Abstract
Nudix (for nucleoside diphosphatases linked to other moieties, X) hydrolases are a diverse family of proteins capable of cleaving an enormous variety of substrates, ranging from nucleotide sugars to NAD+-capped RNAs. Although all the members of this superfamily share a common conserved catalytic motif, the Nudix box, their substrate specificity lies in specific sequence traits, which give rise to different subfamilies. Among them, NADH pyrophosphatases or diphosphatases (NADDs) are poorly studied and nothing is known about their distribution. To address this, we designed a Prosite-compatible pattern to identify new NADDs sequences. In silico scanning of the UniProtKB database showed that 3% of Nudix proteins were NADDs and displayed 21 different domain architectures, the canonical architecture (NUDIX-like_zf-NADH-PPase_NUDIX) being the most abundant (53%). Interestingly, NADD fungal sequences were prominent among eukaryotes, and were distributed over several Classes, including Pezizomycetes. Unexpectedly, in this last fungal Class, NADDs were found to be present from the most common recent ancestor to Tuberaceae, following a molecular phylogeny distribution similar to that previously described using two thousand single concatenated genes. Finally, when truffle-forming ectomycorrhizal Tuber melanosporum NADD was biochemically characterized, it showed the highest NAD+/NADH catalytic efficiency ratio ever described.
Similar content being viewed by others
Introduction
Nudix or NUDT (Nudix-type)1 hydrolases are Mg2+/Mn2+-dependent enzymes active towards nucleoside diphosphates linked to other moieties (X)2 and forming a superfamily distributed throughout the phylogenetic scale with more than 200,000 entries in the UniProtKB database. They share the characteristic conserved sequence required for substrate catalysis named the Nudix box (GX5EX7REUXEEXGU), where U represents a bulky hydrophobic amino acid, usually Ile, Leu or Val2,3,4,5. Nucleotide sugars, diadenosine polyphosphates, nucleoside triphosphates, ADP-ribose, NADH and NAD+ are among their most common substrates6. In addition, some Nudix hydrolases have the ability to degrade protein-conjugated ADP-ribose, as is the case with human NUDT167,8. These compounds participate in crucial processes that require tight regulation. In fact, some Nudix hydrolases are overexpressed following cellular stress in order to recover homeostasis9.
These enzymes can be divided into subfamilies depending on specific sequence traits that are involved in substrate recognition, as first stated by Bessman´s group when they studied four ADP-ribose pyrophosphatases3. Thus, a conserved proline was found 16 amino acids downstream the Nudix box for all members of the ADP-ribose pyrophosphatase subfamily, whereas an invariant tyrosine designated another subfamily, the diadenosine polyphosphate pyrophosphatases3. Finally, an array of eight conserved amino acids (SQPWPFPXS) was seen to be characteristic of NADH pyrophosphatases or diphosphatases (NADDs)3,10. The first member of this last family was biochemically characterized in Escherichia coli as the protein product of the nudC gene, formerly known as orf257, which catalyses the hydrolysis of NAD(H) to AMP and NMN(H)11. Several other NADH pyrophosphatases have been described in different organisms, such as mouse NUDT1312, human NUDT129,13, and Saccharomyces cerevisiae and Caenorhabditis elegans NPY114. In addition, Arabidopsis thaliana present more than one NUDT representative, including NUDT1, NUDT2, NUDT6, NUDT7, NUDT10 and NUDT1910,15,16,17. However, AtNUDT1 activity was negligible at Mn2+ physiological levels18. AtNUDT2, 6, and 7 hydrolyse both ADP-ribose and NADH with almost equal catalytic efficiencies, whereas AtNUDT10 prefers ADP-ribose over NADH, and AtNUDT19, NADPH over NADH10. Of special interest are NADH pyrophosphatases from pathogenic organisms, such as those of Mycobacterium species (M. tuberculosis, M. bovis and M. smegamatis), which have been found to be involved in the degradation of the active forms of anti-tuberculosis drugs, such as isoniazid (INH-NAD) and ethionamide (ETH-NAD)19,20. In addition, NADH pyrophosphatase plays a crucial role in the assimilation of exogenous NAD+ in Salmonella typhimurium21, whereas in Haemophilus influenzae, it has also been described as the enzyme responsible for the growth of the pathogen in NAD+ containing media22. Interestingly, E. coli NudC has also been recently related with the RNA-decapping process, since it was able to efficiently remove the NAD+ cap in different prokaryotic RNAs, hydrolysing the pyrophosphate bond to produce nicotinamide mononucleotide (NMN) and 5′-monophosphate RNA23,24.
However, all the above studies have only provided a partial view of the NADH diphosphatase subfamily diversity. To address this, we have carried out an extensive bioinformatic analysis, taking into account other important amino acids recently described in the EcNudC structure6,23, giving rise to a new Prosite-compatible NADD pattern, which expands the previously published NADH diphosphatase sequence array. This in silico study provides the first phylogenetic distribution of NADDs, and also gives a picture of their domain architectures. The result revealed that NADH diphosphatases represent a small number of sequences (about 3%) of the Nudix superfamily. In addition, bacterial sequences are more abundant than those of eukaryotic organisms. However, among the latter, fungal sequences are profuse and widely distributed among different Classes, including Pezizomycetes, which produce relatively large fruiting bodies (apothecia) from epigeous, semi-hypogeous to hypogeous (truffles) origin25. Some of the last mentioned truffles are highly prized gastronomic delicacies, such as the black truffle of Périgord (Tuber melanosporum Vittad.)26. Of note, and totally unexpected, is the fact that the phylogenetic study made with known Pezizomycetes NADD sequences corroborated a previously reported molecular phylogeny carried out with several hundred conserved concatenated single-copy protein-coding genes27. In addition, the cloning and kinetic characterization of T. melanosporum NADD uncovers a new efficient biocatalyst with the highest NAD+/NADH catalytic efficiency ratio ever described.
