cortical excitability due to tDCS are calcium-dependent14,31, and for LTP induction, calcium concentration within a specific range is required21. It could thus be argued that for the previous studies with relatively low tDCS intensities and/or short durations, these were at the lower limit of respective calcium concentrations. Within this range, enhancing calcium concentration should increase efficacy of LTP induction. The calcium concentration range induced by the stronger protocols in the present study, however, might be within the optimal level, and gradual differences at this range might not have a major impact on the level of LTP induction. Furthermore, higher calcium concentration might activate counteracting homeostatic mechanisms, such as the activation of potassium channels, saturation of NMDA receptors, thus limiting the amount of plasticity22,32,33. These potential mechanisms require further exploration. In principal accordance, studies using different NIBS techniques have also reported some non-linearities with respect to varying intensity of stimulation34,35. Continuous theta burst stimulation (TBS) delivered at an intensity 70% of resting motor threshold (RMT) significantly increased MEP amplitudes, while a decrease of MEP amplitudes was observed when the stimuli were applied at 65% of RMT35. In another study, Moliadze and colleagues found a reduction in MEP amplitudes with 0.4 mA transcranial random noise stimulation (tRNS), but an enhancement at 1 mA34. The trend-wise increase of cortical excitability observed in the intensity domain (1 vs 2, and 3 mA) could be dedicated to more efficient stimulation of larger and deeper cortical areas with increasing intensity.
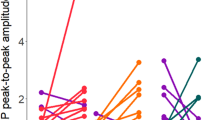
In the current study, we did moreover not observe any significant differences between the effects of different stimulation durations. In accordance, previous studies have shown that increasing tDCS duration does not necessarily enhance magnitude or duration of induced plasticity24,29. Prolonging the duration of stimulation beyond a critical time point might lead to a saturation of the after-effects, caused probably by calcium overflow24. Additionally, we observed that the stimulation duration of 30 minutes, for both, 1 and 2 mA intensities, resulted in delayed enhancements of cortical excitability post-tDCS (Figs. 2 and 3A,B). Previous studies with similar stimulation protocols already described delayed enhancements of cortical excitability post-tDCS23,36. This could reflect longer calcium dynamics at the postsynaptic membrane, causing activation of potassium channels which might have led to transient counterbalancing plasticity induction22,32. The lack of a significant excitability enhancement at some time points in some conditions when compared to their respective baselines and/or the sham stimulation condition, in spite of a uniform trend also in these conditions is probably due to response variability, which has been reported in previous tDCS studies27,37, and is present also in the individual data of the present study (Suppl. Material S1-Fig. 1A–J). This variability is much less obvious in the pooled data, where each epoch relies on a larger number of MEPs (Fig. 3A–C). The significant effects seen at the time point NN for almost all protocols when compared to sham could reflect, at least in part, the significant decrease in MEP observed for sham at this time point, as no other real stimulation condition except 2 mA–20 min showed any enhancement in MEP at this time point when compared to their respective baselines, and should thus be taken with caution.
