Abstract
Clematis crassifolia and Clematis cadmia Buch.-Ham. ex Hook.f. & Thomson are herbaceous vine plants native to China. C. crassifolia is distributed in shaded areas, while C. cadmia mostly grows in bright, sunny conditions in mountainous and hilly landscapes. To understand the potential mechanisms involved in the irradiance responses of C. crassifolia and C. cadmia, we conducted a pot experiment under three irradiance treatments with natural irradiation and two different levels of shading. Various growth, photosynthetic, oxidative and antioxidative parameters and the relative expression of irradiance-related genes were examined. In total, 15 unigenes were selected for the analysis of gene expression. The exposure of C. crassifolia to high irradiance resulted in growth inhibition coupled with increased levels of chlorophyll, increased catalase, peroxidase, and superoxide dismutase activity and increased expression of c144262_g2, c138393_g1 and c131300_g2. In contrast, under high irradiance conditions, C. cadmia showed an increase in growth and soluble protein content accompanied by a decrease in the expression of c144262_g2, c133872_g1, and c142530_g1, suggesting their role in the acclimation of C. cadmia to a high-irradiance environment. The 15 unigenes were differentially expressed in C. crassifolia and C. cadmia under different irradiance conditions. Thus, our study revealed that there are essential differences in the irradiance adaptations of C. crassifolia and C. cadmia due to the differential physiological and molecular mechanisms underlying their irradiance responses, which result from their long-term evolution in contrasting habitats.
Similar content being viewed by others
Introduction
Clematis (Flora of China 6)1 species include diverse groups of perennial woody and herbaceous vine plants that belong to the Ranunculaceae family. Clematis is a large genus within the dicotyledons, and approximately 355 species are known worldwide. China is rich in Clematis diversity, with 147 species listed in the Flora of China2,3 and approximately 110 species widely distributed in Southwest China4.
Due to long-term evolution under diverse habitats that dictate resource availability and successional specificities5, different species of Clematis have adapted to survive in their particular ecological niches. Thus, the entire Clematis genus contains many species that embody extremely different biological characteristics and growth habits6,7.
Clematis species are widespread in the Northern Hemisphere, and most of them have been used extensively in traditional medicines around the world8,9. Clematis has also been extensively used for ornamental purposes in recent years10. A wide range of color and flower shapes are found across many Clematis varieties and species, and therefore, it has been called “the queen of vines”11. For instance, sweet autumn Clematis(C. maximowicziana, C. paniculata) is a vigorous species popular for its masses of fragrant white flowers. Large-flowered Clematis(Clematis patens) is widely used and well known for its large flowers and rich colors10.
C. crassifolia is an evergreen species that is extensively used in urban landscapes and home gardens. It can grow vigorously in cold winter conditions. In southern China, C. crassifolia flowers in winter when the temperature is between 4 °C and 10 °C and the soil is cool and moist, which makes it stand out from many other cultivars and species of Clematis. C. cadmia is loved by gardening professionals for its copious white flowers and excellent tolerance to adverse conditions and could also be exploited as a source of stress-resistant genes for Clematis breeding.
It is well known that different plant species can endure different irradiance stresses. C. cadmia mostly prefer high-light environments in daily production. C. crassifolia is a special Clematis species and usually has a longer and slower growth rate than other Clematis species. This suggests that different light requirements could exist between C. cadmia and C. crassifolia. Thus, it is necessary to research the physiological and biochemical differences between C. cadmia and C. crassifolia under different irradiance environments.
Suboptimal irradiance can be one of the major limiting factors restricting the growth and development of plants12. Plants are under illumination stress when the available light is either in excess or deficient. High irradiance stress can exacerbate ROS production, which overwhelms the ROS scavenging system and generates various secondary messengers, resulting in photoinhibition, photoinactivation and photodamage in plant cells13,14. However, plants grown under insufficient irradiance levels often suffer reduced ribulose-1,5-bisphosphate carboxylase oxygenase (Rubisco) activity and low CO2 assimilation rates, thereby leading to reduced photosynthetic productivity15.
The consequences of irradiance stress on the whole plant are quite complex and relate to physiological and metabolic functions and molecular responses. Mittler16 found that the generation of ROS in plants is usually low under normal growth conditions, whereas enhanced production of ROS occurs under high or low irradiance stress conditions. Increased ROS is known to cause damage to the photosynthetic apparatus as well as to other metabolic systems.
Moreover, in recent years, many genes involved in plant growth and physiological responses to light have been investigated, and stimulus-specific changes in gene expression are often observed when plants acclimate to adverse light environments17,18. Gau et al.19 isolated a single-copy gene (psbY) encoding PsbY-A1 and PsbY-A2, which are present in PS II core complexes and in reaction center complexes, from spinach. They observed that PsbY was involved in photosynthesis and the absorption of light energy in plants, and thus, its expression was significantly affected by light intensity. Evidence shows that irradiance stress conditions induce a primed state in plants that enhances gene expression, including the expression of defensive genes that protect plants against the adverse effects of high or low irradiance, genes that encode signaling proteins related to induced systemic resistance (ISR) pathways and a sequence of genes that are involved in the process of photosynthesis in plants20,21. However, most studies on irradiance have focused on physiological activities in plants, whereas less attention has been paid to the response to irradiance in gene expression.