Results
NADH pyrophosphatases represent a small but diverse group in the Nudix superfamily
E. coli NudC (aka EcNADD) structures (PDB codes 5IW4, 5IW5 and 5ISY) (Supplementary Fig. S1A) and the sequence alignment with other biochemically characterized NADH pyrophosphatases (Fig. 1) were used to determine key sequence features to scan the UniProt database in an attempt to discover new canonical NADD members. The monomeric structure consists of an N-terminal domain (residues 1–92, EcNudC numbering) (aka NUDIX-like) and a C-terminal Nudix domain (residues 126–257, aka NUDIX, Fig. 1), formed by a six-stranded mixed sheet (β11–β16) sandwiched between two perpendicular α-helices (α2 and α3) (Fig. 1, Supplementary Fig. S1B). These two domains are separated by a zinc-binding domain (aka zf-NADH-PPase) (residues 93–125), which takes part in the dimerization of NudC and protrudes from the NTD6,23. The zinc is coordinated with four cysteine residues (C98, C101, C116 and C119) (Fig. 1, triangles, Supplementary Fig. S1B), of which, the first three are mainly conserved in described NADDs with the exception of that of Mycobacterium tuberculosis (Fig. 1). The NAD+ is bound into a pocket mainly found in the Nudix domain and with the participation of two amino acids from the zinc-binding domain, the non-conserved E111 and Y124 (Fig. 1, diamonds; Supplementary Fig. S1C). This last residue is involved in the adenine base binding via a π-π interaction with the aromatic ring of F160 (Fig. 1, star) from subunit A23. The nicotinamide moiety binds in a cavity comprising several bulky hydrophobic residues (I132, W194 and M201), in addition to Q192 and A241 (Fig. 1, circles), the last two residues being involved in the hydrogen bonding with the amide group of nicotinamide. These amino acids together with S199 (Fig. 1, circles), which interacts with the 3-hydroxyl group of the nicotinamide ribose, form the conserved eight-amino acid motif (SQPWPFPXS) downstream from the Nudix box (residues 159–181) (aka NADH signature) (Fig. 1), which has previously been used to classify the NADH hydrolase subfamily3. Based on the relevance of the above mentioned amino acids, the catalytic residues in the Nudix box (E174, E177 and E178) (Fig. 1, squares) and the essential role of NudC dimerization in substrate recognition, the Prosite-compatible pattern [CS]x(2)[CD]x(12,15)[CN]x(5,35)[YF]Px(3)Px(2)Ix(25,32)GFx(4)Ex(7)REx(2)EEx(13,14)Q[PQ]W[PA]xPx(2,9)[QLIMA]M was designed (Fig. 1, red letters; Supplementary Fig. S1C). This pattern covers the distance between C98 and M201, and basically represents the zinc domain ([CS]x(2)[CD]x(12,15)[CN]x(5,35)[YF]) and the conserved amino acids in the Nudix domain without β16 and α3 (Fig. 1), which includes the Nudix Box (GFx(4)Ex(7)REx(2)EE) and part of the conserved eight-amino acid motif, known as NADD signature (Q[PQ]W[PA]xP) (Supplementary Fig. S1D).
Multiple sequence alignment of NADH diphosphatases. The sequence of EcNudC (UniProtKB accession number, P32664) was aligned with its homologs from Salmonella typhimurium (StNADD, Q9L9I5), Haemophilus influenza (HiNADD, Q4QNB3), Mycobacterium tuberculosis (MtNADD, A5U7L9), Caenorhabditis elegans (CeNADD, Q19427), Saccharomyces cerevisiae (ScNADD, P53164), human (hNUDT12, Q9BQG2), Bos taurus (BtNUDT12, Q29RH3) and Tuber melanosporum (TmNADD, D5GP45). The four cysteine residues (C98, C101, C116 and C119) from the zinc-binding domain are marked with inverted triangles (▼). Residues involved in NAD+-binding pocket from the Nudix domain are marked with circles (●), whereas those from the zinc-binding domain with diamonds (♦). Catalytic residues are denoted with squares (■) and F160, involved in the adenine base binding via a π-π interaction, is marked with a star (⋆). The key amino acids of the Prosite pattern are shown in red letters above the alignment.
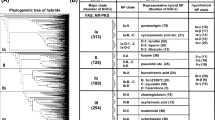
When this pattern was scanned against a Nudix protein sequence database composed of 234,112 sequences obtained using the Prosite pattern PS51462 (Nudix) in the UniprotKB database (including Swiss-Prot and TrEMBL; release 2018–08), 7479 sequences (3,2%) were NADDs. Among them, 90% were from Bacteria, 9.4% from Eukaryota, 0.24% from Archaea and the rest from metagenomic sources. No viral sequences were obtained. In Archaea, they were basically found in the Methanomicrobia Class, whereas in Bacteria, they are almost equally distributed between Gammaproteobacteria (39%), Alphaproteobacteria (30%) and Actinobacteria (23%), with two Orders distinguished by their great number of sequences, Enterobacterales (1008) and Rhizobiales (809). In Eukaryota, the sequences corresponding to mammals (80), birds (35) and bony fishes (26), together with those of roundworms (31), are particularly numerous, although the largest number belongs to Fungi (478). Among them, Ascomycota is the most abundant (86.6%), followed by Basidiomycota (8.3%), Chytridiomycota (1.0%), Mucoromycota (1.8%), Zoopagomycota (1.8%), and one sequence from the fungal sp. No.14919 (0.2%) (Fig. 2).
In addition, although NADDs are distributed among different fungal subphyla, Pezizomycotina (373) and Saccharomycotina (37) are clearly the most important in the retrieved sequences, followed by Agaricomycotina (25) (Fig. 2). Furthermore, within Pezizomycotina, the classes Eurotiomycetes (161) and Sordariomycetes (136) are relevant, since they include several sequences from Fusarium spp. or Aspergillus spp. NADDs are also represented in the Pezizomycotina basal groups, such as Orbiliomycetes and Pezizomycetes, with three and five sequences, respectively (Fig. 2). In particular, they were present in truffle-forming ectomycorrhizal species that are economically important gourmet delicacies, such as the aromatic Périgord black truffle (Tuber melanosporum Vittad.) (UniProtKB accession number: D5GP45), Burgundy truffle (T. aestivum Vittad.) (A0A292PI49), Piedmont white truffle (T. magnatum Pico) (A0A317SZB3) and whitish edible truffle (T. borchii Vittad.) (A0A2T6ZTD3).
