Abstract
Euphorbia heterophylla is a weed species that invades extensive crop areas in subtropical regions of Brazil. This species was previously controlled by imazamox, but the continuous use of this herbicide has selected for resistant biotypes. Two biotypes of E. heterophylla from southern Brazil, one resistant (R) and one susceptible (S) to imazamox, were compared. The resistance of the R biotype was confirmed by dose-response assays since it required 1250.2 g ai ha−1 to reduce the fresh weight by 50% versus 7.4 g ai ha−1 for the S biotype. The acetolactate synthase (ALS) enzyme activity was studied using ALS-inhibiting herbicides from five different chemical families. The R biotype required the highest concentrations to reduce this enzyme activity by 50%. A Ser653Asn mutation was found in the ALS gene of the R biotype. The experiments carried out showed that imazamox absorption and metabolism were not involved in resistance. However, greater 14C-imazamox root exudation was found in the R biotype (~70% of the total absorbed imazamox). Target site mutation in the ALS gene is the principal mechanism that explains the imazamox resistance of the R biotype, but root exudation seems to also contribute to the resistance of this biotype.
Similar content being viewed by others
Introduction
Euphorbia heterophylla L. is a dicotyledonous weed belonging to the Euphorbiaceae family. The species originated in the tropical and subtropical regions of America, where most of the affected crop areas are located1,2,3. Until the 1990s, the presence of this species in cotton, soybean and corn fields was fairly well controlled with acetolactate synthase-inhibiting herbicides (ALS-inhibiting herbicides) (HRAC group B, WSSA group 2). However, due to poor control, the invasion range of E. heterophylla has increased to include more crop areas4,5,6, other countries such as Mexico and the USA7,8 and even other continents such as Europe9, causing great economic losses. This lack of control is due to the evolution of new E. heterophylla biotypes resistant to these herbicides6,10. The first known case of resistance to ALS-inhibiting herbicides in this species was reported in Brazil (1993) and some years later in Paraguay (1995)6. Since then, other E. heterophylla cases with ALS-inhibiting herbicide resistance (including imazamox) have been found in large areas of Brazil (2004), also selecting for resistance to herbicides with other modes of action (MOA)11,12,13,14.
Imazamox [(5-(methoxymethyl)-2-(4-methyl-5-oxo-4-propan-2-yl-1H-imidazol-2-yl) pyridine-3-carboxylic acid)] belongs to the chemical family of imidazolinones within the ALS-inhibiting herbicides. It is a systemic herbicide that acts in early post-emergence stages, causing the inhibition of the ALS enzyme (EC 2.2.1.6), which is involved in the synthesis of the essential branched-chain amino acids isoleucine, leucine and valine15.
To study the basis of herbicide resistance, all the mechanisms should be considered. These mechanisms can be classified as target-site resistance (TSR) and non-target-site resistance (NTSR) mechanisms, depending on whether the target protein is involved or not, respectively16,17. Currently, imazamox resistance is explained by the appearance of point mutations in the ALS gene (TSR mechanism)18,19,20, the lack of herbicide absorption and translocation21,22 and the herbicide metabolism22,23,24 (all these have NTSR mechanisms) in different grass and broadleaf weeds with resistance to ALS-inhibiting herbicides.
Several point mutations are the most frequent mechanisms of resistance to imazamox found in the cases studied across weed species24,25,26,27. Eight point mutations (Ala122, Pro197, Ala205, Asp 376, Arg377, Trp574, Ser653 and Asn654) have been well described28,29, and these mutations show differential cross-resistance patterns to the different chemical families of ALS-inhibiting herbicides. Although TSR mechanisms usually provide high levels of herbicide resistance, some NTSR mechanisms can also provide high levels16,17. In fact, several NTSR mechanisms (alone or together with TSR mechanisms) can influence the resistance level within a single plant.
