Abstract
In angiosperms, sex determination leads to development of unisexual flowers. In Cucumis melo, development of unisexual male flowers results from the expression of the sex determination gene, CmWIP1, in carpel primordia. To bring new insight on the molecular mechanisms through which CmWIP1 leads to carpel abortion in male flowers, we used the yeast two-hybrid approach to look for CmWIP1-interacting proteins. We found that CmWIP1 physically interacts with an S2 bZIP transcription factor, CmbZIP48. We further determined the region mediating the interaction and showed that it involves the N-terminal part of CmWIP1. Using laser capture microdissection coupled with quantitative real-time gene expression analysis, we demonstrated that CmWIP1 and CmbZIP48 share a similar spatiotemporal expression pattern, providing the plant organ context for the CmWIP1-CmbZIP48 protein interaction. Using sex transition mutants, we demonstrated that the expression of the male promoting gene CmWIP1 correlates with the expression of CmbZIP48. Altogether, our data support a model in which the coexpression and the physical interaction of CmWIP1 and CmbZIP48 trigger carpel primordia abortion, leading to the development of unisexual male flowers.
Similar content being viewed by others
Introduction
Most flowering plants are hermaphroditic, producing only bisexual flowers. Sex determination is a developmental process that leads to unisexual flowers. Beside hermaphrodite plants, 10% of the species display unisexual flowers. Monoecious species such as Cucumis melo, here after melon, exhibit male and female flowers on the same plant. Dioecious species, such as Asparagus and date palm, have separate male and female individuals1,2.
In melon, flowers are bisexual at early developmental stages then sex determination occurs by the developmental arrest of either the stamen or the carpel primordia, resulting in unisexual flowers. This sexual organ arrest is genetically governed by the interaction of the andromonoecious (M), androecious gene (A) and gynoecious (G) genes that define the monoecy sex determination pathway in cucurbits3. Genetic analysis revealed a mechanistic model in which expression of the carpel inhibitor, G gene, is dependent on non-expression of A gene and expression of the stamina inhibitor, M gene, is dependent on non-expression of G gene.
The cloning and characterization of the three sex determination genes revealed the identity of M, A and G genes as CmACS-7, CmACS11 and CmWIP1, respectively. CmACS-7 and CmACS11 encode for aminocyclopropane-1-carboxylic acid synthases, an enzyme that catalyse the rate limiting step of ethylene biosynthesis in plants. CmWIP1 belongs to a class of C2H2 zinc finger transcription factors named WIP proteins, based on the presence of a conserved first 3 C-terminal amino acids, WIP4,5. Investigation of sex determination in other cucurbits, including cucumber and watermelon revealed a conserved sex determination pathway that implicates CmACS-7, CmACS11 and CmWIP1 homologs3,6,7,8.
While the molecular mechanisms through which CmACS-7 and CmACS11 control sex determination may be predicted, how CmWIP1 contributes to carpel inhibition is an enigma. In Arabidopsis thaliana, six WIP proteins have been identified and through mutation analysis WIP proteins were associated with seed coat development (AtWIP1)4, transmitting tract growth (AtWIP2)9, leaf vein patterning (AtWIP6)10 and in the early root apical meristem formation11. A common theme and character demonstrated by the WIP zinc finger proteins points to a regulatory role of these genes in organ growth and development. This hypothesis has been further validated by a recent study demonstrating that in Arabidopsis, the ntt/wip4/wip5 triple mutants fail to develop roots11.
BLAST search analysis revealed that WIP proteins are restricted to land plants, including the bryophyte Physcomitrella patens12. No homologous sequences have been found in lower plant-related organisms whose genome is completely sequenced, such as Chlamydomonas reinhardtii or Ostreococcus tauri13,14. Annotation of WIP proteins revealed two domains. The N-terminal region contains degenerative sequence and two to three short conserved motifs with unknown function5. Reverse genetic experiment using TILLING showed that at least one of the conserved motifs is required for the proper function of the protein15. The C-terminal region is highly conserved and contains the zinc fingers and nuclear localization signals and is predicted to be implicated in DNA interaction.
In this study, we aimed to identify novel proteins that interact with CmWIP1 to help identify certain of the important regulatory networks involved in unisexual flower development. The yeast two-hybrid (Y2H) method is a powerful tool for high-throughput protein–protein interaction studies in vivo16. We generated a high-quality cDNA library from unisexual and hermaphrodite flowers and performed a yeast two-hybrid screen using CmWIP1 as bait. We showed that CmWIP1 physically interacts with a basic leucine zipper transcription factor, MELO3C022062_CmbZIP48, and that CmWIP1-CmbZIP48 protein interaction is mediated by the N-terminal part of CmWIP1. The bimolecular fluorescence complementation (BiFC) assays confirmed that CmWIP1 interacts in planta with CmbZIP48. As expected for a transcription factor, the interaction occurs in the nucleus. CmbZIP48 belongs to the S2 subclass of bZIP proteins. We further show that CmWIP1 interacts with MELO3C018916_CmbZIP42 another bZIP S2 subclass member. Thanks to the investigation of the spatiotemporal expression pattern in sex transition mutants, we demonstrated that the expression of the male promoting gene CmWIP1 correlates with the expression of CmbZIP48 in the carpel primordia of male flowers to lead to unisexual male flowers.
