Abstract
Cynomolgus macaque has been used for the evaluation of the zoonotic potential of prion diseases, especially for classical-Bovine Spongiform Encephalopathy (classical-BSE) infectious agent. PrP amino acid sequence is considered to play a key role in the susceptibility to prion strains and only one amino acid change may alter this susceptibility. Macaque and human-PrP sequences have only nine amino acid differences, but the effect of these amino acid changes in the susceptibility to dissimilar prion strains is unknown. In this work, the transmissibility of a panel of different prions from several species was compared in transgenic mice expressing either macaque-PrPC (TgMac) or human-PrPC (Hu-Tg340). Similarities in the transmissibility of most prion strains were observed suggesting that macaque is an adequate model for the evaluation of human susceptibility to most of the prion strains tested. Interestingly, TgMac were more susceptible to classical-BSE strain infection than Hu-Tg340. This differential susceptibility to classical-BSE transmission should be taken into account for the interpretation of the results obtained in macaques. It could notably explain why the macaque model turned out to be so efficient (worst case model) until now to model human situation towards classical-BSE despite the limited number of animals inoculated in the laboratory experiments.
Similar content being viewed by others
Introduction
Transmissible Spongiform Encephalopathies (TSEs) are a group of fatal neurodegenerative diseases, which include Bovine Spongiform Encephalopathy (BSE) in cattle, scrapie in sheep and goats, Chronic Wasting Disease (CWD) in cervids and Creutzfeldt-Jakob disease (CJD) in humans. It is considered that the causative agent of TSEs consists mainly, if not entirely, of PrPSc which is an abnormal isoform of the host encoded prion cellular protein, PrPC 1,2,3,4. TSEs are also called prion diseases. Prion propagation is considered to occur in a two-step process where aggregates of PrPSc interact with PrPC and then induce their conformational change into PrPSc. The conversion in PrPSc results in changes in physical properties as an increase in β-pleated sheet content and resistance to proteolytic degradation and decreased solubility in non-denaturing detergents.
Upon experimental transmission, TSE strains show distinctive biological features (such as incubation periods and neuropathology) which are preserved after sub-passage in the same species. According to the prion hypothesis5, different PrPSc conformations are associated with dissimilar strains. Prion transmission barrier among different species seems to be driven by the differences in the PrP amino acid sequences and by the prion strain being transmitted6,7. While PrP amino acid sequence seems to play a key role in prion transmission susceptibility, host factors other than PrPC can modulate prion strain features7,8. Transgenic mouse models null for murine Prnp gene and expressing the PrPC from another species (such as bovine, ovine, porcine, human, etc), have been used in the prion research field as useful tools to characterise prion strains and to learn about the transmissibility of prion strains to different species6,7,9, and in particular the susceptibility of humans to prions10,11,12,13,14,15,16.
Several studies have been done using non-human primates to study the transmissibility of prion diseases17,18 and more recently, macaque monkeys have been widely used for prion disease transmissions19,20,21,22,23,24,25,26,27,28,29,30. In this sense, non-human primates are considered to be the ultimate model of the human condition with regard to prions, especially for BSE infection19,22. Both macaque and human PrP amino acid sequences are quite similar, but only one amino acid change may alter susceptibility to prions drastically, as occurs with the Met/Val 129 dimorphism in human PrP sequence for classical-BSE prion strain14. The nine amino acid differences between human and macaque PrP (see Fig. 1) may alter prion susceptibility of these two species. In this work, we address this question comparing the susceptibility of transgenic mouse models expressing either human or macaque PrP when inoculated with a panel of diverse prions.
Amino acid comparison of human macaque, cattle and sheep PrP amino acid sequences. Only amino acids 89 to 238 (according to human PrP) are included in the comparison for clarity. Points indicate identical residues. Deletions are indicated by dashes. Amino acid numbering is indicated on the right. Species are named on the left. Amino acid changes in 166 and 168 positions (M/V and E/Q respectively) are boxed.
Results
Macaque PrPC expression in transgenic mice
PrPC expression in brain from homozygous TgMac mice was checked by W estern blot using a specific anti-PrP monoclonal antibody (12B2). Brain PrPC expression levels for the TgMac mice were found to be around half than the PrPC levels found in Hu-Tg340 brains. PrPC from TgMac mice showed a similar electrophoretic profile than the PrPC obtained from the brain of Hu-Tg340 mice (Fig. 2). Neither behavioural defects such as neurological signs, social deficits or alterations in reproduction rates, nor reduction in their lifespan were observed in TgMac mice.
