Abstract
This study reports on the crude oil-sensing using carbon nano structures (CNSs). A mixture of CNSs was obtained by a simple method of preparation using palm cellulose ash and nitric acid as precursors, the powder was characterized by x-ray diffraction and infrared spectroscopy. The optical density of crude oil from Rhoud El-Baguel area (Southeast of Algeria) studied using UV-Vis spectroscopy, before and after adding an amount of CNSs powder to view the CNSs crude oil sensing and therefore a new method to determine the quality of crude oils and the comparison between them. Results show that CNSs prepared from palm cellulose ash have a good crystallinity and it is formed mainly from carbon nano dots (CNDs) with 4.32 Å in layers spacing and 7.4 Å in crystallite size, indicate that CNSs can be used as an excellent crude oil sensor.
Similar content being viewed by others
Introduction
In the petrochemical industry, knowledge of the quality and content of crude oil is a key factor in improving the refining process. Methods of spectroscopy are the most important methods used because they contain important informations regarding the chemical properties of each sample1. The compounds in oil, responsible on optical response named: Fluorophores (Table 1), which are compounds that absorbs in the UV-visible range. This condition is fulfilled for conjugated electron systems, such as polyunsaturated molecules and aromatics2.
Nano carbon structures have a wide range of interest because of their use in applications such as energy storage, tribology, electronics, medicine, catalysis and sensors3,4,5,6,7. Carbon nano structures (CNSs) are an ultra-small photoluminescent (PL) nanomaterial (<10 nm), It has significant optical properties, disposable surface functions, chemical inactivity, high photoresist, simple and inexpensive methods of preparation, and an abundance of raw materials,…8. The preparation of CNSs is a process of mix between two precursor forms, one of which installs the main carbon frame and the other within the structure elements. In this regard, the most prominent synthesis was cellulose ash as a carbon source, while nitrogen acid contains activating molecules9.
CNSs are crystals which can act as a sensor by sparkling at the desired wavelength or color. We emphasize that fluorescent organic molecules are often aromatic or contain multiple bonds, which are alternating single and double bonds, responsible for the high-octane number and therefore the quality of petroleum10. These molecules contain non-bonding electrons that form a cloud around the molecule and are usually prone to excitement and shine in response to the light energy projected on them11.
Expremental and Methods
Extraction of Cellulose from Phoenix Dactylifera L. tree
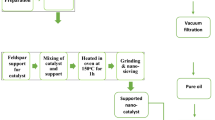
The Phoenix Dactylifera L. leaves were crushed and screened to ensure that the particle size was distributed from 8 meshes to 30 meshes. Leaves were immersed in 5 wt% sodium hydroxide solution at ambient temperature for 12 h. Then they were washed with water for several times and dried in the oven at 80 °C for 24 h. To remove the wax, the debris of leaves were immersed in the solution of methylbenzene and ethyl alcohol (volume ratio of 1:1), and kept boiled for 8 h. The residues were washed with ethyl alcohol several times and then dried in the oven at 80 °C for 24 hours. And to remove lignin, leaves were soaked in hydrogen peroxide (30 vol%) and acetic acid solution (volume ratio of 1:1), and boiled with magnetic stirring at 60 °C for 7 h. Water was used to wash the residue and then filtered until the filter was neutral. The fibers obtained were boiled in 5 wt% of sodium hydroxide solution at 80 °C for 2 h, then, washed with water to neutral and dried in the oven at 80 °C for 24 h. The cellulose fibers from Phoenix Dactylifera L. leaves were obtained12,13.
Synthesis of carbon nanostructures (CNSs)
In order to obtain C-nanostructures, the cellulose extracted previously was carbonized in a muffle furnace directly at 240 °C for 2 h4. About 5 g of fine ashes obtained from cellulose furnace and mixed with concentrated nitric acid (60%) and stay in agitation for 24 hours. The mixture was separated by centrifugation at 12,000 rpm for an hour to separate the residue and supernatant. The latter was heated in a vacuum oven at 200 °C14.