Results and Discussion
CmbZIP48 protein interacts with the N-terminal region of CmWIP1
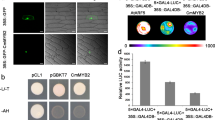
To identify the protein partners of CmWIP1, we used the yeast two-hybrid system (Y2H). Because CmWIP1 is expressed at early stages of the flower development, the cDNA library used as a prey to identify CmWIP1 partners was synthesized from RNA of male, female and hermaphrodite flower meristems at the stage where CmWIP1 is expressed. Out of the 3.5 106 clones screened, 68 positive colonies were obtained after three times re-streaking on selective media. Sequencing and annotation of the positive clones revealed that 17 clones (25%) contain coding sequences of the MELO3C022062 gene in-frame with the GAL4 Activation Domain (Fig. 1a). MELO3C022062 is a single exon gene encoding a basic leucine zipper (bZIP) transcription factor of 179 amino acids, here after CmbZIP48. bZIP transcription factors are characterized by a conserved bZIP protein domain composed of two motifs, a basic region responsible for the DNA binding specificity and a leucine zipper required for protein dimerization (Fig. 1b)17. The bZIP transcription factors regulate crucial biological processes such as organ and tissue differentiation18,19,20, pathogen defence21,22, hormone and sugar signalling23, protection against biotic and abiotic stresses24,25 and flower and seed development17,26. CmbZIP48 shares 54% amino acid identity with AtbZIP42 (AT3G30530) and 50% identity with AtbZIP43 (AT5G38800).
Identification of CmWIP1-CmbZIP48 protein interaction by yeast two hybrid screening. (a) Yeast cells transformed with CmWIP1-AD and CmbZIP48-BD growing on the selective medium. (b) Diagram of the bZIP domain of CmbZIP48. The basic region and the leucine zipper domain are shaded in blue and red, respectively. The sequence of the invariant residues within the bZIP domain of CmbZIP48 is given. (c) Diagram of the CmWIP1 deletion isoforms used for the Y2H interaction assays. The C terminal region of CmWIP1 is black filled. The conserved n1 and n2 domains are shaded in blue and green, respectively.
To further analyse the regions involved in the CmbZIP48-CmWIP1 protein interaction, we generated CmWIP1 deletion mutants (Fig. 1c). To develop a logical approach for constructing CmWIP1 deletion mutants, we analyse the protein sequences of the six members of the WIP family in melon for candidate protein interaction motifs. This analysis revealed that melon WIP proteins can be divided in two domains, a non-conserved N-terminal half and a highly conserved C-terminal half starting with the three amino acids WIP and containing the four C2H2 zinc fingers. The observation that two conserved motifs are present within the non-conserved N-terminal half raises the possibility that these n1 and n2 subdomains may be involved in protein–protein interactions. Similar protein structure has also been reported for the A. thaliana WIP proteins5.
Based on the WIP protein domain conservation, we prepared different deletion mutants, such as CmWIP1_∆C (deletion of the C-terminal region) and CmWIP1_∆N (deletion of the N-terminal region). Interactions assays performed using these deletion mutants demonstrate that the C-terminal half of CmWIP1 is not required for the CmWIP1-CmbZIP48 interaction (Fig. 1c). By contrast the N-terminal region binds CmbZIP48 efficiently and is sufficient for this interaction (Fig. 1c). To map more precisely the subdomains necessary for the interaction with CmbZIP48 within the N-terminal region of CmWIP1, we generated CmWIP1_∆n1 and CmWIP1_∆n2 deletion mutants. We found that deletions of the conserved n1 or n2 domains had no effect on CmWIP1 interaction with CmbZIP48 (Fig. 1c). These results suggested that CmWIP1 interacts with CmbZIP48 through its non-conserved N-terminal regions.
CmWIP1 interacts with CmbZIP48 in planta
Previous studies showed that all WIP proteins contain two monopartite nuclear localisation signals (NLS) located within the second and the fourth C2H2-ZnF domain and localize in the nucleus5. The CmbZIP48 is predicted to contain a monopartite NLS and is expected to localize in the nucleus. To characterize its subcellular localization, we fused the yellow fluorescent protein (YFP) at the N terminus of CmbZIP48 and showed that, as the WIP proteins, CmbZIP48 localized to the nucleus (Fig. 2a).
Nuclear in planta interaction of CmWIP1 and CmbZIP48 by BiFC. (a) The YFP fused at the N terminus of CmbZIP48 localized into the nucleus. (b–i) The BiFC experiments. The constructs combinations are indicated within each panel. Negative (b,c,g,h) and positive (i) controls are shown. Scale bars = 10 µm (a,d–f); 15 µm (g) and 30 µm (b,c,h).