To contextualise the results described above, the distribution of NADDs was also compared with the entire Nudix hydrolase family and another protein family, Peptidase S8 (Subtilisin and Subtilisin-like proteases) (Table 1). The results obtained showed that NADDs were less frequent in Archaea (0.24%) than in Nudix hydrolases (2.5%) and the Peptidase S8 family (4.2%). On the other hand, the percentage of NADDs in Fungi was almost double to that found in the entire Nudix family (6.4% vs. 3.6%), but lower to that found in the Peptidase S8 family (10.5%). The same percentage distribution pattern (0.07% vs 0.03%, 0.09%) was observed in the Pezizomycetes Class (Table 1). Moreover, although the NADD and NUDIX sequences are overrepresented in Bacteria, as they are in the UniProt TrEMBL database (~74%) compared to Eukaryota (~24%) (https://www.uniprot.org/uniprot/?query=reviewed:no#orgViewBy), when the latter kingdom was considered alone, NADDs appeared to be more represented in Fungi (68%) than in the NUDIX (39%) and Peptidase S8 (38%) families, respectively (Table 1). This higher presence was also found in the Ascomycota phylum (59% vs. 28%) and in the Pezizomycetes class (0.7% vs. 0.4%) (Table 1).
NADH pyrophosphatases show multiple domain architectures apart from the canonical
All the above-mentioned truffle NADDs have the canonical NADD domain architecture (i.e. NUDIX-like_zf-NADH-PPase_NUDIX) (Fig. 3, Supplementary Table S1), which is also present in 54% of the fungal sequences (Table 2). The second most abundant NADD domain architecture in fungi is NUDIX-like_NUDIX (36%), followed by zf-NADH-PPase_NUDIX and NUDIX with 4% and 3%, respectively (Fig. 3, Table 2). In addition, there are other fungal minority domain architectures with a linear solenoid structure called ankyrin repeat domains (ANK) involved in protein-protein interactions, such as Ank5_NUDIX-like_zf-NADH-PPase_NUDIX (4), Ank2_NUDIX-like_NUDIX (2), Ank4_NUDIX-like_NUDIX (1) and Ank2_NUDIX-like_zf-NADH-PPase_NUDIX (2) (Fig. 3, Table 2). This last domain organization is similar to that described for human peroxisomal NUDT129. In addition, there are also some unusual domain architectures, one with an ADP ribosylation factor (ARF) domain that function as a regulator of vesicular traffic, three with an actin remodelling motif (NUDIX-like_NUDIX_Arf), one isochorismatase_NUDIX-like_NUDIX and one MULE_NUDIX-like_zf-NADH-PPase_NUDIX (Table 2). The last MULE domain is an all-beta structure that is found in Mutator-like elements (MULE)-encoded transposases, which are related with DNA transposable sequences that can move from one locus to another in the genome28,29.
Phylogenetic analysis of fungal NADDs. The Neighbour-Joining (NJ) tree with 1000 replicates was constructed using MAFFT server. Protein domain architecture is shown beside each protein code: NUDIX-like (red), zf-NADH-PPase (green), NUDIX (blue) and ANK (purple). Fungal sequences are summarized in Supplementary Table S1.
The ranking found in domain architecture in fungal NADD sequences is quite similar to that found in the 7479 NADDs used in this study, i.e. 53% for canonical NUDIX-like_zf-NADH-PPase_NUDIX, 37% for zf-NADH-PPa.5se_NUDIX, 4.5% for NUDIX-like_NUDIX, 2.8% for NUDIX and 1.8% for Ank2_NUDIX-like_zf-NADH-PPase_NUDIX (Fig. 4, Table 2). However, this last domain structure increases to 18.6% in eukaryotes. In the remaining domain architectures, which represent only 2%, are the typical NADDs domains combined with other different domains, such as a transmembrane amino acid transporter protein (Aa-trans), an alpha/beta hydrolase 6 family (Abhydrolase-6), a domain of unknown function (DUF2805), a histidine triad (HIT) present in nucleotide hydrolases that acts on the α-phosphate of ribonucleotides, an ion channel (Ion-trans-2), a domain that binds to NAD+ (TrkA-N), or a putative transposase DNA-binding domain (OrfB-Zn-ribbon) with four conserved cysteines, which could play a similar role to zf-NADH-PPase domain (Fig. 4). Finally, NADD architectures were also compared with that of the Nudix hydrolase family in PFAM (Table 2). Clearly, NUDIX domain alone was the most abundant (83.4%), and the sum of canonical NADD domain plus zf-NADH-PPase_NUDIX accounted for 3.9% of total Nudix hydrolases (Table 2). This percentage is similar to that obtained for the Prosite pattern described in this paper. The rest of the domain architectures are present at less than 0.1% in the NUDIX family, except for NUDIX-like_NUDIX (0.6%) (Table 2).
Domain architecture organization of the NADD subfamily. Domain architectures (21) are shown indicating the UniProtKB accession number of a representative sequence and the number of sequences with each architecture organization. The most important domains are coloured as in Fig. 3.
MISTIC analysis reveals new important hydrophobic amino acids in fungal NADD sequences
Mutual Information (MI) Server to Infer Coevolution (MISTIC) was used to predict positional correlations in the multiple sequence alignment (MSA) corresponding to NADDs sequences in order to guide the identification of structurally or functionally important positions in the fungal NADD family (Fig. 5)30. T. melanosporum NADD sequence (TmNADD, UniProtKB accession number: D5GP45) and its corresponding modelled structure were set as references. Circos representation shows that the information is basically concentrated in three main regions of the protein: residues 203–258 (Zn-binding domain), 292–314 (Nudix box) and 324–332 (NADD signature) (see outer histogram pMI and inner MI connection lines) (Fig. 5). However, previously undescribed regions can be found in the NTD (NUDIX-like domain), such as 108–114 and 172–202, with individual residues (hubs) with high cumulative Mutual Information (cMI) values (i.e., a large number of MI connections), such as L111, G112 and W198 (Fig. 5, solid circles). Similarly, another region (349–363) is found in the Nudix domain, with high cMI values in D353 and E355 (Fig. 5, solid circles). As regards conserved positions (Fig. 5, coloured square boxes of the second circle), the four cysteines in the zinc-binding domain are clearly highlighted (C205, C208, C223 and C244) in red (Fig. 5, triangles), together with three tryptophan residues (W198, W327 and W361) (Fig. 5, squares), pointing to functionally important residues in NADDs that remain to be studied (Fig. 5).
Circos representation of the fungal NADDs.The outer ring shows the amino acid code corresponding to TmNADD (UniProtKB accession number, D5GP45). Coloured rectangular boxes of the second circle indicate the KL (Kullback- Leibler) conservation score (from red to cyan, red: highest; cyan: lowest). The third circle shows the cMI (cumulative Mutual Information score) scores as histograms, which measure the degree of shared mutual information of a given residue. The fourth circle shows the pMI (proximity Mutual Information score), which describes the networks of mutual information in the proximity of a residue. Lines in the centre of the circle connect pairs of positions with MI (Mutational Information) score >6.5. Red lines represent the top 5%, the black lines between 70 and 95%, and the grey lines account for the last 70%. Sequence distribution of Zn-binding domain, Nudix box and NADD signature are shown. The four cysteines from the zinc-binding domain are marked with inverted triangles (▼). Residues with high cMI are marked with circles (●), whereas tryptophan conserved residues are marked with squares (■).