Previous studies on Clematis often focused on the chemical constituents, pharmacology, toxicology and clinical studies of Clematis species4,22,23,24. There are few published studies on growth, physiology and acclimatory variation at the molecular level in Clematis species under different environmental conditions. Our preliminary experiments on the survival and early growth of the genus Clematis indicated that Clematis species growth may be strongly affected by irradiance. The objectives of our study are (i) to investigate the effects of irradiance on photosynthesis, growth and development in C. crassifolia and C. cadmia, (ii) to elucidate how defensive enzymatic activity and gene expression respond to different irradiance levels, and (iii) to determine the irradiance levels that optimize the growth of C. crassifolia and C. cadmia. We focused on various physiological, biochemical and molecular responses in both species under different irradiance levels, which is expected to provide a theoretical foundation for the development of beneficial management practices for C. crassifolia and C. cadmia.
Materials and Methods
Plant materials and growth conditions
A pot experiment was established using shading nets of different thicknesses in a room at the Zhejiang Institute of Subtropical Crops, Zhejiang Province, China (N28°23′, E120°72′). Seeds of one-year-old healthy and uniform Clematis seedlings (C. crassifolia and C. cadmia, 8- to 10-leaf age) were individually transferred to plastic pots (16.5 cm inner diameter, 18 cm height, with holes in the bottom) filled with a substrate mixture of perlite:peat:black soil (2:5:3, v/v/v, 45 kg m−3 of organic manure). After six months, the potted plants were grown under a temperature range of 20–32 °C and three different irradiance levels, (i) natural irradiance (T1), (ii) moderate shade (T2), and (ii) heavy shade (T3) (Fig. 1). The different irradiances were established using different layers of commercial black shading net (resembling square tents, commonly used in agriculture), and the irradiance was measured using a digital lux meter (TES-1339R, Taiwan) for a week and averaged. Planted pots were rotated daily to minimize the effect of the environment and irrigated daily to keep the plants well-watered (the water level was kept at 75% of the field capacity of the soil). After three months of growth, leaves were selected, and a completely randomized design with five replications per treatment was established. Each irradiance treatment included the two species of Clematis, C. crassifolia (C1) and C. Cadmia (C2). The treatments were as follows: T1C1 (C. crassifolia grown under natural irradiance conditions); T2C1 (C. crassifolia grown under moderate shade irradiance conditions); T3C1 (C. crassifolia grown under heavy shade conditions); T1C2 (C. cadmia grown under natural irradiance conditions); T2C2 (C. cadmia grown under moderate shade irradiance conditions); and T3C2 (C. cadmia grown under heavy shade conditions). Thus, the experiment was set up in a split-plot design, in which irradiance levels constituted the main plots and Clematis species were the subplots, and contained five replicates with twenty-five pots each.
Leaf growth analysis
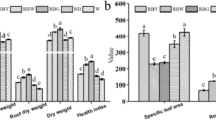
After three months of the differential irradiance treatments, one intact plant from each replication of each treatment was selected to observe and record the growth conditions of the whole plant. The leaf samples of Clematis from all treatments were collected for growth analysis. The leaf mass was weighed by electronic scale, and the leaf area was measured by an LA meter (LI-300, Li-Cor, Lincoln, NE, USA). The leaves were killed by keeping them at 105 °C for 15 min and then dried in an oven at 80 °C until they reached a constant dry weight. Specific leaf weight (SLM) was calculated by leaf dry mass divided by the leaf area for each plant25.
Chlorophyll content analysis
Chlorophyll content was measured as described by Lichtenthaler with a spectrophotometer (Shimadzu UV-2550, Kyoto, Japan) and expressed as mg g−1 fresh weight (FW)26.
Gas exchange measurements
Healthy and fully developed leaves from each treatment were chosen for photosynthetic parameter measurements with a LI-6400 XT portable photosynthesis system (LI-COR Inc., Lincoln, NE, USA) equipped with a 6400-18 RGB LED light source. The measurements were carried out on sunny days from 9:30 to 11:00 am at an air concentration of 21% O2, 1200 µmol m−2s−1 photosynthetically active radiation (PAR) white light, 400 µmol of CO2 mol−1 of dry air, 55% relative humidity and a temperature range of 28–32 °C27.
Cellular membrane damage analysis
Cell membrane stability and integrity were expressed as the relative electrical conductivity (REC) and were measured as previously described28. The membrane peroxidation was expressed as the malondialdehyde (MDA) content and was measured by the method of Hodges et al.29.
Peroxide (H2O2) content and superoxide anion (O−2) production rate analysis
The H2O2 content was determined with the method described by Patterson et al.30 and expressed as mM mg−1 FW. The superoxide anion (O.−2) production rate was determined using the method of Wang and Luo31 and expressed as µM min−1 mg−1 FW.