Importantly, except for the 3 mA–20 min and 2 mA–20 min conditions, none of the stimulation protocols we probed induced relevant late-phase plasticity, which we define as respective enhancements of cortical excitability compared to baseline and sham and lasting for more than a few hours after intervention (Fig. 3A–C). We chose this relatively strict criterion because for one of the late MEP measures (NN) we obtained a significant reduction of MEP amplitudes, which was most probably artefactual (Fig. 2A–C). To compensate for this, we interpreted late-phase MEP amplitude alterations only as relevant if these differed also significantly vs the respective baseline MEPs. It should however be added that though both stimulation conditions induced late-phase plasticity according to this criterion, the amplitudes were moderate. The lack of all other protocols in this study to induce relevant late-phase plasticity is in accordance with other studies in the field14,20, that have demonstrated in animal as well as human models that single session stimulation protocols are not well suited for inducing late-phase plasticity. In animal studies, single plasticity-inducing interventions resulted only in early-phase LTP38,39, whereas repeated intervention within an interval of 30 minutes resulted in late-phase LTP, lasting for several hours longer40,41. tDCS with protocols comparable to those efficient in animal models of late phase plasticity induction, including repetition of stimulation within a critical time window of 30 min, or additional pharmacological interventions, result in late phase effects also in humans. Repetition intervals of 3 or 20 minutes enhanced cortical excitability for at least 24 hours after stimulation24. Likewise, the serotonin and noradrenaline reuptake inhibitors citalopram and reboxetine enhance the after-effects of anodal tDCS for up to 24 hours42,43. Thus, taking the relatively short-lived after-effects of the extended stimulation protocols used in the present study into account, combined interventions might be required, if late-phase plasticity is aimed for. It would nevertheless be interesting to explore if the newly introduced intensified protocols result in more efficient late-phase plasticity, if these are used in respective repeated stimulation designs.
It should be noted that, although we did not find any significant differences between the three intensities and durations tested in the present experiment in healthy participants for the primary motor cortex, this does not exclude that higher intensities, or longer duration of stimulation improve efficacy of stimulation in specific settings, which might include specific brain states, or architecture. 2 and 3 mA tDCS enhanced treatment outcomes compared to 1 mA in patients with schizophrenia44,45, and 2 mA tDCS had a better effect than 1 mA for improving working memory task performance in Parkinson’s disease46. In neuropsychiatric disorders, specific changes of cortical structure and function such as activity alterations of neurotransmitters, cerebral atrophy, and other alterations could shift the parameter range for efficient stimulation, and thus the respective parameter range must be explored systematically in the respective conditions.
This study should be interpreted within the context of a few limitations. Our sample size of 16 young healthy participants is relatively small and homogeneous, thus results obtained from this group cannot be perfectly extrapolated to patient and elderly populations. The results of this study were obtained at the group level, and as shown in the Supplemental Material, interindividual variability was present, similar to previous studies8,9,37. Consequently, results might differ between individuals. We did not investigate mechanistic details of tDCS-induced plasticity in this study, or other factors that might contribute to tDCS-induced neuroplasticity, such as genetic factors. Finally, the transferability of these protocols from the motor to other cortical areas has to be explored in future studies.
In conclusion, we extended the parameter space of anodal tDCS with regard to intensity and duration for up to 3 mA and 30 mins respectively and found tDCS effects in this protocol range (1–3 mA; 15–30 mins) to be relatively similar, and robust to gradual protocol changes. The highest intensity applied in this study (3 mA) showed trend-wise superior effects. The similarity of anodal tDCS effects at the group level encourages the use of protocols within this parameter range. This expansion, and systematic evaluation of the stimulation parameter range thus help to define optimal stimulation protocols for experimental and clinical use.
Methods
Participants
Sixteen non-smoking right-handed healthy volunteers (9 females, mean age 26.5 ± 2.2 (SD) years) participated in the study. Participants were medically examined to ascertain their overall health state. None of the volunteers participating in the experiment had any history of any neurological or psychiatric disorders, or brain implants. During the course of the experiment, participants were instructed not to drink coffee at least for two hours before each session. We used a single-blind study design. The participants were blinded for all conditions of the experiment, and the order of all conditions was randomized. This study was approved by the ethics committee of Leibniz Research Centre for Working Environment and Human Factors (IfADo) and agrees with the provisions of the Helsinki Declaration47. Each participant gave written informed consent after the general objectives of the experiment were explained to them. All participants were naïve to TMS and tDCS prior to participation in this study. Beyond screening of exclusion criteria, no other selection criteria were applied.
