Abstract
Inflammation in the brain and periphery has been associated with stress-related pathology of mental illness. We have shown that prostaglandin (PG) E2, an arachidonic acid-derived lipid mediator, and innate immune receptors Toll-like receptor (TLR) 2/4 are crucial for repeated stress-induced behavioral changes in rodents. However, how the stress induces PGE2 synthesis in the brain and whether TLR2/4 are involved in the PGE2 synthesis remain unknown. Using mice lacking TLR2 and TLR4 in combination, here we show that social defeat stress (SDS) induced the PGE2 synthesis in subcortical, but not cortical, tissues in a TLR2/4-dependent manner. It is known that PGE2 in the brain is mainly derived by monoacylglycerol lipase (MAGL)-mediated conversion of endocannabinoid 2-arachidonoylglycerol to free-arachidonic acid, a substrate for cyclooxygenase (COX) for PGE2 synthesis. We found that TLR2/4 deletion reduced the mRNA expression of MAGL and COX1 in subcortical tissues after repeated SDS. Perturbation of MAGL and COX1 as well as COX2 abolished SDS-induced PGE2 synthesis in subcortical tissues. Furthermore, systemic administration of JZL184, an MAGL inhibitor, abolished repeated SDS-induced social avoidance. These results suggest that SDS induces PGE2 synthesis in subcortical regions of the brain via the MAGL-COX pathway in a TLR2/4-dependent manner, thereby leading to social avoidance.
Similar content being viewed by others
Introduction
Stress is a strain of physical and psychological functions caused by aversive and demanding events. Whereas stress is thought to induce adaptive biological responses to promote survival and well-being, prolonged or excessive stress may cause depression and elevated anxiety as well as cognitive impairments, and can be a risk factor for mental illnesses1,2,3. To understand the mechanism underlying stress-induced behavioral disturbances, various animal models of repeated stress, such as chronic mild stress and repeated social defeat stress (SDS), have been used1,2. Previous studies using these stress models have shown that repeated stress alters neuronal structures and functions in multiple brain areas along with behavioral disturbances3.
Clinical studies have reported altered levels of inflammation-related molecules including prostaglandin (PG) E2 in blood samples of depressive patients4,5,6,7,8. PGE2 is a bioactive lipid derived from free arachidonic acid by sequential actions of cyclooxygenase (COX) and PGE synthase, and binds to its receptors, EP1, EP2, EP3 and EP4, to exert various physiological and pathophysiological actions9. Notably, meta-analyses have shown that treatment with non-steroidal anti-inflammatory drugs (NSAIDs), especially celecoxib, that inhibit PG synthesis has therapeutic effects in depressive patients10,11,12. These observations suggest a crucial role of PGE2 in the pathology of depression. In parallel, rodent studies using repeated stress models have shown the involvement of PGE2 in repeated stress-induced behavioral changes13,14. For example, our previous study indicated that repeated exposure to SDS attenuates dopaminergic response in the medial prefrontal cortex through PGE receptor EP1, thereby leading to social avoidance13. Consistently, repeated SDS increases the PGE2 content in subcortical tissues of the brains13. Furthermore, genetic deletion or pharmacological inhibition of COX1 abolishes repeated SDS-induced social avoidance13. These findings suggest that the COX1-PGE2-EP1 pathway is crucial for repeated SDS-induced behavioral changes. However, how SDS induces the PGE2 synthesis in the brain remains elusive.
Previous studies using immune cells including microglia have shown that cytosolic phospholipase A2 liberates arachidonic acid from the sn-2 position of membrane phospholipid for COX-dependent PGE2 production15. By contrast, it has recently been shown that a major pool, if not all, of free arachidonic acid for PG production in several organs including the brain is derived from the hydrolysis of endocannabinoid 2-arachinodonoylglycerol (2AG) by monoacylglycerol lipase (MAGL)16. Thus, which enzymatic pathway is used for SDS-induced PGE2 production in the brain remains to be determined.
Previous studies have shown that lipopolysaccharide (LPS), a bacterial endotoxin that activates an innate immune receptor Toll-like receptor (TLR) 4, induces PGE2 synthesis in cultured microglia17,18,19,20. TLRs are composed of several isoforms, each of which recognizes specific molecular patterns of microbes including LPS21. It has recently been suggested that endogenous TLR ligands are released from the cells upon cellular damage and induce inflammation and tissue remodeling without apparent infection22,23. Our recent study has demonstrated a crucial role of TLR2 and TLR4 (TLR2/4) for repeated SDS-induced social avoidance and elevated anxiety24. Furthermore, TLR2/4 mediate repeated SDS-induced microglial activation in the medial prefrontal cortex, which underlies concomitant neuronal and behavioral changes24. Thus, whether and how TLR2/4 are involved in SDS-induced PGE2 synthesis in the brain needs to be investigated.