Comparison of prion infection susceptibility in TgMac and Hu-Tg340 mice
TgMac and Hu-Tg340 mice were inoculated through the intracerebral route with a collection of isolates representative of different prion strains (Table 1) from human, sheep and cattle. The susceptibility to prion infection of both mouse lines expressing either human or macaque PrPC was compared using the same inocula.
Transmission of human prions
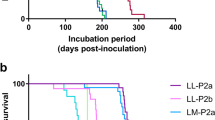
Infection with sCJD prion isolates was not detected in TgMac mice as none of them was scored positive for PrPres after long survival times (Table 2). As expected, sCJD isolates infected efficiently Hu-Tg340 mice with 100% attack rates. By contrary, vCJD isolate transmitted to TgMac mice with 100% attack rates and with a mean survival time similar to the one obtained for Hu-Tg340 mice inoculated with vCJD (537 ± 105 vs 545 ± 146 dpi) (Table 2). Similar antibody labelling and brain-PrPres glycoprofile with a predominant diglycosylated band was revealed by Western blot in both mouse lines for vCJD inoculum, but a very small reduction in the electrophoretic mobility of the macaque-PrPres unglycosylated band was observed when compared to the human-PrPres counterpart (Figs 3 and 4). The predicted molecular mass for macaque-PrP according to its amino acid sequence is slightly higher than for human-PrP (27676 Da and 27661 Da respectively). This minor difference in the molecular mass (15 Da) could explain the small difference observed in the electrophoretic mobility. A different proteinase K cleavage in the brain PrPres from either TgMac or Hu-Tg340 mice seems unlikely as the antibody labelling is similar in all cases (Fig. 3). Neuropathological features (lesion profile and PrPres distribution in Pet blot) observed in brains from vCJD inoculated TgMac mice were similar to those previously described for Hu-Tg340 mice inoculated with vCJD14 showing prominent vacuolation at the cerebellar and hippocampus level (Fig. 5). Immature forms of florid plaques were observed in the brains of TgMac mice inoculated with vCJD (Fig. 6) as previously described in macaques inoculated with vCJD20.
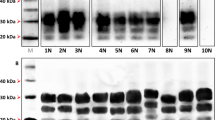
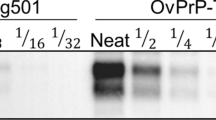
Biochemical comparison of brain PrPres in TgMac or Hu-Tg340 mice. Electrophoresis profiles and antibody labeling of PrPres as detected with mAbs Sha31 (top) and 12B2 (bottom) in brain extracts from TgMac or Hu-Tg340 mice infected with the prion agents indicated in the top. The original isolates either from human or cattle were also included for biochemical comparative purposes. Similar quantities of PrPres were loaded in each lane for a better comparison. Brain extract from Human sCJD 129M/M T1 (sCJD T1) was included as positive control for 12B2 antibody labeling. Both panels were loaded with the same quantities of PrPres extracted from each sample. Molecular weights (in kDa) are shown at the left of the blot.
Biochemical comparison of brain PrPres unglycosylated band in TgMac or Hu-Tg340 mice. Electrophoresis profiles of PrPres as detected by mAb Sha31 in brain extracts from TgMac or Hu-Tg340 mice infected with the prion agents indicated in the top. Brain PrPres were untreated (left panel) or treated (right panel) with PNGaseF. Molecular weights (in kDa) are shown at the left of the blot.
PrPres immunostaining in brains from TgMac and Hu-Tg340 infected with vCJD, BSE or BSE L. All panels show the PrP deposition with Sha31 monoclonal antibody. Cerebral cortex in vCJD, BSE, and non-inoculated control TgMac (A,B and D) or Hu-Tg340 (E, F and H). Thalamus in BSE L inoculated animals (C and G). Immature florid plaque with a dense core of PrP in the cerebral cortex of TgMac inoculated with vCJD (A). Dense plaques in the cerebral cortex of TgMac inoculated with BSE (B). Florid plaques in the cerebral cortex of Hu-Tg340 inoculated with vCJD (E). Florid plaques in the cerebral cortex of Hu-Tg340 inoculated with BSE after second passage54 (F) are shown because in the first passage, with only one positive animal, no plaques were detected. PrPres distributed in a diffuse pattern in the thalamus of TgMac (C) or Hu-Tg340 (G) inoculated with BSE L. No staining was observed in the brain of non-inoculated control mice in these conditions (D and H). Scale bar = 100 μm.
