Abstract
Real-time monitoring of combustion products and composition is critical to emission reduction and efficient energy production. The fuel efficiency in power plants and automobile engines can be dramatically improved by monitoring and controlling the combustion environment. However, the development of novel materials for survivability of oxygen sensors at extreme environments and demonstrated rapid response in chemical sensing is a major hindrance for further development in the field. Gallium oxide (Ga2O3), one among the wide band gap oxides, exhibit promising oxygen sensing properties in terms of reproducibility and long term stability. However, the oxygen sensors based on β-Ga2O3 and other existing materials lack in response time and stability at elevated temperatures. In this context, we demonstrate an approach to design materials based on Ti-doped Ga2O3, which exhibits a rapid response and excellent stability for oxygen sensing at elevated temperatures. We demonstrate that the nanocrystalline β-Ga2O3 films with 5% Ti significantly improves the response time (~20 times) while retaining the stability and repeatability in addition to enhancement in the sensitivity to oxygen. These extreme environment oxygen sensors with a rapid response time and sensitivity represent key advancement for integration into combustion systems for efficient energy conversion and emission reduction.
Similar content being viewed by others
Introduction
Recent attention to climate change and global warming has directed enormous global interest in reducing carbon dioxide (CO2) emissions to the environment. The major contributor to CO2 emission from human activities is from the energy conversion, especially the fossil fuel combustion for electricity, heat and transportation1,2. The strict regulation and mandates of governments to protect environments has also motivated the energy and transportation sectors to research in emission reduction and environmental protection. While transportation industry is trying to meet these stringent CO2 emission standards by reducing vehicle weight3,4,5, extensive research and development of new materials is needed to meet the weight reduction which is highly expensive and only a long-term solution. However, the emissions from fossil fuel combustion can be greatly reduced in both energy and transportation industry if internal engine combustion conditions are efficiently controlled. The optimization of combustion environment requires a reliable and rapid response chemical sensor to monitor the partial pressure of oxygen (pO2) during combustion. Hence, in recent years, the demand of fast and reliable oxygen sensing is rising exponentially6,7. No doubt that the earlier investigations resulted in a great success in fundamental understanding and development of commercialized thin film sensors based on metal oxides, such as SnO2 for domestic gas leak alarms, solid-electrolyte ZrO2 sensors for detecting oxygen concentration in automobile exhaust system, and TiO2-based lambda lean burn sensors8,9. However, addressing the poor stability, reliability, and slow response still remains a technological challenge. Wide band gap β-Ga2O3 has shown promise as a stable extreme environment chemical sensor10,11,12 due to excellent long term thermal stability (melting point ~1780 °C) against interfering gases and humidity13. Due to the high thermal stability of β-Ga2O3, the upper limit for operating temperature is ~1000 °C6. β-Ga2O3 is an n-type semiconductor at elevated temperatures and its conductivity is based on an oxygen deficiency of the crystal lattice. The resulting oxygen vacancies are ionized to form donors14. The material exhibits the same charge carrier mobility in the monocrystalline and in the polycrystalline state which means electron mobility is independent of grain boundaries14. It is also found that the sensors based on β-Ga2O3 films show high reproducibility and stability in the gas-sensitive electrical properties6. Also there has been some reports of β-Ga2O3 thin films operating as surface-control type sensors when exposed to a reducing gas below 900 °C15 and bulk type sensor when exposed to an oxygen above 900 °C16. Despite the advantages, response time (>1 min) for β-Ga2O3 based oxygen sensors (~60–200 s) are still inept for controlling efficient combustion17,18. In this context, in the present work, we demonstrate an approach based on selective doping metal ions into β-Ga2O3 to realize reliable and rapid response high-temperature oxygen sensors for operation in harsh environments.