Using mice lacking TLR2 and TLR4 in combination, here we show a crucial role of TLR2/4 in SDS-induced PGE2 synthesis in the subcortical regions of the brain. We also used JZL184, SC-560 and SC-236 that are inhibitors for MAGL, COX1 and COX2, respectively, and obtained the findings suggesting that the MAGL-COX pathway is responsible for SDS-induced subcortical PGE2 synthesis and social avoidance.
Methods
Animals
Tlr2/4 double knockout (TLR-DKO) mice in a C57BL/6N background were purchased from Oriental Bio Service. Ptgs1 (i.e. the gene encoding COX1) knockout mice (COX1-KO) in a C57BL/6N were purchased from Taconic. To make all the mice congenic to the C57BL/6N strain, these mice were backcrossed with C57BL/6N mice for more than 10 generations. Adult male C57BL/6N mice and male ICR mice retired from breeding were purchased from Japan SLC and CLEA Japan, respectively. After arrival, mice were housed in a group of four mice in a specific pathogen-free and temperature- and humidity-controlled vivarium under a 12-h light, 12-h dark cycle (light on between 0800 and 2000 or between 0600 and 1800) with free access to chow and water. All procedures for animal care and use were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committees of Kyoto University Graduate School of Medicine and Kobe University Graduate School of Medicine.
Social defeat stress
Single and repeated exposure to SDS was applied as described previously with minor modifications24. Briefly, male ICR mice were screened based on their aggressiveness to a male C57BL/6N mouse, as measured by the latency and the number of attacks during the observation period (180 s), and were used as aggressor mice for SDS. Before SDS, 7∼12-week-old male mice were isolated with free access to chow and water for 1 week. Each of the isolated mice to be defeated (i.e. intruder mice) was introduced and kept in the home cage of a resident aggressor ICR mouse for 10 min daily for 1 day (single SDS) or 10 consecutive days (repeated SDS). After the 10 min defeat episode, the mice were returned to their home cages and kept isolated until SDS on the next day. The pairs of defeated and aggressor mice were randomized daily to minimize the variability in the aggressiveness of aggressor mice. SDS was applied between 1600 and 1900 h in a sound-attenuated room in dim light. Naïve mice, which did not receive SDS, were placed in a novel cage for 10 min daily (i.e. cage transfer) for 1 day or 10 consecutive days as a control to compare with those which receive single or repeated SDS, respectively. We included all the data for the analyses without any exclusion. After repeated SDS, the social interaction test and the elevated plus maze test were performed. These behavioral tests were performed as previously described24.
Measurement of PGE2 and IL-1β contents using EIA
Measurement of PGE2 contents in brain homogenates by enzyme immunoassay (EIA) was performed as described previously with minor modifications13. Briefly, a mouse was decapitated immediately after SDS or cage transfer, except that the decapitation was performed 24 h after the last session of repeated SDS in Fig. 1. A brain was removed from the mouse and placed in ice-cold Dulbecco’s modified phosphate-buffered saline (D-PBS) with 25 μM indomethacin to prevent artificial PGE2 production during brain processing. The brain was cut at the coronal plane between the olfactory bulb and the cerebral cortex and at the coronal plane between the midbrain and the cerebellum, and the brain tissue between these coronal planes was used for the measurements. In Figs. 1 and 2, the cortical tissue containing the cerebral cortex and the hippocampus and the remaining brain tissue (i.e. subcortical tissue) were separated. All these procedures were completed within 30 s after decapitation. The brain tissues were snap-frozen in liquid nitrogen and kept at −80 °C until use. For EIA, the brain tissues were homogenized in the homogenization buffer (0.1 M sodium phosphate, pH 7.4, containing 1 mM EDTA and 25 μM indomethacin) using a Polytron homogenizer (Kinematica) or Micro Smash (Tomy). The homogenized solution was centrifuged at 20,000 × g for 15 min, and the supernatants were subjected to PGE2 EIA (Cayman Chemicals) according to the manufacturer’s protocol. The precipitations after the centrifugation were resuspended and sonicated in RIPA buffer (25 mM Tris-HCl, pH 7.6, 150 mM NaCl, 1.0% NP-40, 1.0% sodium deoxycholate, 0.1% SDS), and their protein concentrations were determined using BCA assay (Fujifilm Wako Pure Chemical) according to the manufacturer’s protocol. For the measurement of IL-1β contents in brain homogenates, the same supernatant obtained for PGE2 EIA was subjected to IL-1β EIA (eBioscience). The contents of PGE2 and IL-1β were normalized to the protein concentrations.
