Abstract
Which brain regions contribute to the perceptual awareness of touch remains largely unclear. We collected structural magnetic resonance imaging scans and neurological examination reports of 70 patients with brain injuries or stroke in S1 extending into adjacent parietal, temporal or pre-/frontal regions. We applied voxel-based lesion-symptom mapping to identify brain areas that overlap with an impaired touch perception (i.e., hypoesthesia). As expected, patients with hypoesthesia (n = 43) presented lesions in all Brodmann areas in S1 on postcentral gyrus (BA 1, 2, 3a, 3b). At the anterior border to BA 3b, we additionally identified motor area BA 4p in association with hypoesthesia, as well as further ventrally the ventral premotor cortex (BA 6, BA 44), assumed to be involved in whole-body perception. At the posterior border to S1, we found hypoesthesia associated effects in attention-related areas such as the inferior parietal lobe and intraparietal sulcus. Downstream to S1, we replicated previously reported lesion-hypoesthesia associations in the parietal operculum and insular cortex (i.e., ventral pathway of somatosensory processing). The present findings extend this pathway from S1 to the insular cortex by prefrontal and posterior parietal areas involved in multisensory integration and attention processes.
Similar content being viewed by others
Introduction
The primary somatosensory cortex (S1) in monkeys can be divided into four Brodmann areas: (BA) 1, 2, 3a, and 3b. Each BA consists of a somatotopically organized map that subserves distinct somatosensory functions1,2,3. BA 3b4 and BA 15 receive their main input from cutaneous receptors, while distinct subdomains respond to different sensory stimuli, such as vibration, flutter, or pressure6. Also, BA 3a receives cutaneous inputs, but its activation is predominately driven by muscle receptors4,7. Nevertheless, damage to either BA 3b or BA 3a severely impairs somatosensory perception8. Lesions within BA 2 also cause impaired perception, but rather of kinaesthetic inputs8 since its afferents mainly originate in joints and muscles9. Human lesion studies are in general agreement with these observations, although brain lesions in humans, due to stroke or trauma, do not affect single BAs, so that their distinct functional implementation remains less clear. Like S1 proper in non-human primates, BA 3b in humans seems to receive the main thalamic output10, and lesions comprising BA 3b or adjacent BAs on S1 can cause an impaired perception of shape and space11,12,13.
From S1, somatosensory information is assumed to be projected to the parietal operculum (OP, secondary somatosensory cortex, S2), before terminating in the insular cortex3. In a recent virtual lesion-symptom mapping (VLSM) study, we provided causal proof for the existence of this pathway14. Attenuated perception of touch was associated with lesions comprising the contralateral parietal operculum (OP), predominantly the more frontally located subdivisions OP 4 and 3, together with the insula, the putamen, and white matter projections to the prefrontal cortex. These findings were subsequently replicated in stroke patients with the same MRI-based VLSM methods15.
In the present VLSM study, we addressed the question which brain regions upstream to the parietal operculum, i.e. S2, underpin the perceptual awareness of touch. In line with previous research11,12,13, we hypothesized to identify the four sub-divisions of S1, i.e., BA 1, BA 2, and BA 3a/b, as well as the inferior parietal lobe (i.e., IPL) together with the intraparietal sulcus (i.e., IPS) involved in calibrating somatosensory attention16,17. We did not assume to identify any associations to regions in the motor cortex, such as BA4a. Downstream to S1, we assumed to replicate previous VLSM findings including the parietal operculum, OP 1–4, together with the insular cortex3,14,15.