These NTSR mechanisms can differ depending on the species and MOA. Studies of herbicides with different MOAs16,17,30,31 revealed that variations in the pattern of herbicide absorption and translocation can also provide high resistance levels because they can reduce the herbicide concentration in meristematic tissues to non-toxic levels. Differential herbicide translocation may be caused by different factors, such as the herbicide being retained/sequestered, herbicide metabolism and its metabolites translocating inside the plant32, or large amounts of herbicide being translocated and quickly exuded via the root system, as postulated in the only known case for MCPA in a Raphanus raphanistrum L. biotype33.
The main objective of this work was to study in depth the basis of the high imazamox resistance of one E. heterophylla biotype from Brazil compared to the low resistance of one susceptible biotype of this species, analysing all the possible resistance mechanisms involved, both TSR and NTSR. This research represents the first attempt to unravel the resistance mechanisms to ALS-inhibiting herbicides in this species.
Results
Dose-response assays
The imazamox dose needed to reduce the fresh weight (ED50) by 50% in the R biotype plants was 1250.2 g ai ha−1 versus 7.4 g ai ha−1 for the S biotype (Table 1, Supplementary Fig. S1). These results obtained from the fresh weight showed that the R biotype was 168 times more resistant than the S biotype. Based on the dose to achieve 50% mortality (LD50), the R biotype was 116 times more resistant than the S biotype (Table 1, Supplementary Fig. S1). Considering that the recommended field dose is 40 g ai ha−1, the R biotype can survive more than 50 times this dose, making it impossible to control this biotype with imazamox.
ALS enzyme activity assays
The in vitro activity of the ALS enzyme in the absence of herbicides was similar in the R and S biotypes (220.2 ± 9.2 and 213.4 ± 17.2 nmol of acetoin mg−1 protein h−1, respectively). This enzyme activity was reduced by 50% with 33.7 µM imazamox in the S biotype, while for the R biotype, 538.4 µM (approximately 16 times more herbicide) was necessary. This fact shows that the enzyme plays a very important role in the resistance to imazamox. For the rest of herbicides, the activity was also reduced, but the magnitude depended on the herbicide and biotype (Table 2). The I50 values for the S biotype were very low for bensulfuron and florasulam (<2 μM), indicating that these herbicides are able to stop ALS enzymatic activity for this biotype, while the I50 values for the R biotype were higher than for the S biotype. The RF (resistant factor) values for bensulfuron, bispyribac, florasulam and flucarbazone in the R biotype were 12, 2, 525 and 17, respectively.
ALS sequencing
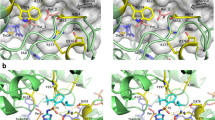
The potential mutation sites known to confer resistance to ALS inhibitors in the ALS gene sequences were amplified in the R and S E. heterophylla biotypes. An amino acid substitution from serine to asparagine was found at position 653 in the ALS gene of the R biotype (Fig. 1).
Foliar retention of imazamox
The R and S E. heterophylla biotypes did not show differences in leaf area or shoot weight (data not presented), but the amount of imazamox solution retained was higher in the R biotype (379.4 ± 28.3 µL g−1 dry weight) than in the S biotype (256.3 ± 32.2 µL g−1 dry weight).
Absorption, translocation, root exudation and visualization of 14C-imazamox applied via foliage
In this assay, one drop (1 µL) of 14C-imazamox was applied to one leaf, and after 3 hours, more than 90% of the 14C-imazamox applied was inside the plants of both biotypes, with no differences between them (Table 3). At this time, more than 80% of the absorbed 14C was located in the treated leaf in both biotypes (Table 3). Translocation from the treated leaf to the rest of the plant increased with time; at 96 HAT, only 23% and 7% of the 14C absorbed remained in the treated leaf for the S and R biotypes, respectively. The images obtained with the phosphor imager at 96 HAT confirmed the higher translocation in the R biotype (Supplementary Fig. S2). However, lower contents were found in the root than in the shoot. The amount of 14C in roots increased until 24 HAT, similar to that in both biotypes, and then decreased until 96 HAT more markedly in the R biotype. At 24 HAT, an increase in the amount of 14C exudated into the nutrient solution was found, reaching at 96 HAT higher values in the R biotype (65%) than in the S biotype (38%) (Table 3).