As demonstrated and reported previously11,19,20,21,22,23,24, doping with carefully chosen metal ions can significantly alter the properties and electrical response of β-Ga2O3, making it suitable for high temperature chemical sensing applications. From an optical properties point of view, doping β-Ga2O3 with Sn, Cr, Cu, Ti, Mo, In, Fe, or W has been reported to induce changes in the optical absorption and band gap11,19,20,21,22,23,24. In literature, there has been some work in improving gas sensing performance of β-Ga2O3 by doping Ce, Sb, W and Zn18. The advantage of using dopants like Ce4+, Sb5+, W6+ is due to similar ionic radii which might allow the substitutional lattice sites resulting in lower sensor resistivity. However, the fastest response from these dopants was still ~1 min (60 s)18, which is unacceptable in managing combustion process efficient. Here in this paper, we report on an innovative approach to tailor the functionality of β-Ga2O3 by doping Ti to dramatically improve the response time of the oxygen sensor while retaining the thermal stability and reliability of β-Ga2O3 for high-temperature sensor applications. In the previous work, we report on the direct, functional relationship between the structural and optical properties of titanium (Ti)-incorporated Ga2O3 (GTO), where tailor-made materials for optical and photocatalytic applications are readily possible19. The hypothesis postulated based on the outcomes is that combining advantages of Ga- and Ti- based oxides through a selective doping of Ti into β-Ga2O3 and/or forming a Ga-Ti-O composite can enhance the sensor performance. The impetus is derived from the following considerations. Titanium, as a dopant, is attracted to oxygen and excess Ti will result in TiO2 phase. TiO2, a wide band gap semiconductor, has also been intensively studied as a key material for fundamental research and numerous technological applications because of its stability, non-toxicity, high abundance and low cost25. Furthermore, similar to β-Ga2O3, TiO2 is also an n-type semiconductor with a potential as a sensor26,27,28. It is noted that oxygen vacancies in transition metal oxides such as TiO2 are much more mobile than cations29, and it is reasonable to consider the movement of oxygen ions (or oxygen vacancies) as opposed to cations30. In addition, Shannon ionic radii31 of Ti4+ ionic radii in both octahedral and tetrahedral coordination closely matches with that of Ga3+. Hence, we propose and demonstrate designing more efficient materials for high-temperature oxygen sensing in harsh environments is readily possible by selectively doping Ti into β-Ga2O3.
Results and Discussion
Sensor performance and mechanism
The temperature dependent electrical data, which serves the baseline characteristics for sensor applications, of GTO samples are shown in Fig. 1. It is generally accepted that barrier formation between grains is responsible for the sensor conductivity and that these barriers have a Schottky-type nature32. Therefore, as a first step, determination of activation energy is quite important for utilization of GTO for sensor applications. The temperature-dependent electrical data (Fig. 1a; Arrhenius plots) of GTO samples were analyzed to obtain the activation energy of the films using the relation:
where R∞ is the resistance at infinite temperature, EA is the activation energy, k is the Boltzmann constant, T is the absolute temperature, and R is the resistance. The EA values determined are presented in Fig. 1b, where the effect of Ti content on the EA values is shown. It can be seen that the EA continuously decrease with increasing Ti in Ga2O3. This observation is imperative since the EA reduction is expected to contribute the enhanced sensitivity. The EA value for intrinsic Ga2O3 films was 1.02 eV, which reduces to a final value of 0.62 eV when the Ti concentration is ~5 at%. Significant reduction in EA is an indication that thermal energy required for oxygen detection becomes smaller by the incorporation of Ti-ions into Ga2O3.
The oxygen sensor response data of GTO films are shown in Fig. 2. The performance characteristics were evaluated at 700 °C in terms of initial sensitivity and response time. The data shown are obtained in the experiments conducted under different pO2 (8–40 Pa) in cyclic periods of pure Ar flow for a constant time followed by Ar/O2 flow for the same time period. The repeatability and stability of the sensors was evaluated (Fig. 2) at 700 °C for several hours as a function of pO2. All of the GTO films demonstrated the stability and repeatability for the extended duration of the tests (≥100 h). No discontinuity or breakdown was observed over the range of pO2 examined. Intrinsic Ga2O3 response also presented changes in the resistance value under Ar exposure, corresponding the polarization effects caused by the ionic conductivity of the films, and the constant increment on the base resistance is attributed to the grain boundary capacitance of the films33. As shown Fig. 2, it can be observed that GTO-0, GTO-20, and GTO-40 responded by the increase in resistance when oxygen is introduced. This response of pure Ga2O3 has been previously observed in the literature17. However, the changes in response behavior becomes evident when the Ti content becomes appreciable. For Ti a concentration of ≥1.5 at%, the sensor response to oxygen flips and resistance now decreases after oxygen is introduced and vice-versa. This remarkable behavior observed for GTO films under pO2 is totally different and has not been achieved or reported earlier for intrinsic Ga2O3. Also, the changes are rapid; the response behavior in GTO films is rather instantaneous and occur rapidly upon oxygen release into the argon stream. However, when the oxygen was cut off, the reversal to original resistance takes bit of a longer time. This phenomenon confirms that out-diffusion of oxygen is relatively slow. To ensure reproducibility, interval between successive measurements is set to ~10 min.
