Abstract
Interactions between cerebral small vessel disease (CSVD) and renal dysfunction (RD) have been reported, but previous studies were mostly retrospective and limited to measurements of estimated glomerular filtration rate (eGFR). In this prospective, longitudinal study of patients with CSVD-related recent small subcortical infarcts (RSSI), we aimed at a comprehensive exploration of markers of early RD and their association with microvascular brain damage. We investigated 101 stroke patients (mean age: 60.2 ± 10.7 years) with an MRI-confirmed RSSI who underwent follow-up brain MRI 15 months post-stroke. Besides serum creatinine and eGFR, we assessed urinary Albumin-Creatinine Ratio and fibroblast growth factor-23 (FGF-23). RD was classified according to recent Kidney Disease: Improving Global Outcomes criteria. We identified 24 patients with RD, only six patients revealed an eGFR <60 mL/min/1.73 m². RSSI patients with RD more often had severe white matter hyperintensities (WMH, 58% vs. 36%, p = 0.04). CSVD progression was not dependent on RD. However, patients in the highest FGF-23 quartile more frequently had new microangiopathic lesions on follow-up MRI (50% vs. 21%, p = 0.03). Early RD was found in a quarter of RSSI patients and associated with WMH severity, but not CSVD progression. High FGF-23 indicates an increased risk for ongoing microvascular brain damage, warranting further studies.
Similar content being viewed by others
Introduction
Approximately a quarter of all ischemic strokes are caused by the occlusion of a small perforating brain artery, formerly termed lacunar stroke, and related to cerebral small vessel disease (CSVD). The neuroimaging correlate is a recent small subcortical infarct (RSSI) and most sensitively diagnosed using diffusion-weighted MRI1. Aside from RSSI, chronic neuroimaging features of CSVD include lacunes as residues of such infarcts, white matter hyperintensities (WMH), microbleeds and enlarged perivascular spaces1.
The pathophysiology of CSVD is incompletely understood. It has been suggested that CSVD may be part of a multisystemic small vessel disease also affecting other vascular beds such as the retina or kidneys2. Especially small vessels of both brain and kidneys share anatomical and functional characteristics, which likely makes them vulnerable to similar risk factors and pathophysiological mechanisms. Shared functional characteristics include requirement of constant high blood flow through a low-resistance vascular system and autoregulation3,4.
While previous investigations consistently showed a higher burden of CSVD-related brain damage (WMH, lacunes, microbleeds and enlarged perivascular spaces) in lacunar stroke patients with renal impairment5,6, a meta-analysis found no difference in the incidence of renal dysfunction (RD) between lacunar versus non-lacunar stroke patients7. However, most studies incorporated in this analysis identified lacunar stroke on the basis of clinical symptomatology, i.e. the presence of a lacunar syndrome, which is rather unreliable compared to radiological classification with diffusion-weighted MRI8. Also, the majority of studies which explored the association of stroke with RD were retrospective and used the estimated glomerular filtration rate (eGFR) as the sole parameter of RD, although current definitions of chronic kidney disease (CKD) demand the inclusion of albuminuria9.
Fibroblast growth factor 23 (FGF-23) is a cell signalling protein that has recently been identified as an early marker of RD10. Moreover, elevated FGF-23 levels have been associated with higher rates of cardiovascular events in patients with RD11,12 and in population-based studies13. Therefore, FGF-23 could be an interesting biomarker in patients with CSVD-related stroke and might be related to their prognosis.
We therefore planned a comprehensive assessment of renal dysfunction (including eGFR, albuminuria and FGF-23) in a prospective cohort of MRI-defined RSSI patients who also underwent control brain MRI at 15-months after stroke. We hypothesized that the incorporation of albuminuria to the definition of RD would increase detection of patients at increased risk for more severe CSVD-related brain changes at baseline and might relate to the progression of CSVD at follow-up. Secondly, we particularly explored the value of FGF-23 as a potential predictor of ongoing CSVD-related brain damage.
Methods
Study participants
From May 2012 to December 2016, all stroke patients ≤75 years with an MRI-confirmed RSSI were invited to participate in this prospective study which has in part already been reported elsewhere14. Exclusion criteria included acute brain infarcts of other aetiologies, pre-stroke modified Rankin Scale >1 and contraindications for MRI (e.g. metal implants or claustrophobia). We asked especially for any vascular events during the follow-up period and confirmed those by medical records.
