Abstract
Natural-product derived lectins can function as potent viral inhibitors with minimal toxicity as shown in vitro and in small animal models. We here assessed the effect of rectal application of an anti-HIV lectin-based microbicide Q-Griffithsin (Q-GRFT) in rectal tissue samples from rhesus macaques. E-cadherin+ cells, CD4+ cells and total mucosal cells were assessed using in situ staining combined with a novel customized digital image analysis platform. Variations in cell numbers between baseline, placebo and Q-GRFT treated samples were analyzed using random intercept linear mixed effect models. The frequencies of rectal E-cadherin+ cells remained stable despite multiple tissue samplings and Q-GRFT gel (0.1%, 0.3% and 1%, respectively) treatment. Whereas single dose application of Q-GRFT did not affect the frequencies of rectal CD4+ cells, multi-dose Q-GRFT caused a small, but significant increase of the frequencies of intra-epithelial CD4+ cells (placebo: median 4%; 1% Q-GRFT: median 7%) and of the CD4+ lamina propria cells (placebo: median 30%; 0.1–1% Q-GRFT: median 36–39%). The resting time between sampling points were further associated with minor changes in the total and CD4+ rectal mucosal cell levels. The results add to general knowledge of in vivo evaluation of anti-HIV microbicide application concerning cellular effects in rectal mucosa.
Similar content being viewed by others
Introduction
The global HIV epidemic continues to significantly impact lives, in 2017 approximately 1.8 million new cases, 1 million AIDS deaths, and 37 million HIV infected individuals were reported1. The main mode of HIV transmission is through sexual contact with a significantly higher transmission rate for receptive and insertive anal versus vaginal sexual intercourse2,3,4. While the vaginal epithelium is multi-stratified, the rectal epithelium is single-layered and thus more vulnerable with underlying HIV target cells easily reachable for penetrating viral particles. Current oral pre-exposure prophylaxis (PrEP) against HIV infection relies on antiretroviral (ARV) drugs. Although PrEP is highly efficient when treatment adherence is sustained5,6,7,8, the ARVs can accumulate systemically causing long-term health consequences, and resistant viruses may develop if persons become infected while on PrEP. There is a high demand to complement the oral PrEP options with a rectal microbicide to give individuals who practice anal sex an alternative option for HIV prevention9. Microbicides for rectal use could potentially be provided in the form of lubricants, creams, fast dissolving rectal tablets, gels, douches or enemas10.
The natural lectin Griffithsin (GRFT) is one of the most potent anti-HIV agents reported to date11. GRFT combined with Carrageenan (another antiviral agent derived from algae) in a fast dissolving vaginal insert formulation, was recently shown to protect rhesus macaques (RMs) from a high-dose vaginal SHIV challenge12. GRFT is here being assessed as a rectal microbicide, as part of the PREVENT (Griffithsin-based rectal microbicides for PREvention of Viral ENTry) program. PREVENT is an integrated preclinical and clinical international program13, with a main goal to deliver a comprehensive set of data on the safety and efficacy profile of GRFT. In vitro investigation using human cells has previously confirmed GRFT’s s outstanding safety and efficacy profile as a microbicide candidate14. GRFT is isolated from a red algae Griffithsia sp.15, and has shown broad spectrum antiviral activity to HIV, herpes simplex type 2 virus, and hepatitis C virus in mid-picomolar IC50 values11,15,16,17. Bulk production of recombinant GRFT was achieved using Nicotiana benthamiana, a plant based protein expression system, offering more than 99% purity, and high stability and activity18,19. The method was further improved to make GRFT manufacturing cost-effective, with high yields and low endotoxin, making it a favorable microbicide candidate. Native GRFT is prone to oxidation and to overcome this inherent property of the lectin, an engineered form of wild type GRFT has been developed, Q-GRFT (M78Q), with an expected improved oxidation resistance. Gel based dosage forms based on polymers such as Carbopol, not causing any reported epithelial damage and/or inflammation, are potential delivery vehicles for such topical microbicides20.
The aim of this study was to evaluate the in vivo effect on mucosal cell populations following rectal application of Q-GRFT gel on the rectal mucosa of healthy RMs. Specifically, the effect on the rectal epithelium (E-cadherin+ cells) and the frequencies of CD4+ HIV target cells and total number of mucosal cells were assessed in a novel fashion by combining in situ immunofluorescence staining and digital image analysis.
Methods
Animals
Six purpose-bred RMs (Macaca mulatta) were used in this study. The RMs were housed at the Center for Disease Control and Prevention (CDC) in Atlanta, GA, USA, in accordance with the Guide for the Care and Use of Laboratory Animals (8th edition) in an AALAC-accredited facility, following the institutional standard operating procedures21. All animal experiments were approved by the CDC Institutional Animal Care and Use Committee (CDC IACUC, protocol 2700SMIMONC).