Phylogenetic analysis of Pezizomycetes NADH pyrophosphatases agrees with their described molecular evolution
In order to explore similar sequences in other Pezizomycetes, the fungal genomes described in the MycoCosm portal were also scanned (https://genome.jgi.doe.gov/mycocosm/home). A total of twenty-four sequences were found, including the five previously used from UniProtKB (Supplementary Table S2). The new phylogenetic NADD tree obtained showed two main clades (Fig. 6A). The first corresponds to the basal clade formed by two main sub-clades, those of Ascobolaceae (1) and Pezizaceae (5) families, respectively, in the last of which, most desert truffles (Tirmania nivea, Terfezia claveryi Chatin, Te. boudieri Chatin and Kalaharituber pfeilii) are found together with the saprophytic cup fungus Peziza echinospora. The second clade shows six sub-clades each corresponding to members of the Ascodesmidaceae (1), Pyronemataceae (6), Sarcosomataceae (1), Sarcoscyphaceae (1), Discinaceae (1), Morchellaceae (3) and Tuberaceae (5) families. The clades obtained with NADD proteins was in accordance with the phylogram generated from maximum likelihood analysis of combined LSU, SSU, ITS, TEF and RPB2 sequence data from 40 pezizalian species25. The latter study also showed Ascobolaceae (1) and Pezizaceae in basal clade 1, Ascodesmidaceae and Pyronemataceae in clade 6, Sarcosomataceae and Sarcoscyphaceae in clade 5, and finally Discinaceae, Morchellaceae and Tuberaceae in clade 425. The above concordance with NADD distribution found for taxa of Pezizomycetes also agreed with the molecular phylogeny obtained using 2,093 concatenated conserved single-copy protein-coding genes from the eight Pezizomycetes27, in which NADD seems to be present in the most recent common ancestor (MRCA) of Pezizomycetes 470 ± 67 million years ago (Ma), starting from Ascobolaceae, and continuing present until Tuberaceae began diverging around 140 ± 10 Ma in the Early Cretaceous27,31. Interestingly, this MRCA seems to have the canonical NADD domain architecture (i.e. NUDIX-like_zf-NADH-PPase_NUDIX), since all the NADDs used in Fig. 6A have this pattern. Of note, in the NADD tree, Choiromyces venosus NADD forms a distinct branch from Tuber spp. within Tuberaceae, and T. melanosporum from the rest of Tuber spp., as previously described in the molecular phylogeny27.
NADD distribution in Pezizomycetes. (A) Phylogenetic analysis of Pezizomycetes NADDs. The Neighbour-Joining (NJ) tree with 1000 replicates was constructed using MAFFT server. Saprotrophic (SAP) or ectomycorrizal (ECM) origin are indicated. Pezizalian sequences are summarized in Supplementary Table S2. (B) Comparative genome map of T. melanosporum gene CAZ86310 and those corresponding to T. magnatum, T. aestivum and T. borchii. Gene codifying the NADD proteins are marked in orange and the terpene cyclase/mutase family member gene in green.
Genome context analysis of the above selected sequences was also carried out, but not all genomes are annotated in Ensembl Fungi (http://fungi.ensembl.org/index.html). However, this is not the case for the above used Tuber spp., whose genome organization reveals a common pattern in NADD flanking genes (Fig. 6B). Thus, the NADD gene is preceded in all cases by a gene whose product is an uncharacterized protein of similar length (1517–1710 amino acids) (Fig. 6B, red), and followed by another gene whose product is a terpene cyclase/mutase family member of 719 amino acids length (Fig. 6B, green), except in the case of T. borchii, whose gene product is an uncharacterized protein of 323 amino acids. This difference in the genomic structure could also be related to the fact that T. borchii forms a sister phylogenetic group with respect to other Tuber spp31.
TmNADD is a highly active NAD+ pyrophosphatase
After sequence-based bioinformatics analysis, the GSTUM_00011698001 gene (aka CAZ86310 in Ensembl Fungi) (Fig. 6B) from T. melanosporum (strain Mel28) (Perigord black truffle) was cloned into pET28a, transformed into E. coli Rosetta 2 and purified in two-steps, which combines an initial Ni2+ affinity chromatography step with a size exclusion in Superdex 200. The enzyme obtained was electrophoretically pure (Supplementary Fig. S2) and showed activity towards NAD+ in the presence of both Mn2+ or Mg2+, but with clear differences depending on the concentrations used (Supplementary Fig. S3), as previously described for EcNADD11. Manganese ion was clearly a better divalent metal ion than Mg2+, since lower concentrations are needed to reach maximal activity (0.5 mM vs 10 mM). In addition, Mn2+ gave rise to a 1.7-fold increase in activity at optimal conditions when compared with Mg2+ (Supplementary Fig. S3). However, at higher concentrations (>0.5 mM), Mn2+ showed an inhibitory effect (Supplementary Fig. S3). This preference for Mn2+ has also been described in human NUDT12 and mouse NUDT13, where it showed a 3-fold increase in activity compared to Mg2+12,13. That preference for Mn2+ seems to be a general characteristic in NADH pyrophosphatases, since it was also described for the E. coli and S. cerevisiae Nudix hydrolases11,32.
The influence of the pH in the reaction catalysed by TmNADD was studied from pH 6.0 to pH 10.0, a clear optimum pH being observed at pH 9.0 (Fig. 7A), with a sharp decrease in activity above and below this value, especially at pH 9.5–10.0. This basic optimum pH was also found in other NADDs, such as hNUDT12 (pH 8.0–9.0), mouse NUDT13 (pH 8.2), EcNudC (pH 8.5) and CeNADD (pH 8.5)11,12,13,14, data which are consistent with the general alkaline nature of most Nudix hydrolases described up to date2,5. In addition, at pH 9.0, TmNADD was also more stable than at any other pH values (Fig. 7C), maintaining above 50% of its activity for 6 hours in glycine buffer (Fig. 7C, inverted triangles), whereas in Tris-HCl at the same pH it was about 8% less stable. This stability decreased gradually from pH 8.0 to pH 7.0, and was completely abolished in 1 hour at pH 6.0 (Fig. 7C, diamonds). However, at pH 10.0, the enzyme maintained 32% of its activity for the same time (Fig. 7C, closed circles). Such pH stability results cannot be compared, since no previous NADD stability studies have been carried out. For comparative purposes, the rest of the biochemical characterization was carried out at pH 8.0, as previously described11,12,13,14.