Stroke work-up and neuroimaging
At the time of stroke (baseline), patients underwent a thorough neurological examination and workup including brain MRI, electrocardiography (ECG), duplex sonography of brain-supplying vessels, 24h-ECG, echocardiography as well as blood and 24-hour urine sampling. In-hospital follow-ups at the stroke outpatient department were carried out at three and 15 months after stroke and included a neurological examination, MRI of the brain and blood sampling.
At baseline, all study patients underwent brain MRI at 1.5 Tesla (Siemens MAGNETOM Espree, Siemens Healthcare, Erlangen, Germany) according to a standard protocol for the workup of patients with suspected cerebrovascular events14. At both follow-ups, brain MRI was performed on a 3 Tesla TimTrio or Prisma scanner (Siemens Healthcare, Erlangen, Germany). All MRI scans were reviewed by a neuroradiological expert (CE) according to the STandards for ReportIng Vascular changes on nEuroimaging (STRIVE)1, blinded to clinical data. Follow-up MRI scans were specifically analysed with regard to changes in cerebrovascular lesions (such as new infarcts, lacunes and WMH progression) as previously described14,15. More specifically, WMH were rated according to the Fazekas scale16,17. Progression of WMH was defined the as occurrence of new white matter hyperintensities or transition to a higher Fazekas scale score (punctate foci to early-confluent lesions, early-confluent lesions to large confluent areas)18.
Renal parameters
We determined creatinine and the eGFR was calculated using the CKD-EPI creatinine equation19. We also analysed albuminuria and the urinary Albumin-Creatinine Ratio (uACR, mg albumin/g creatinine). The uACR has been recommended as the most reliable marker of albuminuria because of known problems with 24-hour urine albuminuria (including problems in proper collection and difficulties in correction towards sex, age, weight, and other parameters)9,20. Therefore, we chose the uACR calculated from the 24-hour urine sample as our main marker of albuminuria. Investigated blood parameters at baseline further included C-terminal FGF-23, which was measured by a 2nd generation enzyme-linked immune-sorbent assay (ELISA) by Immuntopics Inc., San Clemente, CA. Sensitivity of the assay was 1.5 RU/mL, coefficient of intra-assay variation (20 duplicate measurements of two samples) was detected as 1.4 to 2.4%, inter-assay variation (duplicate determinations of 2 samples performed in 10 assays) was 2.4 to 4.7%. Peripheral blood was taken by venepuncture together with the collection of urine samples (mean time from stroke symptom onset to blood sampling: 5.2 ± 2.7 days).
We defined RD/CKD according to the proposed Kidney Disease: Improving Global Outcomes Clinical Practice (KDIGO) guideline (either eGFR <60 mL/min/1.73 m² and/or albuminuria/ACR >30 mg/g). High-risk RD patients were defined as eGFR <45 mL/min/1.73 m², eGFR <60 mL/min/1.73 m² with albuminuria/ACR >30 mg/g or any GFR with albuminuria/ACR >300 mg/g9.
At follow-up examinations, we analysed serum creatinine, and the respective eGFR, as well as FGF-23 from peripheral blood.
Data analysis
For data analysis, we first explored the association of RD with traditional vascular risk factors and the extent of CSVD-related brain damage at baseline in a cross-sectional manner. In a second step, we searched for associations between MRI-confirmed CSVD progression at follow-up with renal parameters both at baseline and follow-up.
Progression of CSVD was defined either as a recurrent RSSI, or the detection of a new lacunes or new WMH on the 15-months-follow-up MRI.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics 22. Continuous variables were evaluated for normal distribution using the Kolmogorov-Smirnov test. Normally distributed continuous variables were compared by the unpaired Student’s t-test, for other distributions the Mann-Whitney U-test was used. For categorical variables, we used the chi-square test and univariate logistic regression. P-values of equal or less than 0.05 were considered statistically significant.
The study was approved by the ethics committee of the Medical University of Graz (ethics committee number: 24–260 ex 11/12) and was carried out in accordance with the relevant guidelines and regulations. All patients gave written informed consent.