Study design
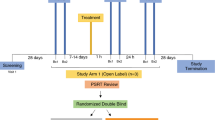
The main goal of the study was to evaluate if a microbicide containing different doses of Q-GRFT formulated in a Carbopol gel affected the epithelial barrier integrity and/or the numbers of HIV target cells in the rectal mucosa. Both the placebo (PL)- and the Q-GRFT-gels were Carbopol-formulated, containing Carbopol 974 P (0.75%), methylparaben, propylparaben, sodium hydroxide, disodium ethylenediaminetetraacetic acid and purified water, and applied in 4 ml per dose. The study design and workflow, in terms of rectal biopsy collection- and treatment-schedule are outlined in Fig. 1. Two rectal biopsies were collected from each RM per time point over a time span of 32 weeks. To assess the baseline cell distribution, seven baseline (BL) samples were collected over a period of 10 weeks. Thereafter, each RM received the PL gel intra-rectally, first in a single-dose one day prior to sampling, followed by multi-dose, i.e. during four consecutive days, prior to sampling on the following day. Subsequently, gradually escalating doses of Q-GRFT gel (0.1%, 0.3% and 1% [wt/vol], respectively) were applied intra-rectally, in a single- and multi-dose procedure, respectively. The resting time, i.e. number of days since previous biopsy sampling, varied between 7–14 days throughout the BL sampling, 15–17 days for the PL single and multi-dose application and Q-GRFT multi-dose application, and 20–32 days for the Q-GRFT single-dose application. Throughout the study the number of previous biopsy procedures increases, so at the last sample time point each RM had been exposed to biopsy sampling 15 times. To discern the effects of treatment from potential effects of sampling (number of previous biopsy samplings) and resting time (days since previous biopsy sampling), a linear mixed effects model was used.
Study outline. Six rhesus macaques were included in the study and two rectal biopsies were collected from each rhesus macaque at each sampling time point. Baseline samples were collected at seven time points during a ten-week period. This was followed by intra-rectal application of the placebo gel in a single-dose setting (4 ml of the gel was applied once and samples were taken on the following day) followed by a multi-dose setting (application of 4 ml placebo gel during four consecutive days prior to sampling on day 5). Thereafter escalating doses of Q-GRFT gel (0.1%, 0.3% and 1% [wt/vol]), in 4 ml per dose, were applied first in the single-dose setting followed by the multi-dose setting as described for the placebo gel. The arrows indicate each gel application and the bars indicate each biopsy sampling point.
Tissue collection and in situ immunofluorescence staining
The rectal biopsies were collected as outlined in Fig. 1. The biopsies were snap frozen in OCT media (Sakura Finetek USA Inc. Torrance, CA) at the CDC, USA. The frozen biopsies were shipped to Karolinska Institutet, Sweden and maintained at −80 °C until sectioning and staining procedures. The cryopreserved rectal biopsies were cut in 8 μm slices using a cryostat, mounted on SuperFrost® Plus Gold slides (Menzel Gläser, Thermo Fischer Scientific, VWR International AB, Spånga, Sweden), air-dried for 1 hr at room temperature (RT), and fixed in 100% methanol for 10 min at RT, which was followed by a wash in PBS.
The immunofluorescence double staining procedure was performed with E-cadherin and CD4 specific antibodies consecutively, and representative images are shown in Fig. 2. The adherence junction protein E-cadherin was detected using purified monoclonal mouse anti-E-cadherin antibody (610182, clone: 36/E-Cadherin, BD Biosciences, Stockholm, Sweden, 1:50 in antibody diluent, Nordic Biosite AB, Täby, Sweden, BCB-20005), followed by a blocking buffer, composed of donkey serum (2%) and BSA-C (0.1%) diluted in washing buffer (1% HEPES and 0.1% Saponin in PBS), and Alexa Fluor 488 conjugated donkey anti-mouse (highly cross absorbed) (A21202, Lot: 1644644, Invitrogen, Thermo Fischer Scientific, Waltham, MA, 1:200 in blocking buffer) secondary antibody for detection. This was followed by an antigen retrieval step using freshly prepared 0.5% hydrogen peroxide in methanol for 10 min at RT. The CD4+ cells were then detected using a purified rabbit anti-CD4 antibody (EPR6855, Abcam, Cambridge, England, 1:200 in antibody diluent), and Alexa Fluor 594 conjugated donkey anti-rabbit (highly cross absorbed, including affinity-purification against mouse immunoglobulins) (A21207, Invitrogen, Thermo Fischer Scientific, 1:400 in antibody diluent) secondary antibody for detection. Tissue sections were counterstained with DAPI (Molecular Probes, Invitrogen, Thermo Fischer Scientific), washed in MilliQ water and thereafter mounted with Fluorescent Mounting Medium (Dako, Carpinteria, CA, USA). Washing buffer was used between each incubation step. Negative controls were included for each tissue section and consisted of incubations in the presence of secondary antibody alone. The stained tissue sections were scanned into digital images using a Pannoramic 250 Flash Slide Scanner (3DHistech Kft., Budapest, Hungary).
In situ staining of E-cadherin and CD4 in rectal tissue. Representative immunofluorescence images of rectal tissue sections from a rhesus macaque stained for E-cadherin (green) and CD4 (red). DAPI (blue) was used as a counterstain for visualization of cell nuclei. The images show staining from a biopsy taken at (a) baseline, (b) after placebo single-dose and (c) after 1% Q-GRFT single-dose. Images in the left column show overview fields of the whole tissue sections (scale bar: 500 μm). Images in the middle column show 20× magnification (scale bar: 100 μm) of the images in the left column. Images in right column show 40× magnification (scale bar: 50 μm) of the regions of interest indicated in the left and middle columns.