Crude oil optic sensing
The oils were excited by ultraviolet rays (300–400 nm) which fluoresce in the visible wavelength range of 400 to 600 nm. The crude oil sample was obtained from Rhoud El-Baguel, close Hassi-Messaoud region, city of Ouargla south eastern of Algeria. To perform optical density measurements, it was required to dilute the sample to obtain a transparent solution to transmit the light. Cyclohexane was chosen as solvent that can optically respond in the range of 350 nm–500 nm, wavelengths used to excite crude oil15,16. Six samples of oil diluted in cyclohexane at different concentrations were used for the measurements. Table 2 shows the different concentrations of prepared samples. Absorption spectra of all samples were measured at room temperature. at 350, 400,450 and 500 nm.
Characterization of carbon nanostructures (CNSs) and optic sensing
The type of the carbon Nano structure was analyzed by X-ray powder diffraction (XRD) using a BENCHTOP PROTO AXRD diffractometer in the range 2θ:10–80°(step: 0.1°) and CuKα1Source (λ = 1,54 Å). Fourier transform infrared spectra were obtained on a SHIMADZU 8400 s (FT-IR) spectrometer whose extent is between 400 and 4000 cm−1. UV/visible absorption spectra were recorded with a UV/VIS 6305 spectrophotometer (JENWAY Company).
Results and Discussion
Characterization of CNSs by XRD
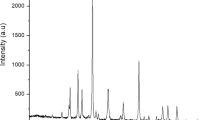
Figure 1 shows the X-ray diffraction pattern of Carbon-Nanostructures (CNSs) produced by one-step thermal carbonization. For carbon nanodots (CNDs), a non-relief reflection band centered on 2θ = 21.68° corresponds the (002) lattice spacing of carbon-based materials with amorphous nature17 or shows a shift down; what is indicates an increase in sp2 layer spacing18.
Crystal planes and a small broad peak to about 2θ = 44.22° and 77.5° correspond to the set (100) and (110) reflections19,20. The spacing between the layers was calculated by applying the Bragg equation and found at approximately 4.23 Å. As long as the average crystallite size, Lc, can be determined using the Scherrer equation:
or:
λ: the wavelength of X-rays (1.54 Å),
β: the width at half height (in radians),
θ: the diffusion angle
and K is the Scherrer constant (0.9)21.
The Lc has been estimated at 7.0 Å.
Infrared spectroscopy FTIR
As shown in Fig. 2, the existence of carbonyl (C=O) causes the peak of about 1696 cm−1. The presence of oxygen-containing carbon structures has been confirmed. The peak at 1528 cm−1 can be attributed to the C=C stretching vibrations. The δ (C=O) vibration band is found at approximately 680 cm−1 22,23. The bands at 1900, 2098.172 and 2334.892 cm−1 can been attributed to inorganic ʋ3CO3, manganese carbonyl stretching frequency and water molecule under strongly hydrogen-bonded conditions24,25,26.
In Fig. 3a it is possible to observe Rhoud El-baguel crude oil optical properties. It appears that the crude concentration varies proportionally with the optical density (OD) in all the domain of UV-Vis. After adding nano carbon, the concentration 0.4 ml/l shows the best OD (Fig. 3b) throughout the UV-Vis range.
The analysis also shows that the samples have a better optical density for a minimum value of wavelength (350 nm) and this for concentrations less than or equal to 0.4 ml/l. While it is less intense for other wavelengths throughout the UV-Vis domain (Fig. 4a). For comparison, the OD increases perfectly according to each concentration and in the whole area at 400 nm (Fig. 4b).
In order to determine clearly the effect of carbon nanostructures on the optical properties of the oil sample, we study the optical density changes in terms of concentration of samples under a constant wave length of 400 nm before and after adding an amount of CNSs powder (Fig. 5).
And we study the optical density changes in terms of UV-Vis wavelengths for 0.4 ml/l concentration of sample before and after adding an amount of CNSs powder (Fig. 6). It is clear that the optical density increases strongly by adding nanocarbon.
Conclusion
Carbon Nanostructures (CNSs) can be synthetized simply with an ash of palm cellulose available locally and maybe used as a very effective tool for sensing and estimating the quality of crude oil and comparing between them.
References
Steffens, J., Landulfo, E., Courrol, L. C. & Guardani, R. Application of fluorescence to the study of crude petroleum. Journal of fluorescence 21(3), 859–864, https://doi.org/10.1007/s10895-009-0586-4 (2011).
