Abstract
Horseshoe crabs are archetypal marine chelicerates with a fossil record extending from the Lower Ordovician to today. The major horseshoe crab groups are thought to have arisen in the middle to late Palaeozoic. Here we present the oldest known limuloid from the lower Carboniferous (Tournaisian stage, c. 350 million years ago) of Scotland: Albalimulus bottoni gen. et sp. nov. A comprehensive phylogenetic analysis supports the placement of A. bottoni as a representative of the extant family Limulidae and 100 million years older than any other limulid taxon. The use of geometric morphometric analyses corroborate the erection of the new taxon and illustrates the exploitation of morphospace by xiphosurids. This new taxon highlights the complex evolutionary history of xiphosurids and the importance of documenting these unique Palaeozoic individuals.
Similar content being viewed by others
Introduction
Horseshoe crabs have a highly conservative and iconic shape: a crescentic prosomal shield, opisthosomal tergites fused into a thoracetron, and a styliform telson1,2. The documentation of fossil and extant3,4,5,6,7,8,9,10 representatives of true horseshoe crabs (Xiphosurida) started in the early 1800’s11,12 and has continued to this day: a research effort resulting in 80 fossil species13. However, this number continues to change as a better understanding of the taphonomic and ontogenetic variation of taxa allows identification of invalid species14,15. Recent phylogenetic works16,17,18 have augmented this research and presented a more complete understanding of the evolutionary history of Xiphosurida. Although horseshoe crabs have a fossil record extending from the Lower Ordovician (c. 480 million years ago) to the present day19,20, Xiphosurida arose close to the base of the Carboniferous21. After this origin, horseshoe crabs diversified and three of the five xiphosurid groups (families) arose: Belinuridae, Paleolimulidae, and Rolfeiidae. Carboniferous belinurids were the most abundant group at this time14,22,23,24,25 with 37 different species arrayed across the genera Alanops Racheboeuf, Vannier & Anderson, 200226, Bellinurus Pictet, 184627, Euproops Meek, 186728, Liomesaspis Raymond, 194423, Prolimulus Frič, 189924, and Stilpnocephalus Selden, Simonetto & Marsiglio, 201925. The four Carboniferous paleolimulid species29,30,31,32 are arrayed across Paleolimulus Dunbar, 192333 and Xaniopyramis Siveter and Selden, 198734. Finally, Rolfeiidae is a monospecific group containing Rolfeia fouldenensis Waterston, 198535. Furthermore, the Superfamily Limuloidea—the group containing Limulidae and Austrolimulidae—is represented in the Carboniferous by Valloisella lievinensis Racheboeuf36. Limulidae, the group of horseshoe crabs containing all extant species, first appears in the Triassic, c. 100 million years later37,38. Given that Limuloidea has a Carboniferous presence, it would be logical to suggest that limulids may also have a deeper origin than previously thought. Here we present a new Carboniferous-aged limuloid from the south of Scotland, Albalimulus bottoni gen. et sp. nov. The morphology of this animal suggests a likely limulid affinity and a comprehensive phylogenetic analysis places this taxon within Limulidae. The identification of this taxon suggests that crown group horseshoe crabs potentially arose much earlier than previously thought: just above the Devonian-Carboniferous boundary (Tournaisian stage).
Specimen History and Geological Setting
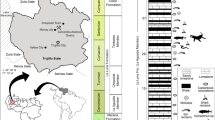
The historical nature of the material (collection year unknown) means that limited information is available regarding its geological and locality setting. The British Geological Survey (Keyworth) specimens, representing a part and counterpart, were collected from a river section of the lower Calciferous Sandstone Series at Whiteadder Water. Unfortunately, the exact horizon in this section is unknown due to lack of collection data. The recorded coordinates of the specimens (55.797878°N, 2.277510°W) give a position slightly east of Duns (Berwickshire, Scotland). The locality known as Crumble Edge, highlighted in the recent literature39,40 (Fig. 1), is along the Whiteadder Water, and has coordinates nearly identical to those reported in the British Geological Survey data (see coordinates of Crumble Edge taken from Kearsey, et al.39, their Fig. 1). We therefore suggest that the British Geological Survey specimen was collected from, or close to, Crumble Edge.
Map showing Tournaisian outcrop and localities close to Duns. (A) Outline of Great Britain. (B) Rectangle in (A), map of northern England, showing Tournaisian outcrop. Redrawn from Smithson, et al.40 with location of Duns identified from Google Maps. Based on BGS Materials © UKRI. (C) Rectangle in (B), map of area surrounding Duns, showing nearby localities. Redrawn using Inkscape. Figures used are from Kearsey, et al.39 and Smithson, et al.40 with location of Duns and river paths added using Google Maps (Map data ©2019 Google). Maps contain Ordnance Survey data © 1028 Crown copyright and database right 2016.
The Ballagan Formation outcrops across the Midland Valley of Scotland and northern England39 (Fig. 1), where it was previously known as the lower part of the Calciferous Sandstone Series and Cementstone Group respectively. The Ballagan Formation extends through the entire Tournaisian (lower Carboniferous), with the Devonian-Carboniferous boundary positioned close to the top of the underlying Kinnesswood Formation41 (Fig. 2). Recent studies exploring the first terrestrialisation of tetrapods have furthered the understanding of the sedimentology, palynology, stratigraphy, and palaeoenvironment of the Ballagan Formation, which consists of a thick succession of red and grey siltstones, fine sandstones, ferroan dolostones, and over 200 separate palaeosol horizons39,40,42,43,44 (Figs 1, 2). These likely record a seasonal climate with a mosaic of closely packed distinct habitats from both coastal floodplain and marginal marine environments39. The fauna is a diverse group of bivalves, ostracods, eumalacostracans, spinicaudatans, scorpions, millipedes, and tetrapods43,44,45,46.