Quantitative image analysis
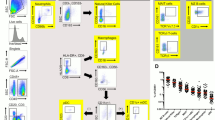
Scanned images of the whole tissue sections were exported as.tif files and each image was split in six smaller images to facilitate image workflow. The pixel-based machine learning software Ilastik22 was used to classify cell nuclei as either epithelial cells or lamina propria (LP) cells. Areas with background noise were also identified. Together with the Ilastik probability maps, the raw.tif images were uploaded to CellProfiler23, where (i) tissue area, (ii) cell nuclei, (iii) LP cells, (iv) epithelial cells, (v) CD4+ cells, (vi) E-cadherin+ cells, were identified and quantified (Fig. 3.) Areas with background noise were excluded from the analysis and samples with background contamination covering >50% of the tissue area were completely removed from the analysis. Based on the nuclear staining, cell outlines were approximated by enlarging the radius of each nucleus 12 pixels. Since all epithelial cells express E-cadherin to different degrees, we used a fixed threshold where epithelial cells with a mean intensity >0.03 intensity units in the green channel were classified as E-cadherin+ cells. This threshold was chosen based on the negative control staining without primary antibody. Classification of CD4+ cells was performed in two steps: (i) positive stained area in the red channel was identified (by applying a tophat filter followed by an intensity threshold of 0.008 to remove diffuse red fluorescence background), and (ii) all cells containing >55 positive red pixels/cell were classified as CD4+. The numbers of E-cadherin+ cells as well as CD4+ cells were normalized by calculating the percentage of positive cells out of total number of respective cell type, in each tissue compartment (i.e. % E-cadherin+ cells out of total epithelial cells, and % CD4+ cells out of total epithelial cells, i.e. intra-epithelial cells, as well as % CD4+ cells out of total LP cells). In addition, the total number of cells per tissue area (cell density), measured as number of cells/mm2 tissue, was assessed in each tissue compartment, as an indicator of influx or efflux of cells. Samples lacking, or with low proportion of epithelium (<20% epithelial cells) were excluded from the epithelial cell analyses, and samples with little or no LP (<20% LP cells) were excluded from the LP cell analyses.
Digital image analysis; work flow. Representative raw and digitalized images of a rectal tissue section stained for E-Cadherin (green), CD4 (red) and DAPI (blue) illustrating the workflow of our customized digital image analysis. (a) A raw fluorescent image of DAPI staining (blue, first column) was used for detection of cells, shown as outlines in the second and third column. Cells were detected using CellProfiler, and the software program Ilastik was used to classify cells as either epithelial cells (second column) or lamina propria (LP) cells (third column). The digitalized images in the fourth column are magnified views of the regions indicated in the second and third columns (epithelial cells upper image and LP cells lower image). Likewise, raw images, in the first column, of immunoflourescent staining of (b) E-cadherin (green), (c) CD4 (red) and (d) E-cadherin, CD4 and DAPI. Images in the second column are digitalized images (exported from CellProfiler and colorized in Matlab) representing analysis results of the images in the first column. The raw- and digitalized images in the third column are magnified views of the regions indicated in the first and second column, respectively. The following color codes were used in (d) E-cadherin positive epithelial cells in green, E-cadherin negative epithelial cells in dark grey, CD4 positive cells in red and CD4 negative submucosal cells in beige.
Statistical analysis
All statistical analyses were performed using R (nlme package version 3.1–131.1, longpower package version 1.0–16.1)24,25,26,27. Variations in the % of E-cadherin+ cells, CD4+ cells and total cell density between BL, PL and Q-GRFT samples, were analyzed using a random intercept linear mixed effect model (lme) with restricted maximum likelihood (REML) estimations. P values < 0.05 were considered significant. A repeated-measures analysis of variance was used to examine the following fixed effects; (1) treatment (PL or Q-GRFT), (2) sampling (number of previous biopsy samplings) and (3) resting time (days since previous biopsy sampling) on the markers, with two ways interactions term between sampling and resting time. Subject (i.e. RM) was included as a random effect in the model to control for variation between subjects.
Power calculations were performed to estimate the minimum fold change detectable at 80% power.
The lme model was used to investigate if treatment, sampling or resting time, had a significant effect on the cells in the mucosa across all time points. If treatment had a significant effect on the outcome variable, the effect of specific treatments was further investigated by comparing treatment groups one-to-one using the lme model. First, to investigate if the gel itself affected the cells in the mucosa, the PL samples were compared to BL samples. Then, to see if the active compound Q-GRFT added any effect to the gel, the different Q-GRFT concentrations (0.1%, 0.3% and 1% [wt/vol], respectively) were compared to the PL treated samples (single- and multi-dose separately). To verify potential effects of Q-GRFT (compared to PL), each Q-GRFT concentration (0.1%, 0.3% and 1% [wt/vol], respectively) and dose (single and multi-dose) was also compared with the BL samples. Furthermore, to assess variation of the frequencies of E-cadherin+ cells, CD4+ cells and total mucosal cells over time, the BL samples alone were analyzed using the lme model with sampling and resting time as fixed effects.
Last, all the Q-GRFT treated samples were assessed separately using a lme model with resting time, dose (single – or multi) and concentration (0.1%, 0.3% and 1% [wt/vol], respectively) as fixed effects to elucidate if dosage or the different concentrations of Q-GRFT had an effect on the frequencies of the % of E-cadherin+ cells, CD4+ cells or the total cell density, respectively.
Results
Basic tissue parameters
A total of 180 rectal biopsies were collected from six healthy RMs, including duplicate samples at each of the 15 sampling time points per animal. By creating a customized workflow of digital image analysis, we could objectively evaluate the in situ immunofluorescence staining of this large set of rectal tissue biopsies (Fig. 3). Overall, 96% of the collected tissue biopsies had good quality and were included in the image analysis. General tissue characteristics, including total tissue area analyzed (medianall samples 3.2 mm2, range 0.4–8.0 mm2), total number of cells analyzed (medianall samples 24,523 cells, range 3,558–63,314 cells), and percentage of epithelial and LP cells out of total number of cells (epithelial: medianall samples 51%, range 30–76%; LP: median all samples 48%, range 23–69%) were retrieved (Table 1).