Results
MRI scans and examination reports were collected by the Clinic of Cognitive Neurology, University Clinic Leipzig, and Max Planck Institute (MPI) for Human Cognitive and Brain Sciences in Leipzig, Germany. According to the inclusion and exclusion criteria (see “Patients” in Methods, Supplementary Table 1), we identified 35 patients (22 men, 13 women; average age = 46.82 ± 12.12 years) with brain lesions in the left hemisphere and 35 patients (28men, 7 women; average age = 49.34 ± 16.05 years) with lesions in the right hemisphere. All patients were investigated at least 10 months after brain injury. In the group of left-sided brain-lesioned patients, we identified 22 out of the 42 patients in whom touch was impaired for the entire right side of the body. For the group with right-sided lesions, 21 out of the 47 patients presented touch impairments of the entire left side of the body. Thirty-eight out of the 43 patients with hypoesthesia additionally presented mild hemiparesis, whereas in patients without hypoesthesia 20 out of the 27 presented mild hemiparesis. Although the chi-square test did not reveal significant differences between patients with and without hypoesthesia with respect to the presence of paresis (p = 0.12), we additionally accounted for potentially confounding influences by a covariate added to both VLSM models (see “Lesion mapping” in Methods). Age, as another potentially confounding factor, was included as a covariate as well.
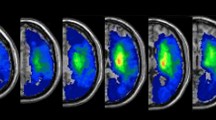
The t-statistics applied to the left or right sided lesion maps alone did not reveal any significant results, probably due to the more conservative statistical model we applied in the present (t-test, p < 0.05, corrected by 10.000 permutations, VLSM 2.5518) as compared to our previous study (Liebermeister measure, corrected false detection rate (FDR) at p < 0.05)14. To increase statistical power in the present study, we next added right-sided lesion maps to the left-sided lesion maps after we flipped left-right orientation. T-statistics revealed significant effects of hypoesthesia that overlapped with lesions in the postcentral gyrus (BA 1, 2, 3a, and 3b). On the primary motor cortex (M1), we identified BA 4p, bordering BA 3b within the central sulcus, but not BA4a. More frontally to S1 and BA 4p, we identified BA 6. The effect in BA 6 reached further downwards into the inferior frontal gyrus (BA 44) and was assigned to the ventral premotor cortex. Posterior to the postcentral gyrus, we identified hypoesthesia-associated effects in the neighboured IPL and IPS (Fig. 1). Downstream to S1 and in line with previous studies14,15, we identified the parietal opercular subdivisions OP 1 to 4, together with the insular cortex (Fig. 1). Comparable findings were observed for the left or right-sided lesion maps alone, but after lowering the threshold to a non-significant level of p = 0.1. To exclude potentially confounding influences due to spatial neglect, we conducted additional t-tests excluding patients with spatial neglect. Findings convincingly overlapped with the lesion maps obtained for the whole sample (Fig. 2). Figure 3 shows comparable group lesion maps for patients with and without spatial neglect.
Brain regions associated with hypoesthesia. Results of the VLSM 2.55 t-statistics for the group of left plus “flipped” right brain-lesioned patients. Shown are axial brain slices indexing T-values of the t-statistics (see colour bar at the bottom, orange to yellow). T-statistics comparing hypoesthesia to no hypoesthesia revealed the primary somatosensory areas BA 1, 2, 3a and 3b, BA 4p on the primary motor cortex, the inferior parietal lobe (IPL, BA 40) and intraparietal sulcus (IPS), as well as BA 6 plus BA 44, together assumed to represent the ventral premotor cortex. Downstream to S1 we identified the parietal opercular subdivisions OP 1 to 4, assumed to represent the secondary somatosensory cortex, together with the insular cortex. Numbers above each axial brain slice indicate corresponding z coordinate in MNI space. The white line in brain slices from z = 27 to z = 52 indexes the central sulcus. Colour bar indicates T-scores across lesion maps and white lines crossing the little brain in the last row index the position of the shown brain slices.
Brain regions associated with hypoesthesia, excluding spatial neglect. Results of the VLSM 2.55 t-statistics excluding patients with right-sided brain lesions and spatial neglect (shown in turquoise). Shown are the same axial brain slices as in Fig. 1. The results of the analysis without neglect patients are superimposed on the statistical map of the whole sample (shown in yellow-orange, same as in Fig. 1). The t-statistics without neglect patients, revealed a smaller topographic extend of hypoesthesia-associated lesions as for the whole group, but nevertheless the same set of brain regions (i.e., primary somatosensory areas BA 1, 2, 3a and 3b, BA 4p on the primary motor cortex, the IPL and IPS, ventral premotor cortex BA 6 plus BA 44, S2-associated parietal opercular subdivisions OP 1 to 4, insular cortex). Numbers above each brain slice indicates corresponding z coordinate in MNI space. The white line in brain slices from z = 27 to z = 52 indexes the central sulcus. Colour bars indicates T-scores across lesion maps (blue to green for lesion maps excluding neglect, red to yellow for the whole sample). White lines crossing the little brain in the last row index the position of the presented brain slices.