Upper Devonian and lower Carboniferous stratigraphy of the Scottish Borders. Redrawn from Marshall, et al.41.
Crumble Edge is a 46 m thick succession exposed in a river cliff near Duns, close to the base of the Ballagan Formation39. It is therefore close to the Devonian-Carboniferous boundary, although its exact correlation with other, more extensive Ballagan Formation outcrops is unknown. It contains 18 palaeosols (mostly Inceptisol) and has been recently logged at high resolution39. A small amount of tetrapod material has been reported from this site40; however, the majority of the recent studies considering tetrapods or invertebrates have used material from other nearby sites42,43,44,45(Fig. 1).
Materials and Methods
The single known specimen is housed within the British Geological Survey (BGS.GSE), Keyworth, where it is curated under two different catalogue numbers for the part (BGS.GSE2028) and counterpart (BGS.GSE9680). BGS.GSE2028/9680 is preserved as a flat impression on a small slab of clayey, grey-black shale. When describing this specimen, we followed the systematic taxonomy16,17 and the anatomical terminology5,38,47 of previous workers. The specimens were coated with ammonium chloride sublimate and photographed under LED lighting using a Canon EOS 5DS digital camera and a Canon MP-E 65 mm 1–5x macro lens housed at the University of New England. Images were stacked using Helicon Focus 7 (Helicon Soft Limited) software.
Following Bicknell, et al.38, a morphometric analysis using landmarks and semilandmarks of 82 specimens was conducted to quantitatively assess the morphology of BGS.GSE2028/9680 relative to other taxa. The examined species were from Austrolimulidae, Belinuridae, Limulidae, Paleolimulidae, Rolfeiidae, and stem xiphosurids (sensu Bicknell, et al.21). Landmarking and semilandmarking was conducted using the Thin-Plate Spline (TPS) suite (http://life.bio.sunysb.edu/morph/index.html). A TPS file was constructed using tpsUtil64 (v.1.7). The TPS file was imported into tspDig2 (v.2.26), which was used to place five landmarks and 50 semi-landmarks along the right prosomal shield and thoracetron (Fig. 3; Table 1). Semilandmarks were placed in a clockwise direction along the most anterior section of the prosomal shield, ending in the most posterior section of the thoracetron. Points were digitised as xy coordinates. The thoracetron was digitised along the right thoracetronic margin, but excluding areas containing moveable and fixed spines, as they are not known to, or preserved in, all horseshoe crab taxa. In cases where the right side was poorly preserved, the left side was used instead, and the data mirrored. These points populated the TPS file with landmark and semilandmark data (Supplementary Information 1). The TPS file was imported into an R environment. The ‘geomorph’ package48 was used to conduct a Procrustes Superimposition and Principal Components Analysis (PCA) of the data. Procrustes Superimposition standardises for size and orientation such that only shape variation was assessed (Supplementary Data 2). The PC data were output and logged in Supplementary Data 3. Only the first two Principal Components (PCs) were considered as they explained the majority of the variation in the data (67.8%).
Approximate semilandmark trajectory (blue arrows and dotted line) and the five digitised landmarks used here. Landmarks are described in Table 1.
To evaluate the phylogenetic position of Albalimulus bottoni gen. et sp. nov., we coded it as an additional taxon in the recently published matrix of Lamsdell17; a matrix that contains a broad sampling fossil and extant euchelicerates (Supplementary Information 4). The analysis was performed under equal-weights parsimony in TNT 1.549 utilising the “New Technology” tree search strategy using random sectorial searches, 1000 iterations of the parsimony ratchet, 50 cycles of drifting and 5 rounds of tree fusing. All multistate characters were considered unordered as in the original analysis.
A further matrix was compiled to align with the morphometric analyses and to explore the impact of involving more than one additional taxon in this matrix. Ten additional taxa were therefore coded into the Lamsdell17 matrix (Supplementary Information 5). This matrix was analysed using the same parameters as the Supplementary Information 4. The maximum parsimony tree produced a large polytomy that collapsed Paleolimulidae, Austrolimulidae and parts of Limulidae. This is not informative for uncovering evolutionary relationships so was not considered at length here.
Systematic palaeontology
Euchelicerata Weygoldt and Paulus, 197950
Order Xiphosurida Latreille, 18024
Suborder Limulina Richter and Richter, 192951
Superfamily Limuloidea Zittel, 188552
Family Limulidae? Zittel, 188552
Genus Albalimulus nov. gen.
Etymology
Albalimulus is a combination of the Gaelic name for Great Britain (Alba) and Limulus, the genus of the extant and iconic North American horseshoe crab and commonly used suffix in generic names of representatives of Limuloidea.
Type species
Albalimulus bottoni, new species.
Distribution
Ballagan Formation, Tournaisian, lower Carboniferous (Figs. 1, 2).
Diagnosis
As for species.
Albalimulus bottoni nov. sp.
Albalimulus bottoni gen. et sp. nov. holotype BGS.GSE2028/9680. (A) Complete specimen of BGS,GSE2028 (part). (B) Complete specimen of BGS.GSE9680 (counter-part). Image mirrored to align with (A). Both specimens were coated with ammonium chloride sublimate and converted to grey-scale. (C) Line drawing of BGS.GSE2028 highlighting important anatomical features. The left prosomal side is preserved less completely than the right side. (D) Idealised reconstruction of A. bottoni. Note that the telson is styliform, reflecting extant taxa; however, the degree of telson elongation cannot be confirmed from the holotype. Abbreviations: gs, genal spine; od, opisthosomal doublure; ops, opisthosoma; pro, prosoma; ps, pustulose cuticle; rim, opisthosomal rim; tn, telson. Image credit (D): Elissa Johnson.