Q-GRFT and sampling procedure did not affect the frequencies of E-cadherin+ cells
E-cadherin is the core membrane protein of the adherence junction protein family, which together with other junction complex members make up a network of epithelial junction proteins. These proteins contribute to a robust mucosal epithelium that can prevent entry of pathogens28. Thus, as a proxy for epithelial integrity, the percentage of E-cadherin+ cells out of total epithelial cells was assessed in the rectal samples.
Immunofluorescence staining combined with digital image analysis was used to measure the frequencies of E-cadherin+ cells in the rectal epithelium. The rectal epithelium is covered in mucus causing a dim autofluorescence background. Thus, to allow an unbiased quantification, we analyzed epithelial cells expressing E-cadherin above a mean intensity/cell of 0.03 intensity units. The frequencies of E-cadherin+ cells varied between 41–94% with a median of 78% across the experimental setup (i.e. all samples) (Fig. 4a, Table 2). To assess if this variation correlated to Q-GRFT gel treatment, a lme-model was used to analyse the fixed effects of treatment, sampling and resting time. Treatment consisted of either applying the PL gel in a single- or multi-dose, or applying different concentrations of Q-GRFT gel (0.1%, 0.3% or 1% formulation, respectively) in a single- or multi-dose fashion. Treatment did not cause a significant change in the frequencies of E-cadherin+ cells in the rectal mucosa. Neither did sampling (number of previous biopsy samplings) or resting time (days since previous biopsy sampling), and no interaction between sampling and resting time was seen (Supplementary Table 1). Thus, Q-GRFT gel treatment did not have a negative impact on the epithelial integrity as defined by E-cadherin+ cell frequencies.
Enumeration of E-cadherin+ cells and CD4+ cells in the rectal mucosa. The plots show the distribution and median ± interquartile range of (a) % of E-cadherin+ cells out of total epithelial cells, (b) % of CD4+intra epithelial cells (i.e. % of CD4+ epithelial cells out of total epithelial cells), and c) % CD4+ Lamina propria (LP) cells (i.e. % of CD4+ LP cells out of total LP cells) in rectal samples. In situ staining and digital image analysis were used to assess the markers in the rectal biopsies collected during baseline, single and multi-dose placebo treatment as well as after escalating concentrations of Q-GFRT (0.1%, 0.3% and 1% [wt/vol], respectively) in a single- and multi-dose setting. Each circle represents a median value from the two biopsies collected at each time point for each RM.
Multi-dose Q-GRFT caused a small increase in the frequencies of rectal CD4+ cells
Cells positive for the HIV receptor CD4 were next assessed as a proxy for HIV target cells. Digital image analysis revealed that the majority of CD4+ cells were localized within the LP of the rectal mucosa, and a few CD4+ cells located within the epithelial cell layer (Fig. 3). To assess if treatment affected the frequencies of CD4+ cells from these two morphologically and spatially distinct sites, the frequencies of CD4+ cells within the epithelium (i.e. CD4+ intra-epithelial cells; % CD4+ cells out of total epithelial cells) and within the LP (% CD4+ cells out of total LP cells) were assessed separately (Fig. 4b,c, Table 2).
The lme model showed that treatment had a significant effect on the frequencies of CD4+ cells, both in the epithelium and in the LP (lmeintra-epithelial: F8,143 = 2.2, p = 0.03, lmeLP: F8,141 = 3.1, p = 0.003) (Table 2, Supplementary Table 1). We then investigated which treatment was causing the effect. The gel itself had no effect on the frequencies of CD4+ cell in the epithelium or the LP, as shown by comparing the PL (single-dose or multi-dose) to the BL samples (Table 2, Supplementary Table 1). Neither did single-dose application of different concentrations of Q-GRFT gel (0.1%, 0.3% or 1% formulation) differ from applying PL gel in a single-dose, with regard to frequencies of CD4+ cells in the epithelium or in the LP (Table 2, Supplementary Table 1). However, the frequencies of intra-epithelial CD4+ cells were significantly higher in samples taken after application of the 1% Q-GRFT gel in multi-dose (median 7%), compared to samples taken after application of the multi-dose PL gel (median 4%) (lmeintra-epithelial: F1,14 = 6.8, p = 0.02). Furthermore, in the LP, all samples taken after application of multi-dose Q-GRFT gel (0.1%, 0.3%, 1%), as compared to samples taken after multi-dose PL gel application, showed significantly higher frequencies of CD4+ cells (multi-dose PL: median 30%; multi-dose Q-GRFT gel: median 36% – 39%, lmeLP: for 0.1% Q-GRFT F1,14 = 21.5, p = 0.0004; for 0.3% Q-GRFT F1,16 = 6.3, p = 0.02; and for 1% Q-GRFT F1,14 = 7.3, p = 0.02) (Table 2, Supplementary Table 1).