Antioxidant enzyme activity analysis
Leaf samples (1.0 g FW) were ground in a mortar containing 8.0 ml of grinding media consisting of 1% polyethylene pyrrole (PVP) and 50 mM phosphate buffer solution (pH 7.4) at 4 °C. Following centrifugation at 10,000 rpm for 15 min at 4 °C, the supernatants were collected to obtain crude enzymes. Total superoxide dismutase (SOD) activity was determined spectrophotometrically at 520 nm and expressed as U g−1 FW32. Catalase (CAT) activity was determined by monitoring the disappearance of H2O2, and specific CAT activity was expressed as U g−1 FW min−1 33. The peroxidase (POD) activity was measured with the method described by Thomas et al., and specific POD activity was expressed as U g−1 FW min−1 34.
Soluble protein content analysis
The soluble protein content was measured with Coomassie brilliant blue staining according to the method described by Bradford and was expressed as mg g−1 FW35.
Related gene expression analysis
We screened various genes related to the irradiance stress response through transcriptome sequencing to analyze gene expression (Table 1). Primer Premier 5 (www.Premierbiosoft.com/primerdesign) was used for designing primers. Table 2 shows the primer information for amplification of the analyzed genes. Total RNA was extracted from frozen and pulverized Clematis leaves using an RNeasy column (Qiagen USA, California, CA), and RNA samples for each replicate were pooled to obtain a single RNA sample for cDNA and cRNA probe preparation and expression profiling. qRT-PCR experiments were conducted as described by Gao et al. using Real Master Mix (SYBR Green) (Roche Applied Science, Mannheim, Germany)36.
Data analysis
Statistical analysis was conducted using a two-factor analysis of variance with SPSS software version 16.0 (SPSS, Chicago, IL, U.S.). Tukey’s multiple range test was applied to detect differences between the means. The data shown are the mean ± standard deviation (SD).
Results
Plant growth and development
The C. crassifolia and C. cadmia grown under different irradiances for three months are shown in Fig. 2. Irradiance had different effects on the leaf growth of C. crassifolia and C. cadmia. Additionally, irradiance levels significantly affected the leaf growth of Clematis (p < 0.05) (Table 3). After three months of irradiance treatment, the total leaf fresh weight per seedling of C. crassifolia plants increased by 8.5% (p < 0.05) and 21.78% (p < 0.05), respectively, under T2 and T3 light treatments compared with that of plants subjected to T1 irradiance treatments. Conversely, the leaf fresh weight of C. cadmia decreased by 25.82% (p < 0.05) and 52.23% (p < 0.05) under T2 and T3 irradiances, respectively, compared with that under T1 treatments. However, both C. crassifolia and C. cadmia exhibited a significant decrease (p < 0.05) in SLW with decreasing irradiance.
Photosynthetic pigments
Irradiance had a notable influence on the photosynthetic pigment content in Clematis leaves (Fig. 3). There were higher Chla, Chlb, and carotenoid concentrations in both C. cadmia and C. crassifolia plants under T2 and T3 light treatments than in those grown under natural sunlight (T1). In C. crassifolia, the Chla and Chlb content increased by 112.6% (p < 0.05) and 181.6% (p < 0.05) and by 138.2% (p < 0.05) and 204.4% (p < 0.05), respectively, under T2 and T3 light treatments compared with the Chla and Chlb content in plants under natural light intensity (T1). While a similar trend appeared in the leaves of C. cadmia plants, all three pigments were at higher levels in C. cadmia than in C. crassifolia across all irradiance conditions. The carotenoid contents in C. cadmia leaves increased by 31.8% (p < 0.05) and 82.8% (p < 0.05), respectively, under T2 and T3 irradiance compared with the carotenoid contents in C. cadmia leaves under T1. Specifically, the Chla/b ratio significantly decreased (p < 0.05) with decreasing irradiance in C. cadmia leaves, while no significant differences in Chla/b ratio were observed in C. crassifolia plants across the different irradiance treatments.
Chlorophyll a (chla), chlorophyll b (chlb), carotenoid (car) and chlorophyll a/b ratio in C. cadmia and C. crassifolia leaves (means ± SD) grown under three different irradiances including natural irradiance (T1), moderate shade (T2); and heavy shade (T3). Different uppercase letters indicate significant difference between different Clematis species under same irradiance treatment at 0.05 levels; lowercase letters indicate significant difference within the same Clematis species under different irradiance treatments at 0.05. The values presented are the means ± SE.
Photosynthetic parameters
The leaf photosynthetic parameters in C. cadmia and C. crassifolia were strikingly affected by the irradiance levels (Table 4). The C. crassifolia plants exposed to natural irradiance (T1) showed less leaf net photosynthesis (Pn) (p < 0.05), stomatal conductance (Gs) (p < 0.05), and intercellular CO2 concentration (Ci) (p < 0.05) and a lower transpiration rate (Tr) (p < 0.05) than those under T2 and T3 irradiance. Pn dramatically increased (p < 0.05) with decreasing irradiance in C. crassifolia plants, whereas a decrease (p < 0.05) in Pn in C. cadmia plants was observed when irradiance decreased from T1 to T3. Even under the same natural irradiance condition (T1), the Pn in C. cadmia was 3.18 times (p < 0.05) that in the C. crassifolia leaves. The variations in Gs, Ci and Tr were similar to that of Pn in the leaves of C. cadmia and C. crassifolia.