Close up of informative and unique features of Albalimulus bottoni gen. et sp. nov. (A) Right ophthalmic ridge (white arrows). (B) Thoracetron-telson joint showing pustules and linear structure (white arrow). (C) Pustulose cuticle ornamentation on thoracetron. Specimens were coated with ammonium chloride sublimate and converted to grey-scale.
Etymology
Trivial name bottoni was chosen in recognition of Mark L. Botton who has contributed extensively to extant horseshoe crab research and conservation of Limulus polyphemus (Linnaeus, 1758)53 across his career.
Holotype by monotypy
BGS.GSE2028/9680 (part/counterpart).
Distribution
Same as for genus.
Type locality and horizon
The Whiteadder Water river, near Duns, Berwickshire, Scotland (55.797878°N, 2.277510°W), likely at or very close to Crumble Edge locality of Kearsey, et al.39 and Smithson, et al.40 (Figs 1, 2).
Diagnosis
Limuloid with pustulose cuticular ornament, most prominent on the thoracetron; well-defined, curved prosomal-opisthosomal hinge; prosomal shield is slightly longer than thoracetron.
Preservation
BGS.GSE2028/9680 is preserved flattened as part and counterpart on a thin slab of siltstone.
Description
BGS.GSE2028/9680 is an articulated prosomal shield, thoracetron, and partial telson preserved as a part and counterpart (Figure 4A–C). Almost no relief is observed. Specimen is 12.5 mm long, including the preserved telson section. Prosoma is semi-circular and 5.4 mm long sagittally. The right side of the prosoma is preserved better than the left side. Prosomal width across the posterior margin of lateral rims is 10.2 mm. A thin prosomal rim is preserved along margins and attains a greatest width of ca. 0.5 mm. No prosomal doublure is visible. Left ophthalmic ridge is preserved as a slight impression that curves out towards the left lateral border. The left ophthalmic ridge is 2.4 mm long and the anterior section is slightly obscured by rock. The right ophthalmic ridge is preserved as a slight impression and curves out towards the right lateral border (Fig. 5A). The right ophthalmic ridge is 2.8 mm long. No lateral compound eyes can be confidently discerned. No cardiac lobe or associated ridges are noted (Fig. 4A). Ocelli are not observed. The left genal spine is not preserved. The right genal spine is completely preserved, 2 mm long and extends posteriorly to 15% of the thoracetron length. The genal spine tip is 6.9 mm from the prosomal midline. The lateral extent between the right genal spine tip and thoracetron is 2.9 mm. Angle between the right genal spine and right side of the thoracetron is 80°. Prosomal-opisthosomal hinge is pronounced, 5.6 mm wide and 0.3 mm long. The hinge curves posteriorly towards the lateral sides of BSG.GSE 2028/9680. The posterior right section of prosoma has pustulose cuticular ornament. No prosomal appendages are preserved.
The thoracetron is trapezoidal, 4.6 mm long and 7.8 mm wide anteriorly, tapering to 2.3 mm posteriorly. The left side is preserved less completely than right side. No axial lobes are noted. No apodemal pits are noted. No definitive evidence for tergal expression is noted. A potential opisthosomal doublure is noted. Doublure is 6.6 mm anteriorly tapering to 3.5 mm posterior (these are minimum values as outer-most section of the left side of the doublure is not preserved). This feature may also reflect compression through preservation. A thin thoracetronic rim is noted on the right side of the thoracetron and only slightly pronounced. The rim is 0.8 mm wide anteriorly tapering to 0.5 mm posteriorly. No fixed or moveable spines are noted. Left side of thoracetron has pustulose cuticular ornament preserved (Fig. 5B,C).
The telson is partly preserved and is articulated with the posterior thoracetronic margin (Fig. 5B). Margin between the thoracetron and telson curves slightly towards the anterior of the specimen. Telson is partly preserved, is 2.8 mm long and 1.9 mm wide anteriorly (minimum values). Telson lacks an axial ridge.
Remarks
Albalimulus bottoni preserves select characters diagnostic of at least Limuloidea: the horseshoe shaped prosoma and trapezoidal thoracetron, non-converging ophthalmic ridges (sensu discussion in Lamsdell17). Other diagnostic and informative features such as appendages, moveable spines, dorsal keel, and compound eyes are not present or preserved. The combined presence of pustulose cuticle, possible lack of pronounced apodemal pits, and pronounced prosomal-opisthosomal hinge are unique and warrant the erection of a new genus and species (Fig. 4D). This outcome is corroborated by the placement of this taxon in a unique position in xiphosurid morphospace (see Geometric Morphometric analysis below).
Belinurina, which includes the Belinuridae, is characterised by the possession of pronounced, concave ophthalmic ridges meeting anteriorly, the presence of ophthalmic spines, a pronounced axial region exhibiting clear segmentation, a marked posterior opisthosomal boss likely associated with powerful telsonic musculature, and a thoracetron bordered by either incised marginal spines, or a flat marginal flange resulting from the fusion of the base of these marginal spines. The general outline of the thoracetron is roughly semi-circular. Since Albalimulus bottoni does not exhibit any of these characters, it can be confidently excluded from Belinurina.