Shown are pairs of the summed lesion maps across patients with and without spatial neglect, superimposed on axial structural T1-weighted MRI images. Left-sided brain slices present the pattern of patients without spatial neglect and the overlapping right-sided brain slices present the pattern of patients with spatial neglect. The colour code indexes number of lesions per voxel. For individual Brodmann areas, age, gender, origin of lesion, and clinical parameters, please refer to Supplementary Table 1. The z-numbers above each brain slice indicates MNI (Montreal Neurological Institute) coordinates.
Discussion
Here, we aimed at deciphering those regions in and adjacent to S1 associated with an impaired perception of touch (i.e., hypoesthesia). In S1, hypoesthesia was associated with lesions in each of the four BAs (i.e., BA 1, 2, 3a, 3b)11,12,13. However, not all patients with lesions in BA 3b, as the assumed main entry site of thalamic projections (i.e., SI proper10), also presented hypoesthesia. This suggests that thalamic projections do not exclusively target 3b19, but probably also neighbouring BAs, such as BA 1, 2, or 3a.
At the anterior border of BA 3b to the primary motor cortex (M1), we identified BA 4p. BA 4p was first described in 1996. Before, M1 has been considered as one structurally and functionally homogeneous area, namely BA 4, occupying the entire precentral gyrus. Geyer et al.20 were the first who found that BA 4 in the human brain divides into two cytoarchitectonically distinct areas, ‘4 anterior’ (4a) and ‘4 posterior’ (4p). They also showed that roughness discrimination activated BA 4p significantly more than self-generated movements suggesting closer associations to neighbouring S1 (i.e., BA 3b) than to BA4a20.
More frontally to BA 4, we identified lesions in the ventral premotor cortex (BA 6, BA 44) associated with hypoesthesia. Electrophysiological recordings from ventral premotor cortex in primates yielded crossmodal receptive fields consisting of multiple body maps21,22,23,24 as required for multisensory integration processes24,25. Also, in humans multi-pattern classification fMRI identified ventral premotor cortex as a multisensory integration region involved in merging crossmodal sensory information from different body maps into a coherent body representation26. Damage to these receptive fields may impair the integration of touch perception into a multisensory concept of the body which clinically may manifest as hypoesthesia.
At the posterior border to the postcentral gyrus, we observed significant effects that spread from S1 into sub-areas of the IPL, namely BA 40, and the IPS. This is in line with findings showing IPL activity in response to many different tactile functions, such as spatial discrimination27, geometrical shape matching28, and detection of deviant tactile stimulations29. Based on these and other reports, the IPL was assigned a role in the calibration of attention required for the perception of provoked or spontaneous tactile sensations16. The neighbouring IPS seems to represent a key area of the dorsal frontoparietal network involved in shifting the attentional focus and calibration attentional weights17,30,31,32. Attention selection and calibration are mandatory prerequisites for accurate evaluation and classification of sensory inputs, which may explain why we found lesions in both, the IPS and the IPL in association with hypoesthesia.
Downstream to S1, we replicated previously reported associations to hypoesthesia in the parietal operculum, consisting of OP 1 to 4 (S2) and the insular cortex3,14,15. As compared to S1, S2 in the parietal operculum consists of larger receptive fields and responds to ipsilateral, as well as contralateral stimuli with greater stimulus selectivity but reduced specificity3,33,34,35,36. But S2 does not only contribute to touch perception. Human lesion studies indicate its role also in the perception of bodily and surrounding space, since lesions in the right more posteriorly located OP1 and OP2, next to the superior temporal gyrus and ventral postcentral gyrus, cause spatial neglect37. To account for potentially confounding influence on group statistics, we additionally analysed lesion maps after excluding patients with spatial neglect. This analysis quarried a VLSM pattern with a smaller topographic extend of hypoesthesia-associated lesions, due to the smaller sample-size, but nevertheless was comparable to the whole-group pattern. This supports the assumption that spatial neglect had no relevant influences on our VLSM analysis.