Relative electrical conductivity (REC) and MDA content
C. crassifolia leaves had 32.19% (p < 0.05) and 62.83% (p < 0.05) lower REC under T2 and T3 irradiance than under natural sunlight (T1), respectively. In C. cadmia leaves, REC increased by 311.4% (p < 0.05) and 400.5% (p < 0.05) under T2 and T3 light intensity, respectively, compared to under natural sunlight (T1) (Fig. 4). There was 30.67% (p < 0.05) and 48.47% (p < 0.05) lower MDA content under T2 and T3 irradiance in the C. crassifolia leaves, respectively, than under natural irradiance (T1), while the opposite trend occurred in the C. cadmia leaves with the decrease in irradiance.
MDA content and relative electrical conductivity (REC) in C. cadmia and C. crassifolia leaves grown under different irradiance conditions. Different uppercase letters indicate significant difference between different Clematis species under same irradiance treatment at 0.05 levels; lowercase letters indicate significant difference within the same Clematis species under different irradiance treatments at 0.05. The values presented are the means ± SE.
H2O2 content, O2 − production rate, and antioxidant enzyme activities
The H2O2 content and O2− production rate were notably different between C. cadmia and C. crassifolia leaves (Fig. 5). The H2O2 content in C. crassifolia leaves decreased by 65.6% (p < 0.05) and 87.03% (p < 0.05) under T2 and T3 irradiance, respectively, compared with that under natural light intensity (T1), whereas H2O2 content increased by 210.3% (p < 0.05) and 679.3% (p < 0.05) in C. cadmia leaves under T2 and T3 irradiance, respectively. (Fig. 5). Similar responses were observed in the O2− production rate.
The O2− production rate and H2O2 content in C. cadmia and C. crassifolia leaves grown under different irradiance conditions. Different uppercase letters indicate significant difference between different Clematis species under same irradiance treatment at 0.05 levels; lowercase letters indicate significant difference within the same Clematis species under different irradiance treatments at 0.05. The values presented are the means ± SE.
A marked decrease was observed in the CAT (p < 0.05) and SOD activity (p < 0.05) in C. crassifolia growing under T2 and T3 light treatments compared with those in the control plants growing under natural irradiance (Fig. 6). Conversely, there were 92.8% (p < 0.05) and 140.4% (p < 0.05) increases in CAT activity and 29.8% (p < 0.05) and 13.5% (p > 0.05) increases in POD activity under T2 and T3 irradiance, respectively, in C. cadmia compared with the activity under natural light intensity (T1). Interestingly, the SOD activity in C. crassifolia leaves grown under natural irradiance (T1) was higher (p < 0.05) than that in the other light treatments, and no obvious differences (p > 0.05) were found between the other irradiance treatments.
Peroxidase (POD), catalase (CAT) and superoxide dismutase (SOD) activity in Clematis leaves grown under three different irradiance conditions. Different uppercase letters indicate significant difference between different Clematis species under same irradiance treatment at 0.05 levels; lowercase letters indicate significant difference within the same Clematis species under different irradiance treatments at 0.05. The values presented are the means ± SE.
Soluble protein content
The soluble protein in the C. cadmia and C. crassifolia leaves differed significantly (p < 0.05) under different light intensities (Fig. 7). A significant increase (p < 0.05) was observed in the soluble protein content from the T1 to the T3 irradiance treatments in C. crassifolia. In contrast, there was a dramatic reduction (by 35.3% (p < 0.05) and 48.87% (p < 0.05)) in the soluble protein content under the T2 and T3 irradiance treatments, respectively, in C. cadmia compared with that under natural light intensity (T1).
Relative gene expression analysis
As shown in Fig. 8, the genes in Clematis showed differential expression in response to various irradiance levels. A total of 15 genes were analyzed for differences in gene expression in C. cadmia and C. crassifolia leaves under three irradiance levels. Three of those genes, including c136757_g1, c144230_g2 and c145729_g1, are involved in the biosynthesis of phytochrome and chlorophyll in chloroplasts. The expression of these genes was significantly different (p < 0.05) in C. cadmia and C. crassifolia under the three irradiance levels. The genes c144230_g2 and c144262_g2, which encode the receptor proteins in the Photosystem II core, were upregulated in C. crassifolia from T1 to T3, while the same genes were upregulated more than 5-fold (p < 0.05) in C. cadmia under T2 irradiance compared their levels under T1. The genes c131300_g2 and c133872_g1, which belong to the abscisic acid receptor family, were differentially upregulated in C. crassifolia and C. cadmia in response to the shaded conditions. A similar trend was observed in c133872_g1, c144154_g3 and c145729_g1 expression. The gene c139448_g1, which is associated with auxin response, first showed upregulation and then downregulation in C. crassifolia but showed no significant difference in C. cadmia. Similarly, the gene c142929_g5, which is also associated with auxin response, first showed upregulation and then downregulation in C. crassifolia and C. cadmia as the irradiance changed from T1 to T3.
Variation in relative gene expression in C. crassifolia (C1) and C. Cadmia (C2) leaves developed under different three irradiance treatments including natural irradiance (T1), moderate shade (T2); and heavy shade (T3). The values presented are the means ± SE. Different uppercase letters indicate significant difference between different Clematis species under same irradiance treatment at 0.05 levels; lowercase letters indicate significant difference within the same Clematis species under different irradiance treatments at 0.05.