TLR2/4 are involved in COX-dependent PGE2 synthesis in subcortical tissues. (a) Schedule of experiments in this study. After social isolation for 1 week, a male wild-type or TLR-DKO mouse was subjected to SDS through an encounter with an ICR aggressor mouse for 10 min daily for 1 day (Day 1) or 10 consecutive days (Day 1 through Day 10). The PGE2 measurement was performed immediately after the SDS exposure on Day 1 or Day 10, or at 24 h after the last SDS exposure (i.e. on Day 11). (b,c) The PGE2 contents in subcortical (b) and cortical (c) tissues of wild-type (WT), TLR-DKO and COX1-KO mice without SDS (naïve), immediately after single SDS (S-SDS), and immediately and at 24 h after the last SDS session of repeated SDS (R-SDS). (d,e) The PGE2 contents in subcortical (d) and cortical (e) tissues of wild-type mice administrated with SC-560 and SC-236 that are COX1 and COX2 inhibitors, respectively, as well as vehicle without SDS and immediately after the last session of repeated SDS (R-SDS). *P < 0.05, **P < 0.01 for Bonferroni’s multiple comparisons test. Data are shown as means ± SEM.
TLR2/4 are involved in the expression of COX1 and MAGL after repeated social defeat stress in subcortical tissues. The relative mRNA expression levels of COX1 (a,b), COX2 (c,d) and MAGL (e,f) in subcortical (a,c,e) and cortical (b,d,f) tissues of wild-type (WT) and TLR2/4 double knockout (TLR-DKO) mice with or without repeated SDS (R-SDS). *P < 0.05, **P < 0.01 for Bonferroni’s multiple comparisons test. Data are shown as means ± SEM.
Measurement of PGE2 and free arachidonic acid contents using liquid chromatography-tandem mass spectrometry
Measurement of PGE2 and free arachidonic acid contents by liquid chromatography-tandem mass spectrometry (LC-MS/MS) was performed as previously described with minor modifications25. Briefly, a mouse was decapitated immediately after the last exposure to repeated SDS or cage transfer. The brain tissues were homogenized in 100% methanol using Micro Smash. The homogenized solution was centrifuged at 15,000 × g for 5 min at 4 °C, and the supernatants were added with 500 pg of d4-prostaglandin E2 and d8-5-hydroxyeicosatetraenoic acid (5-HETE) as deuterated internal standards. Then the samples were subjected to solid-phase extraction on C18 columns, and the extracted lipids were detected with a QTRAP 6500 instrument (SCIEX) equipped with an LC-30AD high-performance LC system (Shimadzu). Quantification was performed on the basis of peak areas on the multiple reaction monitoring chromatograph, with linear calibration curves being obtained with authentic standards for each compound.
Quantitative RT-PCR
The cortical and subcortical tissues obtained from the same mice used for PGE2 measurements were used for quantitative RT-PCR. Quantitative RT-PCR was performed as previously described24. Briefly, total RNA was extracted from these tissues using NucleoSpin RNA/Protein Kit (Macherey-Nagel) according to the manufacturer’s protocol. The resultant RNA was used to obtain cDNA using a High Capacity cDNA Reverse Transcription Kit (Life Technologies). All PCR experiments were conducted in duplicate using SYBR Premix Ex Taq (Takara Bio), and fluorescent SYBR Green signals were automatically detected and analyzed with a CFX384 Touch Real-Time PCR Detection System (Bio-Rad). Primers for PCR are as follows: for mouse 18S ribosomal RNA (18S rRNA), 5′-GTA ACC CGT TGA ACC CCA TT-3′ and 5′-CCA TCC AAT CGG TAG TAG CG-3′; for mouse COX1, 5′-CCT CTT TCC AGG AGC TCA CA-3′ and 5′-TCG ATG TCA CCG TAC AGC TC-3′; for mouse COX2, 5′-GGG AGT CTG GAA CAT TGT GAA-3′ and 5′-TGT CAA TCA AAT ATG ATC TGG ATG T-3′; for mouse MAGL, 5′-GGA ACA AGT CGG AGG TTG AC-3′ and 5′-GCA GCT GTA TGC CAA AGC AC-3′. The values were normalized to those of 18S rRNA in the same cDNA samples. The levels were then normalized to those of the control groups.
Drugs
JZL184, an inhibitor for MAGL (Cayman Chemical), was administered to C57BL/6 N mice, as described previously with minor modifications16. To test its effects on the PGE2 contents, JZL184 dissolved in 18:1:1 mixture of saline, ethanol and Cremophor (Nacalai) or vehicle solution without JZL184 was injected intraperitoneally (i.p.) at 40 mg/kg at least 60 min before single exposure to SDS. To test its effects on repeated SDS-induced behavioral changes, JZL184 was administrated 6 h before each exposure to SDS.
SC-560 (Merck) and SC-236 (Cayman Chemical) that are inhibitors for COX1 and COX2, respectively, were administered to C57BL/6N mice, as described previously with minor modifications13. SC-560 and SC-236 dissolved in DMSO or vehicle solution alone were injected i.p. at 5 mg/kg at 30 min before the last exposure to SDS (i.e. 10th SDS).