Ellingsen, G. & Fery-Forgues, S. Application de la spectroscopie de fluorescence à l'étude du pétrole: le défi de la complexité. Revue de l’Institut Français du Pétrole 53(2), 201–216, https://doi.org/10.2516/ogst:1998019 (1998).
Taylor, T. A. & Patterson, H. H. Excitation resolved synchronous fluorescence analysis of aromatic compounds and fuel oil. Analytical chemistry 59(17), 2180–2187, https://doi.org/10.1021/ac00144a037 (1987).
Ahmed, G. H. G., Laíño, R. B., Calzón, J. A. G. & García, M. E. D. Facile synthesis of water-soluble carbon nano-onions under alkaline conditions. Beilstein journal of nanotechnology 7(1), 758–766, https://doi.org/10.3762/bjnano.7.67 (2016).
Wang, C. et al. Hierarchical CuCo2O4 nickel-cobalt hydroxides core/shell nanoarchitectures for high-performance hybrid supercapacitors. Science Bulletin 62(16), 1122–1131, https://doi.org/10.1016/j.scib.2017.08.014 (2017).
He, W., et al Supercapacitors: Ultrathin and Porous Ni3S2/CoNi2S4 3D‐Network Structure for Superhigh Energy Density Asymmetric Supercapacitors (Adv. Energy Mater. 21/2017). Advanced Energy Materials 7(21), https://doi.org/10.1002/aenm.201770117 (2017).
Wang, C., Sun, P., Qu, G., Yin, J. & Xu, X. Nickel/cobalt based materials for supercapacitors. Chinese Chemical Letters 29(12), 1731–1740, https://doi.org/10.1016/j.cclet.2018.12.005 (2018).
Li, H., Kang, Z., Liu, Y. & Lee, S. T. Carbon nanodots: synthesis, properties and applications. Journal of materials chemistry 22(46), 24230–24253, https://doi.org/10.1039/C2JM34690G (2012).
Gao, F. et al. Rational design of high quality citric acid-derived carbon dots by selecting efficient chemical structure motifs. Carbon 112, 131–141, https://doi.org/10.1016/j.carbon.2016.10.089 (2017).
Kelly, J. J., Barlow, C. H., Jinguji, T. M. & Callis, J. B. Prediction of gasoline octane numbers from near-infrared spectral features in the range 660-1215 nm. Analytical Chemistry 61(4), 313–320, https://doi.org/10.1021/ac00179a007 (1989).
Klonoff, D. C. Overview of fluorescence glucose sensing: a technology with a bright future. Journal of Diabetes Science and Technology 6(6), 1242–1250, https://doi.org/10.1177/193229681200600602 (2012).
Ma, N., Liu, D., Liu, Y. & Sui, G. Extraction and characterization of nanocellulose from Xanthoceras Sorbifolia Husks. Int J Nanosci Nanoeng, 2(6), 43–50, http://www.openscienceonline.com/journal/archive2?journalId=731&paperId=2509 (2015).
Manoj, B., Ashlin, M. R. & Thomas, G. C. Tailoring of low grade coal to fluorescent nanocarbon structures and their potential as a glucose sensor. Scientific reports 8.1, 13891, https://doi.org/10.1038/s41598-018-32371-9 (2018).
Gerrard, D. L. & Maddams, W. F. Solvent effects in uv absorption spectra. I. Phenol in cyclohexane ethanol mixtures. Spectrochimica Acta Part A: Molecular Spectroscopy 34.12, 1205–1211, https://doi.org/10.1016/0584-8539(78)80081-6 (1978).
Pickett, L. W., Margaret Muntz & McPherson, E. M. Vacuum Ultraviolet Absorption Spectra of Cyclic Compounds. I. Cyclohexane, Cyclohexene, Cyclopentane, Cyclopentene and Benzene1. Journal of the American Chemical Society 73.10, 4862–4865, https://doi.org/10.1021/ja01154a116 (1951).
Bourlinos, A. B. et al. Gd(III)-doped carbon dots as a dual fluorescent-MRI probe. J Mater Chem. 22(44), 23327–23330, https://doi.org/10.1039/C2JM35592B (2012).