Imazamox metabolism
The NTSR mechanism involving herbicide metabolism was also investigated in these two biotypes. In this work, metabolites of imazamox were not found in either the biotype of E. heterophylla or in the nutrient solutions after herbicide foliar application (Table 4). This indicated that metabolism was not involved in the imazamox resistance of the R biotype and that the 14C detected inside the plant (in the previous experiment) can be ascribed to 14C-imazamox. However, this assay provides more information than just imazamox metabolism. The total amount of herbicide in the shoot, root and nutrient solution was quantified by LC from 6 to 168 HAT (Table 4), supporting the previous assay (absorption, translocation, root exudation and visualization of 14C-imazamox applied via foliage). The maximum values in the shoot were found at 6 HAT and gradually decreased until 168 HAT, being more pronounced in the R than in the S biotype. At 168 HAT, the amount of herbicide remaining in the shoot was 0.22 µmol for the S biotype and only 0.08 µmol in the R biotype. The amount of herbicide in roots increased until 24 HAT, in a similar way for both biotypes, and then it decreased specially in the R biotype, as occurred in the previous experiment. In the nutrient solution, there was a progressive increase in the herbicide amount with evaluation time that was more important in the R biotype than in the S biotype.
Accumulation, distribution and visualization of 14C-imazamox applied to the roots
In this assay, the exudation of 14C could not be evaluated because there was 14C-imazamox in the nutrient solution. No large differences in accumulation were found between the R and S biotypes, although accumulation was slightly greater in the R biotype (Table 5). Regarding the distribution, at 24 HAT, the herbicide content was more than twice as high in the shoot that in the roots, with the R and S biotypes having a similar distribution. However, at 96 HAT, the % of herbicide decreased in the shoot and increased in the root, making this change much more important in the R biotype. The visualization of 14C at 96 HAT confirmed the higher accumulation of 14C in the R biotype (Supplementary Fig. S3).
Discussion
The high RF value in the dose-response experiments with imazamox for the R E. heterophylla biotype suggested that a TSR mechanism (mutation) may be involved in the resistance to imazamox and that root exudation, a NTSR mechanism, could contribute to it, consistent with the findings of Breccia et al.34 and Ghanizadeh and Harrington16,17.
The presence of a TSR mechanism was demonstrated by enzymatic studies with different ALS-inhibiting herbicides. From the I50 values of the R biotype, it can be deduced that the most effective herbicide should be bensulfuron at the enzymatic level. However, differences in herbicide effectiveness at the plant or field level may vary, as other resistance mechanisms have not been considered. The low inhibition of ALS activity in the R biotype by all tested herbicides revealed cross resistance and suggested that a TSR mechanism, specifically the occurrence of mutation(s) in the ALS gene, may contribute to imazamox resistance since no significant differences in basal enzymatic activity were found with the S biotype; as a result, mechanisms such as differences in copy number or gene expression can be discarded as potential resistance mechanisms to imazamox21,35,36.
Mutations at different positions of the ALS active site have been found in R biotypes of several weed species23,26,37. Amino acid changes in positions that confer cross resistance to imidazolinones and sulfonylureas include Ala-205, Asp-376, Trp-574 and Ser-653. Furthermore, some of these mutations may be responsible for the patterns of cross resistance to other families of ALS inhibitors38,39. In this case, the R E. heterophylla plants presented a Ser653Asn mutation. Amino acid substitutions at Ser653 are more likely to influence IMI binding than SU binding because the first group of herbicides binds at a lower depth in the ALS protein active site than does SU40. Mutations occurring in this position (Ser653Thr) were previously described in Amaranthus powellii and A. retroflexus that conferred resistance only to IMI herbicides41. However, IMI-resistant Setaria viridis and Bromus tectorum populations carrying the Ser653Asn mutation presented cross resistance to the different families of ALS-inhibiting herbicides, with the highest level of IMI and low or moderate levels to the other families of ALS inhibitors42,43. In this study, the highest resistance according to ALS enzyme activity was to TRI, while the levels of resistance to IMI, SU, SUCAR and PYR were low. Therefore, it can be concluded that the Ser653Asn mutation found in the ALS gene of the R biotype may be responsible for the cross-resistance patterns observed in the enzyme activity.