Results
Characteristics of the cohort
We identified 101 RSSI patients who agreed to participate in the study period (mean age 60.2 ± 10.7 years, 73% men). Of those, 98 (97%) completed the 15-month follow-up, while three patients only completed the follow-up investigation at three months. In those three patients, data from their three-month follow-up was used in the prospective analysis.
Baseline characteristics including clinical information, vascular risk factors, laboratory and MRI findings are shown in Table 1.
Renal dysfunction
Average eGFR at baseline was 80.5 ± 15.4 mL/min/1.73 m². Only six patients (5.9%) had an eGFR <60 mL/min/1.73 m², while 22 patients (22.9%) had a pathological uACR. According to the proposed KDIGO criteria, 24 patients (23.8%) thus had RD, and six patients (5.9%) had high-risk RD (Table 1).
Cross-sectional analysis of patients with renal dysfunction
RSSI patients with RD more frequently had diabetes mellitus (33.3% vs. 10.4%) and dyslipidaemia (95.8% vs. 74.0%). All other risk factors were equally distributed among patients with versus without RD (Table 1).
Severe WMH (i.e. early confluent and confluent WMH according to Fazekas scale scores 2 and 3) were more often present in patients with versus without RD (58.4% vs. 36.4%, p = 0.04). The distribution of other neuroimaging markers of CSVD was not different (Table 1). Patients with severe WMH at baseline also had significantly lower eGFR both at baseline (75.6 ± 13.5 vs. 84.0 ± 15.9 mL/min/1.73 m², p < 0.01) and at follow-up (72.9 ± 13.9 vs. 81.7 ± 16.8 mL/min/1.73 m², p < 0.01). FGF-23 levels were not related to WMH severity or the presence of lacunes or microbleeds on brain MRI at baseline (data not shown).
Progression of CSVD
During the 15-months-study period, 12 patients (11.9%) had progression of CSVD (two had a recurrent symptomatic RSSI, seven showed new silent lacunes and three others had new WMH on MRI). Classical vascular risk factors did not predict CSVD progression (Table 2). Patients with severe WMH, microbleeds but also with old cortical infarcts on baseline MRI more frequently had CSVD progression (Table 2).
Patients with RD (as measured by eGFR, uACR and RD risk categories) did not more frequently show progression of CSVD. Similarly, absolute FGF-23 levels were not different between patients with versus without CSVD progression. However, patients in the highest FGF-23 quartile (>112 RU/mL, n = 24) at baseline compared to all other patients more frequently had new CSVD-related ischemic brain changes at follow-up (p = 0.03, OR 3.89). These results were similar when analysing the highest FGF-23 quartile at follow-up (p = 0.03, OR 3.63, Table 2) and remained significant after correction for age and sex (baseline: p = 0.03, follow-up: p = 0.04).
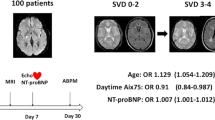
An example of a patient with a high FGF-23 level and CSVD progression is shown in Figure 1. Figure 2 illustrates individual FGF-23 levels at baseline and follow-up, highlighting patients with CSVD progression and recurrent vascular events.
Brain MRI (FLAIR sequence) of a 72-year-old male patient with progression of cerebral small vessel disease during the observation period. The left image shows the baseline MRI (recent infarct not shown), the right image the follow-up MRI after fifteen months depicting a new lacune in the right centrum semiovale (green arrow) and a new white matter hyperintensity in the left centrum semiovale (blue arrow), which occurred clinically asymptomatic. Aside from well-controlled arterial hypertension, this patient had no further vascular risk factors. Baseline eGFR was 68 ml/min/1.73 m², the patient had no increased albuminuria/ACR, but FGF-23 was increased at 159 rU/mL. Follow-up laboratory assessments showed decreasing eGFR (45 ml/min/1.73 m² at fifteen months) and increasing FGF-23 (168 rU/mL at fifteen months).
Recurrent vascular events
During the 15-months observation period, three non-CSVD-related vascular events occurred (one macroangiopathy-related cortical ischaemic infarct, one cortical infarct in the posterior cerebral artery territory and one myocardial infarction).