Statistical analyses
Data are shown as means ± SEM. For comparison between two groups, unpaired Student’s t test was used. For comparison of more than two groups, multiple comparison tests with Bonferroni’s correction were used. The analyses were performed with PRISM 7.0 software (GraphPad). P values less than 0.05 are considered to be significant.
Results
TLR2/4 are crucial for social defeat stress-induced PGE2 synthesis in the brain
To examine whether TLR2/4 are involved in SDS-induced PGE2 synthesis in the brain, we subjected wild-type mice and TLR-DKO mice to repeated SDS (Fig. 3a). An adult male mouse of each genotype (i.e. intruder mouse) was transferred to the home cage of an aggressor ICR mouse for 10 min daily for 10 consecutive days (Fig. 3a). The intruder mouse was immediately attacked by the ICR mouse, and both wild-type and TLR-DKO mice took a submissive posture, a behavioral sign of SDS, in which a mouse stood upright with the abdomen exposed to the ICR mouse, as previously reported24. The brains of the defeated mice were collected immediately after the last SDS session and subjected to PGE2 measurement. SDS increased the PGE2 content in the whole brain, and this increase was significantly reduced in TLR-DKO mice (Fig. 3b). This finding shows a crucial role of TLR2/4 in SDS-induced PGE2 synthesis in the brain. Although we mainly used EIA to measure the PGE2 contents in this study, we confirmed that EIA and LC-MS/MS yield consistent results regarding SDS-induced increase in the PGE2 contents in the brain (see Fig. 1b,c and Supplementary Fig. 1). Since IL-1β has been suggested to be involved in chronic stress-induced depressive-like behavior, we also measured the IL-1β content in the same brain homogenates as those used for the PGE2 measurement. However, since the brains were collected immediately after the last SDS session, neither SDS nor TLR2/4 deficiency altered the IL-1β content in the brain (Fig. 3c).
TLR2/4 are crucial for repeated social defeat stress-induced PGE2 synthesis in the brain. (a) Schedule of experiments in this study. After social isolation for 1 week, a male wild-type or TLR-DKO mouse was subjected to SDS through an encounter with an ICR aggressor mouse for 10 min daily for 10 consecutive days (Day 10). The measurement of PGE2 and IL-1β was performed immediately after the last SDS exposure on Day 10. (b,c) The contents of PGE2 (b) and IL-1β (c) in whole brains of wild-type (WT) and TLR2/4 double knockout (TLR-DKO) mice treated with or without repeated SDS (R-SDS). The contents of PGE2 and IL-1β were normalized to the protein concentrations. *P < 0.05 for Bonferroni’s multiple comparisons test. Data are shown as means ± SEM.
Since we previously reported that SDS-induced inflammatory responses in the medial prefrontal cortex are augmented with repetition of stress, we next compared the PGE2 synthesis upon single vs repeated SDS (Fig. 1a). To examine the PGE2 synthesis between cortical and subcortical regions, we also divided the brains of defeated mice into cortical tissues including the cerebral cortex and the hippocampus and subcortical tissues including the midbrain for the PGE2 measurement. Single exposure to SDS increased the PGE2 content in subcortical tissues (Fig. 1b). This PGE2 increase appeared to be attenuated with repetition of SDS (Fig. 1b), although repeated SDS still significantly increased the PGE2 contents in subcortical tissues (Fig. 1d). TLR2/4 deficiency abolished SDS-induced PGE2 synthesis in subcortical tissues (Fig. 1b). By contrast, neither single nor repeated SDS altered the PGE2 content in cortical tissues of wild-type mice (Fig. 1c). These findings indicate that SDS induces TLR2/4-dependent PGE2 synthesis specifically in subcortical regions. It was noted that the decrease in the PGE2 contents in TLR-DKO mice was not associated with an increase in the contents of free arachidonic acid, a precursor for PGE2 synthesis (Supplementary Fig. 1), suggesting that TLR2/4 may be involved in the supply of free arachidonic acid after repeated SDS.
TLR2/4 is involved in the expression of monoacylglycerol lipase and cyclooxygenase-1 in subcortical tissues after repeated social defeat stress
We previously reported that COX1 is crucial for repeated SDS-induced social avoidance13. It has also been shown that MAGL hydrolyzes 2AG to generate a major pool of free arachidonic acid as a precursor for PG synthesis in the brain16. Thus, we examined whether TLR2/4 affect the mRNA expression of MAGL, COX1 and COX2 with or without repeated SDS. TLR2/4 deficiency reduced the expression of MAGL and COX1 in subcortical tissues, but not in cortical tissues, after repeated SDS (Fig. 2). This reduction was not observed without SDS. The expression of COX2 was not significantly affected by TLR2/4 deficiency. These findings suggest that TLR2/4 are involved in the expression of MAGL and COX1 for SDS-induced PGE2 synthesis in subcortical regions.