The overall morphology of Albalimulus bottoni, showing a trapezoidal outline of the thoracetron, is strongly suggestive of an affinity within the Suborder Limulina. However, A. bottoni lacks a clearly expressed segmented axis and pyramidal cheek nodes, interophthalmic ridges, and prosomal transverse ridge nodes that characterise Paleolimulidae, with its effaced morphology is indicative of Limuloidea. The apparent lack of fixed or moveable marginal spines is shared with Austrolimulidae and Valloisella lievinensis; however, this feature may be absent due to the preservational mode. More specimens are therefore needed to confirm whether the lack of potentially diagnostic features is biological or taphonomic. Regardless, A. bottoni lacks the elongated posterior pleurae forming an embayment surrounding the base of the telson and outwardly directed genal spines, typical of the Austrolimulidae. The results of the phylogenetic analysis (discussed in Phylogenetic Results) favour the placement of A. bottoni in a basal position within Limulidae. However, we are tentative in this placement, due to the singular nature of BGS.GSE 2028/9680, the preservation precluding the identification of all diagnostic characters of the Family, and the poor resolution of phylogenetic analyses involving more taxa (Supplementary Data 5).
A consideration must be given to the linear structure on the posterior section of the thoracetron (Fig. 5B). This lineation is 3.2 mm long and located 2.0 mm posteriorly from the prosomal-opisthosomal hinge. We are unconvinced that it represents a tergite, especially as one would expect to observe at least two structures. We suggest it reflects compression of the fossil, an interpretation supported by the uneven outline. A tergal boundary would be smooth; however, the effaced right side of the fossil may be the reason why the apparent boundary cannot be identified across the entire fossil. Regardless, this potential tergal boundary is not pronounced enough to represent an articulation, and so was unlikely functional. Nonetheless, the presence of tergites would not affect the placement of Albalimulus bottoni in Limuloidea as the group contains taxa that have tergal expression: the austrolimulid Austrolimulus fletcheri Riek, 195554 and the limuloid Valloisella lievinensis55. It does raise doubts about a limulid affinity.
One of the key autapomorphies of Albalimulus bottoni is the pustulose cuticle that is unknown to Xiphosura or Xiphosurida. The feature has been documented in related euchelicerates: arachnids56, chasmataspidids57, and eurypterids58,59, and is also present in other arthropod clades, e.g. aglaspidids and cheloniellids. The pustulose ornament unlikely reflects cuticular secretion, as extant taxa would also commonly exhibit these features60. It has been hypothesised that pustulose ornament potentially provided additional sensory capability and camouflage for benthic euchelicerates61. Albalimulus bottoni would have benefited from additional camouflage as it was so small relative to contemporary predators. Research into pustulose ornament in decapod crustaceans may uncover a functional use for this feature in arthropods.
Morphometric Results
The PCA plots demonstrate Family and generic distribution in morphospace. PC1 describes how laterally extended the genal spine tip is relative to prosoma and whether the thoracetron has a trapezoidal or round shape (Fig. 6). Austrolimulids (Fig. 6A,B) and belinurids dominate positive PC1 space as most of these taxa have large, laterally and posteriorly extended genal spines. Negative PC1 space is dominated primarily by limulids and paleolimulids: taxa with less accentuated genal spines. Notably, a selection of proposed austrolimulids (sensu Lerner, et al.47 and Bicknell62) are located in more negative PC1 space—these taxa have a less pronounced genal spine splay (Fig. 6B). PC2 (24.1% shape variation) describes the degree to which the proximal section of the genal spine is indented into the prosomal shield. Psammolimulus gottingensis Lange, 192363 has the most negative PC2 value as the proximal genal spine section is highly indented (Fig. 6B). Conversely, Bellinurus trilobitoides (Buckland, 1837)64 is located in positive PC2 space as the genal spines are not indented into the prosomal shield (Fig. 6C). Albalimulus bottoni is located close to the origins of both PC axes (PC1 = 0.03, PC2 = 0.08; Figs 6, 7). The genus is located within the outer limits of the Belinuridae and Austrolimulidae convex hulls, at a place in PC space that almost overlays the Limulidae convex hull (Fig. 6). The generic distribution of specimens shows that A. bottoni is not bound by any convex hulls (Fig. 7).
Xiphosurids in Principal Component space. The thin plate spline reconstructions represent factor loadings for PCs 1 and 2. They also demonstrate the extreme morphological variation along these PCs. Austrolimulids and belinurids fall in positive PC1 space; reflecting their extreme genal spine morphologies. Limulids and paleolimulids occupy more negative PC1 space. Albalimulus bottoni falls into the extremes of shape space occupied by Belinuridae and Austrolimulidae, in an area that almost overlays Limulidae. (A) Austrolimulus fletcheri (Australian Museum specimen AM F 38274, holotype). Note the large genal spine splay. (B) Psammolimulus gottingensis (Geowissenschaftliches Zentrum der Georg-August-Universität Geowissenschaftliches Museum specimen GZG INV 15356a 10). Note the indentation of the proximal genal spine section into the prosomal shield. (C) Bellinurus trilobitoides (Natural History Museum UK specimen NHMUK IP In 59324, holotype). Photo credit (A): Josh White; (B): Gerhard Hundertmark.