tDCS
tDCS was applied with a battery-powered stimulator (neuroCare, Ilmenau, Germany) delivering constant direct current (maximum DC output of 4 mA) through a pair of carbonated rubber electrodes covered by saline-soaked sponges, each measuring 35 cm2 (7 × 5 cm). The anode was placed over the left primary motor cortex (M1), over the hotspot of the abductor digiti minimi muscle (ADM) representation, as determined by TMS, while the return electrode was positioned over the right supra-orbital area. Depending on the experimental condition, tDCS was applied for 15, 20, or 30 minutes with intensities of 1, 2, or 3 mA. For each stimulation intensity, current was ramped up for 10 seconds at the beginning and ramped down for 10 seconds at the end of stimulation. For sham stimulation, 1 mA tDCS was applied for 15 seconds (current was ramped up for 10 seconds, 15 seconds of stimulation, after which current was ramped down for 10 seconds), but the tDCS electrodes were kept on the participants’ head for 15 minutes to mimic a true experimental condition.
Cortical excitability measurements
A TMS stimulator (Mag and More, Munich, Germany) delivered magnetic pulses at 0.20–0.25 Hz through a figure-of-8 coil (diameter of 70 mm, maximum magnetic field of 2 Tesla). The coil was held tangentially to the skull with the handle pointing backwards at 45 degrees to the midline. The ‘motor hotspot’, the optimal area of the primary motor cortex representing the ADM was determined as the region where TMS of a given intensity elicited the highest average MEPs. Electromyography (EMG) electrodes attached to the ADM in a belly tendon montage recorded MEPs induced by single pulse biphasic TMS. Analogue EMG signals were then sampled at 5 kHz (CED, Cambridge, UK), amplified and band pass-filtered at 2 Hz–2 kHz (Digimeter, Hertfordshire, UK) using the Signal software (version 4.0), and stored offline for further analyses.
Experimental procedure
Participants were comfortably seated in a reclining chair, with an inflatable pillow wrapped around their necks to stabilize the head position. Each experimental session began in the morning at about 09:30. First, the motor hotspot was identified as described above and marked. TMS stimulus intensity was then adjusted to produce an average peak-to-peak 1 mV MEP amplitude, defined as baseline TMS Intensity, and kept constant throughout the remaining experimental session. 25 MEPs were obtained as baseline cortical excitability measure. tDCS was then applied according to the respective experimental condition. 25 MEPs were recorded with the same baseline TMS intensity immediately after tDCS, and every 5 minutes for up to 30 minutes, and then every 30 minutes until 2 hours after intervention. Additionally, cortical excitability was measured at the same day evening (SE) (approximately 7 hours after tDCS), next morning (NM) (23–24 hours after tDCS), next noon (NN) (about 28 hours after tDCS), and next evening (NE) (about 32 hours after tDCS). To minimize discomfort associated with higher stimulation intensities, a topical analgesic cream, EMLA (2.5% lidocaine and 2.5% prilocaine) was applied to the scalp before each stimulation session.
After each session, a questionnaire48,49 was administered to gain information on how participants rated side-effects of tDCS, and to guess the stimulation intensity they received (sham, 1, 2, or 3 mA).
Calculations and statistical analyses
The peak-to-peak amplitudes of MEPs at each time point post-stimulation were measured, averaged, and normalized to the baseline for each individual. To exclude differences between the baseline MEP and baseline TMS intensity between sessions, a one-way repeated measures ANOVA was conducted with baseline MEP and baseline TMS as dependent variables and session as a within-subject factor.
To investigate the effect of the tDCS parameters – stimulation intensity, and duration - on cortical excitability, a three-way repeated measures ANOVA was conducted with ‘Intensity’, ‘Duration’, and ‘Time’ as within subject factors and mean normalized MEPs as dependent variable. Time refers to the overall stimulation time frame, from baseline up until the next day evening (15 time points). For further analysis, and to compensate for variability between single time bins, we conducted another ANOVA for pooled time bins. Here, post-stimulation time bins were pooled into three time frames – early (0–30 min), late (60–120 min) and very late (SE-NE). Averages were computed over all MEPs of each binned time point: early epoch (0–30 min, 6 time points – including 150 MEP), late epoch (60–120 min, 3 time points – 75 MEP), and very late epoch (SE-NE, 4 time points – 100 MEP). A three-way ANOVA was conducted for the pooled (epoched) data with ‘Intensity’, ‘Duration’ and ‘Epoch’ as within subject factors and mean normalized MEPs as dependent variable.