The response time GTO sensors are determined, using the standard procedures, from the sensor characteristics at various pO2 (Fig. 2). The response time for intrinsic Ga2O3 (GTO-0) is 80 ± 10 s, whereas for the films deposited with 5 at% of Ti-doped Ga2O3 exhibits a rapid response time of only 4 ± 1 s. This is a remarkable result and demonstrates the effect of Ti into Ga2O3 to promote oxygen sensor performance. The sensor response data is presented in Fig. 3a, where the results indicate that increasing Ti content in Ga2O3 decreases the response time i.e., improves the sensor performance. The results indicate that the 5 at% Ti doped Ga2O3 is nearly ~20 times faster as compared to intrinsic Ga2O3. Note that the response time can be influenced by the surface reactions rate or/and the diffusion rate. When pO2 is increased, the oxygen molecules adsorbed at the surface split and occupy the vacancies near the surface. A diffusion process of oxygen inside the film, across the grain boundaries, will reestablish equilibrium with the gas phase15.
The materials that respond to change in pO2 in the upper-temperature range (700 °C and above) are reflecting the equilibria between the atmosphere and their bulk stoichiometry. In general, the relationship between oxygen partial pressure and the electrical conductivity of the mixed valence oxide sensor may be represented34 by:
where A is a constant, pO2 is the partial pressure of oxygen, and m is a parameter determined by the carriers (n or p type) and defects in the oxide34,35 while other parameters were defined already in Eq. (1) The value of |m| is the sensitivity of the sensor; higher the |m| value, higher the sensitivity and vice versa35. The electrical data at various pO2 were used to calculate the m-values, which are presented in Fig. 3b. In intrinsic Ga2O3 films (GTO-0), the m-value is −0.8, which is quite higher than theoretical value (at 1000 °C) of −4 as presented in the literature13,17. However, the Ti doping in Ga2O3 shows the reduction of m value up to −6.92 for GTO-100, i.e., Ti concentration of 5 at%. From Eq. 2, it is clear that the lower value of m value corresponds to a higher sensitivity of sensor in terms of its response to changes in pO2. Also, lower value of EA corresponds to lower sensitivity to the temperature fluctuations. The value of m for pure Ga2O3 (GTO-0) and highest Ti concentration of 5 at% (GTO-100) are −0.8 and −6.92, respectively, where lower m value of GTO-100 demonstrates a better oxygen sensitivity. Similarly, GTO-100 samples with lowest EA (0.62 eV) implies precise measurements of pO2 when employed for oxygen sensing.
Structure
In order to better account for the observed sensor performance and to understand the structural changes with Ti, Transmission Electron Microscopy (TEM) analysis has been performed on GTO-100. The data obtained is shown in Fig. 4. The changes in microstructure due to doping is evident in Fig. 4. Bright-field TEM images reveal multiple layers of the samples indicating a Ga-Ti-O nanocomposite on Si. The top two layers are the Pt deposited using electron beam and ion beam subsequently during FIB lift out process (Fig. 4a). The ring patterns from the selected area electron diffraction (SAED) (Fig. 4b) confirms multiple phases in the sample, where the strongest peaks can be indexed to β-Ga2O3 but weaker intensity peaks are probably mixed with TiO2 (anatase). This overlap of multiple rings makes it impossible to confirm TiO2 phases accurately but confirms the crystallinity of the nanocomposite even with highest Ti doping (5% Ti at%) in β-Ga2O3.