To address the physical interaction of CmbZIP48 and CmWIP1 in planta, we took advantage of the bimolecular fluorescence complementation (BiFC)27,28. We fused the C-terminal portion of the YFP (cYFP) to the full length CmbZIP48. The cYFP was fused to the N-terminus of CmbZIP48. For CmWIP1, we fused the N-terminal portion of YFP (nYFP) to the N-terminus of the full length CmWIP1. The expression of the cYFP-CmbZIP48 and nYFP-CmWIP1 fusions was driven by the constitutive CaMV 35S promoter. The constructs were agro-infiltrated into Nicotiana benthamiana leaves and CmbZIP48-CmWIP1 protein interaction was assayed based on the reconstitution of YFP fluorescence. Infiltration of cYFP-CmbZIP48 paired with nYFP or nYFP-CmWIP1 paired with cYFP yields no YFP fluorescence (Fig. 2b,c). In contrast, when cYFP-CmbZIP48 was assayed with nYFP-CmWIP1, a strong YFP signal was detected in the nucleus indicating that CmbZIP48 and CmWIP1 interact in planta and co-localize in the nucleus (Fig. 2d). Then, we assayed for in planta interaction between cYFP-CmbZIP and nYFP-CmWIP1_∆n1 or nYFP-CmWIP1_∆n2 truncated versions of CmWIP1 (Fig. 2e,f). As shown in yeast, the two CmWIP1 truncated versions were still able to interact with CmbZIP48 in planta in the nucleus (Fig. 2e,f).
To address the specificity of the CmbZIP48-CmWIP1 association, we assayed the interaction of CmWIP1 and CmbZIP48 with CmGLOBOSA and CmDEFICIENS, respectively. GLOBOSA and DEFICIENS are two nuclear MADS-box proteins known to interact in the nucleus (Fig. 2i)29. Fluorescent signal was detected neither for nYFP-CmWIP1 assayed with CmGLOBOSA (Fig. 2g) nor for cYFP-CmbZIP48 assayed with CmDEFICIENS (Fig. 2h), suggesting that the YFP signal observed when nYFP-CmWIP1 and cYFP-CmbZIP48 are co-agroinfiltrated results from the specific CmWIP1-CmbZIP48 protein interaction.
Identification and classification of the bZIP transcription factors in the melon genome
To test whether other melon bZIP proteins could interact with CmWIP1, we performed an extensive genome-wide search using bZIP protein sequences from Arabidopsis, rice, tomato and cucumber as BLASTP queries against the melon genome database30. A total of 63 non-redundant CmbZIP transcription factors were identified (Supplementary Table 1). For convenience, we assigned a unique identifier to the CmbZIPs genes, CmbZIP1 to CmbZIP63, based on their location on chromosomes 1 to 12 (Supplementary Fig. 1 and Supplementary Table 1). Three CmbZIPs mapped to unanchored scaffolds and were numbered CmbZIP61, CmbZIP62 and CmbZIP63 (Supplementary Fig. 1 and Table 1). The CmbZIP proteins range from 129 to 721 aa with an average size of 318 aa and with a range of molecular weights from 15.11 kDa to 78.67 kDa. The CmbZIP names and other details are summurized in Supplementary Table 1.
Compared to other plants, the number of CmbZIP genes and the sizes of CmbZIP proteins are comparable to the bZIP gene families of cucumber (64 bZIPs)31, medicago (65 bZIPs)32, chickpea (59 bZIPs)32, tomato (69 bZIPs)33 and Arabidopsis (77 bZIPs)17.
To analyse the evolutionary history the CmbZIP gene family, an unrooted phylogenetic tree was generated using a multiple sequence alignment of the CmbZIP proteins. The phylogenetic tree subdivided the melon bZIP genes family into 10 groups (A to I and S groups based on the Arabidopsis nomenclature in 17) with well-supported boostrap values (Supplementary Fig. 2). As expected, CmbZIP family members from the same group clustered together into the same clade (Supplementary Fig. 2). The two-species phylogenetic analysis of bZIPs among melon and Arabidopsis showed that all the clades contain melon and Arabidopsis bZIP proteins and no melon specific bZIP clade was observed (Supplementary Fig. 3). This interspecies tree also suggests the existence of homologous bZIP genes among melon and Arabidopsis and that melon bZIP proteins have been conserved during evolution.
The intron-exon organization of the members of a multigene family is an imprint of their evolution. To get a deeper insight into the evolution of the melon bZIP genes, we investigated the intron-exon structure of the 63 CmbZIP genes. Among all, we detected 15 (23%) intronless CmbZIP genes and most of them (14 genes) were clustered in the group S (Supplementary Fig. 2). Similar proportion of intronless bZIP genes has been reported in Arabidopsis, cucumber, tomato and legume plants. For the CmbZIP genes containing introns, the number of introns ranged from 1 to 11 and as expected CmbZIP genes from the same group tended to share similar gene structures (Supplementary Fig. 2). For example, the CmbZIP genes of group D and G contain 7 to 11 introns and 10 to 11 introns, respectively. Interestingly, all the CmbZIP genes of group C have 5 introns.
To physically map the CmbZIPs genes, we determined their chromosome locations (Supplementary Table 1). Among all, 3 CmbZIP genes were mapped on unanchored scaffolds and 60 CmbZIP genes were mapped on the 12 melon chromosomes and were distributed unevenly (Supplementary Fig. 1). Chromosomes 3, 1, 4 and 7 contain the largest numbers of CmbZIPs with 11, 9, 8 and 7 members, respectively, whereas chromosomes 2, 5, 9, and 12 contain only two members (Supplementary Fig. 1).
Tandem gene duplications are important mechanisms that drive the expansion of a gene family. In melon, we detected only four pairs of tandem duplication, CmbZIP6/7, CmbZIP29/30, CmbZIP34/35 and CmbZIP57/58, on chromosomes 1, 4, 6 and 11, respectively (Supplementary Table 1), suggesting a marginal contribution of tandem duplications to the CmbZIP gene family expansion.