Monoacylglycerol lipase and cyclooxygenases are crucial for social defeat stress-induced PGE2 synthesis in the brain
Since TLR2/4 are involved in the COX1 expression after repeated SDS, we examined the PGE2 contents in subcortical and cortical tissues of COX1-KO mice. In COX1-KO mice, the PGE2 content after repeated SDS was reduced to negligible levels in both subcortical and cortical tissues (Fig. 1b,c), indicating the role of COX1 in SDS-induced PGE2 synthesis. To test the roles of COX1 as well as COX2 at the exact time of SDS exposure, we administered SC-560 and SC-236 that are inhibitors for COX1 and COX2, respectively, immediately before the last exposure to repeated SDS, and measured the PGE2 contents immediately after that. Both inhibitors abolished SDS-induced increase in the PGE2 contents in subcortical tissues (Fig. 1d,e), suggesting the involvement of both COX1 and COX2. By contrast, the PGE2 contents in cortical tissues were not significantly affected. We also examined whether MAGL is involved in SDS-induced PGE2 synthesis in the brain. JZL184, an MAGL selective inhibitor, was intraperitoneally administered to mice at least 60 min prior to SDS. Treatment with JZL184 decreased the PGE2 content immediately after SDS in subcortical, but not cortical, tissues (Fig. 4). Collectively, these findings indicate that MAGL and COX are crucial for SDS-induced PGE2 synthesis in subcortical regions.
JZL184, an inhibitor for MAGL, suppresses social defeat stress-induced PGE2 synthesis in subcortical tissues. The PGE2 contents in subcortical (a) and cortical (b) tissues of wild-type mice treated with vehicle or JZL184 (40 mg/kg, i.p.) at least 60 min prior to single SDS. Brains were obtained immediately after single SDS and subjected to the PGE2 measurement. *P < 0.05 for unpaired t test. Data are shown as means ± SEM.
Inhibition of monoacylglycerol lipase abolishes repeated social defeat stress-induced social avoidance
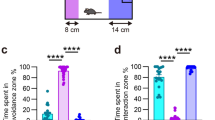
Finally, we examined whether MAGL-mediated PGE2 synthesis is involved in repeated SDS-induced behavioral changes. We systemically administered JZL184, a selective inhibitor for MAGL, or its vehicle throughout the course of repeated SDS (Fig. 5a), and found that the JZL184 administrations abolished repeated SDS-induced social avoidance (Fig. 5b,c). By contrast, the elevated anxiety induced by repeated SDS was spared (Fig. 5d). These findings suggest that MAGL-mediated PGE2 synthesis is involved in a subset of repeated SDS-induced behavioral changes.
JZL184, an inhibitor for MAGL, suppresses social defeat stress-induced social avoidance. (a) Schedule of experiments. After social isolation for 1 week, a male wild-type mouse was subjected to SDS through an encounter with an ICR aggressor mouse for 10 min daily for 10 consecutive days (Day 1 through Day 10). JZL184 or vehicle was administered at 6 h before each SDS exposure. The social interaction test and the elevated anxiety test were performed after repeated SDS to analyze repeated SDS-induced social avoidance and elevated anxiety, respectively. (b,c) The effect of JZL184 on repeated SDS-induced social avoidance. The durations in the interaction (b) or avoidance (c) zone without or with an ICR mouse during the social interaction test were measured for wild-type mice without SDS (naïve) or with repeated SDS (R-SDS) which received i.p. injection of either vehicle or JZL184. (d) The effect of JZL184 on repeated SDS-induced elevated anxiety. The proportions of the time spent in the closed arms, open arms and center zone in the elevated plus maze test were measured for the same mice analyzed for the social interaction test. #P < 0.1, *P < 0.05, **P < 0.01 for Bonferroni’s multiple comparison test. Data are shown as means ± SEM.
Discussion
Whereas PGE2 signaling is crucial for repeated SDS-induced behavioral changes, how SDS increases the PGE2 synthesis in the brain remains unknown. In the present study, SDS increased the PGE2 content in subcortical, but not cortical, tissues in a TLR2/4-dependent manner. Our findings indicate that MAGL and COX mediate SDS-induced PGE2 synthesis in subcortical tissues, and that TLR2/4 is involved in their expression after repeated SDS. Furthermore, pharmacological inhibition of MAGL abolished repeated SDS-induced social avoidance. These findings suggest that the MAGL-COX pathway that is upregulated by TLR2/4 mediates SDS-induced PGE2 synthesis in subcortical regions for the induction of social avoidance (Supplementary Fig. 2).