To investigate if the real stimulation conditions altered excitability relative to sham, a two-way repeated measures ANOVA was conducted with ‘Condition’ (10 levels) and ‘Time’ (15 levels) as within-subject factors and normalized mean MEP amplitude values at each time point as dependent variable. Additionally, a two-way repeated measure ANOVA was conducted for pooled data, with ‘Condition’ and ‘Epoch’ as within-subject factors and mean normalized MEPs as dependent variable, as described above.
For all ANOVAs, Mauchly’s test of sphericity was conducted, and where necessary, the Greenhouse-Geisser correction was applied. In case of significant results of the ANOVAs, exploratory post-hoc Student’s t-tests (paired samples, two-tailed, p < 0.05, not corrected for multiple comparisons) were conducted to determine significant differences between baseline and post-tDCS MEPs, between conditions of active stimulation and sham, and between respective active conditions of stimulation. Exploratory post-hoc tests were not corrected for multiple comparisons to avoid a relevant enhancement of type II errors, according to Perneger50, and Feise51. This is also in accordance with other studies in the field, and thus increases inter-study comparability52,53.
All analyses were carried out using SPSS, version 24 (IBM SPSS Statistics, New York, USA).
Calculations and statistics for the side-effect, and blinding questionnaires are described in the Supplemental Material.
References
Polania, R., Nitsche, M. A. & Ruff, C. C. Studying and modifying brain function with non-invasive brain stimulation. Nat Neurosci 21, 174–187, https://doi.org/10.1038/s41593-017-0054-4 (2018).
Buch, E. R. et al. Effects of tDCS on motor learning and memory formation: A consensus and critical position paper. Clin Neurophysiol 128, 589–603, https://doi.org/10.1016/j.clinph.2017.01.004 (2017).
Kuo, M. F., Paulus, W. & Nitsche, M. A. Therapeutic effects of non-invasive brain stimulation with direct currents (tDCS) in neuropsychiatric diseases. Neuroimage 85(Pt 3), 948–960, https://doi.org/10.1016/j.neuroimage.2013.05.117 (2014).
Shin, Y. I., Foerster, A. & Nitsche, M. A. Transcranial direct current stimulation (tDCS) - application in neuropsychology. Neuropsychologia 69, 154–175, https://doi.org/10.1016/j.neuropsychologia.2015.02.002 (2015).
Fregni, F. et al. A randomized, sham-controlled, proof of principle study of transcranial direct current stimulation for the treatment of pain in fibromyalgia. Arthritis Rheum 54, 3988–3998, https://doi.org/10.1002/art.22195 (2006).
Floel, A. tDCS-enhanced motor and cognitive function in neurological diseases. Neuroimage 85(Pt 3), 934–947, https://doi.org/10.1016/j.neuroimage.2013.05.098 (2014).
Boggio, P. S. et al. A randomized, double-blind clinical trial on the efficacy of cortical direct current stimulation for the treatment of major depression. Int J Neuropsychopharmacol 11, 249–254, https://doi.org/10.1017/S1461145707007833 (2008).
Nitsche, M. A. & Paulus, W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol 527(Pt 3), 633–639 (2000).
Nitsche, M. A. & Paulus, W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology 57, 1899–1901 (2001).
Nitsche, M. A. et al. Level of action of cathodal DC polarisation induced inhibition of the human motor cortex. Clin Neurophysiol 114, 600–604 (2003).