Transmission of cattle prions
Neither TgMac nor Hu-Tg340 mice were scored positive for the transmission of the disease when inoculated with atypical-BSE H. However, atypical-BSE L transmitted efficiently in both mouse lines (100% mice were scored positive; Table 2) showing similar long survival times (550 ± 86 vs 607 ± 13). Neuropathological features observed in brains from atypical-BSE L inoculated TgMac mice were similar to those observed in Hu-Tg340 mice inoculated with the same prion isolate (Figs 5 and 6). There were not significant differences in antibody labelling and the brain-PrPres glycoprofile in Western blot in both mouse lines inoculated with atypical-BSE L (Fig. 3).
Remarkably, while only one out of eight of the Hu-Tg340 mice inoculated with classical-BSE was scored positive for the transmission of the disease, all the TgMac mice inoculated with classical-BSE were positive for brain PrPres after 518 ± 106 dpi (Table 2). A similar antibody labelling and brain-PrPres glycoprofile was observed in both mouse lines inoculated with classical-BSE (Fig. 3). This glycoprofile was also similar to that observed after inoculation of both mouse lines with vCJD. As previously observed in both lines inoculated with vCJD, a slight reduction in the electrophoretic mobility of the macaque-PrPres unglycosylated band was observed when compared to the human-PrPres counterpart after inoculation with classical-BSE or atypical-BSE L (Figs 3 and 4). Neuropathological features (lesion profile and PrPres distribution in Pet blot) observed in brains from classical-BSE inoculated TgMac mice were similar to those previously reported for Hu-Tg340 mice after infection with classical-BSE16. Dense plaques representing immature forms of florid plaques were observed in the brains of TgMac mice inoculated with classical-BSE (Fig. 6) as previously described in macaques inoculated with classical-BSE20.
Transmission of sheep prions
In accordance with previous studies31, none of the Hu-Tg340 mice inoculated with scrapie was scored positive for the disease while two out of six TgMac mice were scored positive for the disease at 273 and 392 dpi when inoculated with Sheep-Sc PS21 scrapie isolate (Table 2). Both TgMac mice scored positive showed sparse PrPres accumulation in their brain. None of the TgMac mice inoculated with Sheep-Sc198-9 was scored positive for the disease.
Discussion
In this work, the transmission features of a collection of TSE isolates, representing a panel of diverse prion strains, is compared in two transgenic mouse experimental models expressing either macaque-PrPC or human-PrPC. Human and macaque transmission barriers for different prions are compared in identical conditions in the same context. The main differences in both transgenic models are included in the PrP amino acid sequence expressed in each transgenic mouse line. The use of macaque species as a model for prion transmission in humans32 remarks the importance of a deep analysis of the differences in the prion transmission barrier of human versus macaque PrP amino acid sequences.
Results presented here showed a strain dependent susceptibility of both mouse models to the panel of inoculated prion isolates. Atypical-BSE showed similar transmission features in both Hu-Tg340 and TgMac, suggesting that the amino acid differences between macaque and human-PrPC are irrelevant for the transmissibility of both atypical-BSE strains. The ability of atypical BSE-L prion strain to infect both transgenic mouse lines expressing human or macaque-PrPC is in agreement with previous experiments published either in transgenic mice expressing human-PrPC 33,34,35 or in macaques23. In parallel, the inability of atypical BSE-H prion strain to infect both transgenic mouse lines expressing human or macaque-PrPC is in agreement with previous experiments in transgenic mice expressing human-PrPC 33 or in macaques28.
By contrast, different transmission features were observed in Hu-Tg340 and TgMac inoculated with other prion strains such as classical-BSE. The susceptibility observed in mice expressing macaque PrPC is higher than in those expressing human PrPC in terms of attack rate. This information must be considered when macaque species is used for the evaluation of the transmissibility of animal prions to human species. It could notably explain why the macaque model turned out to be so efficient (worst case model) until now to model human situation towards BSE despite the limited number of animals inoculated in the laboratory experiments. In a similar way, Hu-Tg340 and TgMac mice show identical transmission features for vCJD (Table 2). This behavior beside the anatomical similarities of human and macaques supports the suitability of macaques as a valuable tool for modeling vCJD secondary transmissions in humans.
Table 3 shows a compilation of data from different experiments using macaque species for the evaluation of the transmissibility of different prions21,23,28. These data should be compared with caution, as there are experimental differences in the inocula used, inoculation route, etc. Anyway, in most cases, the data shows a comparable transmissibility in both macaque and transgenic mice expressing macaque PrP.