Since MAGL is crucial for SDS-induced PGE2 synthesis in subcortical tissues, the synthesis of 2AG could be involved. Synaptic/neuronal activity induces 2AG synthesis at postsynaptic sites26, so that the site of 2AG synthesis could determine a brain region in which PGE2 is synthesized. PGE2 attenuates the dopaminergic pathway to the medial prefrontal cortex via EP1 receptor for repeated SDS-induced social avoidance13. We reported that EP1 receptor is located at the GABAergic synapses that are formed on midbrain dopamine neurons and augments inhibitory inputs to these neurons27. Thus, 2AG synthesized from midbrain dopamine neurons or other neurons in the vicinity could be used for MAGL-mediated PGE2 synthesis. It should be noted that MAGL inhibition could also increase the 2AG level and thus CB1 activity, which may contribute to the suppression of repeated SDS-induced social avoidance together with PGE2. Indeed, stressful stimuli induce 2AG synthesis in subcortical regions, such as the amygdala, and attenuates stress-induced behavioral changes via CB1 receptor28,29. The balance between 2AG and PGE2, which is regulated by MAGL activity, could determine stress susceptibility. Whereas our findings favor a role of MAGL in SDS-induced PGE2 synthesis, we cannot exclude a possible role of cytosolic phospholipase A2, which warrants future investigation.
MAGL expression has been reported in various cell types in the brain including neurons, astrocytes and microglia30,31. MAGL in neurons and astrocytes has been shown to be responsible for 2AG hydrolysis in the brain without or with LPS stimulation30. Since COX1 expression is enriched in microglia in the brain13, free arachidonic acid derived from 2AG could be transported from neurons and/or astrocytes to microglia for COX1-mediated PGE2 synthesis. Free arachidonic acid has been shown to be shuttled between different cell types at least in culture30. Alternatively, since COX2 is expressed in amygdala neurons32, COX2 could utilize free arachidonic acid derived from MAGL in the same neurons for the PGE2 synthesis in subcortical regions. Cell type-specific deletion of MAGL and COX is useful to address which cell types are involved in the MAGL-COX pathway upon SDS exposure.
Since both SC-560 and SC-236 abolished SDS-induced PGE2 synthesis in subcortical tissues, both COX1 and COX2 appear to be involved. Which COX isoform directly mediates this PGE2 synthesis remains to be determined. By contrast, we reported that SC-560, but not SC-236, at the same doses abolished repeated SDS-induced social avoidance13. Thus, PGE2 synthesis could cooperate with other bioactive lipids derived from COX1 for the behavioral change. A lipidomics approach is useful for comprehensive analysis of bioactive lipids involved in repeated SDS-induced behavioral changes.
Our findings indicate a role of TLR2/4 in the MAGL-COX pathway for SDS-induced PGE2 synthesis. Consistently, free arachidonic acid derived from 2AG by MAGL is used as a precursor for the PGE2 synthesis in the brain induced by LPS, a TLR4 agonist16. Previous studies using animal models of chronic inflammation have identified several candidates for endogenous TLR2/4 ligands24,33,34. Since single and repeated SDS increased PGE2 synthesis immediately in subcortical tissues, endogenous TLR2/4 ligand(s) released upon SDS could immediately stimulate PGE2 synthesis. In addition, TLR2/4 are involved in the mRNA expression of MAGL and COX1 in subcortical regions after repeated SDS. Thus, TLR2/4-induced transcriptional activation could also increase MAGL-mediated hydrolysis of 2AG to free arachidonic acid and subsequent COX1-mediated PGE2 synthesis.
In conclusion, the present study showed crucial roles of TLR2/4, MAGL and COX in SDS-induced PGE2 synthesis and their behavioral relevance using knockout mice and selective inhibitors. To elucidate roles and actions of these molecules in SDS-induced neural and behavioral changes paves the way for understanding the stress-related pathology of mental illnesses and promoting therapeutic development for mental illnesses.
Data availability
The datasets generated and/or analyzed during the present study are available from the corresponding author upon reasonable request.
References
Duman, R. S. & Aghajanian, G. K. Synaptic dysfunction in depression: potential therapeutic targets. Science 338, 68–72, https://doi.org/10.1126/science.1222939 (2012).
Krishnan, V. & Nestler, E. J. The molecular neurobiology of depression. Nature 455, 894–902, https://doi.org/10.1038/nature07455 (2008).
McEwen, B. S. & Morrison, J. H. The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron 79, 16–29, https://doi.org/10.1016/j.neuron.2013.06.028 (2013).
Schiepers, O. J., Wichers, M. C. & Maes, M. Cytokines and major depression. Prog Neuropsychopharmacol Biol Psychiatry 29, 201–217, https://doi.org/10.1016/j.pnpbp.2004.11.003 (2005).
Berthold-Losleben, M. & Himmerich, H. The TNF-alpha system: functional aspects in depression, narcolepsy and psychopharmacology. Curr Neuropharmacol 6, 193–202, https://doi.org/10.2174/157015908785777238 (2008).