Surface and Interface Chemistry
To probe the chemical changes and better understand the dopant Ti chemistry in β-Ga2O3 and identify the TiO2 phase which was inconclusive in TEM, X-ray Absorption Near Edge Structure (XANES) was employed. In XANES, the fine structure observed above the edge, so-called white line, reflects the local site symmetry around the X-ray absorbing atom, the analysis of this region is for the fingerprint of the elements. It is well known that β-Ga2O3 has a spinel structure, containing Ga atoms in both tetrahedral and octahedral sites36,37. The Ga L-edge spectra shown in Fig. 5a is due to excitation of electrons in the 2p3/2 orbital of Ga, leading to sharp increase in absorption of Ga-containing materials at 1116.4 eV38. However, the edge for Ga in Ga2O3 is shifted to 1120 eV due to valence dependency which causes edges to shift39. Therefore, the first peak labeled A (or edge) in Ga L edge spectrum is assigned to Ga atoms in octahedral sites while subsequent peak labeled B is assigned to Ga in tetrahedral sites37. As the Ti concentration is steadily increased the signature peak of tetrahedral Ga atoms is diminished, which is presumably due to the replacement of Ga atoms in tetrahedral sites by Ti atoms.
In Fig. 5b, O K-edge spectra of GTO samples is shown along with the TiO2 standard. The O K-edge spectra for TiO2 standard matches signature features of anatase40 which confirms the TiO2 phase. In anatase (TiO2 standard), first two peaks at low energy (I and II) is due to dipole transitions to band states of t2g-eg symmetry separated by the “ligand-field splitting” of O 2p states hybridized with the Ti 3d states localized at the Ti sites41. The next two peaks (III and IV) in TiO2 standard is due to transitions to oxygen 2p hybridized with Ti 4p states of respective local symmetry b3 and e in their octahedral environment40. The O K-edge XANES spectrum of the Ga2O3 (GTO-0) nanostructure sample is quite different from that of the TiO2 standard. For the GTO-0 sample, XANES spectrum exhibits two main peaks at ~535 eV and ~542 eV (A and B. respectively), similar to that of the β-Ga2O342. Also from the crystal structure, it can be deduced that oxygen also resides in both octahedral and tetrahedral sites. Here, the difference of the O K-edge XANES spectra shown in Fig. 5b between the two samples (TiO2 standard and GTO-0) is the pre-edge feature due to Ti doping after GTO-60. This pre-edge (~530 eV) arise from the existence of an energy state in the band gap. In Fig. 5c, Ti L-edge XANES spectra of GTO samples are shown, but there was no titanium detected by XANES below for GTO-20. For anatase (TiO2) standard and all GTO samples, two sets of peaks are observed which are separated by ~6 eV. This splitting is due to core-hole spin-orbit splitting of the 2p levels41,43. It is believed that the structure of these two sets of peaks is from splitting of the d-derived final states into t2g- and eg-like levels44,45. This two set of the peak is also seen in GTO samples where titanium is above the detection limit in XANES. This XANES study basically suggests Ti doping in β-Ga2O3 substitutes at tetrahedral position but at higher concentration Ti phase separates in TiO2 (anatase) as shown in illustration in Fig. 5d.
The quality and atomic scale defects and clustering were further examined by atom probe tomography (APT) of the sample with highest Ti concentration, i.e., 5 at% (GTO-100). Specifically, APT allows us to verify dopant clustering (if any) at the highest concentration. The APT specimens were lifted out using focused ion beam (FIB) using Omni Probe and mounted on the micro-post for annular milling. The APT reconstructions of GTO-100 specimen are shown in Fig. 6 with Ga atoms in red and Ti in blue. The APT reconstruction shows a 37.78 × 35.98 × 119.1 nm3 volume. From the APT reconstruction, Ti is evenly distributed through the entire film thickness and no noticeable cluster formation of Ti atoms even for the highest Ti concentration. Thus, the XANES and APT data accounts for the structural and chemical quality of the GTO films.