Macaque-PrP presents an important transmission barrier for human prion strains such as type 1 or type 2 sCJD as none of these strains was able to generate a detectable infection in transgenic mice expressing macaque-PrP within the time frame of these experiments. Previous data evidenced that macaques can be infected with type 1 sCJD showing incubation periods around twice than in the case of vCJD prion strain (56 months versus 25, 30, 32 and 37 months21). Given that the survival time observed for vCJD in mice expressing macaque-PrP is close to the end point of these experiments, the transmission barrier for sCJD would impede the detection of type 1 sCJD in the mouse model. When compared with human-PrP, macaque-PrP amino acid sequence presents the R to K change in 220 position (see Fig. 1). It is remarkable that 220 amino acid position is adjacent to the E219K polymorphism described in humans, which has been linked to protecting humans against sCJD in epidemiological studies in Asiatic populations36,37. E219K polymorphism has been associated to perturbations in surface change distribution and structural rearrangements (mainly localized at the β2-α2 loop region). Similarly, we can speculate that the R220K change observed in macaque-PrP would alter the indicated zone, providing a protective effect to macaques for sCJD infection. Moreover, when compared with human-PrP, macaque-PrP presents two amino acid changes at the 166 and 168 positions also present in cattle and sheep PrP sequences (M/V and E/Q respectively, see Fig. 1). Both 166 and 168 amino acid changes are in the β2-α2 loop of PrP. Changes in the β2-α2 loop are deeply related with alterations in the susceptibility to prion strains38,39,40,41. In particular, the amino acid changes observed in 166 and 168 positions are included in sequence elements implicated in both resistance to CWD in humans and in delaying the disease progression of sCJD42. The conservation of both V166 and Q168 amino acids in macaque, cattle and sheep PrP, three species highly susceptible to classical-BSE infection, allows as to propose these residues as determinant elements for the susceptibility to classical-BSE.
Finally, macaque PrP presents a differential susceptibility for several scrapie isolates, providing a tool discriminating scrapie prion strains. Previous studies in macaques report susceptibility to scrapie from 3 of 4 scrapie inocula (see Table 3) suggesting that different results may be obtained in animals expressing macaque-PrP due to differences in the inoculated scrapie strain18,28,43.
Overall, although both Tg-Mac and Hu-Tg340 mice showed a similar susceptibility to vCJD, Tg-Mac mice present an important transmission barrier to other human prion strains such as type 1 or type 2 sCJD suggesting a strain dependent susceptibility of Tg-Mac to human prions. On the other hand, the similar transmission properties observed in both mouse models when inoculated with most of the animal prion strains tested here (BSE-L, BSE-H and sheep scrapie) suggest that macaque species is an adequate model for the evaluation of human susceptibility to them. However, macaque-PrP is more proficient for classical-BSE prion replication than human-PrP. The use of macaques to model the human condition with regard to classical-BSE infection would take advantage of this property of macaque PrP.
Methods
Ethics statement
This study was carried out in accordance with the Directive 2010/63/EU. All protocols were approved by the Committee on the Ethics of Animal Experiments (CEEA) of the Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria (INIA); Permit Number CEEA 2012/002.
Generation of transgenic mice expressing Macaca fascicularis PrPC
The transgenic mouse line expressing Macaca fascicularis PrPC was obtained as previously described with minor modifications7. The open reading frame (ORF) of the macaque PrP gene was isolated by PCR amplification from macaque DNA using primers that created a AscI restriction enzyme site adjacent to the translation start and stop sites (5′-GGCGCGCCATGGCGAACCTTGGCTGCTGGATGCTG-3′ and 5′-GGCGCGCCTCATCCCACTATCAGGAAGATGAG-3′). The PCR fragment was subcloned into vector containing 6.2 kb of the Prp mouse promoter region and the DNA segment from exon I to exon II, which is directly fused to exon III of the Prp gene44,45, and the insert was sequenced to confirm no difference in the inferred amino acid sequence with respect to previously sequenced macaque PrP ORF (GenBank accession number NM_001287629). The PrP ORF was excised from the final construct using restriction endonuclease NotI and SalI to yield 12.2 kb DNA fragments. The construct was then purified using QIAEX II Gel Extraction Kit (Qiagen). The DNA was resuspended in Tris-EDTA at a final concentration of 2 μg/ml and then microinjected into 237 pronuclear-stage oocytes that were collected from superovulated FVB/N females. The presence of macaque-PrP transgene in the offspring was determined by PCR assay using specific primers for the macaque-PrP ORF: 5′-TTATAGTTGCTGAGCGTCGTCAGGGA-3′ and 5′-TGGGATTCGGTTCCTCCAGGAG-3′ pair and 5′-AGAACAACTTTGTGCACGACTGCGTC-3′ and 5′-CGAAGGAACAAGCAGGAAGGCG-3′ pair.