Hodes, G. E., Ménard, C. & Russo, S. J. Integrating Interleukin-6 into depression diagnosis and treatment. Neurobiol Stress 4, 15–22, https://doi.org/10.1016/j.ynstr.2016.03.003 (2016).
Ohishi, K., Ueno, R., Nishino, S., Sakai, T. & Hayaishi, O. Increased level of salivary prostaglandins in patients with major depression. Biol Psychiatry 23, 326–334 (1988).
Wohleb, E. S., Franklin, T., Iwata, M. & Duman, R. S. Integrating neuroimmune systems in the neurobiology of depression. Nat Rev Neurosci 17, 497–511, https://doi.org/10.1038/nrn.2016.69 (2016).
Narumiya, S. Physiology and pathophysiology of prostanoid receptors. Proc Jpn Acad Ser B Phys Biol Sci 83, 296–319, https://doi.org/10.2183/pjab/83.296 (2007).
Mendlewicz, J. et al. Shortened onset of action of antidepressants in major depression using acetylsalicylic acid augmentation: a pilot open-label study. Int Clin Psychopharmacol 21, 227–231 (2006).
Müller, N. et al. The cyclooxygenase-2 inhibitor celecoxib has therapeutic effects in major depression: results of a double-blind, randomized, placebo controlled, add-on pilot study to reboxetine. Mol Psychiatry 11, 680–684, https://doi.org/10.1038/sj.mp.4001805 (2006).
Akhondzadeh, S. et al. Clinical trial of adjunctive celecoxib treatment in patients with major depression: a double blind and placebo controlled trial. Depress Anxiety 26, 607–611, https://doi.org/10.1002/da.20589 (2009).
Tanaka, K. et al. Prostaglandin E2-mediated attenuation of mesocortical dopaminergic pathway is critical for susceptibility to repeated social defeat stress in mice. J Neurosci 32, 4319–4329, https://doi.org/10.1523/JNEUROSCI.5952-11.2012 (2012).
Guevara, C., Fernandez, A. C., Cardenas, R. & Suarez-Roca, H. Reduction of spinal PGE2 concentrations prevents swim stress-induced thermal hyperalgesia. Neurosci Lett 591, 110–114, https://doi.org/10.1016/j.neulet.2015.02.035 (2015).
Shimizu, T. Lipid mediators in health and disease: enzymes and receptors as therapeutic targets for the regulation of immunity and inflammation. Annu Rev Pharmacol Toxicol 49, 123–150, https://doi.org/10.1146/annurev.pharmtox.011008.145616 (2009).
Nomura, D. K. et al. Endocannabinoid hydrolysis generates brain prostaglandins that promote neuroinflammation. Science 334, 809–813, https://doi.org/10.1126/science.1209200 (2011).
Wu, Y., Zhong, L., Yu, Z. & Qi, J. Anti-neuroinflammatory effects of tannic acid against lipopolysaccharide-induced BV2 microglial cells via inhibition of NF-κB activation. Drug Dev Res, https://doi.org/10.1002/ddr.21490 (2019).
Yu, Z. et al. Capillarisin Suppresses Lipopolysaccharide-Induced Inflammatory Mediators in BV2 Microglial Cells by Suppressing TLR4-Mediated NF-κB and MAPKs Signaling Pathway. Neurochem Res 40, 1095–1101, https://doi.org/10.1007/s11064-015-1567-4 (2015).
Wang, H. Y. et al. Protocatechuic Acid Inhibits Inflammatory Responses in LPS-Stimulated BV2 Microglia via NF-κB and MAPKs Signaling Pathways. Neurochem Res 40, 1655–1660, https://doi.org/10.1007/s11064-015-1646-6 (2015).
Matsui, T. et al. Release of prostaglandin E(2) and nitric oxide from spinal microglia is dependent on activation of p38 mitogen-activated protein kinase. Anesth Analg 111, 554–560, https://doi.org/10.1213/ANE.0b013e3181e3a2a2 (2010).
Akira, S. Toll-like receptor signaling. J Biol Chem 278, 38105–38108, https://doi.org/10.1074/jbc.R300028200 (2003).
Rivest, S. Regulation of innate immune responses in the brain. Nat Rev Immunol 9, 429–439, https://doi.org/10.1038/nri2565 (2009).
Iwasaki, A. & Medzhitov, R. Regulation of adaptive immunity by the innate immune system. Science 327, 291–295, https://doi.org/10.1126/science.1183021 (2010).
Nie, X. et al. The Innate Immune Receptors TLR2/4 Mediate Repeated Social Defeat Stress-Induced Social Avoidance through Prefrontal Microglial Activation. Neuron 99, 464–479.e467, https://doi.org/10.1016/j.neuron.2018.06.035 (2018).