Conclusions
An approach based on selective doping of Ti into β-Ga2O3 is demonstrated to realize reliable and rapid response high-temperature oxygen sensors for utilization in power plants and transportation systems. In Ti doped β-Ga2O3, a rapid response time of ~ 4 s is noted for oxygen sensor operating at 700 °C. This is remarkably high (~20 times) compared to the intrinsic Ga2O3 or existing other oxygen sensors. Ti-doped β-Ga2O3 exhibits lowest activation energy, low m-value, good recovery time, and excellent repeatability. The structural and chemical analyses indicate the role of Ti in β-Ga2O3 to promote the sensor performance. We believe that there may be further options available to further tune and improve the sensor performance while the underlying science and mechanisms may be applicable to a large class of “doped nanostructured oxides for sensor applications.”
Methods
Sample preparation
Gallium oxide and Ti-incorporated Ga2O3 films were deposited onto silicon (Si) and quartz with thin film Pt interdigitated finger electrodes with 100 μm lines substrates by radio-frequency magnetron sputtering. Co-sputtering of the Ga2O3 (99.999%) and Ti (99.95%) targets (2 in. diameter; Plasmaterials Inc.) was performed to produce GTO films. All the substrates were thoroughly cleaned and dried with nitrogen. The Ga2O3-and Ti targets were placed on 2-in. sputter guns, which were placed at 8 cm from the substrate. The base pressure was ~10−6 Torr. A sputtering power of 25 W was initially applied to the targets while introducing high-purity argon (Ar) into the chamber to ignite the plasma. Once the plasma was ignited, the sputtering power was increased to the desired or set values for Ga2O3 and Ti targets, respectively. A constant sputtering power of 100 W was maintained for the Ga2O3 target. The Ti-target sputtering power was varied in the range of 0–100 W to vary the Ti concentration (x) in the films. For clarity purposes, the sample identification is made with the sputtering power employed for deposition. Thus, the samples with variable Ti content are named GTO-0, GTO-20, GTO-40, GTO-60, GTO-80, and GTO-100, respectively, where the end numbers (0–100) represent sputtering power applied to the Ti target. The flows of argon and oxygen were controlled using MKS mass flow meters. Before each deposition, the Ga2O3 target was pre-sputtered for 10 min with the shutter above the gun closed. The samples were deposited at a substrate temperature (Ts) of 500 °C, which is optimum for producing nanocrystalline, β-phase Ga2O3 films19,46. The substrates were heated by resistive heating, and the desired Ts was controlled by an Athena X25 controller. The time of deposition was kept constant at 3 hours. The doping of titanium on gallium oxide is done by substitutional of gallium site with titanium and creates gallium vacancies as shown below by Kroger-Vink notation:
The solubility of titanium in Ga2O3 can be simply explained by the comparing its ionic radii, i.e., Ga3+ has ionic radii of 0.047 nm and 0.062 nm for the coordination number of 4 and 6 while Ti4+ has ionic radii of 0.042 nm and 0.06 nm for the coordination number of 4 and 635,47. They (Ga and Ti) also have similar electronegativity (1.6 Pau and 1.5 Pau respectively)48.
X-ray absorption near edge structure (XANES)
The XANES experiments were carried out at beamline 6.3.1 of Advanced Light Source (ALS) at Lawrence Berkeley National Laboratory (LBNL). Beamline 6.3.1 utilizes a variable line space (VLS) monochromator on a bend magnet source to provide photons with energies from 100 to 2200 eV into a multi-purpose end-station that is like the end-station on Beamline 6.3.249. Reference Ti L-edge, Mg K-edge, and O K-edge spectra were also collected from TiO2 and MgO standards for calibration for different gratings used. Spectra were collected at room temperature in the total electron yield (TEY) mode with the signal obtained from the sample drain current. No substantial charging problems were detected. Ga L-edge, O K-edge, and Ti L-edge were collected for all samples.
Atom probe tomography (APT)
APT samples were also prepared using the FEI Quanta dual beam FIB. The APT specimen preparation method by site-specific FIB lift-out and annular milling50,51. A CAMECA LEAP 4000XHR system equipped with pulsed UV laser (355 nm wavelength) was used to perform APT experiments using 40pJ laser pulse energy at the 100 kHz frequency with specimen temperature of 40 K. The APT results were reconstructed and analyzed using Interactive Visualization and Analysis Software (IVAS) 3.6.8 using standard reconstruction procedure51.