Phylogenetic Results
A phylogenetic analysis resulted in nine most parsimonious trees (CI: 0.468, RI: 0.877, tree length 743). The overall topology of a strict consensus tree produced from these trees (Fig. 8) reflects previous phylogenies that used the same matrix (see17,18,21,62). Our phylogenetic analysis placed Albalimulus bottoni within Limulidae, in a polytomy close to the base of the group. This polytomy contains Tarracolimulus rieki Romero and Via Boada, 197765, Mesolimulus crespelli Vía Boada, 198766, M. walchi (Desmarest, 1822)11, and the stem leading to extant taxa. Most notably, the node leading to Mesolimulus Størmer, 19528 observed in Lamsdell17 is collapsed into the polytomy in Fig. 8. A phylogenetic matrix that coded an additional 10 taxa collapsed many established families and resulted in a large polytomy (Supplemental Data 5).
Xiphosurid phylogeny produced using the Lamsdell17 phylogenetic matrix. Albalimulus bottoni (in bold and underlined) is located close to the base of Limulidae. Major groups are colour coded to match Fig. 5. Other clades coded into this phylogenetic matrix (Supplemental Data 4) were not presented as their placement in the phylogeny is unchanged and they do not relate directly to the current research. Abbreviation: Pal., Paleolimulidae.
Discussion
The systematic and phylogenetic placement of Albalimulus bottoni indicates that the Limuloidea, and potentially Limulidae, had evolved by the early Carboniferous (Tournaisian). If A. bottoni does indeed represent the oldest representative of Limulidae, it demonstrates that four xiphosurid groups had a Carboniferous origin and supports previous theories exploring this idea17. This also potentially extends the temporal range of the limulids by around 100 million years from the Triassic67 to the Mississippian and ultimately demonstrates that xiphosurid evolution is more complex than previously thought. The major limulid evolutionary events after A. bottoni are increased size, aligning with Cope’s Law68, and the maintenance of an overall morphology similar to A. bottoni. This suggests that evolutionary conservation within the group may have persisted over 350 million years69. Curiously, the key autapomorphy of A. bottoni—the pustulose cuticle ornament—was not retained, suggesting that it represents a derived trait in this taxon, or was lost in younger taxa.
Bicknell, et al.38 highlighted that geometric morphometric analyses of horseshoe crabs are strongly influenced by extreme prosomal morphologies; as exemplified by austrolimulids. The placement of morphologies with hypertrophied genal spines in very positive PC1 space reconfirms this observation. The possession of extremely pronounced genal spines is also associated with the habitation of freshwater conditions (explored in Anderson70 and more recently in Bicknell62). PC1 may therefore record a morphological response to palaeoenvironmental conditions and associated life modes. What then is the advantage of the larger genal spine splay? Fisher71 suggested that large genal spines might be used in sub-aerial activity and potentially be used as a defensive feature—an observation that aligns with evidence for predation thereon72. Alternatively, the hypertrophied genal spines may have served to reduce the impact of uni-directional currents in freshwater conditions, having a stabilising effect70. This thesis can be tested using computational fluid flow—a method that was recently used to model the functional impact of fluid on the horseshoe crab carapace73. Extending this method to analysing three-dimensional reconstructions of taxa with overdeveloped genal spine morphologies therefore represents a key direction for uncovering the impact (if any) that genal spines had on water-flow about the dorsal carapace.
As Albalimulus bottoni is the oldest known limuloid, and likely the oldest limulid, the life mode of the fossil species can be suggested by comparing to extant taxa7,74,75,76,77,78. Extant horseshoe crabs are omnivorous marine organisms that use gnathobases on the walking legs to masticate prey such as small molluscs, crustaceans and polychaete worms74,75,79. BGS.GSE 2028/9680 was collected from a succession that includes coastal floodplain, and marginal marine deposits; this indicates that the new taxon was likely marine, with a mode of life similar to that of extant taxa. It may also have experienced an infaunal life mode similar the comparably sized, immature extant individuals, as a detrital feeder7,80.
Conclusion
Albalimulus bottoni from the lower Carboniferous (Tournaisian) of Scotland represents the oldest known limuloid. Furthermore, the phylogenetic and geometric morphometric analyses presented here suggest that the taxon is most likely a limulid. These findings highlight that Limuloidea, and potentially Limulidae, had a deeper origin than previously documented and conforms to phylogenetic estimates from previous authors. If the phylogenetic placement of A. bottoni in Limulidae is correct, this discovery also shows that four of five xiphosurid groups had an origin in the Carboniferous. Regardless, the new specimen demonstrates that horseshoe crabs had a far more complex and diverse evolutionary history than previously noted and that further work is now needed to fill in the gap between A. bottoni and more recent limulids.
References
Rudkin, D. M. & Young, G. A. In Biology and Conservation of Horseshoe Crabs (eds John T. Tanacredi, Mark L. Botton, & David R. Smith) 25–44 (Springer, 2009).
Rudkin, D. M., Young, G. A. & Nowlan, G. S. The oldest horseshoe crab: a new xiphosurid from Late Ordovician Konservat‐Lagerstätten deposits, Manitoba, Canada. Palaeontology 51, 1–9 (2008).
Müller, O. F. Entomostraca seu Insecta Testacea, quae in aquis Daniae et Norvegiae reperit, descripsit et iconibus illustravit, (Symtibus Bibliopolii J.G. Mülleriani, Müller, O. F. Lipsiae et Havniae, 1785).
Latreille, P. A. Histoire naturelle, générale et particulière, des crustacés et des insectes. Vol. 3, 467 (Dufart, 1802).
Bicknell, R. D. C., Klinkhamer, A. J., Flavel, R. J., Wroe, S. & Paterson, J. R. A 3D anatomical atlas of appendage musculature in the chelicerate arthropod Limulus polyphemus. PLoS ONE 13, e0191400 (2018).