Colas, R. A., Shinohara, M., Dalli, J., Chiang, N. & Serhan, C. N. Identification and signature profiles for pro-resolving and inflammatory lipid mediators in human tissue. Am J Physiol Cell Physiol 307, C39–54, https://doi.org/10.1152/ajpcell.00024.2014 (2014).
Castillo, P. E., Younts, T. J., Chávez, A. E. & Hashimotodani, Y. Endocannabinoid signaling and synaptic function. Neuron 76, 70–81, https://doi.org/10.1016/j.neuron.2012.09.020 (2012).
Furuyashiki, T. & Narumiya, S. Stress responses: the contribution of prostaglandin E(2) and its receptors. Nat Rev Endocrinol 7, 163–175, https://doi.org/10.1038/nrendo.2010.194 (2011).
Shen, C. J. et al. Cannabinoid CB. Nat Med 25, 337–349, https://doi.org/10.1038/s41591-018-0299-9 (2019).
Bluett, R. J. et al. Endocannabinoid signalling modulates susceptibility to traumatic stress exposure. Nat Commun 8, 14782, https://doi.org/10.1038/ncomms14782 (2017).
Viader, A. et al. Metabolic Interplay between Astrocytes and Neurons Regulates Endocannabinoid Action. Cell Rep 12, 798–808, https://doi.org/10.1016/j.celrep.2015.06.075 (2015).
Kouchi, Z. Monoacylglycerol lipase promotes Fcγ receptor-mediated phagocytosis in microglia but does not regulate LPS-induced upregulation of inflammatory cytokines. Biochem Biophys Res Commun 464, 603–610, https://doi.org/10.1016/j.bbrc.2015.07.019 (2015).
Yamagata, K., Andreasson, K. I., Kaufmann, W. E., Barnes, C. A. & Worley, P. F. Expression of a mitogen-inducible cyclooxygenase in brain neurons: regulation by synaptic activity and glucocorticoids. Neuron 11, 371–386, https://doi.org/10.1016/0896-6273(93)90192-t (1993).
Krishnan, V. et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 131, 391–404, https://doi.org/10.1016/j.cell.2007.09.018 (2007).
Yu, L., Wang, L. & Chen, S. Endogenous toll-like receptor ligands and their biological significance. J Cell Mol Med 14, 2592–2603, https://doi.org/10.1111/j.1582-4934.2010.01127.x (2010).
Acknowledgements
We thank Atsushi Mizutani, Nodoka Asamoto, Hiroko Iwamura and Tatsuya Yamagiwa for animal care, Tae Arai, Akiko Washimi and Misako Takizawa for secretarial help, and Kimiko Nonomura for technical assistance. This study was supported in part by a CREST grant from AMED (JP18gm0910012 to T.F.), Grants-in-Aids for Scientific Research from the Japan Society for the Promotion of Science (16H05132, 17K19457 to T.F. and 17K08593 to S.K.), Grants-in-Aids for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology in Japan (17H06057, 17H05572, 18H05429 to T.F. and 19H05021 to S.K.), research grants from the Uehara Memorial Foundation (T.F.), the Sumitomo Foundation (T.F.), the Naito Foundation (T.F.), the Astellas Foundation for Research on Metabolic Disorders (T.F.), the Takeda Science Foundation (T.F.), the Ono Medical Research Foundation (T.F.) and the Suzuken Memorial Foundation (T.F.).
Author information
Authors and Affiliations
Contributions
X.N. and T.F. designed the study. X.N., S.K. and M.S. performed the experiments. X.N., S.K., A.K., S.N. and T.F. analyzed and interpreted the results. X.N. and T.F. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nie, X., Kitaoka, S., Shinohara, M. et al. Roles of Toll-like receptor 2/4, monoacylglycerol lipase, and cyclooxygenase in social defeat stress-induced prostaglandin E2 synthesis in the brain and their behavioral relevance. Sci Rep 9, 17548 (2019). https://doi.org/10.1038/s41598-019-54082-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-54082-5
This article is cited by
-
Stress-induced vagal activity influences anxiety-relevant prefrontal and amygdala neuronal oscillations in male mice
Nature Communications (2024)
-
Emphasizing the Crosstalk Between Inflammatory and Neural Signaling in Post-traumatic Stress Disorder (PTSD)
Journal of Neuroimmune Pharmacology (2023)
-
Unique brain endothelial profiles activated by social stress promote cell adhesion, prostaglandin E2 signaling, hypothalamic–pituitary–adrenal axis modulation, and anxiety
Neuropsychopharmacology (2022)
-
Chronic social defeat stress increases the amounts of 12-lipoxygenase lipid metabolites in the nucleus accumbens of stress-resilient mice
Scientific Reports (2022)
-
Molecular and neurocircuitry mechanisms of social avoidance
Cellular and Molecular Life Sciences (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.