Stagg, C. J. & Nitsche, M. A. Physiological basis of transcranial direct current stimulation. Neuroscientist 17, 37–53, https://doi.org/10.1177/1073858410386614 (2011).
Woods, A. J. et al. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin Neurophysiol 127, 1031–1048, https://doi.org/10.1016/j.clinph.2015.11.012 (2016).
Liebetanz, D., Nitsche, M. A., Tergau, F. & Paulus, W. Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain 125, 2238–2247 (2002).
Nitsche, M. A. et al. Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J Physiol 553, 293–301, https://doi.org/10.1113/jphysiol.2003.049916 (2003).
Nitsche, M. A. et al. Consolidation of human motor cortical neuroplasticity by D-cycloserine. Neuropsychopharmacology 29, 1573–1578, https://doi.org/10.1038/sj.npp.1300517 (2004).
Stafford, J., Brownlow, M. L., Qualley, A. & Jankord, R. AMPA receptor translocation and phosphorylation are induced by transcranial direct current stimulation in rats. Neurobiol Learn Mem 150, 36–41, https://doi.org/10.1016/j.nlm.2017.11.002 (2018).
Martins, C. W., de Melo Rodrigues, L. C., Nitsche, M. A. & Nakamura-Palacios, E. M. AMPA receptors are involved in prefrontal direct current stimulation effects on long-term working memory and GAP-43 expression. Behav Brain Res 362, 208–212, https://doi.org/10.1016/j.bbr.2019.01.023 (2019).
Nitsche, M. A. et al. GABAergic modulation of DC stimulation-induced motor cortex excitability shifts in humans. Eur J Neurosci 19, 2720–2726, https://doi.org/10.1111/j.0953-816X.2004.03398.x (2004).
Stagg, C. J. et al. Polarity-sensitive modulation of cortical neurotransmitters by transcranial stimulation. J Neurosci 29, 5202–5206, https://doi.org/10.1523/JNEUROSCI.4432-08.2009 (2009).
Malenka, R. C. & Bear, M. F. LTP and LTD: an embarrassment of riches. Neuron 44, 5–21, https://doi.org/10.1016/j.neuron.2004.09.012 (2004).
Lisman, J. E. Three Ca2+ levels affect plasticity differently: the LTP zone, the LTD zone and no man’s land. J Physiol 532, 285 (2001).
Misonou, H. et al. Regulation of ion channel localization and phosphorylation by neuronal activity. Nat Neurosci 7, 711–718, https://doi.org/10.1038/nn1260 (2004).
Batsikadze, G., Moliadze, V., Paulus, W., Kuo, M. F. & Nitsche, M. A. Partially non-linear stimulation intensity-dependent effects of direct current stimulation on motor cortex excitability in humans. J Physiol 591, 1987–2000, https://doi.org/10.1113/jphysiol.2012.249730 (2013).
Monte-Silva, K. et al. Induction of late LTP-like plasticity in the human motor cortex by repeated non-invasive brain stimulation. Brain Stimul 6, 424–432, https://doi.org/10.1016/j.brs.2012.04.011 (2013).
Esmaeilpour, Z. et al. Incomplete evidence that increasing current intensity of tDCS boosts outcomes. Brain Stimul 11, 310–321, https://doi.org/10.1016/j.brs.2017.12.002 (2018).
Jamil, A. et al. Systematic evaluation of the impact of stimulation intensity on neuroplastic after-effects induced by transcranial direct current stimulation. J Physiol 595, 1273–1288, https://doi.org/10.1113/jp272738 (2017).
Ammann, C., Lindquist, M. A. & Celnik, P. A. Response variability of different anodal transcranial direct current stimulation intensities across multiple sessions. Brain Stimul 10, 757–763, https://doi.org/10.1016/j.brs.2017.04.003 (2017).
Bikson, M. et al. Safety of Transcranial Direct Current Stimulation: Evidence Based Update 2016. Brain Stimul 9, 641–661, https://doi.org/10.1016/j.brs.2016.06.004 (2016).