The insular cortex, as the assumed interface for bottom-up cognitive signals, seems to underpin affective processing and awareness of sensory signals38,39, hence contributing to the sense of ownership and agency40. The insula is divided by the central sulcus into an anterior and a posterior part. While the posterior part seems to subserve bodily awareness41, the anterior part appears to process viscerosensory42 and interoceptive signals43. Our findings generally support these assumptions and agree with functional brain imaging studies yielding insula activity in response to nocuous events44, vibrations45,46, affective touches47, and non-painful mechanical stimuli48.
Conclusions
In line with our a-priori hypotheses, we identified regions, together assumed to constitute a path of perception and recognition that leads through S1 and S2, in the parietal operculum, to the insula3,14,15. Posteriorily to S1, we found the IPL and IPS, as expected; regions implemented in attention selection and calibration processes16,17. In our previous lesion-mapping study14, we focussed on brain regions downstream to S1 and additionally identified the putamen as a subcortical structure involved in the perception of touch. Since our group statistics in the present study did not reveal the putamen, we investigated the corresponding area voxel by voxel. We found that most patients with lesions in the putamen (mean across the putamen: n = 10) did not present hypoesthesia, whereas only few patients (mean across the putamen: n = 2) presented hypoesthesia. This finding is not in line with our previous study. Together with the rather low number of lesioned voxels per se, the discrepancies between both our studies question whether lesions in the putamen contribute to hypoesthesia.
Besides expected effects in S1, S2, the insular cortex3,14,15, the IPL and the IPS16,17, present lesion-mapping additionally quarried BA 4p - a primary motor area involved in motor control but which also proved to be sensitive to tactile stimulations20. More ventrally, we moreover identified the ventral premotor cortex; a brain region assumed to contain neuronal ensembles involved in whole-body perception26. To rule out, that these effect in motor cortices were caused by differences in the presence of hemiparesis, we compared hemiparesis between patients with and without hypoesthesia and revealed no significant differences. We also analysed lesion maps without accounting for the presence of paresis which resembled the same hypoesthesia-associated brain pattern as shown in this article (not shown to avoid redundancies). This supports the interpretation that hypoesthesia-associated effects in motor (BA 4p) and premotor cortex (BA 6/44) were not just driven by additional motor impairments but by hypoesthesia. We also addressed potentially confounding influences by spatial neglect by two t-tests, one including patients with neglect (Fig. 1), and the other one excluding patients with neglect (Fig. 2). Maps of both t-tests convincingly overlapped. To exclude age as another confounding factor, we added age as a covariate. Resulting brain maps, however, showed an almost similar pattern as the maps revealed without regressing out age.
Together, our findings are in agreement with intracerebral recordings in humans showing that more than 10% of the cortical surface appears to be involved in the processing of somatosensory stimuli. The identified cortical network convincingly overlaps with the network presented in the present study, including not only S1, S2 and insular areas, but also neighbouring motor, premotor, and inferior parietal areas49. These and the present findings emphasize the role of those regions in the perception of touch. Whether the impairment in touch perception results from an impairment in perceptual awareness, attention or multisensory integration processes required for proper body perception remains speculative. With respect to the existing body of evidence26, we assume that lesions in the ventral premotor cortex impair multisensory integration processes required for the correct integration of tactile stimuli into a whole-body concept required for correct perceptual awareness. Lesions in the IPL and the IPS may in turn impair correct attentional selection and calibration processes16,17, required to accurately evaluate the intensity and location of touches applied to the skin. The loss in associated capacities may trigger or facilitate the impression of hypesthesia when being touched. Lesion studies with isolated lesions in each of those regions would be required to further disentangle their true functional anatomical associations, but suitable patients are rare and currently available statistical methods are unsuitable to handle small samples or single cases.