Transmission electron microscopy (TEM)
TEM samples were prepared using lamella lift out procedure using dual beam FIB (FEI Helios) and mounted on copper half grids. The TEM was done using FEI Tecnai TEM using field emission gun operating with an accelerating voltage of 200 kV.
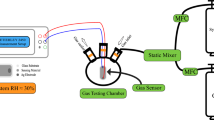
Oxygen sensor testing and evaluation
The sensor performance was evaluated at a temperature of 700 °C; the electrical measurements were recorded using Keithley 6514 electrometer, and the input current (10 nA) was supplied via a Keithley 220 Programmable Current Source. Two gas tanks were employed for this investigation, a 99.99% Ar as the baseline gas, and a 99.99% O2 as the analyte gas; both gasses were controlled using an MKS mass flow controller to achieve different partial pressures of oxygen. All the sensor performance responses were recorded as a function of Ti sputtering power and Ti concentration and feature such sensitivity and time response are presented.
The activation energy was calculated using Eq. 1. The experiments were conducted at different partial pressures. Each partial pressure was subjected to 6 cycles (N = 6) for repeatability. Each and every fitting procedure is carried out until the best fit (R is greater than 0.9 or better) is achieved. The R values were obtained from fitting for each partial pressure along with the standard deviation which was used to calculate the final value of activation energy. The error of fitting is as reflected in the data graphs presented, where the error estimation and statistical standard deviation is also considered.
Data availability
The necessary data required is included in the manuscript. The data available in the literature has been cited as needed and appropriate.
References
Gurney, K. R. et al. High Resolution Fossil Fuel Combustion CO2 Emission Fluxes for the United States. Environmental Science & Technology 43, 5535–5541 (2009).
Rao, A. B. & Rubin, E. S. A Technical, Economic, and Environmental Assessment of Amine-Based CO2 Capture Technology for Power Plant Greenhouse Gas Control. Environmental Science & Technology 36, 4467–4475, https://doi.org/10.1021/es0158861 (2002).
Devaraj, A. et al. A low-cost hierarchical nanostructured beta-titanium alloy with high strength. Nature communications 7 (2016).
Raupach, M. R. et al. Global and regional drivers of accelerating CO2 emissions. Proceedings of the National Academy of Sciences 104, 10288–10293 (2007).
Canadell, J. G. et al. Contributions to accelerating atmospheric CO2 growth from economic activity, carbon intensity, and efficiency of natural sinks. Proceedings of the national academy of sciences 104, 18866–18870 (2007).
Eranna, G., Joshi, B., Runthala, D. & Gupta, R. Oxide materials for development of integrated gas sensors—a comprehensive review. Critical Reviews in Solid State and Materials Sciences 29, 111–188 (2004).
Kiasari, N. M., Soltanian, S., Gholamkhass, B. & Servati, P. Sketching functional, ubiquitous ZnO nano-sensors on paper. RSC Advances 4, 19663–19667 (2014).
Yun, D. H., Kim, D. & Park, C. O. YSZ sensor for lean burn combustion system. Sensors and Actuators B: Chemical 13, 114–116 (1993).
Comini, E. et al. Quasi-One dimensional metal oxide semiconductors: Preparation, characterization and application as chemical sensors. Progress in Materials Science 54, 1–67 (2009).
Rubio, E. et al. Tungsten Incorporation into Gallium Oxide: Crystal Structure, Surface and Interface Chemistry, Thermal Stability, and Interdiffusion. The Journal of Physical Chemistry C 120, 26720–26735 (2016).
Rubio, E. & Ramana, C. Tungsten-incorporation induced red-shift in the bandgap of gallium oxide thin films. Applied Physics Letters 102, 191913 (2013).
Fleischer, M., Höllbauer, L. & Meixner, H. Effect of the sensor structure on the stability of Ga2O3 sensors for reducing gases. Sensors and Actuators B: Chemical 18, 119–124 (1994).
Frank, J., Fleischer, M., Meixner, H. & Feltz, A. Enhancement of sensitivity and conductivity of semiconducting Ga2O3 gas sensors by doping with SnO2. Sensors and Actuators B: Chemical 49, 110–114 (1998).