Tremblay, S. et al. Systematic assessment of duration and intensity of anodal transcranial direct current stimulation on primary motor cortex excitability. Eur J Neurosci 44, 2184–2190, https://doi.org/10.1111/ejn.13321 (2016).
Kidgell, D. J. et al. Different current intensities of anodal transcranial direct current stimulation do not differentially modulate motor cortex plasticity. Neural Plast 2013, 603502, https://doi.org/10.1155/2013/603502 (2013).
Grundey, J. et al. Nicotine modulates human brain plasticity via calcium-dependent mechanisms. J Physiol 596, 5429–5441, https://doi.org/10.1113/JP276502 (2018).
An, W. F. et al. Modulation of A-type potassium channels by a family of calcium sensors. Nature 403, 553–556, https://doi.org/10.1038/35000592 (2000).
Lau, C. G. & Zukin, R. S. NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nature reviews. Neuroscience 8, 413–426, https://doi.org/10.1038/nrn2153 (2007).
Moliadze, V., Atalay, D., Antal, A. & Paulus, W. Close to threshold transcranial electrical stimulation preferentially activates inhibitory networks before switching to excitation with higher intensities. Brain Stimul 5, 505–511, https://doi.org/10.1016/j.brs.2011.11.004 (2012).
Doeltgen, S. H. & Ridding, M. C. Low-intensity, short-interval theta burst stimulation modulates excitatory but not inhibitory motor networks. Clin Neurophysiol 122, 1411–1416, https://doi.org/10.1016/j.clinph.2010.12.034 (2011).
Kuo, H. I. et al. Comparing cortical plasticity induced by conventional and high-definition 4 x 1 ring tDCS: a neurophysiological study. Brain Stimul 6, 644–648, https://doi.org/10.1016/j.brs.2012.09.010 (2013).
Wiethoff, S., Hamada, M. & Rothwell, J. C. Variability in response to transcranial direct current stimulation of the motor cortex. Brain Stimul 7, 468–475, https://doi.org/10.1016/j.brs.2014.02.003 (2014).
Rohan, J. G., Carhuatanta, K. A., McInturf, S. M., Miklasevich, M. K. & Jankord, R. Modulating Hippocampal Plasticity with In Vivo Brain Stimulation. J Neurosci 35, 12824–12832, https://doi.org/10.1523/jneurosci.2376-15.2015 (2015).
Ranieri, F. et al. Modulation of LTP at rat hippocampal CA3-CA1 synapses by direct current stimulation. J Neurophysiol 107, 1868–1880, https://doi.org/10.1152/jn.00319.2011 (2012).
Reymann, K. G. & Frey, J. U. The late maintenance of hippocampal LTP: requirements, phases, ‘synaptic tagging’, ‘late-associativity’ and implications. Neuropharmacology 52, 24–40, https://doi.org/10.1016/j.neuropharm.2006.07.026 (2007).
Vickers, C. A., Dickson, K. S. & Wyllie, D. J. Induction and maintenance of late-phase long-term potentiation in isolated dendrites of rat hippocampal CA1 pyramidal neurones. J Physiol 568, 803–813, https://doi.org/10.1113/jphysiol.2005.092924 (2005).
Kuo, H. I. et al. Chronic Enhancement of Serotonin Facilitates Excitatory Transcranial Direct Current Stimulation-Induced Neuroplasticity. Neuropsychopharmacology 41, 1223–1230, https://doi.org/10.1038/npp.2015.270 (2016).
Kuo, H. I. et al. Acute and chronic effects of noradrenergic enhancement on transcranial direct current stimulation-induced neuroplasticity in humans. J Physiol 595, 1305–1314, https://doi.org/10.1113/jp273137 (2017).