Methods
Patients
We included patients with brain lesions in the postcentral gyrus extending into the parietal, temporal and/or pre-/frontal lobe in the same brain hemisphere. Patients with malformations or lesions in the contralateral brain hemisphere were excluded. Cognitive and severe communication deficits were exclusion criteria too. The study was approved by the ethics committee of the University Clinic Leipzig and conducted according to the ethical guidelines of the Declaration of Helsinki. All patients gave written informed consent.
Besides a high-resolution whole-brain 3D standard T1-weighted anatomical image, we used a T2-weighted fluid-attenuated inversion-recovery (FLAIR) image in parallel to better delineate the borders of stroke lesions. Data acquisition was done with a 3 T Bruker MedSpec 100 System (Bruker, Ettlingen, Germany), a 3 T Tim TRIO, or a 3 T VERIO MRI scanner (Siemens, Erlangen, Germany).
Examination of touch impairments (i.e., hypoesthesia)
The examination procedures were the same as described elsewhere14. The standardized examinations were conducted by physiotherapists of the MPI Clinic for Cognitive Neurology. Patients lay supine on an examination bed. They were told to close their eyes and the room was darkened. A paintbrush (6 mm tip width) was used to apply strokes to the fingers or toes. From there, strokes were directed further proximally to the upper limb and from there to the trunk and the face. With each stroke, the patient was asked whether the sensation was felt as normal or attenuated (i.e., hypoesthesia). Distal and proximal strokes were compared, as well as the left and the right side of the body. To identify the extension of hypoesthesia, strokes were next directed from the body part with hypoesthesia to neighbouring body parts using a paintbrush with a 14 mm tip-width. This was repeated on the dorsal body site.
Lesion mapping
We used MRIcroN (http://www.sph.sc.edu/comd/rorden/mricron) to manually delineate the lesion on every single transversal T1- weighted image slice. The two researchers (S.P. and B.P.) who created the 3D lesion maps were unaware of the clinical deficits. They first delineated the lesions independently of each other. Afterwards, both examiners reviewed all maps together. In case of discrepancies between both examiners, the lesion map was jointly revised. Lesion maps together with the T1-weighted image were spatially normalized to the MNI-template (i.e, Montreal Neurological Institute) using SPM12 (http://www.fil.ion.ucl.ac.uk/spm/).
To assess the relationship between impaired touch perception (i.e., hypoesthesia) and brain lesions, normalized lesion maps were applied to the VLSM software 2.55 (https://langneurosci.mc.vanderbilt.edu/resources.html) for Matlab R2017b (Natick, Massachusetts, USA, https://mathworks.com) that divides patients according to whether they present a lesion in a given voxel or not. These two groups are then statistically compared (i.e., t-test) according to whether they present a symptom, like hypoesthesia18. The analysis is corrected for the lesion extend by regressing out the number of lesioned voxels.
To assess the influence of neglect on group results, we conducted two analyses: The first analysis included all patients with lesions in the left hemisphere. In patients with lesions in the right hemisphere, we first flipped right/left orientation before adding the lesion masks. The second analysis was conducted in the same way after excluding patients with spatial neglect. In both analyses, presence of paresis (either present or not) was added as a covariate to account for the corresponding variance. Voxels surviving the alpha level of p < 0.05 and 10.000 permutations were considered significant.
To identify lesion-associated Brodmann areas in each patient, we superimposed them to the Brodmann template offered by MRIcroN (Supplementary Table 1). The assignment of the group findings to their corresponding BAs was done by superimposing the group maps on the cytoarchitectonic maps as implemented in the Anatomy toolbox for SPM50 (Fig. 1). Insula cortex, BA 6 and BA 44 were allocated using the WFU_PickAtlas by Joseph Maldjian from the Functional MRI Laboratory at the Wake Forest School of Medicine (see http://www.nitrc.org/projects/wfu_pickatlas).