Owen, R. On the anatomy of the American King-crab (Limulus polyphemus, Latr.). Transactions of the Linnean Society of London 28, 459–506 (1872).
Shuster, C. N. Jr. A pictorial review of the natural history and ecology of the horseshoe crab Limulus polyphemus, with reference to other Limulidae. Progress in Clinical and Biological Research 81, 1–52 (1982).
Størmer, L. Phylogeny and taxonomy of fossil horseshoe crabs. Journal of Paleontology 26, 630–640 (1952).
Fisher, D. C. Phylogenetic and macroevolutionary patterns within the Xiphosurida. Proceedings of the Third North American Paleontological Convention 1, 175–180 (1982).
Selden, P. A. & Siveter, D. J. The origin of the limuloids. Lethaia 20, 383–392 (1987).
Desmarest, A.-G. In Histoire naturelle des crustacés fossiles, sous les rapports zoologiques et geologiques (eds A Brongniart & A. -G. Desmarest) 67–142 (F.-G. Levrault, 1822).
Desmarest, A.-G. Crustacés fossiles, in Société de Naturalistes et d’Agriculteurs, Nouveau Dictionnaire d’Histoire naturelle, appliquée aux Arts, à l’Agriculture, à l’Économie rurale et domestique, à la Médecine, etc. Vol. 7, 495–519 (1817).
Dunlop, J. A., Penney, D. & Jekel, D. In World Spider Catalog, version 19.5 Vol. 12 (Natural History Museum Bern, 2019).
Anderson, L. I. Xiphosurans from the Westphalian D of the Radstock Basin, Somerset Coalfield, the South Wales Coalfield and Mazon Creek. Illinois. Proceedings of the Geologists’ Association 105, 265–275 (1994).
Haug, C. et al. A holomorph approach to xiphosuran evolution—a case study on the ontogeny of Euproops. Development Genes and Evolution 222, 253–268 (2012).
Lamsdell, J. C. Revised systematics of Palaeozoic ‘horseshoe crabs’ and the myth of monophyletic Xiphosura. Zoological Journal of the Linnean Society 167, 1–27 (2013).
Lamsdell, J. C. Horseshoe crab phylogeny and independent colonizations of fresh water: ecological invasion as a driver for morphological innovation. Palaeontology 59, 181–194 (2016).
Selden, P. A., Lamsdell, J. C. & Qi, L. An unusual euchelicerate linking horseshoe crabs and eurypterids, from the Lower Devonian (Lochkovian) of Yunnan, China. Zoologica Scripta 44, 645–652 (2015).
Van Roy, P., Briggs, D. E. G. & Gaines, R. R. The Fezouata fossils of Morocco; an extraordinary record of marine life in the Early Ordovician. Journal of the Geological Society 172, 541–549 (2015).
Van Roy, P. et al. Ordovician faunas of Burgess Shale type. Nature 465, 215–218 (2010).
Bicknell, R. D. C., Lustri, L. & Brougham, T. Revision of ‘Bellinurus’ carteri (Chelicerata: Xiphosura) from the Late Devonian of Pennsylvania, USA. Comptes Rendus Palevol (2019).
Haug, C. & Rötzer, M. A. I. N. The ontogeny of the 300 million year old xiphosuran Euproops danae (Euchelicerata) and implications for resolving the Euproops species complex. Development Genes and Evolution 228, 63–74 (2018).
Raymond, P. E. Late Paleozoic xiphosurans. Bulletin of the Museum of Comparative Zoology 94, 475–508 (1944).
Frič, A. Preliminary note on Prolimulus woodwardi, Fritsch, from the Permian Gaskohle at Nyřan, Bohemia. Geological Magazine 6, 57–58 (1899).
Selden, P. A., Simonetto, L. & Marsiglio, G. An effaced horseshoe crab (Arthropoda: Chelicerata: Xiphosura) from the Upper Carboniferous of the Carnic Alps (Friuli, NE Italy). Rivista Italiana di Paleontologia e Stratigrafia 125, 333–342 (2019).
Racheboeuf, P. R., Vannier, J. & Anderson, L. I. A new three‐dimensionally preserved xiphosuran chelicerate from the Montceau‐Les‐Mines Lagerstätte (Carboniferous, France). Palaeontology 45, 125–147 (2002).
Pictet, F. J. Traite élémentaire de paléontologie. Vol. 4 (Langlois et Leclercq 1846).
Meek, F. B. Notes on a new genus of fossil Crustacea. Geological Magazine, Decade 4, 320–321 (1867).
Chernyshev, B. I. Arthropoda from the Urals and other regions of the USSR. Materials of the Central Scientific and Prospecting Institute Paleontology and Stratigraphy 1, 15–25 (1933).
Schram, F. R. Limulines of the Mississippian Bear Gulch Limestone of Central Montana, USA. Transactions of the San Diego Society of Natural History 19, 67–74 (1979).
Babcock, L. E., Merriam, D. F. & West, R. R. Paleolimulus, an early limuline (Xiphosurida), from Pennsylvanian‐Permian Lagerstätten of Kansas and taphonomic comparison with modern Limulus. Lethaia 33, 129–141 (2000).
Babcock, L. E. & Merriam, D. F. Horseshoe crabs (Arthropoda: Xiphosurida) from the Pennsylvanian of Kansas and elsewhere. Transactions of the Kansas Academy of Science 103, 76–94 (2000).
Dunbar, C. O. Kansas Permian insects, Part 2, Paleolimulus, a new genus of Paleozoic Xiphosura, with notes on other genera. American Journal of Science 5, 443–454 (1923).