Regarding the other resistance mechanisms, metabolism as a mechanism of NTSR to imazamox has been described in Triticum aestivum, where the main identified metabolites were imazamox-OH and imazamox-glucose22,23,44. This behaviour was also observed in other species, such as Papaver rhoeas24, where imazamox metabolism is involved in its resistance. However, in this work, imazamox metabolites were not found in R or S E. heterophylla plants or in the nutrient solution, indicating that metabolism was not involved in the imazamox resistance of the R biotype. The amounts of herbicide detected in the shoot, root and nutrient solution were proportional to those quantified in the foliar absorption and translocation assays. These results corroborated the hypothesis that the substance moving within both R and S plants corresponded to the herbicide.
Resistance or tolerance can also be related to differences in herbicide leaf retention, an NTSR mechanism, between different species45,46,47,48 and different biotypes49,50,51. In relation to herbicide retention capacity, the leaf area has also been related to ALS inhibitor resistance in a few cases. For example, in Amaranthus powellii, the biotype resistant to ALS-inhibiting herbicides (IMI and SU) produced a 58% smaller leaf area than did the S biotype36. However, in sulfonylurea-resistant Kochia scoparia, the R and S biotypes produced similar leaf areas and dry weights35. Seemingly, E. heterophylla R and S biotypes also produced comparable leaf areas and dry weights in this study. In this study, the greater retention in the biotype R contrasts greater resistance because a greater amount of herbicide is available to be absorbed. Therefore, retention capacity did not contribute to the higher imazamox resistance of the R biotype.
The exudation could contribute to the imazamox resistance in this biotype as an NTSR mechanism. Consistent with this idea, in the R biotype, lower 14C-imazamox contents were found in the root than in the untreated parts of the shoot. This result could be explained by the exudation of the herbicide by the root being more important in the R biotype. Moreover, large amounts of 14C-imazamox were found in the nutritive solution of both R and S plants. Accordingly, at 96 HAT via root application, the shoot content decreased, and the root content increased, with these changes being more intense in the R biotype. These results could be interpreted as a much higher retranslocation (upwards from roots to shoots and then downwards from shoots back to roots) in the R biotype, in agreement with results obtained in the assays with foliar herbicide application. Phosphorous images for both foliar and root applications also supported the hypothesis that imazamox could be eliminated from R plants through the roots. This is the first known case in which herbicide root exudation could contribute to imazamox resistance in E. heterophylla.
The only acknowledged previous case in which a similar NTSR mechanism has been described, that is, herbicide root exudation, was for an R biotype of Raphanus raphanistrum treated with MCPA, in which the herbicide was quickly translocated and high levels were exuded by the roots33. Herbicides can move inside the plant by diffusion, active transport and bulk transport. The third process is responsible for long-distance transport inside plants52. Considering the high rates of herbicide translocation and exudation by the roots, bulk transport was presumably responsible for imazamox movement. Differential herbicide transport between R and S plants can be attributed to ATP-dependent [ATP-binding cassette (ABC)] transporters53, which move the molecule into the vacuole or extracellular space30,54,55,56. Changes in the expression of NTSR genes, related to herbicide-metabolizing enzyme(s) or transporter proteins, can lead to an increase in herbicide degradation or translocation, respectively57,58.