References
Kaas, J. H., Nelson, R. J., Sur, M., Lin, C. S. & Merzenich, M. M. Multiple representations of the body within the primary somatosensory cortex of primates. Science 204, 521–523 (1979).
Chen, L. M., Friedman, R. M. & Roe, A. W. Optical imaging of SI topography in anesthetized and awake squirrel monkeys. J. Neurosci. 25, 7648–7659, https://doi.org/10.1523/JNEUROSCI.1990-05.2005 (2005).
Dijkerman, H. C. & de Haan, E. H. Somatosensory processes subserving perception and action. Beh. Brain Sci. 30, 189–201; discussion 201–139, https://doi.org/10.1017/S0140525X07001392 (2007).
Tanji, J. & Wise, S. P. Submodality distribution in sensorimotor cortex of the unanesthetized monkey. J. Neurophysiol. 45, 467–481, https://doi.org/10.1152/jn.1981.45.3.467 (1981).
Whitsel, B. L., Dreyer, D. A. & Roppolo, J. R. Determinants of body representation in postcentral gyrus of macaques. J. Neurophysiol 34, 1018–1034, https://doi.org/10.1152/jn.1971.34.6.1018 (1971).
Friedman, R. M., Chen, L. M. & Roe, A. W. Modality maps within primate somatosensory cortex. Proc. Natl. Acad. Sci. USA 101, 12724–12729, https://doi.org/10.1073/pnas.0404884101 (2004).
Phillips, C. G., Powell, T. P. & Wiesendanger, M. Projection from low-threshold muscle afferents of hand and forearm to area 3a of baboon’s cortex. J. Physiol. 217, 419–446 (1971).
Randolph, M. & Semmes, J. Behavioral consequences of selective subtotal ablations in the postcentral gyrus of Macaca mulatta. Brain Res. 70, 55–70 (1974).
Merzenich, M. M., Kaas, J. H., Sur, M. & Lin, C. S. Double representation of the body surface within cytoarchitectonic areas 3b and 1 in “SI” in the owl monkey (Aotus trivirgatus). J. Comp. Neurol. 181, 41–73, https://doi.org/10.1002/cne.901810104 (1978).
Kaas, J. H. What, if anything, is SI? Organization of first somatosensory area of cortex. Physiol. Rev. 63, 206–231, https://doi.org/10.1152/physrev.1983.63.1.206 (1983).
Corkin, S., Milner, B. & Rasmussen, T. Somatosensory thresholds–contrasting effects of postcentral-gyrus and posterior parietal-lobe excisions. Arch. Neurol. 23, 41–58 (1970).
Pause, M., Kunesch, E., Binkofski, F. & Freund, H. J. Sensorimotor disturbances in patients with lesions of the parietal cortex. Brain 112(Pt 6), 1599–1625 (1989).
Roland, P. E. Somatosensory detection in patients with circumscribed lesions of the brain. Exp. Brain Res. 66, 303–317 (1987).
Preusser, S. et al. The perception of touch and the ventral somatosensory pathway. Brain 138, 540–548, https://doi.org/10.1093/brain/awu370 (2015).
Meyer, S. et al. Voxel-based lesion-symptom mapping of stroke lesions underlying somatosensory deficits. NeuroImage Clin. 10, 257–266, https://doi.org/10.1016/j.nicl.2015.12.005 (2016).
Bauer, C. C., Barrios, F. A. & Diaz, J. L. Subjective somatosensory experiences disclosed by focused attention: cortical-hippocampal-insular and amygdala contributions. PloS one 9, e104721, https://doi.org/10.1371/journal.pone.0104721 (2014).
Goltz, D. et al. Connections between intraparietal sulcus and a sensorimotor network underpin sustained tactile attention. J. Neurosci. 35, 7938–7949, https://doi.org/10.1523/JNEUROSCI.3421-14.2015 (2015).
Bates, E. et al. Voxel-based lesion-symptom mapping. Nat. Neurosci. 6, 448–450, https://doi.org/10.1038/nn1050 (2003).