Discussion
Excessive or deficient irradiance is the most common environmental stress factor that affects a series of physiological, developmental and biochemical changes in plants, including molecular and cellular responses37. However, plants are also known to cope with these stress conditions by adjusting their metabolism and physiology and making specific changes in gene expression38. Differences in plant growth, carbon allocation, physiology and gene expression have been investigated for species suffering from different levels of irradiance stress39. In this study, we have illustrated the growth, physiological mechanisms and changes in some gene expression patterns of C. cadmia and C. crassifolia in response to different irradiance levels.
Irradiance that is too intense can be stressful, resulting in reduced growth, leaf fading and foliage yellowing, as shown in Fig. 2, where the C. crassifolia plants under natural irradiance (T1) experienced growth inhibition accompanied by lower leaf fresh weight. This is probably because higher irradiance can lead to photoinhibition and other adverse reactions in C. crassifolia. However, C. cadmia showed increased growth vigor and leaf fresh weight under higher irradiance conditions, which demonstrated that C. crassifolia and C. cadmia prefer entirely different light environments. This difference was largely caused by long-term adaptation to their contrasting ecological niches. Moreover, the specific leaf weight in the two Clematis species decreased with the decrease in irradiance. This is a common response in species suffering from low irradiance, indicating that plants increase light energy capture by increasing dry weight per unit leaf area; this strategy has been observed in Torreya grandis25, Camptotheca acuminata40, and wheat41.
Plant leaf systems possess numerous fundamental functions for adapting to different irradiances, including chlorophyll content changes and photosynthesis. The changes in pigment content in Clematis are shown in Fig. 3. The decreasing irradiance elevated the content of carotenoids, chlorophyll a and chlorophyll b in both species of Clematis but reduced the Chla/b ratio in C. cadmia. These findings were consistent with observations in C. acuminata40, suggesting that a long exposure to high light caused pigments to degrade in C. crassifolia, while the content of chlorophyll b, as an antenna chlorophyll, increased in C. cadmia to absorb more light energy for photosynthesis; thus, the Chla/b ratio underwent a sharp decline in C. cadmia40. Photosynthesis is correlated with chlorophyll content in plants. In our study, decreasing irradiance significantly increased the Pn, Ci, Tr, and Gs values in C. crassifolia but lowered those values in C. cadmia. These results indicated that C. cadmia could take full advantage of the energy under high light (T1) and suitable growth conditions, but higher light intensity (T1) would lead to an immediate interruption of growth and excessive light energy accumulation in C. crassifolia42.
Accordingly, excessive light energy could impair the photosynthetic electron transport chain and interrupt PSII electron transport. Excess ROS can be produced by the direct transfer of excitation energy from chlorophyll, causing membrane permeability dysfunction and lipid peroxidation43. In this study, as C. crassifolia and C. cadmia were grown under different irradiance levels for three months, we observed that high light caused higher H2O2 content and a higher O2− production rate in C. crassifolia, thereby resulting in a sharp increase in MDA content and coincident increases in the relative electrical conductivity (REC); however, C. cadmia showed the opposite response. This phenomenon clearly suggests that an inability to adapt to light intensity could lead to either a photooxidative or an inadequate stress response in plants, closely accompanied by ROS overproduction and ultimately leading to damage to plant membranes44. This result was in agreement with the effects of photoinactivation and lipid peroxidation described by Takahama and Nishimura45. Substantial evidence has indicated that plants can alleviate and repair the damage caused by ROS through antioxidant enzymes, including SOD, POD, and CAT40,46,47. Our research confirmed this point in Clematis and showed that the POD, SOD and CAT enzyme activity increased when plants suffered from irradiance stress.
Proteins encoded by various plant genes, including most of the known enzymes (such as POD, APX and GST) and some compatible modulators of metabolic processes, perform many biological functions48. Our study found that light intensity significantly impacted the soluble protein content in Clematis; the soluble protein content increased in C. crassifolia grown under low irradiance but decreased in C. cadmia under the same conditions. Similar results were reported in microalgae49 and wheat50, which suggests that soluble protein content could represent the physiological status of plants to some extent51.
In general, plants respond to stresses with changes in the expression of related genes that encode proteins that provide protective effects in plants52. Our study focused on the responses of C. cadmia and C. crassifolia to different irradiances and screened 15 light-related genes to analyze their expression (Fig. 8). Overall, the 15 genes were differentially expressed under irradiance stress in C. cadmia and C. crassifolia. After three months of irradiance treatment, low light induced upregulated gene expression of numerous genes in C. crassifolia, which are involved in pigment biosynthesis, Photosystem II core protein synthesis, glycometabolism and auxin response protein synthesis. Our results in C. crassifolia are consistent with previous observations of some shade-tolerant plants, such as Anoectochilus formosanus38. This suggested that these genes contribute to adaptation to low light in C. crassifolia. PYL receptors perceive ABA intracellularly and, as a result, form ternary complexes initiating ABA signaling53,54. In this study, the PYL receptors were upregulated in C. cadmia grown under low light, which indicated that the C. cadmia plants were under stress when growing in low light (T2, T3) and that low light could activate ABA-responsive gene expression in the protoplasts, contributing to the adjustment of C. cadmia to the adverse environment. Interestingly, the superoxide dismutase [Cu-Zn] pathway gene was also upregulated in C. cadmia under low light, which indicated that C. cadmia increases the biosynthesis of SOD under low light. However, C. crassifolia showed the opposite response.