Siveter, D. J. & Selden, P. A. A new, giant xiphosurid from the lower Namurian of Weardale, County Durham. Proceedings of the Yorkshire Geological Society 46, 153–168 (1987).
Waterston, C. D. Chelicerata from the Dinantian of Foulden, Berwickshire, Scotland. Earth and Environmental Science Transactions of The Royal Society of Edinburgh 76, 25–33 (1985).
Racheboeuf, P. R. Valloisella lievinensis n. g. n. sp.: nouveau Xiphosure carbonifère du Nord de la France. Neues Jahrbuch für Geologie und Paläontologie, Monatshefte 6, 336–342 (1992).
Lamsdell, J. C. & McKenzie, S. C. Tachypleus syriacus (Woodward)—a sexually dimorphic Cretaceous crown limulid reveals underestimated horseshoe crab divergence times. Organisms Diversity &. Evolution 15, 681–693 (2015).
Bicknell, R. D. C. et al. A new limulid genus from the Strelovec Formation (Middle Triassic, Anisian) of northern Slovenia. Geological Magazine, 1–14 (2019).
Kearsey, T. I. et al. The terrestrial landscapes of tetrapod evolution in earliest Carboniferous seasonal wetlands of SE Scotland. Palaeogeography, Palaeoclimatology, Palaeoecology 457, 52–69 (2016).
Smithson, T. R., Richards, K. R. & Clack, J. A. Lungfish diversity in Romer’s Gap: reaction to the end‐Devonian extinction. Palaeontology 59, 29–44 (2016).
Marshall, J. E. A. et al. Reinterpreting the age of the uppermost ‘Old Red Sandstone’and Early Carboniferous in Scotland. Earth and Environmental Science Transactions of the Royal Society of Edinburgh, 1–14 (2019).
Smithson, T. R., Wood, S. P., Marshall, J. E. A. & Clack, J. A. Earliest Carboniferous tetrapod and arthropod faunas from Scotland populate Romer’s Gap. Proceedings of the National Academy of Sciences 109, 4532–4537 (2012).
Clack, J. A. et al. Phylogenetic and environmental context of a Tournaisian tetrapod fauna. Nature Ecology & Evolution 1, 0002 (2017).
Clack, J. A. et al. A Tournaisian (earliest Carboniferous) conglomerate-preserved non-marine faunal assemblage and its environmental and sedimentological context. PeerJ 6, e5972 (2019).
Ross, A. J. et al. A new terrestrial millipede fauna of earliest Carboniferous (Tournaisian) age from southeastern Scotland helps fill ‘Romer’s Gap’. Earth and Environmental Science Transactions of the Royal Society of Edinburgh 108, 99–110 (2018).
Williams, M., Stephenson, M., Wilkinson, I. P., Leng, M. J. & Miller, C. G. Early Carboniferous (Late Tournaisian–Early Viséan) ostracods from the Ballagan Formation, central Scotland, UK. Journal of Micropalaeontology 24, 77–94 (2005).
Lerner, A. J., Lucas, S. G. & Lockley, M. First fossil horseshoe crab (Xiphosurida) from the Triassic of North America. Neues Jahrbuch für Geologie und Paläontologie-Abhandlungen 286, 289–302 (2017).
Adams, D. C. & Otárola‐Castillo, E. geomorph: an R package for the collection and analysis of geometric morphometric shape data. Methods in Ecology and Evolution 4, 393–399 (2013).
Goloboff, P. A. & Catalano, S. A. TNT version 1.5, including a full implementation of phylogenetic morphometrics. Cladistics 32, 221–238 (2016).
Weygoldt, P. & Paulus, H. F. Untersuchungen zur Morphologie, Taxonomie und Phylogenie der Chelicerata. Zeitschrift für zoologische Systematik und Evolutionsforschung 17, 85–115, 177–200 (1979).
Richter, R. & Richter, E. Weinbergina opitzi ng, n. sp., ein Schwertträger (Merost., Xiphos.) aus dem Devon (Rheinland). Senckenbergiana 11, 193–209 (1929).
Zittel, K. A. V. Handbuch der Palaeontologie. I. Abtheilung, Palaeozoologie. (R. Oldenbourg, 1885).
Linnaeus, C. Systema naturæ per regna tria naturæ, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. 10 edn, (1758).
Riek, E. F. A new xiphosuran from the Triassic sediments at Brookvale, New South Wales. Records of the Australian Museum 23, 281–282 (1955).
Anderson, L. I. & Horrocks, C. Valloisella lievinensis Racheboeuf, 1992 (Chelicerata: Xiphosura) from the Westphalian B of England. Neues Jahrbuch für Geologie und Paläontologie, Monatshefte 11, 647–658 (1995).
Dunlop, J. A. & Selden, P. A. Scorpion fragments from the Silurian of Powys, Wales. Arachnology 16, 27–33 (2013).
Marshall, D. J., Lamsdell, J. C., Shpinev, E. & Braddy, S. J. A diverse chasmataspidid (Arthropoda: Chelicerata) fauna from the Early Devonian (Lochkovian) of Siberia. Palaeontology 57, 631–655 (2014).
Tetlie, O. E. Like father, like son? Not amongst the eurypterids (Chelicerata) from Beartooth Butte, Wyoming. Journal of Paleontology 81, 1423–1431 (2007).
Waterston, C. D. Problems of functional morphology and classification in stylonuroid eurypterids (Chelicerata, Merostomata), with observations on the Scottish Silurian Stylonuroidea. Earth and Environmental Science Transactions of The Royal Society of Edinburgh 70, 251–322 (1979).