If we extended the group with CSVD progression by those patients with recurrent vascular events, that group more frequently had high-risk RD (20% vs. 3.5%, OR 6.92, p = 0.01) and more often belonged to the highest FGF-23 quartile (53.3% vs. 18.8%, OR 4.93, p = 0.003), while traditional vascular risk factors were not different.
Progression of renal dysfunction
Overall, 23 patients (22.8%) developed a significant decrease of renal function (GFR reduction by more than 10 mL/min/1.73 m²) at the 15-month-follow-up. While not statistically significant, patients with worsening renal function more often had CSVD progression (21.7 vs. 9.0%, OR 2.82, p = 0.10) or any vascular endpoint event (26.1 vs. 11.5%, OR 2.71, p = 0.09). Also, those patients more frequently were in the highest FGF-23 quartile at follow-up (36.0% vs. 17.3%, OR 2.68, p = 0.05) and tended to be in the highest FGF-23 quartile at baseline (33.3% vs. 18.4%, OR 2.21, p = 0.12).
Discussion
In our series of patients aged ≤75 years with an MRI-confirmed RSSI, about one quarter had evidence for early stage renal dysfunction according to the recently proposed KDIGO criteria. This was associated with an increased frequency of diabetes mellitus and dyslipidaemia, and patients with RD more often had early confluent to confluent WMH. Progression of CSVD as visible on MRI during a follow-up period of 15 months was not related to conventional parameters of RD; however, higher FGF-23 levels were associated with accumulating microangiopathic brain damage within the follow-up period as well as with vascular events in general.
Extending previous work, which mainly assessed eGFR, we here applied the recently proposed KDIGO definition of RD/CKD by integrating both eGFR and albuminuria. This helps identifying patients with early stage RD - a subgroup that would particularly profit from preventive strategies21. While only six patients in our study cohort had an eGFR <60 mL/min/1.73 m², 22 patients had a pathological uACR, resulting in 24 patients with mainly mild RD. The cross-sectional analysis at baseline revealed that even those patients with mild RD already more often had severe WMH. These results are in line with a study of lacunar stroke patients showing a higher total burden of CSVD in patients with RD and an investigation of young stroke patients which reported a lower eGFR in patients with more severe WMH5,22. A larger recent study investigating patients with different stroke subtypes including transient ischemic attacks also corroborates these findings in that WMH were significantly more severe in patients with RD younger than 60 years, which was the mean patient age in our study23.
The association of severe WMH and RD could be a consequence of exposure to same vascular risk factors known to affect the cerebral and renal microvasculature (e.g. lifetime hypertension)4. Hypertension was also highly prevalent in our patients but not to a significantly different extent between patients with and without RD, while we found such differences for diabetes mellitus and dyslipidaemia. Conversely, a number of direct mechanisms acting on both organs have also been suggested, including nitric oxide deficiency in RD24, hyperphosphatemia and associated arterial calcification25, deficiency of calcification inhibitors like Klotho, a co-receptor for FGF-2326, and others3. The presence of lacunes and microbleeds were not associated with RD in our study. This may be a consequence of mild RD and a weaker association between RD and those two CSVD markers than with WMH as shown in a previous study23. As usual, lacunes and microbleeds also occurred more rarely in our patients in general which may have prohibited adequate statistical assessment. We did not investigate enlarged perivascular spaces, which have been shown to be associated with markers of RD in a previous study5.
CSVD progression was observed especially in patients with severe WMH, microbleeds and old cortical infarcts, which is in line with earlier observations of this association27. Interestingly, CSVD progression at 15 months was not different when comparing patients with and without RD or when analysing the various conventional parameters of RD separately. Again, this is likely a consequence that RD was mostly mild in our patients. However, elevated FGF-23 was associated with the occurrence of new CSVD-related brain changes in longitudinal analysis. Patients within the highest FGF-23 quartile also experienced an overall higher proportion of vascular events and accumulating cerebral ischemic damage.