Hoy, K. E., Arnold, S. L., Emonson, M. R., Daskalakis, Z. J. & Fitzgerald, P. B. An investigation into the effects of tDCS dose on cognitive performance over time in patients with schizophrenia. Schizophrenia research 155, 96–100, https://doi.org/10.1016/j.schres.2014.03.006 (2014).
Andrade, C. Transcranial direct current stimulation for refractory auditory hallucinations in schizophrenia. J Clin Psychiatry 74, e1054–1058, https://doi.org/10.4088/JCP.13f08826 (2013).
Boggio, P. S. et al. Effects of transcranial direct current stimulation on working memory in patients with Parkinson’s disease. J Neurol Sci 249, 31–38, https://doi.org/10.1016/j.jns.2006.05.062 (2006).
World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. Jama 310, 2191–2194, https://doi.org/10.1001/jama.2013.281053 (2013).
Brunoni, A. R. et al. A systematic review on reporting and assessment of adverse effects associated with transcranial direct current stimulation. Int J Neuropsychopharmacol 14, 1133–1145, https://doi.org/10.1017/s1461145710001690 (2011).
Poreisz, C., Boros, K., Antal, A. & Paulus, W. Safety aspects of transcranial direct current stimulation concerning healthy subjects and patients. Brain Res Bull 72, 208–214, https://doi.org/10.1016/j.brainresbull.2007.01.004 (2007).
Perneger, T. V. What’s wrong with Bonferroni adjustments. BMJ (Clinical research ed.) 316, 1236–1238, https://doi.org/10.1136/bmj.316.7139.1236 (1998).
Feise, R. J. Do multiple outcome measures require p-value adjustment? BMC medical research methodology 2, 8 (2002).
Ryan, K. et al. 1H MR spectroscopy of the motor cortex immediately following transcranial direct current stimulation at 7 Tesla. PLoS One 13, e0198053, https://doi.org/10.1371/journal.pone.0198053 (2018).
O’Shea, J. et al. Predicting behavioural response to TDCS in chronic motor stroke. Neuroimage 85(Pt 3), 924–933, https://doi.org/10.1016/j.neuroimage.2013.05.096 (2014).
Acknowledgements
This work was supported by a research grant from the German Federal Ministry of Research and Education (BMBF) (GCBS grant 01EE1501, TRAINSTIM grant 01GQ1424E). The publication of this article was funded by the Open Access Fund of the Leibniz Association.
Author information
Authors and Affiliations
Contributions
D.A. wrote the main manuscript text and planned the experiments. M.M.S. assisted with measurements. M.K. and A.J. supervised the project. M.A.N. conceived the original idea, revised the final version of the manuscript and supervised the project. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
Prof. Michael A. Nitsche is member of Advisory Board of Neuroelectrics. Mr. Desmond Agboada, Mr. Mohsen Mosayebi Samani, Dr. Asif Jamil, and Dr. Min-Fang Kuo declare no potential conflicts of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Agboada, D., Mosayebi Samani, M., Jamil, A. et al. Expanding the parameter space of anodal transcranial direct current stimulation of the primary motor cortex. Sci Rep 9, 18185 (2019). https://doi.org/10.1038/s41598-019-54621-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-54621-0
This article is cited by
-
Effects of anodal tDCS on resting state eeg power and motor function in acute stroke: a randomized controlled trial
Journal of NeuroEngineering and Rehabilitation (2024)
-
TDCS over PPC or DLPFC does not improve visual working memory capacity
Communications Psychology (2024)
-
Exploring the impact of intensified multiple session tDCS over the left DLPFC on brain function in MCI: a randomized control trial
Scientific Reports (2024)
-
Non-linear dose response effect of cathodal transcranial direct current stimulation on muscle strength in young healthy adults: a randomized controlled study
BMC Sports Science, Medicine and Rehabilitation (2023)
-
Frontal two-electrode transcranial direct current stimulation protocols may not affect performance on a combined flanker Go/No-Go task
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.