It is not clear why this mechanism occurs and which phases are involved. However, it is known that the exudation of toxic compounds can be stimulated by abiotic and biotic stresses59. This is not the first time that the herbicide distribution and amount have been related to its effectiveness. In 1985, Turnbull and Stephenson60 began to relate the herbicide distribution and amount to the effectiveness of the herbicide, and in 2009, Bukun et al.61 proposed the biological activity of the herbicide as most important, followed by the amount and distribution. It could be hypothesized that this mechanism could eliminate high amounts of herbicide from the cell medium and contribute to resistance. Regardless of the chemical form (herbicide or metabolites) in which the herbicide is exuded, moving the substance outside of the plant (exudation) is synonymous with detoxification. In fact, herbicide effectiveness depends on the amount of herbicide that is able to reach the target protein in the plant49 On the other hand, considering that the enzyme is mutated in R plants, the herbicide could not bind to it and would remain free in tissues. This may allow enhanced translocation to other parts of the plant, including roots and exudation (supported by Supplementary Fig. S2). Therefore, it cannot be discarded that higher root exudation in the R biotype might be a side effect of the altered target site. To confirm that exudation contributes to resistance, non-mutated plants with root exudation and plants with only ALS mutations but no root exudation should be compared with this biotype. Unfortunately, these E. heterophylla biotypes do not exist yet, to the best of our knowledge.
This research described for the first time the resistance mechanisms to ALS inhibiting herbicides in this species. The principal mechanism that can explain the resistance in the R biotype is the mutation Ser653Asn (TSR). However, root exudation via the NTSR mechanism could also contribute to resistance by removing the toxic compound from the inside of the plant.
Materials and Methods
Plant material
Seeds of E. heterophylla from two biotypes, one resistant (R) and one susceptible (S) to ALS-inhibiting herbicides, were kindly provided by Dr. L. Vargas in 201512 (area of Nova Boa Vista Li Perau, Brazil). One hundred seeds of each biotype were germinated and grown in 40 × 80 × 15 cm trays with a 2:1 (v/v) mixture of fertilized peat (COMPO SANA Universal, COMPO Ibérica, Spain) and sandy soil in a growth chamber at 28/18 °C (day/night) under a 16 hours photoperiod and an irradiance of 850 µmol m−2 s−1. When the plants had produced four fully extended leaves, they were treated with commercially formulated imazamox (Pulsar® 40, 4% w/v ai, BASF, Germany) at 40 g ai ha−1 in mixture with the adjuvant Dash® (34.5% w/v methyl oleate/methyl palmitate, BASF, Germany) at a dose of 1.25 L ha−1 using a treatment chamber (Devries Manufacturing, Hollandale, MN, USA) equipped with a TeeJet 8002 EVS flat fan nozzle calibrated to deliver 250 L ha−1 at 200 kPa at a height of 50 cm above plants. Only half of the plants from each biotype were treated with imazamox as described above. After 21 days, the R plants treated with imazamox exhibited 95% survival and no visual damage by the herbicide, while the S plants exhibited 100% mortality. The individuals of each biotype showed a high level of homogeneity in the response. Then, 20 R plants treated with the herbicide and 20 S plants not treated with herbicide were transplanted separately in the field (University of Córdoba) such that there was no possibility for cross pollination between them. When the plants reached maturity, seeds were collected and dried at 25 °C in the laboratory, labelled as being R or S to imazamox and later stored in a cold chamber at 4 °C. These F1 seeds were used in all subsequent trials.
Growing conditions
Seeds were germinated in Petri dishes containing filter paper moistened with distilled water and placed in a growth chamber at 28/18 °C (day/night) with a photoperiod of 16 h, an irradiance of 850 µmol m−2 s−1 and a relative humidity of 80%. Seedlings were transplanted either into pots or into a hydroponic system, depending on the assay. Pots with a 500 mL volume contained fertilized peat (COMPO SANA Universal, COMPO Ibérica, Spain) and sand (2:1, v/v) as substrate and 1 plant/pot. In the hydroponic system, each plant was grown in an opaque container with 20 mL of continuously aerated nutrient solution. The nutrient solution had the following composition: 2 mM Ca(NO3)2, 0.75 mM K2SO4, 0.65 mM MgSO4, 0.5 mM KH2PO4, 50 μM KCl, 10 μM Fe-EDDHA, 10 μM H3BO3, 1 μM MnSO4, 0.5 μM CuSO4, 0.5 μM ZnSO4, and 0.05 μM (NH4)6Mo7O24. The pH was adjusted to 6.0 with 0.1 N KOH. All plants were grown in a growth chamber at 26/18 °C (day/night) under a 16 h photoperiod.