Kalberlah, C., Villringer, A. & Pleger, B. Dynamic causal modeling suggests serial processing of tactile vibratory stimuli in the human somatosensory cortex-an fMRI study. NeuroImage 74, 164–171, https://doi.org/10.1016/j.neuroimage.2013.02.018 (2013).
Geyer, S. et al. Two different areas within the primary motor cortex of man. Nature 382, 805–807, https://doi.org/10.1038/382805a0 (1996).
Fogassi, L. et al. Coding of peripersonal space in inferior premotor cortex (area F4). J. Neurophysiol. 76, 141–157, https://doi.org/10.1152/jn.1996.76.1.141 (1996).
Iwamura, Y. Hierarchical somatosensory processing. Curr. Opin. Neurobiol. 8, 522–528 (1998).
Rizzolatti, G., Scandolara, C., Matelli, M. & Gentilucci, M. Afferent properties of periarcuate neurons in macaque monkeys. I. Somatosensory responses. Beh. Brain Res. 2, 125–146 (1981).
Graziano, M. S. & Gandhi, S. Location of the polysensory zone in the precentral gyrus of anesthetized monkeys. Exp. Brain Res. 135, 259–266, https://doi.org/10.1007/s002210000518 (2000).
Graziano, M. S., Hu, X. T. & Gross, C. G. Visuospatial properties of ventral premotor cortex. J. Neurophysiol. 77, 2268–2292, https://doi.org/10.1152/jn.1997.77.5.2268 (1997).
Gentile, G., Bjornsdotter, M., Petkova, V. I., Abdulkarim, Z. & Ehrsson, H. H. Patterns of neural activity in the human ventral premotor cortex reflect a whole-body multisensory percept. NeuroImage 109, 328–340, https://doi.org/10.1016/j.neuroimage.2015.01.008 (2015).
Akatsuka, K., Noguchi, Y., Harada, T., Sadato, N. & Kakigi, R. Neural codes for somatosensory two-point discrimination in inferior parietal lobule: an fMRI study. NeuroImage 40, 852–858, https://doi.org/10.1016/j.neuroimage.2007.12.013 (2008).
Miquee, A. et al. Neuronal substrates of haptic shape encoding and matching: a functional magnetic resonance imaging study. Neuroscience 152, 29–39, https://doi.org/10.1016/j.neuroscience.2007.12.021 (2008).
Yamashiro, K. et al. Effect of changes in stimulus site on activation of the posterior parietal cortex. Brain Top. 28, 261–268, https://doi.org/10.1007/s10548-014-0378-2 (2015).
Corbetta, M. & Shulman, G. L. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 3, 201–215, https://doi.org/10.1038/nrn755 (2002).
Molenberghs, P., Mesulam, M. M., Peeters, R. & Vandenberghe, R. R. Remapping attentional priorities: differential contribution of superior parietal lobule and intraparietal sulcus. Cereb. Cortex 17, 2703–2712, https://doi.org/10.1093/cercor/bhl179 (2007).
Vandenberghe, R., Molenberghs, P. & Gillebert, C. R. Spatial attention deficits in humans: the critical role of superior compared to inferior parietal lesions. Neuropsychologia 50, 1092–1103, https://doi.org/10.1016/j.neuropsychologia.2011.12.016 (2012).
Disbrow, E., Roberts, T., Poeppel, D. & Krubitzer, L. Evidence for interhemispheric processing of inputs from the hands in human S2 and PV. J. Neurophysiol. 85, 2236–2244, https://doi.org/10.1152/jn.2001.85.5.2236 (2001).
Lin, Y. Y. & Forss, N. Functional characterization of human second somatosensory cortex by magnetoencephalography. Beh. Brain Res. 135, 141–145 (2002).
Ruben, J. et al. Somatotopic organization of human secondary somatosensory cortex. Cerebral cortex 11, 463–473 (2001).
Sinclair, R. J. & Burton, H. Neuronal activity in the second somatosensory cortex of monkeys (Macaca mulatta) during active touch of gratings. J. Neurophysiol. 70, 331–350, https://doi.org/10.1152/jn.1993.70.1.331 (1993).