Effect of pH and temperature on TmNADD activity. (A) Effect of pH. Assay conditions were 0.5 mM MnCl2, 5 mM NAD+ and 0.11 μM of TmNADD in different 50 mM buffers at 37 °C. The activity was measured by HPLC for 15 min, as described in Materials and Methods. The buffers used were sodium phosphate pH 6.0–7.5, Tris-HCl pH 8.0–8.5, and glycine pH 9.0–10.0. (B) Effect of temperature. Assay conditions were the same as above but at pH 8.0 and different temperatures (20–70 °C). (C) Effect of pH on enzyme stability. TmNADD was incubated at 37 °C for different periods of time (0–360 min) at different pHs, and the activity was measured in the above standard reaction conditions. The buffers used (50 mM) were sodium phosphate pH 6.0 (●), pH 7.0 (■), Tris-HCl pH 8.0 (▲), pH 9.0 (○), and glycine pH 9.0 (▼), pH 10.0 (♦). (D) Effect of temperature on enzyme stability. The enzyme was incubated at pH 8.0 under the above standard conditions for different periods of time (0–360 min) at different temperatures [20 °C (●), 37 °C (■), 50 °C (▲) and 60 °C (▼)], and the activity was measured in the above standard reaction conditions. Data are the average of triplicate measurements.
The temperature also affected TmNADD activity, with an optimal temperature between 30 °C and 37 °C at pH 8.0, and a steady decrease in activity above 40 °C, although maintaining 36% maximal activity at 70 °C (Fig. 7B). This optimal temperature was similar to that described for Mycobacterium bovis BCG NADD (MbNADD), which has an optimal temperature of 40 °C19. TmNADD displayed a half-life of about five hours at 37 °C, whereas at 20 °C it increased up to six hours (Fig. 7D). However, at temperatures above 37 °C, the enzyme half-life rapidly decreased to less than 60 minutes (Fig. 7D), only 2% remaining at 60 °C after 30 minutes.
As regards the substrate specificity, the enzyme showed a Michaelis-Menten kinetic towards both substrates (NADH and NAD+), but with a clear preference for NADH. Thus, the obtained KM for NADH was 0.12 ± 0.01 mM, with a kcat of 10.7 ± 1.8 s−1 and a kcat/KM of 89.2 ± 19.8 mM−1 s−1, which is almost 10-fold higher than the catalytic efficiency shown towards NAD+ (kcat/KM of 9.3 ± 1.6 mM−1 s−1) (Table 3). When comparing individually, the activity towards NAD+ and NADH, TmNADD showed the second best catalytic efficiency described to date towards both NAD+ and NADH, after human NUDT12 (Table 3). As regards NADH, it expressed higher catalytic efficiency than ScNADD and CeNADD (16- and 28-fold, respectively)14, but 11-fold lower than hNUDT1213. However, when NAD+ was considered, these differences increases up to 93-fold compared to ScNADD and CeNADD14, and decreased to 6-fold with respect to hNUDT1213. These results clearly indicate the preference of the above NADDs for NADH over NAD+13,14. However, no data are available in the literature about the reaction time needed to attain the full conversion of NAD+ into NMN and AMP. When TmNADD was assayed with 5 mM NAD+, complete conversion was obtained after 16 h (Supplementary Fig. S4).
Discussion
Nudix hydrolases are a vast and diverse family of proteins involved in the regulation of cellular responses and homeostasis, which are widely distributed in all kingdoms of life9. The Nudix box acts as an essential catalytic site of these enzymes, in which the glutamine residues (in bold) found in its sequence (GX5EX7REUXEEXGU) bind indispensable divalent cations, such as Mg2+ or Mn2+10. Their substrate specificity is associated with additional sequences to this box, giving rise to the several subfamilies3. Among them, NADH pyrophosphatases or diphosphatases have been poorly studied, especially in enzymes of fungal origin. Taking advantage of the large number of new sequences emanating from genomic sequencing projects and their translation into the corresponding public protein databases, this work provides a complete in silico analysis of this subfamily, finding that NADDs represent 3.2% of the Nudix proteins in the UniProtKB database. Bacterial sequences are the most abundant, followed by eukaryotic, archaeal and metagenomic, without any viral representative. Curiously, fungal NADDs, distributed among different subphyla, account for 6.4% of NADDs and 68% of eukaryotic sequences, the most relevant being Pezizomycotina and Saccharomycotina (Fig. 2, Table 1). This abundance in Fungi is almost double those of the eukaryotic sequences of entire Nudix and Peptidase S8 families, as described in the Results section (Table 1). In addition, when these NADDs are compared within CATH Superfamily 3.90.79.10 (Supplementary Fig. S1D), which includes Nudix hydrolases (http://www.cathdb.info/version/v4_2_0/superfamily/3.90.79.10), fungal NADDs are also the most representative within structural cluster 3.90.79.10/3 (SC:3), where the nucleoside triphosphatases NudI, the ADP-ribose pyrophosphatases, the mitochondrial putative pre-mRNA cleavage factors, and the bifunctional NMN adenylyltransferases/Nudix hydrolases are also found. In fact, only one sequence from Nosema bombycis (strain CQ1 / CVCC 102059) (R0KWY1) was found in the nucleoside triphosphatase nudI sub-family when CATH sequences were retrieved from UniProt database.
NADDs are also found in Pezizomycetes basal groups, and in particular, in five economically important ectomycorrhizal truffle species, including the aromatic Périgord black truffle (T. melanosporum). The sequences of these truffle NADDs show the canonical domain architecture (i.e. NUDIX-like_zf-NADH-PPase_NUDIX) (Fig. 3), which is also the most common in both the fungal (54%) (Table 2) and in the 7479 NADDs (53%) sequences retrieved from UniProtKB (Fig. 4). This canonical architecture was also found in all the Pezizomycetes NADDs obtained from the MycoCosm portal, whose phylogenetic tree corroborates well with both the phylogram generated from the maximum likelihood analysis of combined LSU, SSU, ITS, TEF and RPB2 sequence data from 40 pezizalian species25 and the Pezizomycetes taxa distribution found in its molecular phylogeny obtained using 2,093 concatenated conserved single-copy protein-coding genes from the eight Pezizomycetes27. This suggests that NADD seems to be present in the most recent common ancestor (MRCA) of Pezizomycetes, and has been maintained as an essential protein from Ascobolaceae to Tuberaceae27,31.