In summary, the present results confirm that the promotion of C. crassifolia growth under low irradiance may be related to the changes in gene expression in response to irradiance, the increased chlorophyll content and photosynthetic rate and the decreased ROS content. In addition, C. crassifolia and C. cadmia exhibit different physiological and metabolic characteristics, as well as antioxidant defense and gene expression patterns, under different irradiance conditions. This difference was largely due to the contrasting irradiance conditions in the habitats of the different species. Long-term evolution led to the differences between the species. Therefore, we proposed the hypothesis that irradiance might regulate the expression patterns of genes in these plants and might be an important environmental factor that affects the direction of plant evolution. The hypothesis will be tested in future work.
References
Wang, W. T. & Bartholomew, B. Clematis Linnaeus. In: Flora of China (eds ZY Wu, PH Raven, DY Hong). Beijing Science Press & St. Louis Botanical Garden Press, 6, 333–386 (2001).
Snoeijer, W. A suggested classification for the genus Clematis. Clematis, 7–20 (1992).
Kizu, H. & Tommori, T. Studies on the constituents of Clematis species. V. On the saponins of the root of Clematis chinensis OSBECK.(5). Chemical and Pharmaceutical Bulletin 30(9), 3340–3346, https://doi.org/10.1248/cpb.30.3340 (1982).
Chawla, R., Kumar, S. & Sharma, A. The genus Clematis (Ranunculaceae): chemical and pharmacological perspectives. Journal of ethnopharmacology 143(143(1)), 116–150, https://doi.org/10.1016/j.jep.2012.06.014 (2012).
Bolnick, D. I. et al. Ecological release from interspecific competition leads to decoupled changes in population and individual niche width. Proceedings of the Royal Society B: Biological Sciences 277(1689), 1789–1797, https://doi.org/10.1098/rspb.2010.0018 (2010).
Kizu, H., Shimana, H. & Tomimori, T. Studies on the constituents of Clematis species. VI. The constituents of Clematis stans Sieb. et Zucc. Chemical and pharmaceutical bulletin 43(12), 2187–2194 (1995).
Habibi, M. et al. A new species of Clematis L.(Ranunculaceae) from Iran. Phytotaxa 162(2), 99–106, https://doi.org/10.11646/phytotaxa.162.2.4 (2014).
Zeng, Y.-X. et al. Comparative analysis of volatile components from Clematis species growing in China. Analytica Chimica Acta 595(1–2), 328–339, https://doi.org/10.1016/j.aca.2006.12.022 (2007).
Shi, S. et al. Triterpene saponins from Clematis mandshurica. Journal of natural products 69(11), 1591–1595, https://doi.org/10.1021/np060287z (2006).
Starbuck, C. J. Selecting landscape plants: ornamental vines. horticultural, https://hdl.handle.net/10355/51102 (2000).
Wang, W. A revision of Clematis sect. Clematis (Ranunculaceae). Acta phytotaxonomica sinica 41(1), 1–62 (2003).
Dai, Y. J. et al. Effects of shade treatments on the photosynthetic capacity, chlorophyll fluorescence, and chlorophyll content of Tetrastigma hemsleyanum Dielset Gilg. Environ.Exp.Bot 65, 77–182, https://doi.org/10.1016/j.envexpbot.2008.12.008 (2009).
Cheirsilp, B. & Torpee, S. Enhanced growth and lipid production of microalgae under mixotrophic culture condition: effect of light intensity, glucose concentration and fed-batch cultivation. Bioresource technology 110, 510–516, https://doi.org/10.1016/j.biortech.2012.01.125 (2012).
Quiles, M. J. Stimulation of chlororespiration by heat and high light intensity in oat plants. Plant, Cell & Environment 29, 8:1463–1470, https://doi.org/10.1111/j.1365-3040.2006.01510.x (2006).
Wittmann, C., Guido, A. & Hardy, P. Leaf and twig photosynthesis of young beech (Fagus sylvatica) and aspen (Populus tremula) trees grown under different light regime. Basic and Applied Ecology 2(2), 145–154, https://doi.org/10.1078/1439-1791-00047 (2001).
Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends in plant science 7(9), 405–410, https://doi.org/10.1016/S1360-1385(02)02312-9 (2002).
Hihara, Y. et al. DNA microarray analysis of cyanobacterial gene expression during acclimation to high light. The Plant Cell 13, 4:793–806, https://doi.org/10.1105/tpc.13.4.793 (2001).
Ishida, A. et al. Light activates the adrenal gland: timing of gene expression and glucocorticoid release. Cell metabolism 2(5), 297–307, https://doi.org/10.1016/j.cmet.2005.09.009 (2005).