Previous studies have shown a higher occurrence of recurrent cardiovascular events in the highest quartile of FGF-23 in patients with acute coronary syndrome as well as worse outcomes in patients with known RD, including increased frequency of heart failure and mortality11,12,28. A population-based study of stroke-free individuals found that FGF-23 is a risk factor for subsequent stroke independent of RD29, an association in CSVD with progressing vascular disease has not yet been reported. FGF-23 concentrations in our study (median 76 RU/ml) were higher compared to population-based cohorts (median 43–74 RU/ml) but lower compared to studies on patients with CKD (median 102–392 RU/ml). Most previous investigations on FGF-23 were therefore performed using quartiles for comparison as we have done13. To the best of our knowledge, this is the first investigation of FGF-23 in patients with CSVD. Our finding of a worse disease course in RSSI patients with high FGF-23 levels demands additional investigations, especially with regard to potential cut-off values for indicating an increased risk for progressive (micro)vascular changes.
Strengths of our study include the precise definition of renal dysfunction according to recently proposed criteria also incorporating early disease stages as well as the MRI-based definition of RSSI and CSVD according to current guidelines9. Furthermore, repeated MRI at prospectively defined follow-up investigations allowed for exact identification of neuroimaging markers of CSVD and their progress. However, our study also has some relevant limitations. These come mainly from the relatively small patient number with few outcome events which prohibited accounting for covariates in multivariate analysis. Therefore, the findings of our study need to be regarded as exploratory and hypothesis-generating. As our study design aimed for very high follow-up completion rates and to minimize the influence of concomitant neurodegenerative brain changes, we decided to exclude patients above 75 years of age. This also explains why our study cohort contained primarily mildly affected patients, as vascular risk factors, RD and the incidence of cardiovascular events are strongly affected by age30. On the other hand this could be viewed as even strengthening the impact of our observations which thus were made already in the presence of mostly mild RD and FGF-23 elevations. Studies of larger, unselected study cohorts with longer follow-up periods using volumetric WMH quantification tools in comparison with well-established visual WMH progression scales18,27,31 will now be needed for confirmation.
Data availability
The datasets generated and/or analysed during this study are not publicly available, but are available from the corresponding author on reasonable request.
References
Wardlaw, J. M. et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 12, 822–838 (2013).
Toyoda, K. Cerebral Small Vessel Disease and Chronic Kidney Disease. J. Stroke 17, 31 (2015).
Lau, W. L., Huisa, B. N. & Fisher, M. The Cerebrovascular-Chronic Kidney Disease Connection: Perspectives and Mechanisms. Transl. Stroke Res 8, 67–76 (2017).
Toyoda, K. & Ninomiya, T. Stroke and cerebrovascular diseases in patients with chronic kidney disease. Lancet Neurol. 13, 823–833 (2014).
Xiao, L. et al. Chronic Kidney Disease in Patients With Lacunar Stroke: Association With Enlarged Perivascular Spaces and Total Magnetic Resonance Imaging Burden of Cerebral Small Vessel Disease. Stroke 46, 2081–2086 (2015).
Yang, S. et al. Association Between Serum Cystatin C Level and Total Magnetic Resonance Imaging Burden of Cerebral Small Vessel Disease in Patients With Acute Lacunar Stroke. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 26, 186–191 (2017).
Makin, S. D. J., Cook, F. A. B., Dennis, M. S. & Wardlaw, J. M. Cerebral Small Vessel Disease and Renal Function: Systematic Review and Meta-Analysis. Cerebrovasc. Dis. 39, 39–52 (2015).
Doubal, F., Jackson, C., Sudlow, C., Dennis, M. & Wardlaw, J. Associations of clinical stroke misclassification (‘clinical-imaging dissociation’) in acute ischemic stroke. Cerebrovasc. Dis. Basel Switz 29, 395–402 (2010).
Stevens, P. E. & Levin, A., Kidney Disease. Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann. Intern. Med. 158, 825–830 (2013).
Wetmore, J. B. The rise of FGF23: should insights from population-based studies inform future clinical trials? Kidney Int. 90, 477–479 (2016).
Scialla, J. J. et al. Fibroblast Growth Factor-23 and Cardiovascular Events in CKD. J. Am. Soc. Nephrol. 25, 349–360 (2014).
Seiler, S. et al. Associations of FGF-23 and sKlotho with cardiovascular outcomes among patients with CKD stages 2-4. Clin. J. Am. Soc. Nephrol. CJASN 9, 1049–1058 (2014).