The bioinformatic study also showed for the first time a total of twenty-one different architectures for the NADDs used in this work (Fig. 4), whereas only eleven were found in fungal sequences (Table 2). The human peroxisomal NUDT12 domain architecture (Ank2_NUDIX-like_zf-NADH-PPase_NUDIX) (Fig. 3, Table 2)9 accounts for 18.6% of the NADD domain architectures in eukaryotes, but only 0.42% in Fungi, in particular in the soil fungi Bifiguratus adelaidae (A0A261XXG3) and Syncephalastrum racemosum (A0A1X2H7S1). The latter fungus has occasionally been described as being the causative agent in toenail onychomycosis33. In addition, while the Ank-2 motif is found in 225,525 sequences with about 12,570 different Pfam domain architectures (http://pfam.xfam.org/family/Ank_2#tabview=tab0), the Ank-2_NUDIX-like_zf-NADH-PPase_NUDIX motif is only found in 88 sequences (http://pfam.xfam.org/family/zf-NADH-PPase#tabview=tab1) plus the two sequences found in this paper. This represents only 0.7% of the architectures where the Ank-2 motif is found. Its possible role associated with NADD is still unknown, although this Ank-2 domain is one of the most common protein-protein interaction platforms in nature. However, the presence of ankyrin domains (Ank-2, Ank-4 and Ank-5) in NADDs is relatively frequent both in fungal and eukaryotes, but totally absent in bacteria and archaea.
TmNADD is the first ectomycorrhizal Nudix NADH diphosphatase to be cloned and kinetically characterized, similar catalytic properties to those described previously for others NADDs being identified, but with some interesting particular features. As regards divalent cations, TmNADD had a preference for Mn2+ rather than Mg2+, with an optimal concentration (500 µM) higher than that described for hNUDT12 (50 µM) but similar to that of S. cerevisiae (300 µM)13,32. However, the optimal Mg2+ concentration was in the mM range (5–10 mM), similar to that described for E. coli, S. cerevisiae and C. elegans, but higher than that of hNUDT12 (0.4–2 mM)11,13,14. Its alkaline optimal pH was also a common feature of other NADDs, maintaining more than 50% activity for 6 hours in glycine buffer pH 9.0 at its optimum temperature (30–37 °C). Kinetically, TmNADD is 10 times more active towards NADH than NAD+. This ratio is lower than those of E. coli (120), S. cerevisiae (60), C. elegans (30) and even lower than that of hNUDT12 (20)11,13,14, meaning that the NAD+/NADH catalytic efficiency ratio is twice as high in TmNADD as in hNUDT12, making TmNADD the enzyme with the highest NAD+/NADH ratio ever described (Table 3). This observation, together with the fact that NAD+ is fully converted to NMN (Supplementary Fig. S4), and its easy expression and purification compared to hNUDT12, makes TmNADD a promising biocatalyst for the production of NMN from relatively cheap NAD+. NMN is a well-known NAD+-booster, capable of increasing the intracellular level of NAD+ after administration, increasing both life span and health in a number of animal models, and which is now being trialled in humans34,35.
Materials and Methods
Protein expression and purification
Tuber melanosporum Mel28 GSTUM_00011698001 gene, which was used for the cloning of the NADH pyrophosphatase/diphosphatase (TmNADD, UniprotKB accession number; D5GP45), was purchased from Genscript (NJ, USA) and inserted into pET28a vector. The TmNADD-pET28a construct was transformed into Escherichia coli Rosetta2(DE3) and induced using 0.25 mM isopropyl-β-D-thiogalactoside (IPTG) for 16 h at 20 °C with constant shaking. The culture was centrifuged and the pellet resuspended in lysis buffer (50 mM Tris-HCl pH 7.5, 150 mM NaCl). Cells were disrupted using a Bead Beater homogenizer (Biospec). After ultracentrifugation (40000 g, 40 min), the supernatant was loaded at 4 °C onto a HiPrep IMAC 16/10 FF column (GE Lifesciences, Spain) coupled to a FPLC chromatography system (ÄKTA Prime Plus, GE Lifesciences). The enzyme containing fractions were pooled, desalted and loaded into a Superdex 200 HiLoad 16/600 column (GE Lifesciences), obtaining an electrophoretically pure enzyme. TmNADD was stored at −20 °C with 10% glycerol.
Characterization of the purified enzyme
Reactions towards NAD+ were carried out under standard NADD activity assay that have been described previously11. Reactions at 37 °C were prepared in 50 mM Tris-HCl pH 8.0 in the presence of 0.5 mM MnCl2, 5 mM NAD+ and 0.11 μM of TmNADD, unless otherwise stated. At regular intervals during the 15 min that the assay lasted, aliquots were taken, stopped at pH 3.0 with TFA (1% final concentration), kept on ice for 10 min and centrifuged for 10 min at 12000 g. NAD+ conversion into NMN and AMP was followed by HPLC in a reverse phase C18 column (Gemini C18 250 × 4.6 mm, Phenomenex) and a method involving buffer A (10 mM tetrabutylammonium bromide, 10 mM potassium phosphate pH 7.0 and 0.25% methanol) running at 1 mL min−1 in a gradient from 0 to 100% of buffer B (2.8 mM tetrabutylammonium bromide, 100 mM potassium phosphate pH 5.5 and 30% methanol) for 21 minutes. In these conditions, the reaction products NMN and AMP had a retention time of 5.1 and 13.1 minutes, respectively, and NAD+ had a retention time of 10.7 minutes. NAD+ diphosphatase activity was determined following the increase in the area of the AMP peak. Reactions towards NADH (5 mM) were carried out under the same conditions for 5 min, but using 0.02 μM of TmNADD. The reactions were stopped at regular intervals by the addition of 100 mM EDTA at pH 8.06. Under these conditions, the reaction products NMNH and AMP had retention times of 15.2 and 13.1 minutes, respectively, while that of NADH was 19.1 minutes. NADH diphosphatase activity was also determined following the increase in the area of the AMP peak.
The optimal pH of TmNADD was carried out by performing the standard activity assay in various buffers with different pH values: 6.0–10.0. The buffers used were as follows: sodium phosphate buffer (pH 6.0–7.5), Tris-HCl buffer (pH 8.0–9,0), and glycine-NaOH buffer (pH 9.0–10.0). All buffers were at a concentration of 50 mM. Next, optimum temperature for TmNADD was determined using the standard NADD activity assay described above at different temperatures ranging from 20 to 60 °C.