Karnath, H. O., Ferber, S. & Himmelbach, M. Spatial awareness is a function of the temporal not the posterior parietal lobe. Nature 411, 950–953, https://doi.org/10.1038/35082075 (2001).
Craig, A. D. How do you feel? Interoception: the sense of the physiological condition of the body. Nat. Rev. Neurosci. 3, 655–666, https://doi.org/10.1038/nrn894 (2002).
Craig, A. D. Human feelings: why are some more aware than others? Trends Cogn. Sci. 8, 239–241, https://doi.org/10.1016/j.tics.2004.04.004 (2004).
Farrer, C. et al. Modulating the experience of agency: a positron emission tomography study. NeuroImage 18, 324–333 (2003).
Karnath, H. O., Baier, B. & Nagele, T. Awareness of the functioning of one’s own limbs mediated by the insular cortex? J. Neurosci. 25, 7134–7138, https://doi.org/10.1523/JNEUROSCI.1590-05.2005 (2005).
Oppenheimer, S. M., Gelb, A., Girvin, J. P. & Hachinski, V. C. Cardiovascular effects of human insular cortex stimulation. Neurology 42, 1727–1732 (1992).
Craig, A. D. How do you feel–now? The anterior insula and human awareness. Nat. Rev. Neurosci. 10, 59–70, https://doi.org/10.1038/nrn2555 (2009).
Apkarian, A. V., Bushnell, M. C., Treede, R. D. & Zubieta, J. K. Human brain mechanisms of pain perception and regulation in health and disease. Eur. J. Pain 9, 463–484, https://doi.org/10.1016/j.ejpain.2004.11.001 (2005).
Burton, H., Videen, T. O. & Raichle, M. E. Tactile-vibration-activated foci in insular and parietal-opercular cortex studied with positron emission tomography: mapping the second somatosensory area in humans. Somatosens. Mot. Res. 10, 297–308 (1993).
Soros, P. et al. Functional MRI of working memory and selective attention in vibrotactile frequency discrimination. BMC Neurosci. 8, 48, https://doi.org/10.1186/1471-2202-8-48 (2007).
Olausson, H. et al. Unmyelinated tactile afferents signal touch and project to insular cortex. Nat. Neurosci. 5, 900–904, https://doi.org/10.1038/nn896 (2002).
Lui, F. et al. Touch or pain? Spatio-temporal patterns of cortical fMRI activity following brief mechanical stimuli. Pain 138, 362–374, https://doi.org/10.1016/j.pain.2008.01.010 (2008).
Avanzini, P. et al. Four-dimensional maps of the human somatosensory system. Proc. Natl. Acad. Sci. USA 113, E1936–1943, https://doi.org/10.1073/pnas.1601889113 (2016).
Eickhoff, S. B. et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage 25, 1325–1335, https://doi.org/10.1016/j.neuroimage.2004.12.034 (2005).
Acknowledgements
Funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)– Projektnummer 122679504 – SFB 874 to BP. This study was also supported by the the Bernstein Focus, State Dependencies of Learning 01GQ0975, Project 18GL4DW4 to B.P., IFB AdiposityDiseases to B.P. and the nutriCARD initiative to B.P. (http://www.nutricard.de), Federal Ministry of Education and Research (BMBF), Germany, FKZ: 01E01001 (http://www.bmbf.de), and the German Research Foundation (DFG) (http://www.dfg.de), within the framework of the CRC 1052 Obesity Mechanisms to BP and MR.
Author information
Authors and Affiliations
Contributions
B.P. and S.P. conceived the study. S.P. collected the data. S.P. and B.P. created individual lesion maps, M.R. conducted all analyses, B.P. wrote the paper, all authors edited the final version of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rullmann, M., Preusser, S. & Pleger, B. Prefrontal and posterior parietal contributions to the perceptual awareness of touch. Sci Rep 9, 16981 (2019). https://doi.org/10.1038/s41598-019-53637-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-53637-w
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.