Dose-response assays
E. heterophylla plants at the 4-leaf growth stage were treated with imazamox and the adjuvant as described above. The doses of imazamox for the S biotype were 0, 2.5, 5, 10, 20, 40, and 80 g ai ha−1, and those for the R biotype were 0, 25, 50, 100, 200, 400, 600, 1200 and 2400 g ai ha−1. The dose-response assays were performed using a randomized design with five repetitions per herbicide dose. Twenty-one days after treatment (DAT), the plants were cut at ground level and weighed to determine the fresh weight reduction (ED) and plant mortality (LD)62,63. A previous assay was performed to select the adequate dose range for both biotypes. The results of this assay were similar to those obtained in the present work (data not shown).
ALS enzyme activity assays
The activity of the ALS enzyme was determined in vitro following the methodology from Shaner et al.64 and modified by Hatami et al.65 with two steps (extraction and enzymatic activity). To evaluate possible cross resistance patterns, the following ALS-inhibiting herbicides were tested: imazamox (imidazolinone, IMI), bensulfuron (sulfonylurea, SU), bispyribac (pyrimidinyl–thio–benzoate, PYR), florasulam (triazolopyridine, TRI) and flucarbazone (sulfonylamino-carbonyltriazolinone, SUCAR).
Extraction step
Samples of 3 g of young leaf tissue of each biotype from untreated plants at the 4-leaf growth stage were cut, identified, wrapped in aluminium foil and stored in liquid nitrogen. The samples were ground in a porcelain mortar using liquid nitrogen until a fine and homogeneous powder was obtained, to which 0.5 g of polyvinylpyrrolidone (PVPP) was added. The extraction buffer used at a ratio of 1:2 (g foliar tissue: mL buffer) was composed of 1 M K-phosphate buffer (pH of 7.5), 10 mM sodium pyruvate, 5 mM magnesium chloride, 50 mM thiamine pyrophosphate, 100 μM flavin adenine dinucleotide, 12 mM dithiothreitol, and 1:9 (v/v) glycerol:distilled water. The suspension was stirred for 10 min at 4 °C and subsequently filtered using four layers of cheesecloth. The filtered sample was centrifuged (15 min at 15,000 rpm and 4 °C). The supernatant was used immediately for enzymatic activity assays.
Enzymatic activity step
For in vitro bioassays of the enzyme, reactions were prepared in 2 mL Eppendorf tubes. Analytical-grade herbicides were used for this assay and purchased from Sigma Aldrich (St. Louis, MO USA). The herbicide concentrations used were 0, 0.1, 0.5, 1, 10, 100, 500, 1000 and 5000 µM. Twenty microliters of distilled H2O was used for the positive controls (100% ALS activity), and 50 μL of a solution of 1:50 (v/v) H2SO4-distilled H2O was added to the other Eppendorf tubes for the negative controls (0% ALS activity). Ninety microliters of the enzyme extract was added to each Eppendorf tube together with 110 μL of reaction buffer and herbicide concentrations. The mixture was incubated at 37 °C for 1 h, and the reaction was stopped by the addition of 50 μL of H2SO4 to the tubes, except for the negative control. A second incubation was performed at 60 °C for 15 min. To decarboxylate acetolactate in acetoin, 250 μL of creatine and 250 μL of naphthol were added. The tubes were again incubated for 15 min at 60 °C. The absorbance was measured at 520 nm with a spectrophotometer (mod. DU-640, Beckman, Fullerton, CA, USA). The total protein content was determined by the Bradford method66. The experiment was performed twice with three repetitions for each concentration of herbicide. The variance stability tests of enzymatic activity data (herbicide x concentration) showed no differences between replicates, and they were pooled within a single data set, i.e., the sample size was six repetitions per herbicide concentration.