Seven different lines (founders) of macaque PrPC (macPrP) were obtained. Mouse line with highest levels of macaque PrPC expression was selected for further experiments. Homozygous TgMac mouse line was established backcrossing these animals with homozygous null animals MuPrP−/− 46 to obtain a null murine PrP background (PrP mu−/− mac+/−). Interbreeding within these animals was performed to obtain homozygosis for the macaque-PrP transgene within a murine PrP background (PrP mu−/− mac+/+). The absence of murine PrP gene was determined by PCR using specific primers: 5′-TAGATGTCAAGGACCTTCAGCC-c and 5′-GTTCCACTGATTATGGGTACC-3′.
Transmission studies
Hu-Tg340 transgenic mice expressing human PrPC (four fold levels than human brain previously described in16) and TgMac mice generated here were challenged with a collection of “natural” isolates of distinct origin representative of different TSE agents (see Table 1 for a description of the isolates) as previously described7. For that, groups of 5 to 9 individual identified mice (6 to 7 week-old) were inoculated by intra-cerebral route with 20 μl of 10% brain homogenate. As a control, 6 animals of each mouse line were inoculated with healthy cattle brain. All inocula were prepared from brain tissues as 10% (w/v) homogenates in 5% glucose. After inoculation, mice were observed daily and their neurological status assessed twice a week. Animals were killed for ethical reasons when progression of the disease was evident or at the set end point of the experiment, 650 days post inoculation (dpi). Once euthanized, necropsy was performed. A part of the brain was fixed by immersion in 10% buffered formalin for histopathological studies and the other was frozen at −20 °C to determine presence of PrPres by Western blot.
Survival times were calculated as mean ± SD of the dpi of all the mice scored positive for PrPres. Attack rate was determined as the ratio of PrPres-positive mice among all the inoculated mice.
Western blot analysis of brain PrPC expression in transgenic mice
PrPC expression was analysed as previously described7. Briefly, whole brains from each mouse line were homogenized in extraction buffer (0.5% NP-40, 1% sodium deoxycholate, 10 mM in phosphate-buffered saline pH7.4, with Complete inhibitor cocktail (Roche). Samples were precleared by centrifugation at 2,000 × g for 5 min, after which an equal volume of 2× SDS reducing sample loading buffer was added, and boiled for 5 min before loading onto an 12% Bis-Tris Gel (Criterion XT, BioRad). For immunobloting experiments, the monoclonal antibody 12B247 was used at a concentration of 0.1 µg/mL. 12B2 recognizes 89-WGQGG-93 epitope of the macaque/human PrPC sequence.
Immunocomplexes were detected incubating the membranes for 1 hour with horseradish peroxidase conjugated anti mouse IgG (GE Healthcare Amersham Biosciences). Immunoblots were developed with enhanced chemiluminescence ECL Select (GE Healthcare Amersham Biosciences). Images were captured using ChemiDoc XRS+ System (Bio-Rad) and images were processed using Image Lab 5.2.1 Software (Bio-Rad). All the original Western blot images are presented as Supplementary information.
Western blot analysis of brain PrPres in transgenic mice
Brain PrPres was analysed as previously described7,14. Briefly, 175 ± 20 mg of frozen brain tissue were homogenized in 5% glucose in distilled water in grinding tubes (Bio-Rad) adjusted to 10% (w/v) using a TeSeETM Precess 48TM homogenizer (Bio-Rad) following manufacturer instructions. Presence of PrPres in transgenic mice brains was determined by Western blot, following the procedure described below and using the reagents of the ELISA commercial test (TeSeE, Bio-Rad). Based on a previously described protocol48 10–100 μl of a 10% (w/v) brain homogenate were treated with proteinase K and the resulting samples were loaded in 12% Bis-Tris Gel (Criterion XT, BioRad). Proteins were transferred electrophoretically onto PVDF membranes (Millipore). For immunoblotting, monoclonal antibodies 12B2 and Sha3149 were used at a concentration of 1 µg/mL. Sha31 recognizes 145-YEDRYYRE-152 epitope of the macaque/human PrPC sequence. Immunocomplexes were detected as described above for brain PrPC analysis. N-glycosidase F (PNGaseF, New England Biolabs) was used according to manufacturer’s instructions with minor modifications.