Two members of the CmbZIP S2 sub-group, CmbZIP48 and CmbZIP42, interact with CmWIP1
In the Y2H and BiFC experiments, we identified and confirmed that CmbZIP48 interacts with CmWIP1 (Figs 1 and 2). CmbZIP48 belongs to the group S of bZIP transcription factors (Supplementary Fig. 3 and Table 1). The phylogenetic analysis in melon and Arabidopsis revealed that the members of the group S can be divided into 3 sub-groups, S1, S2 and S3 and that CmbZIP48 is part of the S2 sub-group (Fig. 3a). Global protein alignment shows that all the S2 bZIP proteins from melon and Arabidopsis share the typical bZIP protein domain, N-x7-R-x9-L-x6-L-x6-L (Supplementary Fig. 4).
Targeted Y2H and expression pattern of the S2 CmbZIP. (a) Phylogenetic analysis of group S bZIP proteins in melon and Arabidopsis. The scale bar indicates 0.1 amino acid substitution per site. (b) Targeted Y2H assays between CmWIP1 and the five CmbZIP of the S2 sub-group. (c) Gene expression pattern of CmWIP1 and the five S2 CmbZIP genes in the carpel and stamen primordia of female and male flowers of a monoecious plant.
To test whether other bZIP proteins of the S2 sub-group, here after, CmbZIP14, CmbZIP42, CmbZIP49 and CmbZIP52, could interact with CmWIP1, we used targeted Y2H assays (Fig. 3b). Yeast lines coexpressing CmbZIP14, CmbZIP49 or CmbZIP52 with CmWIP1 were unable to grow on selective media, suggesting no physical interactions (Fig. 3b). In contrast, yeast lines coexpressing CmbZIP42 and CmWIP1 were able to grow, as did yeast lines coexpressing CmWIP1 and CmbZIP48 (Fig. 3b). Interestingly, among the five melon S2 bZIP proteins, CmbZIP42 is the CmbZIP48-closest homolog, sharing 71% amino acid identity.
CmWIP1 and CmbZIP48 are coexpressed in carpel primordia of male flowers
To weigh the biological significance of the physical interaction between two proteins, it is admitted that if the two interacting genes have also correlated expressions across various tissues, they are likely to control the same functions34. Thus, we compared the expression profiles of CmWIP1 to CmbZIP42 and CmbZIP48 genes. For comparison we also analysed the expression profiles of CmbZIP14, CmbZIP49 and CmbZIP52, the three other S2 sub-group bZIP proteins that do not interact physically with CmWIP1.
Using in situ hybridization (ISH), we previously demonstrated that the spatial expression of CmWIP1 is restricted to the carpel primordia of male flowers and no expression was detected in carpel primordia of buds determined to develop a carpel15. To obtain an in-depth resolution of the S2 CmbZIP spatial expression pattern, we isolated carpel and stamen primordia tissues from male and female flowers from a monoecious plant using laser capture microdissection (LCM). To check whether the quantitative real-time (qRT) gene expression analysis using microdissected tissues can be used as an alternative to the ISH, we first assayed the expression of CmWIP1. As for the ISH analysis, CmWIP1 is mainly expressed in the carpel primordia of male flowers. Weak to no expression was detected in the stamen primordia of male flowers and carpel and stamen primordia of female flowers (Fig. 3c). This result demonstrate that the LCM-qRT gene expression analysis corroborate the results obtained using ISH experiment and thus can be used to determine the spatial gene expression pattern. Expression analysis of CmbZIP14, CmbZIP49 and CmbZIP52 showed weak to no expression in the carpel primordia of male flowers where CmWIP1 is expressed (Fig. 3c). CmbZIP42 was found not expressed in any sexual primordia of male or female flowers, explaining why the CmWIP1-CmbZIP42 protein interaction was not detected in the non-targeted Y2H screen (Fig. 3c). Interestingly, CmbZIP48 was found mimicking the expression pattern of CmWIP1 in male flowers, at the developmental stage where CmWIP1 inhibits carpel development. Indeed, we found both genes highly expressed in the carpel primordia and weakly expressed in the stamina primordia of male flowers. In contrast, in female flowers, CmbZIP48 is expressed in both carpel and stamen primordia, whereas CmWIP1 is not expressed (Fig. 3c). Altogether, these results highlight that CmWIP1 and CmbZIP48 share a similar expression pattern and are spatially coexpressed in the stamen primordia of male flowers providing the plant organ context for the CmWIP1-CmbZIP48 protein interaction.
Integration of the CmbZIP48 in the sex determination model
We previously demonstrated that sex determination in melon relies on the interplay between alleles of three sex determination genes, M, G and A. In monoecious plants, male flowers results from non-expression of CmACS11, that permits CmWIP1 expression. Female flowers develop on at the youngest nodes of the growing vines expressing CmACS11, which represses the expression of CmWIP1, and thus, releasing the expression of CmACS-7 inhibiting stamina development. If nonfunctional CmACS-7 is expressed, hermaphrodite, instead of female, flowers develop. Androecious plants result from a loss-of-function of CmACS11 leading to expression of CmWIP1 in all flowers on a plant. Gynoecious plants are obtained by inactivation of CmWIP1 function and hermaphrodite plants are obtained by inactivation of CmWIP1 and CmACS-73,6,15.