The effect of pH and temperature on enzyme stability were determined by incubating TmNADD at 37 °C at different pHs, or at pH 8.0 at different temperatures for increasing periods of time (0–360 min). The residual activity after the incubation period was measured in the standard reaction conditions.
The substrate specificity of TmNADD was determined at 37 °C and in 50 mM Tris-HCl pH 8.0 using various substrates. The Michaelis-Menten constant (KM) and maximal velocity (Vmax) were estimated using plots of initial rates vs. NADH or NAD+ concentrations, respectively. The reported values represent means ± SE of the fits of the curves to the Michaelis-Menten equation by non-linear regression based on triplicate experiments. The catalytic constant (kcat) and the catalytic efficiency (kcat /KM) were deduced from the obtained KM and Vmax values.
In silico analysis
Protein sequences were obtained from the UniProtKB database (https://www.uniprot.org/), using the Prosite pattern PS51462 (NUDIX) as a query. Incomplete sequences and duplicate were removed, rendering the sequences used in the study. The NADD-like sequences were identified using the ScanProsite application (https://prosite.expasy.org/scanprosite/) and the pattern designed in this work. Sequences were aligned with the default parameters using MAFFT server (https://mafft.cbrc.jp/alignment/server/) and displayed using ESPript 3.036. Subsequently, the tree was built on the same web server using the Neighbour-Joining (NJ) method, JTT substitution model, heterogeneity between ignored sites (α = ∞) and a bootstrap of 1000 replicates to increase the reliability of the tree obtained. Mutation correlation analysis was carried out with the retrieved fungal NADD sequences using MISTIC (Mutual Information Server to Infer Coevolution) web server (http://mistic.leloir.org.ar/)30. The domain architectures of retrieved sequences were obtained from the Pfam (https://pfam.xfam.org/) and visualized with iTOL (https://itol.embl.de/).
References
McLennan, A. G., Cartwright, J. L. & Gasmi, L. The human NUDT family of nucleotide hydrolases. Enzymes of diverse substrate specificity. Advances in experimental medicine and biology 486, 115–118, https://doi.org/10.1007/0-306-46843-3_23 (2000).
Mildvan, A. S. et al. Structures and mechanisms of Nudix hydrolases. Archives of biochemistry and biophysics 433, 129–143, https://doi.org/10.1016/j.abb.2004.08.017 (2005).
Dunn, C. A., O’Handley, S. F., Frick, D. N. & Bessman, M. J. Studies on the ADP-ribose pyrophosphatase subfamily of the nudix hydrolases and tentative identification of trgB, a gene associated with tellurite resistance. The Journal of biological chemistry 274, 32318–32324 (1999).
Nguyen, V. N. et al. Substrate specificity characterization for eight putative nudix hydrolases. Evaluation of criteria for substrate identification within the Nudix family. Proteins 84, 1810–1822, https://doi.org/10.1002/prot.25163 (2016).
Srouji, J. R., Xu, A., Park, A., Kirsch, J. F. & Brenner, S. E. The evolution of function within the Nudix homology clan. Proteins 85, 775–811, https://doi.org/10.1002/prot.25223 (2017).
Zhang, D. et al. Structural basis of prokaryotic NAD-RNA decapping by NudC. Cell research 26, 1062–1066, https://doi.org/10.1038/cr.2016.98 (2016).
Daniels, C. M., Thirawatananond, P., Ong, S. E., Gabelli, S. B. & Leung, A. K. Nudix hydrolases degrade protein-conjugated ADP-ribose. Scientific reports 5, 18271, https://doi.org/10.1038/srep18271 (2015).
Palazzo, L. et al. Processing of protein ADP-ribosylation by Nudix hydrolases. Biochemical Journal 468, 293–301, https://doi.org/10.1042/bj20141554 (2015).
Carreras-Puigvert, J. et al. A comprehensive structural, biochemical and biological profiling of the human NUDIX hydrolase family. Nature communications 8, 1541, https://doi.org/10.1038/s41467-017-01642-w (2017).
Yoshimura, K. & Shigeoka, S. Versatile physiological functions of the Nudix hydrolase family in Arabidopsis. Biosci Biotechnol Biochem 79, 354–366, https://doi.org/10.1080/09168451.2014.987207 (2015).
Frick, D. N. & Bessman, M. J. Cloning, purification, and properties of a novel NADH pyrophosphatase. Evidence for a nucleotide pyrophosphatase catalytic domain in MutT-like enzymes. The Journal of biological chemistry 270, 1529–1534 (1995).
Abdelraheim, S. R., Spiller, D. G. & McLennan, A. G. Mouse Nudt13 is a mitochondrial nudix hydrolase with NAD(P)H pyrophosphohydrolase Activity. The protein journal 36, 425–432, https://doi.org/10.1007/s10930-017-9734-x (2017).
Abdelraheim, S. R., Spiller, D. G. & McLennan, A. G. Mammalian NADH diphosphatases of the Nudix family: cloning and characterization of the human peroxisomal NUDT12 protein. The Biochemical journal 374, 329–335, https://doi.org/10.1042/BJ20030441 (2003).
Xu, W., Dunn, C. A. & Bessman, M. J. Cloning and characterization of the NADH pyrophosphatases from Caenorhabditis elegans and Saccharomyces cerevisiae, members of a Nudix hydrolase subfamily. Biochemical and biophysical research communications 273, 753–758, https://doi.org/10.1006/bbrc.2000.2999 (2000).
Dobrzanska, M., Szurmak, B., Wyslouch-Cieszynska, A. & Kraszewska, E. Cloning and characterization of the first member of the Nudix family from Arabidopsis thaliana. The Journal of biological chemistry 277, 50482–50486, https://doi.org/10.1074/jbc.M205207200 (2002).
Ogawa, T., Ueda, Y., Yoshimura, K. & Shigeoka, S. Comprehensive analysis of cytosolic Nudix hydrolases in Arabidopsis thaliana. The Journal of biological chemistry 280, 25277–25283, https://doi.org/10.1074/jbc.M503536200 (2005).
Ogawa, T. et al. Molecular characterization of organelle-type Nudix hydrolases in Arabidopsis. Plant physiology 148, 1412–1424, https://doi.org/10.1104/pp.108.128413 (2008).