Miller, J. The sensory fields and life mode of Phacops rana (Green, 1832) (Trilobita). Earth and Environmental Science Transactions of The Royal Society of Edinburgh 69, 337–367 (1976).
Lamsdell, J. C. Selectivity in the evolution of Palaeozoic arthropod groups, with focus on mass extinctions and radiations: a phylogenetic approach, University of Kansas, (2014).
Bicknell, R. D. C. Xiphosurid from the Upper Permian of Tasmania confirms Palaeozoic origin of Austrolimulidae. Palaeontologia Electronica 22, 1–13 (2019).
Lange, W. Über neue Fossilfunde aus der Trias von Göttingen. Zeitschrift der deutschen geologischen Gesellschaft 74, 162–168 (1923).
Buckland, W. The Bridgewater treatises on the power, wisdom and goodness of God as manifested in the creation. Treatise IV. Geology and mineralogy with reference to natural theology., Vol. 2 (William Pickering, 1837).
Romero, P. A. & Via Boada, L. “Tarracolimulus rieke” nuevo Limulido del Triasico de Montral-Alcover (Tarragona). Cuadernos Geologia Iberica 4, 239–246 (1977).
Vía Boada, L. Artropodos fosiles Triasicos de Alcover-Montral. II. Limulidos. Cuadernos Geología Ibérica 11, 281–294 (1987).
Hu, S. et al. Exceptional appendage and soft-tissue preservation in a Middle Triassic horseshoe crab from SW China. Scientific Reports 7, 14112 (2017).
Benson, R. B. J., Hunt, G., Carrano, M. T. & Campione, N. Cope’s rule and the adaptive landscape of dinosaur body size evolution. Palaeontology 61, 13–48 (2018).
Bicknell, R. D. C. et al. On the appendicular anatomy of the xiphosurid Tachypleus syriacus and the evolution of fossil horseshoe crab appendages. The Science of Nature 106, 38 (2019).
Anderson, L. I. Taphonomy and taxonomy of Palaeozoic Xiphosura PhD thesis, University of Manchester, (1996).
Fisher, D. C. In Mazon Creek Fossils (ed. Matthew H. Nitecki) 379–447 (Elsevier, 1979).
Bicknell, R. D. C., Pates, S. & Botton, M. L. Abnormal xiphosurids, with possible application to Cambrian trilobites. Palaeontologia Electronica 21, 1–17 (2018).
Davis, A. L., Hoover, A. P. & Miller, L. A. Lift and drag acting on the shell of the American horseshoe crab (Limulus polyphemus). Bulletin of Mathematical Biology, 1–20 (2019).
Bicknell, R. D. C. et al. Computational biomechanical analyses demonstrate similar shell-crushing abilities in modern and ancient arthropods. Proceedings of the Royal Society B: Biological Sciences 285, 20181935 (2018).
Bicknell, R. D. C., Paterson, J. R., Caron, J.-B. & Skovsted, C. B. The gnathobasic spine microstructure of Recent and Silurian chelicerates and the Cambrian artiopodan Sidneyia: Functional and evolutionary implications. Arthropod Structure & Development 47, 12–24 (2018).
Razak, M. R. M. & Kassim, Z. Feeding mechanisms of adult tropical horseshoe crab, Tachypleus gigas toward feeds’ conditions. ASM Science Journal 11, 76–85 (2018).
Razak, M. R. M. & Kassim, Z. Food intake, gut transit time and defecation pattern of Asian horseshoe crab, Tachypleus gigas. ASM Science Journal 11, 56–66 (2018).
Botton, M. L. Diet and food preferences of the adult horseshoe crab Limulus polyphemus in Delaware Bay, New Jersey, USA. Marine Biology 81, 199–207 (1984).
Akbar John, B., Kamaruzzaman, B. Y., Jalal, K. C. A. & Zaleha, K. Feeding ecology and food preferences of Carcinoscorpius rotundicauda collected from the Pahang nesting grounds. Sains Malaysiana 41, 855–861 (2012).
Gaines, E. F., Carmichael, R. H., Grady, S. P. & Valiela, I. Stable isotopic evidence for changing nutritional sources of juvenile horseshoe crabs. The Biological Bulletin 203, 228–230 (2002).
Acknowledgements
This research was supported by funding from a University of New England Postdoctoral Research Fellow (to R.D.C.B.), a Charles Schuchert and Carl O. Dunbar Grants-in-Aid Award (to R.D.C.B.), a James R Welch Scholarship (to R.D.C.B), and an Alexander Agassiz Postdoctoral Fellowship (to S.P.). This research was published by a grant from the Wetmore Colles Fund. We thank the BGS.GSE for sending the specimens to R.D.C.B. We thank David Marshall for his insightful ideas regarding pustulose cuticle. We thank Peter Van Roy for his review of a pervious draft; his comments thoroughly improved the text. We thank Josh White and Gerhard Hundertmark for images of specimens. We thank Elissa Johnson for her stunning reconstruction of Albalimulus bottoni. TNT is made available through a subsidy by the Willi Hennig Society. Finally, we thank David Marshall and an anonymous referee for their insightful reviews that helped direct and improve the text.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bicknell, R.D.C., Pates, S. Xiphosurid from the Tournaisian (Carboniferous) of Scotland confirms deep origin of Limuloidea. Sci Rep 9, 17102 (2019). https://doi.org/10.1038/s41598-019-53442-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-53442-5
This article is cited by
-
A reappraisal of Paleozoic horseshoe crabs from Russia and Ukraine
The Science of Nature (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.