To integrate the role of the CmWIP1-CmbZIP48 interaction in our sex determination model, we investigated the expression of CmACS-7, CmWIP1 and CmbZIP48 genes in flowers of androecious, gynoecious and hermaphrodite melon mutants (Fig. 4a). As expected, CmACS-7 is highly expressed in the carpel primordia of female and hermaphrodite carpel-bearing flowers whereas weak to no CmACS-7 expression was detected in the stamen primordia of female and hermaphrodite flowers and carpel and stamen primordia of male flowers (Fig. 4b). CmWIP1 was found highly expressed in the carpel primordia of male flowers. In the female and hermaphrodite flowers, CmWIP1 was not expressed (Fig. 4c). CmbZIP48 expression was not affected by the flower sexual type and was found strongly expressed in the carpel primordia of female, hermaphrodite and male flowers (Fig. 4d).
Genetic model integrating CmWIP1 and bZIP48 interaction. (a) Schematic representation of the monoecious, androecious, gynoecious and hermaphrodite sexual morphs in melon. (b–d) Quantitative RT-PCR of CmACS-7, CmWIP1 and CmbZIP48 in carpel and stamen primordia of female, hermaphrodite and male flowers of gynoecious, hermaphrodite and androecious melons, respectively. Shown are the mean + − SD of three biological replicates. GC: gynoecious carpel primordia, GS: gynoecious stamen primordia, HC: hermaphrodite carpel primordia, HS: hermaphrodite stamen primordia, AC: androecious carpel primordia, AS: androecious stamen primordia. (e,f) Model of the sex-determination pathway in melon integrating the CmWIP1-CmbZIP48 co-expression and protein interaction. (e) When CmWIP1 and CmbZIP48 are coexpressed, CmWIP1-CmbZIP48 complex forms and represses carpel development leading to the rise of a male flower. (f) When CmWIP1 is not expressed, the expression of the genes involved in carpel development leads to a female flower.
Altogether, these data support a model in which the coexpression and protein-protein interaction of CmWIP1 and CmbZIP48 turn on the carpel primordia abortion leading the development of a male flower. The lack of CmWIP1 expression yields carpel development and leads to the raising of a female flower (Fig. 4e,f). This model also suggests that CmbZIP48 expression is independent of CmWIP1 expression, indicating that CmbZIP48 may recruit other transcription factors required for carpel development. In Arabidopsis, the bZIP transcription factor HY5 was identified as a negative regulator of ethylene biosynthesis. HY5 activates the expression of the transcriptional repressor AtERF11 which further represses the expression of AtACS2 and AtACS535. This may suggest that CmWIP1-CmbZIP48 complex recruit a melon ERF gene to repress the expression of ACS genes. In this scenario, the repression of CmACS-7 leads to the stamina development and the repression of CmACS11 maintain CmWIP1 expression that blocks the development of the carpel thus yielding a male flower. Furthermore, the integration of the CmbZIP48 into the genetic model of sex determination sheds light on the molecular mechanisms of how CmWIP1 inhibits the carpel development.
Methods
The yeast-two hybrid system
The standardized yeast-two hybrid protocol36 was used to perform the screen in which CmWIP1 protein served as bait and cDNA library as a prey in order to identify interacting protein partners of CmWIP1. Total RNA was extracted from male, female and hermaphrodite flowers just before and after the arrest of the inappropriate organ37 using the Trizol reagent (Invitrogen). The cDNA library was synthesized from 6 µg of total RNA using the cDNA Library Construction Kit (Invitrogen). The CmWIP1 bait gene was fused to the GAL4 DNA binding domain into pDESTTM32 vector. The cDNA library was fused to the GAL4 transcriptional activation domain cloned into the pDESTTM22 vector. For the two-hybrid assays, yeast cells MaV203 (Invitrogen) were cotransformed with the two constructs according to the protocol of Dohmen et al.38. The ability to drive the HIS3 reporter gene was assessed by growing transformants on selective medium lacking tryptophan, leucine, and histidine and supplemented with 60 mM 3-amino-19,29,49-triazole (3-AT). Handling of yeast cultures and plate growth assays were performed as described in the Yeast Protocols Handbook (Clontech).
Bimolecular fluorescence complementation (BiFC)
Visualization of the interaction between two proteins of interest in living Nicotiana benthamiana plant cells was achieved through the utilization of standard BiFC protocols39. The coding sequence of the genes of interest were cloned into the vectors pBiFC2 (YFPN), and pBiFC3 (YFPC). The two target proteins fused to the YFP fragment, either YFPN or YFPC, were transiently expressed in leaves of 3-week-old N.benthamiana plants by infiltration as described in Voinnet et al.40. Upon interaction between the two target proteins, the YFPN and YFPC fragments restore fluorescence. YFP fluorescence was detected 3 days after infiltration by using the 514-nm laser line of a LSM 710 confocal laser scanning microscope (Carl Zeiss) equipped with an argon laser. Fluorescent images of interactions or absence of fluorescence in tobacco leaf cells were captured using the ZEN software.
Bioinformatic and phylogenetic analysis
Multiple sequence alignment of the S2 bZIP protein sequences of melon and Arabidopsis was performed using the ClustalW (http://www.ebi.ac.uk/Tools/clustalw)2. Phylogenetic trees were constructed using the Neighbor-Joining method by MEGA 7.0 (http://www.megasoftware.net/index.html).