Gau, A. E. et al. PsbY, a novel manganese-binding, low-molecular-mass protein associated with photosystem II. Molecular and General Genetics MGG 260(1), 56–68, https://doi.org/10.1007/s004380050870 (1998).
Vanderauwera, S. et al. Genome-wide analysis of hydrogen peroxide-regulated gene expression in Arabidopsis reveals a high light-induced transcriptional cluster involved in anthocyanin biosynthesis. Plant Physiology 139(2), 806–821, https://doi.org/10.1104/pp.105.065896 (2005).
Baena-González, E. et al. Chloroplast transcription at different light intensities. Glutathione-mediated phosphorylation of the major RNA polymerase involved in redox-regulated organellar gene expression. Plant physiology 127(3), 1044–1052, https://doi.org/10.1104/pp.010168 (2001).
Trémolières, M. et al. Changes in foliar nutrient content and resorption in Fraxinus excelsior L., Ulmus minor Mill. and Clematis vitalba L. after prevention of floods. Annals of forest science 56(8), 641–650, https://doi.org/10.1051/forest:19990802 (1999).
Liu, X. B. et al. An in vivo and in vitro assessment of the anti-inflammatory, antinociceptive, and immunomodulatory activities of Clematis terniflora DC. extract, participation of aurantiamide acetate. Journal of ethnopharmacology 169, 287–294, https://doi.org/10.1016/j.jep.2015.04.009 (2015).
Fu, Q. et al. Triterpenoid saponins from Clematis chinensis and their inhibitory activities on NO production. Phytochemistry Letters 21, 206–210, https://doi.org/10.1016/j.phytol.2017.07.004 (2017).
Tang, H. et al. Growth, photosynthetic and physiological responses of Torreya grandis seedlings to varied light environments. Trees 29(4), 1011–1022, https://doi.org/10.1007/s0046 (2015).
Lichtenthaler, H. K. Chlorophylls and carotenoids - pigments of photosynthetic biomembranes. Methods Enzymol 148, 350–382, https://doi.org/10.1016/0076-6879(87)48036-1 (1987).
Li, T. et al. Salicylic acid alleviates the adverse effects of salt stress in Torreya grandis cv. Merrillii seedlings by activating photosynthesis and enhancing antioxidant systems. PLOS one 9(10), e109492, https://doi.org/10.1371/journal.pone.0109492 (2014).
Deshmukh, P. S., Sairam, R. K. & Shukla, D. S. Measurement of ion leakage as a screening technique for drought resistance in wheat genotypes. Plant Physiol 35, 89–911, (1991) (Indian).
Hodges, D. M. et al. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207(4), 604–611, https://doi.org/10.1007/s004250050524 (1999).
Patterson, B. D., Elspeth, A. M. & Ian, B. Ferguson. Estimation of hydrogen peroxide in plant extracts using titanium (IV). Analytical biochemistry 139(2), 487–492, https://doi.org/10.1016/0003-2697(84)90039-3 (1984).
Wang, A. G. & Luo, G. H. Quantitative relation between there action of hydroxylamine and superoxid eanion radicals in plants. Plant Physiol. Commun 6, 55–57, (1990) (in Chinese).
Favaretto, V. F. et al. Differential responses of antioxidant enzymes in pioneer and late-successional tropical tree species grown under sun and shade conditions. Environmental and Experimental Botany 70(1), 20–28, https://doi.org/10.1016/j.envexpbot.2010.06.003 (2011).
Díaz-Vivancos, P. et al. Alteration in the chloroplastic metabolism leads to ROS accumulation in pea plants in response to plum pox virus. Journal of experimental botany 59(8), 2147–2160, https://doi.org/10.1093/jxb/ern082 (2008).
Thomas, R. L., Jen, J. J. & Morr, C. V. Changes in soluble and bound peroxidase-IAA oxidase during tomato fruit development. Journal of Food Science 47(1), 158–161, https://doi.org/10.1111/j.1365-2621.1982.tb11048.x (1982).
Bradford, M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical biochemistry 72(1–2), 248–254, https://doi.org/10.1016/0003-2697(76)90527-3 (1976).
Gao, L. et al. Transcriptome profiling of Clematis apiifolia: insights into heat-stress responses. DNA and cell biology 36(11), 938–946, https://doi.org/10.1089/dna.2017.3850 (2017).
Hu, L. et al. Moderate ammonium: nitrate alleviates low light intensity stress in mini Chinese cabbage seedling by regulating root architecture and photosynthesis. Scientia Horticulturae 186, 143–153, https://doi.org/10.1016/j.scienta.2015.02.020 (2015).
Kreps, J. A. et al. Transcriptome changes for Arabidopsis in response to salt, osmotic, and cold stress. Plant physiology 130(4), 2129–2141, https://doi.org/10.1104/pp.008532 (2002).
Valladares, F. et al. The greater seedling high-light tolerance of Quercus robur over Fagus sylvatica is linked to agreater physiological plasticity. Trees 16(6), 395–403, https://doi.org/10.1007/s00468-002-0184-4 (2002).
Ma, X. et al. Growth, physiological, and biochemical responses of Camptotheca acuminata seedlings to different light environments. Frontiers in plant science 6, 321, https://doi.org/10.3389/fpls.2015.00321 (2015).