Comparing Q-GRFT gel vs. all BL samples showed comparable results (as comparing Q-GRFT gel vs. PL samples). I.e. applying the Q-GRFT gel in single-dose had no effect on the frequencies of CD4+ cells in the epithelium or in the LP, apart from the single-dose 0.1% Q-GRFT gel which displayed significantly higher frequencies of CD4+ cells in LP (BL: median 34%, single-dose 0.1% Q-GRFT: median 38%. lmeLP: F1,70 = 5.0, p = 0.03). Furthermore, 1% multi-dose Q-GRFT gel significantly affected the frequencies of intra-epithelial CD4+ cell (BL: median 5%; multi-dose 1% Q-GRFT: median 7%, lmeintra-epithelial: F1,71 = 7.2, p = 0.009), and all concentrations of multi-dose Q-GRFT gel (0.1%, 0.3%, 1%), significantly increased the frequencies of CD4+ cell in the LP (BL: median 34%; multi-dose Q-GRFT: median 36% – 39%, lmeLP: for 0.1% Q-GRFT F1,68 = 9.7, p = 0.003, for 0.3% Q-GRFT F1,70 = 4.5, p = 0.04, and for 1% Q-GRFT F1,68 = 5.7, p = 0.02) (Table 2, Supplementary Table 1).
All the Q-GRFT gel samples were next assessed to tease out if dosage (single or multi), or different concentrations of Q-GRFT gel (0.1%, 0.3% and 1%), affected the frequencies of the CD4+ cells. The lme model showed that neither dose nor concentration affected the frequencies of intra-epithelial CD4+ cell or the frequencies of CD4+ cell in the LP (Supplementary Table 1). A significant interaction (p = 0.02) was seen between dose and concentration for the frequencies of intra-epithelial CD4+ cells, but not for CD4+ cell in the LP. No interaction was seen between resting time and dose (Supplementary Table 1).
Thus, a slight increase in CD4+ cell numbers in the rectal epithelium and LP was detected following multi-dose Q-GRFT gel treatment as compared to PL as well as BL samples.
Sampling procedures affected the frequencies of CD4+ cells in the rectal mucosa
Our lme model was set up to account for potential effects caused by resting time interval and biopsy sampling. The model revealed that the fixed effect of resting time reduced the CD4+ cell numbers significantly in both the epithelium and the LP (lmeintra-epithelial: resting time F1,143 = 21.7, p = 0.00001, lmeLP: resting time F1,141 = 9.5, p = 0.003) and that sampling reduced the CD4+ cell numbers significantly in the LP (lmeLP: sampling F1,141 = 4.1, p = 0.04). No interaction between resting time and sampling was seen (Supplementary Table 1). To investigate this effect further, the BL samples were assessed alone, to exclude any interference from PL or Q-GRFT gel treatment. Indeed, comparable results were seen for the BL samples, as for taking all samples into account. (lmeintra-epithelial: resting time F1,62 = 18.2, p = 0.0001; lmeLP: resting time F1,59 = 10.5, p = 0.002; sampling F1,59 = 4.7, p = 0.03). BL samples collected after a resting period of 7 days had a median of intra-epithelial CD4+ cells of 6% and of CD4+ cells in the LP of 36%, while samples collected after a resting period of 13/14 days had a median of intra-epithelial CD4+ cells of 4% and of CD4+ cells in the LP of 31% (Table 2). The CD4+ cell frequencies in the LP declined after multiple biopsy samplings from the first BL time point (median of 36% CD4+ cells), to the last BL time point (median of 27% CD4+ cells in the LP).
Lastly all Q-GFRT gel treated samples were assessed and resting time also affected the frequencies of CD4+ cells in both compartments significantly in this set of data (lmeintra-epithelial: F1,55 = 11.2, p = 0.001; lmeLP: F1,56 = 7.8, p = 0.007 (Supplementary Table 1).
These data collectively show a reduced CD4+ cell frequency (both in the epithelium and in the LP) in relation to longer resting time as well as reduced CD4+ cell numbers in the LP in relation to multiple sampling.
Resting time but not Q-GFRT treatment affected the total cell numbers in the rectal mucosa
In addition to evaluating CD4+ cell frequencies as a proxy for HIV target cells, we assessed if PL and/or Q-GRFT gel treatment affected the total cell density in the tissue (number of cells/mm2). Local irritation/inflammation due to sampling and treatment can potentially lead to influx of other cells than CD4+ cells into the tissue. The lme model showed that neither PL nor Q-GRFT gel treatment (0.1%, 0.3%, 1% formulation) had any significant effect on the total number of mucosal cells per tissue area, nor when these cells were divided into epithelial or LP compartments (Table 3, Supplementary Table 2). The lme model further showed that resting time caused a small but significant decrease in the total cell density (lmetot: resting time F1,140 = 4.0, p = 0.05) and in the cell density in the LP (lmeLP: resting time F1,141 = 6.6, p = 0.01), while having no effect on the cell density in the epithelial compartment (Supplementary Table 2). No effect was seen for sampling and there was no interaction between sampling and resting time.
To avoid any interference from treatment, the BL samples were examined separately, showing that no effect was seen for sampling. Resting time did not have an effect of the total cell density or the cell density in the epithelium, but showed a significant decrease in the cell density in the LP (lmeLP: resting time F1,59 = 4.9, p = 0.03), (Supplementary Table 2). Thus, a longer resting time (14 vs. 7 days) might induce a small decrease of the total cell density.
Discussion
We here evaluated effects on critical cell populations of a topically applied rectal Q-GRFT microbicide gel in a dose-escalating model in the rectal mucosa of RMs. As a measure of epithelial tissue barrier integrity and HIV target immune cell density, rectal biopsies were assessed for E-cadherin+ cells, CD4+ cells and total mucosal cell numbers, respectively. Neither the placebo gel nor the different concentrations of Q-GRFT gel affected the frequencies of E-cadherin+ cells. Furthermore, neither did the PL gel as such, nor the Q-GRFT gel treatment in a single-dose setting compared to the PL gel cause any change in frequencies of CD4+ cells or total mucosal cells in the rectal mucosa. However, Q-GRFT gel treatment at the highest concentration (1%) in the multi-dose setting caused a minor but statistically significant increase of the intra-epithelial CD4+ cell numbers. Q-GRFT gel treatment (0.1–1%) in the multi-dose setting also caused a minor but statistically significant increase in the frequencies of CD4+ cells in LP. Q-GRFT gel treatment did not affect the total number of mucosal cells; neither in the epithelium nor in the LP, indicating that the increase of CD4+ counts may represent upregulation of the CD4 marker rather than an influx of CD4+ cells.