Zhang, H. B., Lin, G. D., Zhou, Z. H., Dong, X. & Chen, T. Raman spectra of MWCNTs and MWCNT-based H2-adsorbing system. Carbon 40(13), 2429–2436, https://doi.org/10.1016/S0008-6223(02)00148-3 (2002).
Suárez-Garcıa, F., Martınez-Alonso, A. & Díez Tascón, J. M. Pyrolysis of apple pulp: chemical activation with phosphoric acid. Journal of Analytical and Applied Pyrolysis 63.2, 283–301, https://doi.org/10.1016/S0165-2370(01)00160-7 (2002).
Vinod Gupta and Tawfik A. Saleh (July 20th 2011). Syntheses of Carbon Nanotube-Metal Oxides Composites; Adsorption and Photo-degradation, Carbon Nanotubes - From Research to Applications, Stefano Bianco, IntechOpen. Availablefrom, https://www.intechopen.com/books/carbon-nanotubes-from-research-to-applications/syntheses-of-carbon-nanotube-metal-oxides-composites-adsorption-and-photo-degradation, https://doi.org/10.5772/18009.
Smilgies, D.-M. Scherrer grain-size analysis adapted to grazing-incidence scattering with area detectors. Journal of applied crystallography 42.6, 1030–1034, https://doi.org/10.1107/S0021889809040126 (2009).
Dhenadhayalan, N. & Lin, K. C. Chemically induced fluorescence switching of carbon-dots and its multiple logic gate implementation. Sci Rep. 5, 10012, https://doi.org/10.1038/srep10012 (2015).
Moon, B. J. et al. Facile and purification-free synthesis of nitrogenated amphiphilic graphitic carbon dots. Chem. Mater. 28(5), 1481–1488, https://doi.org/10.1021/acs.chemmater.5b04915 (2016).
Glišić, S., Nikolić, G., Cakić, M. & Trutić, N. Spectroscopic study of copper (II) complexes with carboxymethyl dextran and dextran sulfate. Russian Journal of Physical Chemistry A 89(7), 1254–1262, https://doi.org/10.1038/srep10012 (2015).
Brangule, A. & Gross, K. A. Importance of FTIR spectra deconvolution for the analysis of amorphous calcium phosphates. In IOP Conference Series: Materials Science and Engineering (Vol. 77, No. 1, p. 012027). IOP Publishing, https://doi.org/10.1088/1757-899x/77/1/012027 (2015).
Orgel, L. E. The Infrared Spectra of Substituted Metal Carbonyls. Inorganic Chemistry 1(1), 25–29, https://doi.org/10.1021/ic50001a007 (1962).
Furutani, Y., Terakita, A., Shichida, Y. & Kandori, H. FTIR studies of the photoactivation processes in squid retinochrome. Biochemistry 44(22), 7988–7997, https://doi.org/10.1021/bi050219w (2005).
Acknowledgements
This paper is supported by the PRFU project of Algerian ministry of high education and scientific research under the ID number A16N01UN390120180002.
Author information
Authors and Affiliations
Contributions
1 Chouaib Fethiza Tedjani: The first author and he is the PhD student who has done laboratory work and getting results. 2- Dr. Omar Ben Mya: The second author, the supervisor and he is the author of the manuscript (the corresponding author). 3- Dr. Abdelkrim Rebiai: The third author, cosupervisor and who assist the student in his work. 4- Abdelhamid Khachkhouche: Master’s student that assist first author in experimental part. 5- Abdelhakim Dehbi: Master’s student that assist first author in experimental part. 6- Nacereddine Méchara: Master’s student that assist first author in experimental part.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fethiza Tedjani, C., Ben Mya, O., Rebiai, A. et al. Crude Oil Sensing using Carbon Nano Structures Synthetized from Phoenix Dactylifera L. Cellulose. Sci Rep 9, 17806 (2019). https://doi.org/10.1038/s41598-019-54417-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-54417-2
This article is cited by
-
Effect of precursors on structural, optical and surface properties of ZnO thin film prepared by spray pyrolysis method: efficient removal of Cu (II) from wastewater
Transition Metal Chemistry (2024)
-
Extraction of pure cellulose from palm residues using alkaline treatment method and its performance in PVC polymer matrix composite
Biomass Conversion and Biorefinery (2023)
-
Experience of virtual commissioning of a process control system for the production of high-paraffin oil
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.