Bos, H. J. & Neuteboom, J. A. N. H. Morphological analysis of leaf and tiller number dynamics of wheat (Triticum aestivum L.): responses to temperature and light intensity. Annals of Botany 81(1), 131–139, https://doi.org/10.1006/anbo.1997.0531 (1998).
Argyroudi-Akoyunoglou, J. H. et al. Reorganization of the Photosystem II unit in developing thylakoids of higher plants after transfer to darkness: Changes in chlorophyllb, light-harvesting chlorophyll protein content, and grana stacking. Plant physiology 70(5), 1242–1248, https://doi.org/10.1104/pp.70.5.1242 (1982).
Downs, K. M. et al. The effects of photoperiod length, light intensity, and feed energy on growth responses and meat yield of broilers. Journal of applied poultry research 15(3), 406–416, https://doi.org/10.1093/japr/15.3.406 (2006).
Gill, S. S. & Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant physiology and biochemistry 48(12), 909–930, https://doi.org/10.1016/j.plaphy.2010.08.016 (2010).
Liang, W., Wang, M. & Ai, X. The role of calcium in regulating photosynthesis and related physiological indexes of cucumber seedlings under low light intensity and suboptimal temperature stress. Scientia Horticulturae 123(1), 34–38, https://doi.org/10.1016/j.scienta.2009.07.015 (2009).
Takahama, U. & Nishimura, M. Formation of singlet molecular oxygen in illuminated chloroplasts. Effects on photoinactivation and lipid peroxidation. Plant and Cell Physiology 16(4), 737–748, https://doi.org/10.1093/oxfordjournals.pcp.a075194 (1975).
Verhagen, J. et al. Climate change and drought risks for agriculture. The impact of climate change on drylands. Springer, Netherlands, pp 49–59, https://doi.org/10.1007/1-4020-2158-5_6 (2004).
Shao, Q. et al. Effects of shade treatments on photosynthetic characteristics, chloroplast ultrastructure, and physiology of Anoectochilus roxburghii. PLoS One 9(2), e85996, https://doi.org/10.1371/journal.pone.0085996 (2014).
Bond, D. M. et al. Infiltration-RNAseq: transcriptome profiling of Agrobacterium-mediated infiltration of transcription factors to discover gene function and expression networks in plants. Plant methods 12(1), 41, https://doi.org/10.1186/s13007-016-0141-7 (2016).
Ruiz, J. et al. Towards industrial products from microalgae. Energy & Environmental Science 9(10), 3036–3043, https://doi.org/10.1039/C6EE01493C (2016).
Chen, Y. E. et al. Responses of photosystem II and antioxidative systems to high light and high temperature co-stress in wheat. Environmental and Experimental Botany 135, 45–55, https://doi.org/10.1016/j.envexpbot.2016.12.001 (2017).
Doganlar, Z. B. et al. Effects of salt stress on pigment and total soluble protein contents of three different tomato cultivars. African Journal of Agricultural Research 5(15), 2056–2065, https://doi.org/10.5897/AJAR10.258 (2010).
Powar, P. V. Studies on tissue cultures of Celastrus paniculatus. Diss. RGUHS, http://hdl.handle.net/123456789/3465 (2010).
González-Guzmán, M. et al. PYR/PYL/RCAR receptors play a major role for quantitative regulation of stomatal aperture and transcriptional response to abscisic acid. Plant cell, https://doi.org/10.1105/tpc.112.098574 (2012).
Acknowledgements
This project was supported by the Zhejiang Province Major Science and Technology Project for new variety breeding of agriculture (forest)–Collection, conservation, evaluation and utilization of clematis vine flower resources (2016C02056-13-4), Zhejiang Province Public Welfare Project–Collection and identification of germplasm resources of Evergreen Clematis, cold resistance evaluation and protection and utilization (LGN18C150006), Wenzhou City Seed Seedling Project Study on Germplasm Innovation and Breeding of New Variety of Grandiflora Clematis (Z20170001), Wenzhou City Forestry Four New Technology Promotion Demonstration Project (F-DY201509170025) and Zhejiang Academy of Agricultural Sciences–Study on the regulation mechanism of bHLH transcription factor in the formation of lotus flower color (2018R26R08E01) and Excavation and Protection of Endangered Plants-Resource Conservation and Excavation of the Rare and Endangered Ornamental Plants of Chimonanthus praecox, Magnolia denudata, Carpinus tientaiensis and Clematis in Zhejiang Province (2019C02036).
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments, performed the experiments and wrote the manuscript: Xiaohua Ma and Renjuan Qian;Xiaohua Ma and Renjuan Qian contributed equally to this work.Help to performed the experiments: Xule Zhang;helped perform the analysis data: Qingdi Hu.Revised the manuscript: Hongjian Liu.Approved the final version:Jian Zheng
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ma, X., Qian, R., Zhang, X. et al. Contrasting growth, physiological and gene expression responses of Clematis crassifolia and Clematis cadmia to different irradiance conditions. Sci Rep 9, 17842 (2019). https://doi.org/10.1038/s41598-019-54428-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-54428-z
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.