The observed increase in CD4+ cell numbers was further evaluated for any confounding effects because we noted fluctuations of the frequencies of CD4+ cells within the BL samples, prior to any gel treatment (PL or Q-GRFT). Previous studies suggest that not only specific microbicides such as Nonoxynol-9, but also experimental sampling procedures as such, may induce short-term (hrs) rectal epithelial damage in non-human primates29,30,31. Remarkably, in our present study, as the number of BL samplings increased over time, the frequencies of CD4+ cells decreased, both in the epithelium and in the LP. This decrease was dependent on the resting time between sampling. The extended resting time in the BL samples, 13 or 14 days, caused a reduction in the frequencies of CD4+ cells as compared to the BL samples with 7 days resting time. Furthermore, an extended resting time also caused a reduction in total mucosal cells in the LP. A caveat with this study is that the RMs, in addition to the tissue samples obtained for the present study, were further sampled for rectal fluid and/or swabs, frequently within the first 24 hours, and daily in the multi-dose setting, prior to collection of the biopsies. These additional samplings may be a confounder in our analysis. Thus, multiple sampling and resting time are potential factors that may need to be considered when designing experimental trials. Due to the combined effect of non-homogeneous resting time throughout the study (ranges 7–14 days during BL, and 14–32 days during treatment), and gel treatment, it is challenging to interpret the impact of resting time alone in the entire sample set. Further studies will thus be needed to address a more detailed phenotypic analysis of these CD4+ cells, and especially if they represent the activated CD4+CCR5+ phenotype in the rectal mucosa which has been shown to correlate to SIV acquisition in RMs, in an experimental vaccine model32.
The biologic relevance of the minor increase of CD4+ cells observed in the rectal mucosa with multiple Q-GRFT dosing is likely to be insignificant. We have previously evaluated a hyperosmolar lubricant for its effect on rectal mucosa and SHIV acquisition33. Despite findings of focal infiltrates of mononuclear cells and rectal lavages showing cytokine surges, bleeding and sloughed epithelial cells, we did not observe any increased SHIV acquisition in RMs as compared to placebo treatment. Thus, it is less likely that the minor increases of CD4+ cells, together with the stable numbers of E-cadherin+ cells and total mucosal cells, in the present study, would be associated with an increased risk of HIV/SIV infection. In the LP, the increase in CD4+ cells was slightly less with the higher concentrations than with 0.1% gel, a surprising finding as an inflammatory effect would likely be dose-dependent. However, Q-GRFT was assessed in a narrow range of concentrations (a 10-fold change between the lowest (0.1%) and the highest (1%) concentration). To further understand the biological relevance of the CD4 observations, it would be important to evaluate a wider range of product concentrations, a mechanical control (mock gel), and a positive control known to increase HIV or SIV acquisition in vivo, such as imiquimod or Nonoxynol-9. Interestingly, ARV-based microbicides, such as TFV, have been found safe in clinical trials despite numerous mucosal molecular changes including altered epidermal protein expression, up-regulated inflammatory proteins, inhibited wound-healing and T-cell proliferation34,35,36.
The observation in the present study of stable E-cadherin+ cell counts, and visually intact epithelial linings in the tissue sections, despite PL or Q-GRFT treatment, indicates a non-toxic effect on the rectal epithelium. Moreover, the human and non-human primate colonic mucosa undergoes rapid restitution following acute injuries30,37. The timing of sampling in relation to placebo or microbicide application is thus critical since possible effects such as cytokine responses, microbiome changes and subsequent immune activation and influx of cells follow different kinetic patterns. It will thus be interesting to expand these findings to additional HIV target cell and epithelial junction protein markers as well as sampling time points closer in time to application of the product (placebo or microbicide).
Earlier studies have reported the outstanding safety profile of GRFT including in vitro models, explant models and small animal models14,38. In a pilot study using a rectal gel containing 0.1% (wt/vol) wild-type GRFT (a less oxidation-resistant compound as compared to Q-GFRT) we have also evaluated the effects of single-dose PL and GRFT gel by assessing proteome and microbiome data from rectal samples from RMs39. Remarkably, the GRFT microbicide gel, when formulated in either HEC or Carbopol, was not associated with any significant changes in protein levels, but with an increased abundance of two common and beneficial microbial taxa related to application of the HEC-GRFT gel. However, both placebo formulations (HEC and Carbopol) were associated with temporary alterations in expression of proteins involved in proteolysis, and in activation of the immune response and inflammation. Thus, application of topical rectal compounds may induce an inflammatory response.