Histopathology
Mouse brains were analysed as previously described50,51 with minor modifications. Briefly, samples were fixed in neutral-buffered 10% formalin (4% formaldehide) before paraffin embedding. After deparaffinization, 4 μm-thick tissue sections were stained with haematoxylin and eosin. Lesion profiles of the brains were established according the standard method described by Fraser and Dickinson52. For paraffin-embedded tissue (PET) blots, the protocol described by Andréoletti et al.53 was used. We conducted PrPress immunohistochemistry as previously described51. Briefly, 4 μm-thick sections were deparaffinized before antigen retrieval. Briefly, sections were immersed in 98% formic acid for 7 min at room temperature and washed in running tap water before being immersed in 4M guanidine isothiocyanate for 1 h at 4 °C. Guanidine isothiocyanate treatment was followed by proteinase K digestion: TBS (50 mM Tris-HCl, 150 mM NaCl, pH 7.6) containing 20 μg/ml of proteinase K for 15 min at 37 °C. Primary antibody incubation was conducted overnight at 4 °C using the Sha31 mAb at 1/1000 dilution. A secondary goat anti-mouse IgG biotinylated antibody (DAKO) diluted 1/200 in 10% normal goat serum was incubated for 30 min at room temperature, and an avidin-biotin-peroxidase complex (Pierce) was applied using diaminobenzidine (DAB) as a substrate. Finally, sections were counterstained with Mayer’s hematoxylin for 1 min, dehydrated, and routinely mounted. Serial sections from positive controls and appropriate negative controls were included in each immunohistochemistry run.
All the procedures described in this section involving prion infected materials were performed within a biosafety level 3 laboratory.
References
Prusiner, S. B. Novel proteinaceous infectious particles cause scrapie. Science 216, 136–144 (1982).
Castilla, J., Saa, P., Hetz, C. & Soto, C. In vitro generation of infectious scrapie prions. Cell 121, 195–206 (2005).
Wang, F., Wang, X., Yuan, C. G. & Ma, J. Generating a prion with bacterially expressed recombinant prion protein. Science 327, 1132–1135 (2010).
Makarava, N. et al. Recombinant prion protein induces a new transmissible prion disease in wild-type animals. Acta Neuropathol 119, 177–187 (2010).
Peretz, D. et al. A change in the conformation of prions accompanies the emergence of a new prion strain. Neuron 34, 921–932 (2002).
Collinge, J. & Clarke, A. R. A general model of prion strains and their pathogenicity. Science 318, 930–936, https://doi.org/10.1126/science.1138718 (2007).
Espinosa, J. C. et al. PrPC Governs Susceptibility to Prion Strains in Bank Vole, While Other Host Factors Modulate Strain Features. J Virol 90, 10660–10669, https://doi.org/10.1128/JVI.01592-16 (2016).
Crowell, J., Hughson, A., Caughey, B. & Bessen, R. A. Host Determinants of Prion Strain Diversity Independent of Prion Protein Genotype. J Virol 89, 10427–10441, https://doi.org/10.1128/JVI.01586-15 (2015).
Watts, J. C. & Prusiner, S. B. Mouse Models for Studying the Formation and Propagation of Prions. J Biol Chem, https://doi.org/10.1074/jbc.R114.550707 (2014).
Asante, E. A. et al. BSE prions propagate as either variant CJD-like or sporadic CJD-like prion strains in transgenic mice expressing human prion protein. Embo J 21, 6358–6366 (2002).
Beringue, V. et al. Prominent and persistent extraneural infection in human PrP transgenic mice infected with variant CJD. PLoS One 3, e1419, https://doi.org/10.1371/journal.pone.0001419 (2008).
Berry, D. B. et al. Drug resistance confounding prion therapeutics. Proc Natl Acad Sci USA 110, E4160–4169, https://doi.org/10.1073/pnas.1317164110 (2013).
Bishop, M. T. et al. Predicting susceptibility and incubation time of human-to-human transmission of vCJD. Lancet Neurol 5, 393–398 (2006).
Fernandez-Borges, N. et al. Protective Effect of Val129-PrP against Bovine Spongiform Encephalopathy but not Variant Creutzfeldt-Jakob Disease. Emerg Infect Dis 23, 1522–1530, https://doi.org/10.3201/eid2309.161948 (2017).
Kong, Q. et al. Chronic wasting disease of elk: transmissibility to humans examined by transgenic mouse models. J Neurosci 25, 7944–7949 (2005).
Padilla, D. et al. Sheep and Goat BSE Propagate More Efficiently than Cattle BSE in Human PrP Transgenic Mice. PLoS Pathog 7, e1001319 (2011).
Gibbs, C. J. Jr. & Gajdusek, D. C. Transmission of scrapie to the cynomolgus monkey (Macaca fascicularis). Nature 236, 73–74 (1972).