Klaus, S. M. et al. A nudix enzyme removes pyrophosphate from dihydroneopterin triphosphate in the folate synthesis pathway of bacteria and plants. The Journal of biological chemistry 280, 5274–5280, https://doi.org/10.1074/jbc.M413759200 (2005).
Wang, X. D. et al. Comparative analysis of mycobacterial NADH pyrophosphatase isoforms reveals a novel mechanism for isoniazid and ethionamide inactivation. Molecular microbiology 82, 1375–1391, https://doi.org/10.1111/j.1365-2958.2011.07892.x (2011).
Elitas, M. Isoniazid killing of Mycobacterium smegmatis NADH pyrophosphatase mutant at single-cell level using microfluidics and time-lapse microscopy. Scientific reports 7, 10770, https://doi.org/10.1038/s41598-017-11503-7 (2017).
Park, U. E., Roth, J. R. & Olivera, B. M. Salmonella typhimurium mutants lacking NAD pyrophosphatase. Journal of bacteriology 170, 3725–3730 (1988).
Schmidt-Brauns, J. et al. Is a NAD pyrophosphatase activity necessary for Haemophilus influenzae type b multiplication in the blood stream? International journal of medical microbiology: IJMM 291, 219–225, https://doi.org/10.1078/1438-4221-00122 (2001).
Hofer, K. et al. Structure and function of the bacterial decapping enzyme NudC. Nature chemical biology 12, 730–734, https://doi.org/10.1038/nchembio.2132 (2016).
Cahova, H., Winz, M. L., Hofer, K., Nubel, G. & Jaschke, A. NAD captureSeq indicates NAD as a bacterial cap for a subset of regulatory RNAs. Nature 519, 374–377, https://doi.org/10.1038/nature14020 (2015).
Ekanayaka, A. H., Hyde, K. D., Jones, E. B. G. & Zhao, Q. Taxonomy and phylogeny of operculate discomycetes: Pezizomycetes. Fungal Diversity 90, 161–243, https://doi.org/10.1007/s13225-018-0402-z (2018).
Thomas, P. & Buntgen, U. A risk assessment of Europe’s black truffle sector under predicted climate change. Sci Total Environ 655, 27–34, https://doi.org/10.1016/j.scitotenv.2018.11.252 (2019).
Murat, C. et al. Pezizomycetes genomes reveal the molecular basis of ectomycorrhizal truffle lifestyle. Nature ecology & evolution, https://doi.org/10.1038/s41559-018-0710-4 (2018).
Liu, K. & Wessler, S. R. Transposition of Mutator-like transposable elements (MULEs) resembles hAT and Transib elements and V(D)J recombination. Nucleic acids research 45, 6644–6655, https://doi.org/10.1093/nar/gkx357 (2017).
Ferguson, A. A., Zhao, D. & Jiang, N. Selective acquisition and retention of genomic sequences by Pack-Mutator-like elements based on guanine-cytosine content and the breadth of expression. Plant physiology 163, 1419–1432, https://doi.org/10.1104/pp.113.223271 (2013).
Simonetti, F. L., Teppa, E., Chernomoretz, A., Nielsen, M. & Marino Buslje, C. MISTIC: Mutual information server to infer coevolution. Nucleic acids research 41, W8–14, https://doi.org/10.1093/nar/gkt427 (2013).
Bonito, G. et al. Historical biogeography and diversification of truffles in the Tuberaceae and their newly identified southern hemisphere sister lineage. PloS one 8, e52765, https://doi.org/10.1371/journal.pone.0052765 (2013).
Cartwright, J. L., Gasmi, L., Spiller, D. G. & McLennan, A. G. The Saccharomyces cerevisiae PCD1 gene encodes a peroxisomal nudix hydrolase active toward coenzyme A and its derivatives. The Journal of biological chemistry 275, 32925–32930, https://doi.org/10.1074/jbc.M005015200 (2000).
Pavlovic, M. D. & Bulajic, N. Great toenail onychomycosis caused by Syncephalastrum racemosum. Dermatol Online J 12, 7 (2006).
Marinescu, G. C., Popescu, R. G., Stoian, G. & Dinischiotu, A. Beta-nicotinamide mononucleotide (NMN) production in Escherichia coli. Scientific reports 8, 12278, https://doi.org/10.1038/s41598-018-30792-0 (2018).
Marinescu, G. C., Popescu, R. G. & Dinischiotu, A. Size exclusion chromatography method for purification of nicotinamide mononucleotide (NMN) from bacterial cells. Scientific reports 8, 4433, https://doi.org/10.1038/s41598-018-22806-8 (2018).
Robert, X. & Gouet, P. Deciphering key features in protein structures with the new ENDscript server. Nucleic acids research 42, W320–324, https://doi.org/10.1093/nar/gku316 (2014).
Acknowledgements
This study was partially supported by Spanish grants from MINECO-FEDER (BIO2013-45336-R, AGL2017-86526P, AEI/FEDER), UE (CGL2016-78946-R) and from the Ayudas a los Grupos y Unidades de Excelencia Científica de la Región de Murcia, Fundación Séneca- Agencia de Ciencia y Tecnología de la Región de Murcia (19893/GERM/15, Programa de Apoyo a la Investigación 2014 and 20866/PI/18). AGGS was supported by a pre-doctoral contract (FPU-UMU) from the University of Murcia. RZP is supported by a postdoctoral grant from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No. 840110. We are grateful to F. Martin and the Mycorrhizal Genomics Initiative consortium for access to genome data (MycoCosm portal). The genome sequence data were produced by the US Department of Energy Joint Genome Institute in collaboration with the user community.
Author information
Authors and Affiliations
Contributions
A.G.G.-S., A.S.-F. conceived and designed the experiments. A.G.G.-S., R.Z.-P., A.B.M.-M. performed the experiments. A.G.G.-S., R.Z.-P., A.M., M.P.-G., A.S.-F. analysed the data. A.G.G.-S., J.F.H., A.S.-F. contributed to the bioinformatic studies; A.S.-F. devised the project; A.G.G.-S., A.M., M.P.-G., A.S.-F. wrote the manuscript with the contribution of all the authors. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
García-Saura, A.G., Zapata-Pérez, R., Martínez-Moñino, A.B. et al. The first comprehensive phylogenetic and biochemical analysis of NADH diphosphatases reveals that the enzyme from Tuber melanosporum is highly active towards NAD+. Sci Rep 9, 16753 (2019). https://doi.org/10.1038/s41598-019-53138-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-53138-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.