Identification of bZIP proteins in melon
To identify candidate bZIP proteins from melon, the melon database (http://www.melonomics.net/) was searched first using the keywords ‘bZIP’. In addition, Arabidopsis, rice, tomato and cucumber bZIP protein sequences were downloaded from The Arabidopsis Information Resource (http://www.arabidopsis.org/), the Rice Genome Annotation Project (http://rice.plantbiology.msu.edu/), the Sol Genomics Network (http://solgenomics.net/) and the Cucurbit Genomics Database (http://cucurbitgenomics.org/), respectively. These sequences were used to identify homologous peptides from melon by performing a BLASTP search at melon genome v3.5 database (http://www.melonomics.net/)30. The BLAST E-value was set to 1e−3. The retrieved sequences were searched using SMART (http://smart.embl-heidelberg.de/), and Pfam (http://pfam.sanger.ac.uk/) databases for the presence of the conserved bZIP domain. Finally, repeated and incomplete sequences were removed manually and the non-redundant CmbZIP sequences were subjected to further analyses.
Chromosomal distribution and gene structure
The CmbZIP genes were mapped onto the corresponding chromosomes by BLASTP against the melon genome v3.5 database (http://www.melonomics.net/) using default settings. The CmbZIP genes were plotted onto the melon chromosomes according to their ascending physical position (bp). Tandem duplications were characterized as adjacent genes of same sub-family located within 10 predicted genes apart or within 30 kbp of each other.
The exon-intron structure of the CmbZIP genes was determined using the Gene-Structure Display Server (gsds.cbi.pku.edu.cn) through comparison of their coding sequence (CDS) with their corresponding genomic sequence.
Laser capture microdissection
Tissue embedding
Flowers buds were fixed in RCL2 (Excilone) with 0.01% triton, vacuum 4 times for 15 min and kept in the fixative overnight at 4 °C. Tissues were dehydrated at 4 °C in a graded series of ethanol (70% for 30 min, 96% for 30 min, 100% for 3 × 30 min), followed by a graded series of ethanol:histoclear bath (3:1, 1:1, 1:3 for 1 h each). Histoclear was then substituted by Surgipath Paraplast plus tissue embedding media (Leica Biosystems) overnight at 60 °C. Finally, flowers were poured into paraffin blocks, cooled and stored at −20 °C.
Laser-assisted microdissection
Longitudinal flowers sections of 8 µm were cut using a Rotary microtome (HM 3555 Microtom). Ribbons were stretched on UV-treated, 1 mm PEN-membrane covered slides (Arcturus Bioscience, Excilone). Each slide corresponding to 15–25 sections of flowers at bisexual stage. Side were de-paraffined, and laser capture microdissection was immediately conducted with a Palm DIC FLUO Microdissection System (Zeiss). The contour of stamen or carpel primordia were cut with the laser and target regions were automatically catapulted into Adhesive cap 500 clear (Zeiss).
RNA extraction
Cells were lysed immediately after dissection, using the PicoPure® RNA Isolation Kit (Arcturus Bioscience, Excilone) and stored at −20 °C before RNA extraction. RNA quality and concentration were evaluated with a Bioanalyser 2100 (Agilent Technologies) on Agilent RNA Pico chips. RNA recovery was from 500–1000 pg/µl, with a RIN at 7.
Preparation of cDNA libraries
2 ng of total RNA was used for each cDNA library preparation using the SMARTer Ultra Low RNA Kit for Illumina Sequencing from Clontech according to manufacturer’s instructions (16 cycles of PCR were used for cDNA amplification). cDNA were used to assessed the expression of the S2 bZIP and CmWIP1 genes by qPCR experiments.
Reverse transcription polymerase chain reaction (RT-PCR) and qPCR
Total RNA was extracted from frozen flowers with the Trizol reagent (Invitrogen). Contaminating DNA was removed by DNaseI treatment (Invitrogen). First strand cDNA was synthesized from 2 μg of total RNA with the Superscript® III reverse transcriptase (Invitrogen). Primer design was performed with the Primer3 software (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi). Primers sequences are listed in Supplementary Table 2. To check specificity of the designed primers, all amplicons were sequenced and blasted against NCBI database. Polymerase chain reactions were performed in an optical 384-well plate with the Bio-Rad CFX96 Real-time PCR apparatus, with qPCR MasterMix Plus for SYBR® Green I w/o ROX (Eurogentec) and according to manufacturer’s instructions. PCR amplification specificity was verified by a dissociation curve (55 °C to 95 °C). A negative control without cDNA, technical replicates on three independent synthesis of cDNA (derived from the same RNA sample), and three independent biological experiments were performed in all cases. To compare data from different PCR runs and cDNA samples, CT values for CmbZIP49, CmbZIP14, CmbZIP52, CmbZIP42, CmbZIP48 and CmWIP1 were normalized to the CT value of CmActin2 (primers shown in Supplementary Table 2). The gene relative expressions were determined as described in6.
Data availability
All the data supporting the results of this paper are present in the paper and/or the supplementary materials. All relevant data are available from the corresponding author on request.
References
Ainsworth, C., Parker, J. & Buchanan-Wollaston, V. Sex determination in plants. Current topics in developmental biology 38, 167–223 (1998).