Frank, J., Fleischer, M. & Meixner, H. Electrical doping of gas-sensitive, semiconducting Ga2O3 thin films. Sensors and Actuators B: Chemical 34, 373–377 (1996).
Fleischer, M. & Meixner, H. Sensing reducing gases at high temperatures using long-term stable Ga2O3 thin films. Sensors and Actuators B: Chemical 6, 257–261 (1992).
Fleischer, M. & Meixner, H. Gallium oxide thin films: a new material for high-temperature oxygen sensors. Sensors and Actuators B: Chemical 4, 437–441 (1991).
Baban, C., Toyoda, Y. & Ogita, M. Oxygen sensing at high temperatures using Ga2O3 films. Thin Solid Films 484, 369–373 (2005).
Li, Y., Trinchi, A., Wlodarski, W., Galatsis, K. & Kalantar-zadeh, K. Investigation of the oxygen gas sensing performance of Ga2O3 thin films with different dopants. Sensors and Actuators B: Chemical 93, 431–434, https://doi.org/10.1016/S0925-4005(03)00171-0 (2003).
Manandhar, S. & Ramana, C. Direct, functional relationship between structural and optical properties in titanium-incorporated gallium oxide nanocrystalline thin films. Applied Physics Letters 110, 061902 (2017).
López, I. et al. Influence of Sn and Cr Doping on Morphology and Luminescence of Thermally Grown Ga2O3 Nanowires. The Journal of Physical Chemistry C 117, 3036–3045 (2013).
Zhang, Y. et al. Optical and structural properties of Cu-doped β- Ga2O3 films. Materials Science and Engineering: B 176, 846–849, https://doi.org/10.1016/j.mseb.2011.04.014 (2011).
Zinkevich, M. et al. Microstructural and thermodynamic study of γ- Ga2O3. Zeitschrift für Metallkunde 95, 756–762 (2004).
Roy, S. & Ramana, C. V. Effect of Thermochemical Synthetic Conditions on the Structure and Dielectric Properties of Ga1.9Fe0.1O3 Compounds. Inorganic Chemistry, https://doi.org/10.1021/acs.inorgchem.7b02363 (2018).
Zavabeti, A. et al. A liquid metal reaction environment for the room-temperature synthesis of atomically thin metal oxides. Science 358, 332–335 (2017).
Chen, N. et al. TiO2 nanoparticles functionalized by Pd nanoparticles for gas-sensing application with enhanced butane response performances. Scientific Reports 7, 7692 (2017).
Wu, H. et al. One-step Synthesis of Ordered Pd@TiO2 Nanofibers Array Film as Outstanding NH3 Gas Sensor at Room Temperature. Scientific Reports 7, 14688 (2017).
Lu, H. F. et al. Amorphous TiO2 nanotube arrays for low-temperature oxygen sensors. Nanotechnology 19, 405504 (2008).
Lin, S., Li, D., Wu, J., Li, X. & Akbar, S. A selective room temperature formaldehyde gas sensor using TiO2 nanotube arrays. Sensors and Actuators B: Chemical 156, 505–509 (2011).
Waser, R. & Aono, M. Nanoionics-based resistive switching memories. Nature Materials 6, 833–840 (2007).
Moon, H. et al. Interfacial chemical bonding-mediated ionic resistive switching. Scientific Reports 7, 1264 (2017).
Shannon, R. D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta. Crystallography A 32, 751–767 (1976).
Schipani, F., Aldao, C. & Ponce, M. Schottky barriers measurements through Arrhenius plots in gas sensors based on semiconductor films. AIP Advances 2, 032138 (2012).
Fleischer, M., Wagner, V., Hacker, G. & Meixner, H. Comparision of ac and dc measurement techniques using semiconducting Ga2O3 sensors. Sensors and Actuators B: Chemical 26, 85–88 (1995).
Moseley, P. Materials selection for semiconductor gas sensors. Sensors and Actuators B: Chemical 6, 149–156 (1992).
Ramamoorthy, R., Dutta, P. & Akbar, S. Oxygen sensors: materials, methods, designs and applications. Journal of materials science 38, 4271–4282 (2003).
Geller, S. Crystal Structure of β‐Ga2O3. The Journal of Chemical Physics 33, 676–684 (1960).