ALS sequencing
Leaf tissues (±100 mg per sample) of the R and S plants were used for DNA extraction with the Speedtools DNA Extraction Plant Kit (Biotools B and M Labs. S.A). The plants were selected by a previous herbicide treatment at a lethal dose. Only the surviving plants were selected. Two pairs of primers, ALS3B/ALS3F (5′-TCARTACTWAGTGCKACCATC-3′ and 5′-GGRGAAGCCATTCCTCC-3′, respectively) and P1/P2 (5′-GAAGCCCTCGARCGTCAAGG-3′ and P2 5′-ATAGGTTGWTCCCARTTAG-3′), were used to amplify fragments of 501 and 639 bp, respectively, of the CAD and BE domains of the ALS gene67. The polymerase chain reactions (PCRs) were performed with Certamp Complex Enzyme Mix (Biotools BandM Labs, Madrid, Spain) following the manufacturer’s instructions (50 ng of DNA, 0.2 mM of each primer, 1 µL of dNTPs at 10 mM each, 1 µL of 50 mM MgCl2, 2.5 µL of 10X buffer, 1 µL of Certamp complex enzyme mix, and sterile bidistilled water up to complete 25 µL the reaction). PCR conditions consisted of one denaturation cycle at 94 °C for 1 min, followed by 35 cycles of 94 °C for 1 min (denaturation), 52 °C for 30 s (annealing), and 72 °C for 1 min (extension step) and a final extension cycle (5 min at 75 °C)67. PCR product sizes were confirmed on 1% agarose gels by viewing them under UV light. Ten PCR products per biotype were sequenced by Sanger technology. ALS sequences were verified and assembled using SeqMan Pro 11 (DNASTAR, WI, USA) and Geneious 8.1.8 (Biomatters Ltd., Auckland, New Zealand) software, respectively. This study was performed in 10 plants of each biotype, with 2–3 PCR products per plant.
Foliar retention of imazamox
For this experiment, the methodology described by Domínguez-Méndez et al.23 was followed. Imazamox was applied to potted plants at the 4-leaf growth stage under the conditions described in the dose-response assays. The solution contained 40 g ai ha−1 imazamox plus the adjuvant and 100 mg L−1 Na-fluorescein. After 30 min, once the treated leaves had dried, the shoots were cut and immediately placed into test tubes containing 50 mL of 5 mM NaOH and agitated for 30 seconds. The wash solution was recovered, and the fluorescein absorbance was measured in a spectrofluorometer at λexc of 490 nm and λemi of 510 nm. For the determination of dry matter, the shoots were placed in an oven at a temperature of 60 °C and dried for 72 h, and the weight of each sample was recorded. The retention was expressed in μL of herbicide per gram of dry matter. The experiment was conducted with a completely randomized delineation with seven repetitions per biotype and herbicide concentration.