Marthi, A. et al. Fibroblast Growth Factor-23 and Risks of Cardiovascular and Noncardiovascular Diseases: A Meta-Analysis. J. Am. Soc. Nephrol. JASN 29, 2015–2027 (2018).
Gattringer, T. et al. Serum neurofilament light is sensitive to active cerebral small vessel disease. Neurology 89, 2108–2114 (2017).
Pinter, D. et al. Longitudinal MRI dynamics of recent small subcortical infarcts and possible predictors. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 271678X18775215, https://doi.org/10.1177/0271678X18775215 (2018).
Fazekas, F., Chawluk, J., Alavi, A., Hurtig, H. & Zimmerman, R. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. Am. J. Roentgenol 149, 351–356 (1987).
Kapeller, P. et al. Visual Rating of Age-Related White Matter Changes on Magnetic Resonance Imaging. Stroke 34, 441–445 (2003).
Schmidt, R., Fazekas, F., Kapeller, P., Schmidt, H. & Hartung, H. P. MRI white matter hyperintensities: three-year follow-up of the Austrian Stroke Prevention Study. Neurology 53, 132–139 (1999).
Levey, A. S. et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 150, 604–612 (2009).
Rodby, R. A. Timed Urine Collections for Albumin and Protein: ‘The King Is Dead, Long Live the King!’. Am. J. Kidney Dis. 68, 836–838 (2016).
Wouters, O. J., O’Donoghue, D. J., Ritchie, J., Kanavos, P. G. & Narva, A. S. Early chronic kidney disease: diagnosis, management and models of care. Nat. Rev. Nephrol 11, 491–502 (2015).
Steinicke, R. et al. Kidney function and white matter disease in young stroke patients: analysis of the stroke in young fabry patients study population. Stroke 43, 2382–2388 (2012).
Liu, B. et al. Age-Specific Associations of Renal Impairment With Magnetic Resonance Imaging Markers of Cerebral Small Vessel Disease in Transient Ischemic Attack and Stroke. Stroke 49, 899–904 (2018).
Baylis, C. Nitric oxide deficiency in chronic kidney disease. Am. J. Physiol. Renal Physiol 294, F1–9 (2008).
Hruska, K. A., Mathew, S., Lund, R., Qiu, P. & Pratt, R. Hyperphosphatemia of chronic kidney disease. Kidney Int. 74, 148–157 (2008).
Hu, M. C. et al. Klotho deficiency causes vascular calcification in chronic kidney disease. J. Am. Soc. Nephrol. JASN 22, 124–136 (2011).
Gouw, A. A. et al. Progression of white matter hyperintensities and incidence of new lacunes over a 3-year period: the Leukoaraiosis and Disability study. Stroke 39, 1414–1420 (2008).
Bergmark, B. A. et al. Association of Fibroblast Growth Factor 23 With Recurrent Cardiovascular Events in Patients After an Acute Coronary Syndrome: A Secondary Analysis of a Randomized Clinical Trial. JAMA Cardiol 3, 473–480 (2018).
Wright, C. B. et al. Plasma FGF23 and the risk of stroke: the Northern Manhattan Study (NOMAS). Neurology 82, 1700–1706 (2014).
Sniderman, A. D. & Furberg, C. D. Age as a modifiable risk factor for cardiovascular disease. The Lancet 371, 1547–1549 (2008).
Prins, N. D. et al. Measuring progression of cerebral white matter lesions on MRI: visual rating and volumetrics. Neurology 62, 1533–1539 (2004).
Author information
Authors and Affiliations
Contributions
F.F. and T.G. designed the study. C.E., D.P., B.O. and A.R. contributed to the study conception. S.F., M.K., D.P., S.E. and T.G. acquired the data. C.E. rated the MRI scans. S.F., F.F. and T.G. drafted the manuscript, all other authors reviewed it for important intellectual content and agreed to its final version. The study was supervised by T.G., C.E. and F.F.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fandler-Höfler, S., Enzinger, C., Kneihsl, M. et al. Early renal dysfunction and fibroblast growth factor-23 in patients with small vessel disease-related stroke. Sci Rep 9, 15410 (2019). https://doi.org/10.1038/s41598-019-51965-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-51965-5
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.