In conclusion, this study represents a first step in the in vivo evaluation of a non ARV-based microbicide product with regards to cellular effects in situ in the rectal mucosa. The study highlights that the frequencies of rectal E-cadherin+ cells remain stable despite multiple rectal tissue samplings and local Q-GRFT treatment, whereas the application of Q-GRFT treatment (multi-dose) results in minor increases in the frequencies of rectal mucosal CD4+ cells. The resting time between sampling points were further associated with minor changes in the total and CD4+ rectal mucosal cell levels and needs to be observed in similar future studies. Although statistically significant, the biological relevance of the minor changes in CD4+ cell counts for mucosal susceptibility to virus infection is most likely negligible, but needs attention in a future clinical trial. Furthermore, a novel customized digital image analysis workflow was developed for assessment of these cell populations for both rectal epithelium and lamina propria, respectively. Using this image analyses technique, we ultimately aim to build a platform for safety trials in which the number and location of several types of HIV target cells (T cells, dendritic cells and monocytes and their distinct phenotypes) and epithelial junction markers can be assessed. The analysis platform could thus complement traditional methods of safety evaluations by highlighting markers of relevance for HIV transmission. The technique is especially interesting for larger clinical trials since the computerized analysis can handle a large set of samples in an objective and high-throughput manner. Lastly, these results also add to general knowledge of RM rectal mucosal histology and should be relevant for other types of experimental studies in non-human primates.
Data availability
All relevant data are presented within the paper and in the supplementary files and are furthermore available upon request from the corresponding author.
References
UNAIDS. 2017 GLOBAL HIV STATISTICS, http://www.unaids.org/sites/ default/files/media_asset/UNAIDS_FactSheet_en.pdf2018. (2018).
Baggaley, R. F. et al. Does per-act HIV-1 transmission risk through anal sex vary by gender? An updated systematic review and meta-analysis. Am J Reprod Immunol 80, e13039, https://doi.org/10.1111/aji.13039 (2018).
Patel, P. et al. Estimating per-act HIV transmission risk: a systematic review. AIDS 28, 1509–1519, https://doi.org/10.1097/QAD.0000000000000298 (2014).
Shaw, G. M. & Hunter, E. HIV transmission. Cold Spring Harb Perspect Med 2, https://doi.org/10.1101/cshperspect.a006965 (2012).
Baeten, J. M. et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 367, 399–410, https://doi.org/10.1056/NEJMoa1108524 (2012).
Grant, R. M. et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 363, 2587–2599, https://doi.org/10.1056/NEJMoa1011205 (2010).
Molina, J. M. et al. On-Demand Preexposure Prophylaxis in Men at High Risk for HIV-1 Infection. N Engl J Med 373, 2237–2246, https://doi.org/10.1056/NEJMoa1506273 (2015).
Thigpen, M. C. et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med 367, 423–434, https://doi.org/10.1056/NEJMoa1110711 (2012).
Riddell, Jt, Amico, K. R., Mayer, K. H. & HIV Preexposure Prophylaxis: A Review. JAMA 319, 1261–1268, https://doi.org/10.1001/jama.2018.1917 (2018).
Patel, M. M. Formulation and development of di-dependent microparticulate system for colon-specific drug delivery. Drug Deliv Transl Res 7, 312–324, https://doi.org/10.1007/s13346-017-0358-7 (2017).
O’Keefe, B. R. et al. Scaleable manufacture of HIV-1 entry inhibitor griffithsin and validation of its safety and efficacy as a topical microbicide component. Proc Natl Acad Sci USA 106, 6099–6104, https://doi.org/10.1073/pnas.0901506106 (2009).
Derby, N. et al. Griffithsin carrageenan fast dissolving inserts prevent SHIV HSV-2 and HPV infections in vivo. Nat Commun 9, 3881, https://doi.org/10.1038/s41467-018-06349-0 (2018).
Grantome. Griffithsin-based Rectal Microbicides for PREvention of Viral ENTry (PREVENT) NIH/U19-AI113182-01, http://grantome.com/grant/NIH/U19-AI113182-05 (2015).
Kouokam, J. C. et al. Investigation of griffithsin’s interactions with human cells confirms its outstanding safety and efficacy profile as a microbicide candidate. PLoS One 6, e22635, https://doi.org/10.1371/journal.pone.0022635 (2011).
Mori, T. et al. Isolation and characterization of griffithsin, a novel HIV-inactivating protein, from the red alga Griffithsia sp. J Biol Chem 280, 9345–9353, https://doi.org/10.1074/jbc.M411122200 (2005).
Meuleman, P. et al. Griffithsin has antiviral activity against hepatitis C virus. Antimicrob Agents Chemother 55, 5159–5167, https://doi.org/10.1128/AAC.00633-11 (2011).
Nixon, B. et al. Griffithsin protects mice from genital herpes by preventing cell-to-cell spread. J Virol 87, 6257–6269, https://doi.org/10.1128/JVI.00012-13 (2013).
Fuqua, J. L., Hamorsky, K., Khalsa, G., Matoba, N. & Palmer, K. E. Bulk production of the antiviral lectin griffithsin. Plant Biotechnol J 13, 1160–1168, https://doi.org/10.1111/pbi.12433 (2015).
Fuqua, J. L., Wanga, V. & Palmer, K. E. Improving the large scale purification of the HIV microbicide, griffithsin. BMC Biotechnol 15, 12, https://doi.org/10.1186/s12896-015-0120-5 (2015).
Dezzutti, C. S. et al. Development of HIV-1 rectal-specific microbicides and colonic tissue evaluation. PLoS One 9, e102585, https://doi.org/10.1371/journal.pone.0102585 (2014).
Council, N. R. Guide for the Care and Use of Laboratory Animals: Eighth Edition. (The National Academies Press, 2011).
Sommer, C., Strähle, C., Köthe, U. & Hamprecht, F. A. In Eighth IEEE International Symposium on Biomedical Imaging (ISBI). Proceedings, (2011), 230-233 (2011).