Gibbs, C. J. Jr. & Gajdusek, D. C. Experimental subacute spongiform virus encephalopathies in primates and other laboratory animals. Science 182, 67–68 (1973).
Lasmezas, C. I. et al. BSE transmission to macaques. Nature 381, 743–744 (1996).
Lasmezas, C. I. et al. Adaptation of the bovine spongiform encephalopathy agent to primates and comparison with Creutzfeldt–Jakob disease: implications for human health. Proc Natl Acad Sci USA 98, 4142–4147 (2001).
Herzog, C. et al. PrPTSE Distribution in a Primate Model of Variant, Sporadic, and Iatrogenic Creutzfeldt-Jakob Disease. J Virol 79, 14339–14345 (2005).
Lasmezas, C. I. et al. Risk of oral infection with bovine spongiform encephalopathy agent in primates. Lancet 365, 781–783 (2005).
Comoy, E. E. et al. Atypical BSE (BASE) transmitted from asymptomatic aging cattle to a primate. PLoS One 3, e3017, https://doi.org/10.1371/journal.pone.0003017 (2008).
Ono, F. et al. Atypical L-type bovine spongiform encephalopathy (L-BSE) transmission to cynomolgus macaques, a non-human primate. Japanese journal of infectious diseases 64, 81–84 (2011).
Ono, F. et al. Experimental transmission of bovine spongiform encephalopathy (BSE) to cynomolgus macaques, a non-human primate. Japanese journal of infectious diseases 64, 50–54 (2011).
Comoy, E. E. et al. Evaluation of the zoonotic potential of transmissible mink encephalopathy. Pathogens 2, 520–532, https://doi.org/10.3390/pathogens2030520pathogens2030520 (2013).
Race, B. et al. Chronic wasting disease agents in nonhuman primates. Emerg Infect Dis 20, 833–837, https://doi.org/10.3201/eid2005.130778 (2014).
Comoy, E. E. et al. Transmission of scrapie prions to primate after an extended silent incubation period. Sci Rep 5, 11573, https://doi.org/10.1038/srep11573 (2015).
Comoy, E. E. et al. Experimental transfusion of variant CJD-infected blood reveals previously uncharacterised prion disorder in mice and macaque. Nature communications 8, 1268, https://doi.org/10.1038/s41467-017-01347-0 (2017).
Race, B. et al. Lack of Transmission of Chronic Wasting Disease to Cynomolgus Macaques. Journal of virology 92, e00550–00518, https://doi.org/10.1128/JVI.00550-18. (2018).
Cassard, H. et al. Evidence for zoonotic potential of ovine scrapie prions. Nature communications 5, 5821, https://doi.org/10.1038/ncomms6821 (2014).
Comoy, E. E., Mikol, J. & Deslys, J. P. Unexpected prion phenotypes in experimentally transfused animals: predictive models for humans? Prion, 1–8, https://doi.org/10.1080/19336896.2018.1505399 (2018).
Beringue, V. et al. Transmission of atypical bovine prions to mice transgenic for human prion protein. Emerg Infect Dis 14, 1898–1901 (2008).
Kong, Q. et al. Evaluation of the human transmission risk of an atypical bovine spongiform encephalopathy prion strain. J Virol 82, 3697–3701, https://doi.org/10.1128/JVI.02561-07 (2008).
Wilson, R. et al. Presence of subclinical infection in gene-targeted human prion protein transgenic mice exposed to atypical bovine spongiform encephalopathy. J Gen Virol 94, 2819–2827, https://doi.org/10.1099/vir.0.052738-0 (2013).
Shibuya, S., Higuchi, J., Shin, R. W., Tateishi, J. & Kitamoto, T. Codon 219 Lys allele of PRNP is not found in sporadic Creutzfeldt-Jakob disease. Ann Neurol 43, 826–828 (1998).
Soldevila, M. et al. Prion susceptibility and protective alleles exhibit marked geographic differences. Hum Mutat 22, 104–105 (2003).
Bett, C. et al. Structure of the beta2-alpha2 loop and interspecies prion transmission. FASEB J 26, 2868–2876, https://doi.org/10.1096/fj.11-200923 (2012).
Billeter, M. et al. Prion protein NMR structure and species barrier for prion diseases. Proc Natl Acad Sci USA 94, 7281–7285 (1997).
Kurt, T. D. et al. Prion transmission prevented by modifying the beta2-alpha2 loop structure of host PrPC. J Neurosci 34, 1022–1027, https://doi.org/10.1523/JNEUROSCI.4636-13.2014 (2014).