Renner, S. S. The relative and absolute frequencies of angiosperm sexual systems: dioecy, monoecy, gynodioecy, and an updated online database. American journal of botany 101, 1588–1596, https://doi.org/10.3732/ajb.1400196 (2014).
Boualem, A. et al. A cucurbit androecy gene reveals how unisexual flowers develop and dioecy emerges. Science 350, 688–691, https://doi.org/10.1126/science.aac8370 (2015).
Sagasser, M., Lu, G. H., Hahlbrock, K. & Weisshaar, B. A. thaliana TRANSPARENT TESTA 1 is involved in seed coat development and defines the WIP subfamily of plant zinc finger proteins. Genes & development 16, 138–149, https://doi.org/10.1101/gad.212702 (2002).
Appelhagen, I. et al. Weird fingers: functional analysis of WIP domain proteins. FEBS letters 584, 3116–3122, https://doi.org/10.1016/j.febslet.2010.06.007 (2010).
Boualem, A. et al. A conserved mutation in an ethylene biosynthesis enzyme leads to andromonoecy in melons. Science 321, 836–838, https://doi.org/10.1126/science.1159023 (2008).
Boualem, A. et al. A conserved ethylene biosynthesis enzyme leads to andromonoecy in two cucumis species. PloS one 4, e6144, https://doi.org/10.1371/journal.pone.0006144 (2009).
Boualem, A. et al. The Andromonoecious Sex Determination Gene Predates the Separation of Cucumis and Citrullus Genera. PloS one 11, e0155444, https://doi.org/10.1371/journal.pone.0155444 (2016).
Crawford, B. C., Ditta, G. & Yanofsky, M. F. The NTT gene is required for transmitting-tract development in carpels of Arabidopsis thaliana. Current biology: CB 17, 1101–1108, https://doi.org/10.1016/j.cub.2007.05.079 (2007).
Petricka, J. J., Clay, N. K. & Nelson, T. M. Vein patterning screens and the defectively organized tributaries mutants in Arabidopsis thaliana. The Plant journal: for cell and molecular biology 56, 251–263, https://doi.org/10.1111/j.1365-313X.2008.03595.x (2008).
Crawford, B. C. et al. Plant development. Genetic control of distal stem cell fate within root and embryonic meristems. Science 347, 655–659, https://doi.org/10.1126/science.aaa0196 (2015).
Lang, D. et al. The Physcomitrella patens chromosome-scale assembly reveals moss genome structure and evolution. The Plant journal: for cell and molecular biology 93, 515–533, https://doi.org/10.1111/tpj.13801 (2018).
Derelle, E. et al. Genome analysis of the smallest free-living eukaryote Ostreococcus tauri unveils many unique features. Proceedings of the National Academy of Sciences of the United States of America 103, 11647–11652, https://doi.org/10.1073/pnas.0604795103 (2006).
Merchant, S. S. et al. The Chlamydomonas genome reveals the evolution of key animal and plant functions. Science 318, 245–250, https://doi.org/10.1126/science.1143609 (2007).
Martin, A. et al. A transposon-induced epigenetic change leads to sex determination in melon. Nature 461, 1135–1138, https://doi.org/10.1038/nature08498 (2009).
Fields, S. & Song, O. A novel genetic system to detect protein-protein interactions. Nature 340, 245–246, https://doi.org/10.1038/340245a0 (1989).
Jakoby, M. et al. bZIP transcription factors in Arabidopsis. Trends in plant science 7, 106–111 (2002).
Walsh, J., Waters, C. A. & Freeling, M. The maize gene liguleless2 encodes a basic leucine zipper protein involved in the establishment of the leaf blade-sheath boundary. Genes & development 12, 208–218, https://doi.org/10.1101/gad.12.2.208 (1998).
Chuang, C. F., Running, M. P., Williams, R. W. & Meyerowitz, E. M. The PERIANTHIA gene encodes a bZIP protein involved in the determination of floral organ number in Arabidopsis thaliana. Genes & development 13, 334–344, https://doi.org/10.1101/gad.13.3.334 (1999).
Abe, M. et al. FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science 309, 1052–1056, https://doi.org/10.1126/science.1115983 (2005).
Thurow, C. et al. Tobacco bZIP transcription factor TGA2.2 and related factor TGA2.1 have distinct roles in plant defense responses and plant development. The Plant journal: for cell and molecular biology 44, 100–113, https://doi.org/10.1111/j.1365-313X.2005.02513.x (2005).
Kaminaka, H. et al. bZIP10-LSD1 antagonism modulates basal defense and cell death in Arabidopsis following infection. The EMBO journal 25, 4400–4411, https://doi.org/10.1038/sj.emboj.7601312 (2006).
Nieva, C. et al. Isolation and functional characterisation of two new bZIP maize regulators of the ABA responsive gene rab28. Plant molecular biology 58, 899–914, https://doi.org/10.1007/s11103-005-8407-x (2005).
Liu, C., Wu, Y. & Wang, X. bZIP transcription factor OsbZIP52/RISBZ5: a potential negative regulator of cold and drought stress response in rice. Planta 235, 1157–1169, https://doi.org/10.1007/s00425-011-1564-z (2012).
Ying, S. et al. Cloning and characterization of a maize bZIP transcription factor, ZmbZIP72, confers drought and salt tolerance in transgenic Arabidopsis. Planta 235, 253–266, https://doi.org/10.1007/s00425-011-1496-7 (2012).