Lamprecht, M. R., Sabatini, D. M. & Carpenter, A. E. CellProfiler: free, versatile software for automated biological image analysis. Biotechniques 42, 71–75, https://doi.org/10.2144/000112257 (2007).
Diggle, P. J., Heagerty, P. J., Liang, K. & L., Z. S. Analysis of Longitudinal Data, Second Edition. (Oxford University Press, 2002).
Donohue, M. C., Gamst, A. C. & D., E. S. Package ‘longpower’, https://cran.r-project.org/web/packages/longpower/longpower.pdf2018. (2018).
Liu, G. & Liang, K. Y. Sample size calculations for studies with correlated observations. Biometrics 53, 937–947 (1997).
Pinheiro, J., Bates, D., DebRoy, S., Sarkar, D. & Team, R. C. nlme: Linear and Nonlinear Mixed Effects Models, https://cran.r-project.org/web/ packages/nlme/nlme.pdf2018. (2018).
Niessen, C. M. Tight junctions/adherens junctions: basic structure and function. J Invest Dermatol 127, 2525–2532, https://doi.org/10.1038/sj.jid.5700865 (2007).
Fuchs, E. J. et al. Hyperosmolar sexual lubricant causes epithelial damage in the distal colon: potential implication for HIV transmission. J Infect Dis 195, 703–710, https://doi.org/10.1086/511279 (2007).
Patton, D. L., Sweeney, Y. T. & Paul, K. J. A summary of preclinical topical microbicide rectal safety and efficacy evaluations in a pigtailed macaque model. Sex Transm Dis 36, 350–356, https://doi.org/10.1097/OLQ.0b013e318195c31a (2009).
Phillips, D. M., Taylor, C. L., Zacharopoulos, V. R. & Maguire, R. A. Nonoxynol-9 causes rapid exfoliation of sheets of rectal epithelium. Contraception 62, 149–154 (2000).
Carnathan, D. G. et al. Activated CD4+CCR5+ T cells in the rectum predict increased SIV acquisition in SIVGag/Tat-vaccinated rhesus macaques. Proc Natl Acad Sci USA 112, 518–523, https://doi.org/10.1073/pnas.1407466112 (2015).
Vishwanathan, S. A. et al. Rectal application of a highly osmolar personal lubricant in a macaque model induces acute cytotoxicity but does not increase risk of SHIV infection. PLoS One 10, e0120021, https://doi.org/10.1371/journal.pone.0120021 (2015).
Hladik, F. et al. Mucosal effects of tenofovir 1% gel. Elife 4, https://doi.org/10.7554/eLife.04525 (2015).
Rodriguez-Garcia, M. et al. Tenofovir Inhibits Wound Healing of Epithelial Cells and Fibroblasts from the Upper and Lower Human Female Reproductive Tract. Sci Rep 8, 45725, https://doi.org/10.1038/srep45725 (2017).
Romas, L. et al. Rectal 1% Tenofovir Gel Use Associates with Altered Epidermal Protein Expression. AIDS Res Hum Retroviruses 32, 1005–1015, https://doi.org/10.1089/AID.2015.0381 (2016).
Feil, W. et al. Rapid epithelial restitution of human and rabbit colonic mucosa. Gastroenterology 97, 685–701 (1989).
Lusvarghi, S. & Bewley, C. A. Griffithsin: An Antiviral Lectin with Outstanding Therapeutic Potential. Viruses 8, https://doi.org/10.3390/v8100296 (2016).
Girard, L. et al. Impact of the griffithsin anti-HIV microbicide and placebo gels on the rectal mucosal proteome and microbiome in non-human primates. Sci Rep 8, 8059, https://doi.org/10.1038/s41598-018-26313-8 (2018).
Acknowledgements
This project was supported by a grant from the National Institutes of Health (grant #: U19AI113182). G.G. was sponsored by the Physician against AIDS Foundation and K.B. by the Swedish Research Council (KB). The authors thank the PREVENT consortium. The findings and conclusions in this paper are those of the authors and do not necessarily represent the views of the National Institutes of Health or the official position of the Centers for Disease Control and Prevention. Open access funding provided by Karolinska Institute.
Author information
Authors and Affiliations
Contributions
K.E.P. provided overall PREVENT study conception, D.G., A.B., N.M., J.McN., K.E.P., A.T. and K.B. planned and designed the present study. D.G., P.G., J.M., J.McN. performed the non-human primate experiments. G.G. and A.T. designed the in situ staining experiments and G.G. performed the in situ staining. G.E. and C.W. developed the digital image workflow and G.E. performed the digital image analysis. G.E., A.T., L.N.-R. and A.M. performed the data analyses. L.W. and L.C.R. provided gels used for the study. G.G., G.E., A.T. and K.B. drafted the manuscript. All authors contributed to interpretation of data, revisions of the manuscript, and have seen and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Günaydın, G., Edfeldt, G., Garber, D.A. et al. Impact of Q-Griffithsin anti-HIV microbicide gel in non-human primates: In situ analyses of epithelial and immune cell markers in rectal mucosa. Sci Rep 9, 18120 (2019). https://doi.org/10.1038/s41598-019-54493-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-54493-4
This article is cited by
-
A topical rectal douche product containing Q-Griffithsin does not disrupt the epithelial border or alter CD4+ cell distribution in the human rectal mucosa
Scientific Reports (2023)
-
Status of mannose-binding lectin (MBL) and complement system in COVID-19 patients and therapeutic applications of antiviral plant MBLs
Molecular and Cellular Biochemistry (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.