Shimizu, K.-i. et al. Influence of local structure on the catalytic activity of gallium oxide for the selective reduction of NO by CH4. Chemical Communications, 1827–1828 (1996).
Calvin, S. XAFS for Everyone. (CRC press, 2013).
Agarwal, B. & Verma, L. A rule for chemical shifts of X-ray absorption edges. Journal of Physics C: Solid State Physics 3, 535 (1970).
Wu, Z., Ouvrard, G., Gressier, P. & Natoli, C. Ti and OK edges for titanium oxides by multiple scattering calculations: Comparison to XAS and EELS spectra. Physical Review B 55, 10382 (1997).
De Groot, F. et al. Oxygen 1s x-ray-absorption edges of transition-metal oxides. Physical Review B 40, 5715 (1989).
Tourtin, F., Armand, P., Ibanez, A., Tourillon, G. & Philippot, E. Gallium phosphate thin solid films: structural and chemical determination of the oxygen surroundings by XANES and XPS. Thin Solid Films 322, 85–92 (1998).
Van Der Laan, G. Polaronic satellites in x-ray-absorption spectra. Physical Review B 41, 12366 (1990).
Ruus, R. et al. Ti 2p and O 1s X-ray absorption of TiO2 polymorphs. Solid State Communications 104, 199–203 (1997).
Crocombette, J. & Jollet, F. Ti 2p x-ray absorption in titanium dioxides (TiO2): the influence of the cation site environment. Journal of Physics: Condensed Matter 6, 10811 (1994).
Kumar, S. S. et al. Structure, morphology, and optical properties of amorphous and nanocrystalline gallium oxide thin films. The Journal of Physical Chemistry C 117, 4194–4200 (2013).
Shannon, R. & Prewitt, C. Coordination and volume changes accompanying high-pressure phase transformations of oxides. Materials Research Bulletin 4, 57–62 (1969).
Dakhel, A. A. Investigation of opto-dielectric properties of Ti-doped Ga2O3 thin films. Solid State Sciences 20, 54–58, https://doi.org/10.1016/j.solidstatesciences.2013.03.009 (2013).
Underwood, J. H. & Gullikson, E. M. High-resolution, high-flux, user friendly VLS beamline at the ALS for the 50–1300eV energy region. Journal of electron spectroscopy and related phenomena 92, 265–272 (1998).
Thompson, K. et al. In situ site-specific specimen preparation for atom probe tomography. Ultramicroscopy 107, 131–139 (2007).
Larson, D. J., Prosa, T. J., Ulfig, R. M., Geiser, B. P. & Kelly, T. F. Local electrode atom probe tomography. NY: Springer, New York (2013).
Acknowledgements
The authors acknowledge, with pleasure, support from the National Science Foundation (NSF) with grant # ECCS-1509653. The APT characterizations were carried out at the Environmental Molecular Sciences Laboratory (EMSL), a National Scientific User Facility located at the Pacific Northwest National Laboratory (PNNL) and supported by the U.S. DOE Office of Biological and Environmental Research. PNNL is operated by Battelle Memorial Institute for the U.S. DOE under contract DE-AC 06-76RLO 1830. XAFS measurement was carried out at beamline at Advance Light Source (ALS) in Lawrence Berkeley National Laboratory (LBNL). CVR also acknowledges the NSF-PREM grant #DMR-1827745.
Author information
Authors and Affiliations
Contributions
C.V.R. conceived and supervised the research. S.M. and A.K.B. deposited the samples and fabricated sensors. S.M., A.K.B. and C.V.R. characterized the samples. A.D. and S.M. characterized the samples by A.P.T., S.T. and S.M. performed the XANES measurements. CVR coordinated the project and scientific contributions from all the authors. All authors were involved in data analyses, designed figures, and wrote the manuscript. S.M. and C.V.R. coordinated to collect the comments and feedback from all co-authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Manandhar, S., Battu, A.K., Devaraj, A. et al. Rapid Response High Temperature Oxygen Sensor Based on Titanium Doped Gallium Oxide. Sci Rep 10, 178 (2020). https://doi.org/10.1038/s41598-019-54136-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-54136-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.