Sigurdson, C. J. et al. A molecular switch controls interspecies prion disease transmission in mice. J Clin Invest 120, 2590–2599, https://doi.org/10.1172/JCI42051 (2010).
Kurt, T. D. et al. Human prion protein sequence elements impede cross-species chronic wasting disease transmission. J Clin Invest 125, 1485–1496, https://doi.org/10.1172/JCI79408 (2015).
Gajdusek, D. C. Unconventional viruses and the origin and disappearance of kuru. Science 197, 943–960 (1977).
Fischer, M. et al. Prion protein (PrP) with amino-proximal deletions restoring susceptibility of PrP knockout mice to scrapie. EMBO J 15, 1255–1264 (1996).
Weber, P., Metzger, D. & Chambon, P. Temporally controlled targeted somatic mutagenesis in the mouse brain. Eur J Neurosci 14, 1777–1783 (2001).
Manson, J. C. et al. 129/Ola mice carrying a null mutation in PrP that abolishes mRNA production are developmentally normal. Mol Neurobiol 8, 121–127 (1994).
Yull, H. M. et al. Detection of type 1 prion protein in variant creutzfeldt-jakob disease. Am J Pathol 168, 151–157 (2006).
Torres, J. M. et al. Classical bovine spongiform encephalopathy by transmission of H-type prion in homologous prion protein context. Emerg Infect Dis 17, 1636–1644, https://doi.org/10.3201/eid1709.101403 (2011).
Feraudet, C. et al. Screening of 145 Anti-PrP Monoclonal Antibodies for Their Capacity to Inhibit PrPSc Replication in Infected Cells. J. Biol. Chem. 280, 11247–11258 (2005).
Andreoletti, O. et al. PrP(Sc) accumulation in placentas of ewes exposed to natural scrapie: influence of foetal PrP genotype and effect on ewe-to-lamb transmission. J Gen Virol 83, 2607–2616 (2002).
Espinosa, J. C. et al. Sheep-passaged bovine spongiform encephalopathy agent exhibits altered pathobiological properties in bovine-PrP transgenic mice. J Virol 81, 835–843, https://doi.org/10.1128/JVI.01356-06 (2007).
Fraser, H. & Dickinson, A. G. The sequential development of the brain lesion of scrapie in three strains of mice. J Comp Pathol 78, 301–311 (1968).
Andreoletti, O. et al. PrP(Sc) accumulation in myocytes from sheep incubating natural scrapie. Nat Med 10, 591–593 (2004).
Torres, J. M. et al. Elements modulating the prion species barrier and its passage consequences. PLoS One 9, e89722, https://doi.org/10.1371/journal.pone.0089722 (2014).
Acknowledgements
We declare no competing financial interests. We thank the following providers of tissues: Basque Biobank (Bilbao, Spain), Animal and Plant Health Agency (New Haw, Addlestone, Surrey U.K.), Biobanco Hospital Universitario Fundación Alcorcón (Alcorcón, Spain), National Veterinary Research Institute (Pulawy, Poland), French National Institute for Agricultural Research (Nouzilly, France) and Instituto Superiore di Sanitá (Rome, Italy). The TgMac mice line was established at the Mouse Clinical Institute (Institut Clinique de la Souris, MCI/ICS) in the Genetic Engineering and Model Validation Department with funds from ANR (ANR-10-BLAN-1330 01) and internal specific grant from INSERM. This work was supported by grants from the Spanish Ministerio de Economía y Competitividad [AGL2016-78054-R (AEI/FEDER, UE), AGL2012-37988-C04-04 and RTA2012-00004-00-00 and fellowship BES-2010-040922 to P.A.C.], the Alliance Biosecure Foundation (FABS201403), and the Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria (fellowship FPI-SGIT-2015-02 to A.M.M.).
Author information
Authors and Affiliations
Contributions
J.C.E. and J.M.T. designed the experiments. J.C.E., E.E.C., A.M.M., P.A.C., M.C.B., J.L.P., J.P.D. and J.M.T. conducted the experiments. J.C.E., E.E.C. and J.M.T. wrote the manuscript. J.C.E., E.M.C., A.M.M., P.A.C. and J.M.T. revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Espinosa, J.C., Comoy, E.E., Marin-Moreno, A. et al. Transgenic mouse models expressing human and macaque prion protein exhibit similar prion susceptibility on a strain-dependent manner. Sci Rep 9, 15699 (2019). https://doi.org/10.1038/s41598-019-52155-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-52155-z
This article is cited by
-
Non-human primates in prion diseases
Cell and Tissue Research (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.