Zou, M., Guan, Y., Ren, H., Zhang, F. & Chen, F. A bZIP transcription factor, OsABI5, is involved in rice fertility and stress tolerance. Plant molecular biology 66, 675–683, https://doi.org/10.1007/s11103-008-9298-4 (2008).
Bracha-Drori, K. et al. Detection of protein-protein interactions in plants using bimolecular fluorescence complementation. The Plant journal: for cell and molecular biology 40, 419–427, https://doi.org/10.1111/j.1365-313X.2004.02206.x (2004).
Citovsky, V., Gafni, Y. & Tzfira, T. Localizing protein-protein interactions by bimolecular fluorescence complementation in planta. Methods 45, 196–206, https://doi.org/10.1016/j.ymeth.2008.06.007 (2008).
Trobner, W. et al. GLOBOSA: a homeotic gene which interacts with DEFICIENS in the control of Antirrhinum floral organogenesis. The EMBO journal 11, 4693–4704 (1992).
Garcia-Mas, J. et al. The genome of melon (Cucumis melo L.). Proceedings of the National Academy of Sciences of the United States of America 109, 11872–11877, https://doi.org/10.1073/pnas.1205415109 (2012).
Baloglu, M. C., Eldem, V., Hajyzadeh, M. & Unver, T. Genome-wide analysis of the bZIP transcription factors in cucumber. PloS one 9, e96014, https://doi.org/10.1371/journal.pone.0096014 (2014).
Wang, Z. et al. Genome-wide analysis of the basic leucine zipper (bZIP) transcription factor gene family in six legume genomes. BMC genomics 16, 1053, https://doi.org/10.1186/s12864-015-2258-x (2015).
Li, D., Fu, F., Zhang, H. & Song, F. Genome-wide systematic characterization of the bZIP transcriptional factor family in tomato (Solanum lycopersicum L.). BMC genomics 16, 771, https://doi.org/10.1186/s12864-015-1990-6 (2015).
Zhang, B. & Horvath, S. A general framework for weighted gene co-expression network analysis. Statistical applications in genetics and molecular biology 4, Article17, https://doi.org/10.2202/1544-6115.1128 (2005).
Li, Z. et al. The ethylene response factor AtERF11 that is transcriptionally modulated by the bZIP transcription factor HY5 is a crucial repressor for ethylene biosynthesis in Arabidopsis. The Plant journal: for cell and molecular biology 68, 88–99, https://doi.org/10.1111/j.1365-313X.2011.04670.x (2011).
Schwikowski, B., Uetz, P. & Fields, S. A network of protein-protein interactions in yeast. Nature biotechnology 18, 1257–1261, https://doi.org/10.1038/82360 (2000).
Bai, S. L. et al. Developmental analyses reveal early arrests of the spore-bearing parts of reproductive organs in unisexual flowers of cucumber (Cucumis sativus L.). Planta 220, 230–240, https://doi.org/10.1007/s00425-004-1342-2 (2004).
Dohmen, R. J., Strasser, A. W., Honer, C. B. & Hollenberg, C. P. An efficient transformation procedure enabling long-term storage of competent cells of various yeast genera. Yeast 7, 691–692, https://doi.org/10.1002/yea.320070704 (1991).
Walter, M. et al. Visualization of protein interactions in living plant cells using bimolecular fluorescence complementation. The Plant journal: for cell and molecular biology 40, 428–438, https://doi.org/10.1111/j.1365-313X.2004.02219.x (2004).
Voinnet, O., Rivas, S., Mestre, P. & Baulcombe, D. An enhanced transient expression system in plants based on suppression of gene silencing by the p19 protein of tomato bushy stunt virus. The Plant journal: for cell and molecular biology 33, 949–956 (2003).
Acknowledgements
We thank P. Audigier and F. Vion for plant handling, the cell biology imaging platform and research facilities provided by the Institute of Plant-Science Paris-Saclay (IPS2). This work was supported by the Plant Biology and Breeding department in INRA, the grants Program LabEx Saclay Plant Sciences-SPS (ANR-10-LABX-40-SPS), EPISEX (ANR-17-CE20-00019) and the European Research Council (ERC-SEXYPARTH).
Author information
Authors and Affiliations
Contributions
J.S.Y.E., A.H., B.M., C.C., D.R. and H.M. performed the experiments and analyzed the data; C.D., A. Bo. and A. Be. conceived and designed the study; J.S.Y.E., A. Bo. and A. Be. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Eleblu, J.S.Y., Haraghi, A., Mania, B. et al. The gynoecious CmWIP1 transcription factor interacts with CmbZIP48 to inhibit carpel development. Sci Rep 9, 15443 (2019). https://doi.org/10.1038/s41598-019-52004-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-52004-z
This article is cited by
-
Ethylene produced in carpel primordia controls CmHB40 expression to inhibit stamen development
Nature Plants (2023)
-
A comprehensive RNA-Seq-based gene expression atlas of the summer squash (Cucurbita pepo) provides insights into fruit morphology and ripening mechanisms
BMC Genomics (2021)
-
Screening and identification of miRNAs related to sexual differentiation of strobili in Ginkgo biloba by integration analysis of small RNA, RNA, and degradome sequencing
BMC Plant Biology (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.