Absorption, translocation, root exudation and visualization of 14C-imazamox applied via foliage
Plants grown in a hydroponic system at the 4-leaf growth stage were treated with a solution of 14C-imazamox (1637 MBq/mmol) + commercially formulated imazamox + 1.25 L ha−1 adjuvant containing a specific activity of 1.67 kBq µL−1 (100,000 dpm) and an imazamox concentration of 40 g ai ha−1. One droplet (1 µL) of this solution was applied on the adaxial surface of a leaf of the second pair using a micropipette (LabMate) (Fig. 2). The time intervals studied were 3, 6, 12, 24, 48 and 96 hours after treatment (HAT). Three plants per biotype and evaluation time were used in a completely randomized design. At each sampled time, the treated leaf was washed twice with a water:methanol [10:90 v/v] solution to recover the unabsorbed 14C-herbicide. Each wash solution was mixed with 2 mL of scintillation liquid (Ultima Gold, Perkin-Elmer, BV BioScience Packard) and analysed by scintillation liquid spectrometry (LSS) (Scintillation counter, Beckman LS 6500). Whole-treated plants were carefully removed from the container and separated into the treated leaf, the rest of the shoot and the roots. Each sample was stored in a filter paper cone for combustion and dried at 60 °C for 72 h. The samples were individually combusted in a biological oxidizer (Packard Tri Carb 307, Packard Instrument Co., Downers Grove, IL, USA). The 14CO2 from the combustion was recovered in 18 mL of a mixture of Carbo-Sorb E and PermaFluor (1:1 v/v) (Perkin-Elmer, BV Bioscience Packard). The radioactivity was also quantified by LSS. The percentage of 14C recovered was estimated from the radioactive values as follows:
The 14C translocation was visualized in another group of plants using a phosphor imager (Cyclon, Perkin-Elmer, Packard Bioscience BV). Whole plants, treated as described previously, were gently dried and fixed on filter paper (25 × 12.5 cm) at room temperature. Subsequently, the plants were placed on phosphor storage film (Storage Phosphor System: Cyclone, Perkin–Elmer Packard BioScience BV) for a period of 4 h to determine the distribution of the radiolabelled herbicide. Three plants were used per biotype and time to observe the translocation of 14C. In addition, to quantify the 14C exuded via the root system, 20 mL of nutrient solution was added, and then 1 mL was taken, mixed with 2 mL of scintillation liquid and quantified by LSS.
Imazamox metabolism
At the 4-leaf stage, plants were treated with the herbicide via foliage as described for the dose-response assays but grown in a hydroponic system. In this case, the dose was 40 g ai−1 ha−1 imazamox + 1.25 L ha−1 adjuvant. Plants were collected at 6, 12, 24, 48, 96 and 168 HAT. There were three plants per biotype and sampled time. The following methodology was described by Rojano et al.32. At each time point, plants were extracted from the container and divided into aerial parts and roots, and the nutrient solution was also conserved. The aerial part and the root were individually ground into powder in a porcelain mortar using liquid nitrogen. The samples were transferred to recipient tubes with 5 mL of methanol/water [95:5 (v/v)] and then sonicated at 70 W for 15 min with a duty cycle of 70% (0.7 s/s). The extract was isolated by centrifugation (15 min at 12,000 rpm). The resulting solid was subjected to extraction two more times, using 5 mL of methanol/water [95:5 (v/v)] each time. After obtaining 15 mL of solution, the samples were evaporated to dryness under a stream of nitrogen. The solid residue was reconstituted in 0.5 mL of methanol/water [95:5 (v/v)] and filtered using a syringe (2.5 mL) through a nylon filter (45 μm pore size and 13 mm internal diameter). For the nutrient solution, all the mixture in the container was evaporated and then reconstituted in 0.5 mL of methanol/water.
The samples were analysed using a 15 Gold HPLC System from Beckman Coulter (Fullerton, USA) equipped with a 26 System Gold Diode Array detector (wavelength range of 190–600 nm). A hydrophilic interaction liquid chromatography column (20 × 4.6 cm, 3 μm particle size) was used to separate the desired compounds. Fifty microliters of the reconstituted sample was injected into the liquid chromatograph, with 1% (v/v) acetic acid in water as mobile phase A and pure methanol as mobile phase B. Imazamox and its metabolites were determined by LC-UV absorption analysis at a wavelength of 240 nm. The elution program started with 5% mobile phase B, followed by a linear gradient of step 1 (5% to 20% methanol for 10 min), step 2 (20% to 80% methanol for 10 minutes), step 3 (80% to 100% methanol for 5 minutes) and step 4 (100% to 5% methanol for 10 minutes). The temperature and constant flow rate of the column were 40 °C and 1.0 mL min−1, respectively. The quantification of imazamox metabolites was based on the calibration model prepared with commercial imazamox. The chromatographic peaks represented in LC-UV were assigned in accordance with the retention times.
Accumulation, distribution and visualization of 14C-imazamox applied to the roots
Plants were grown under the hydroponic system described above, and when they were at the 4-leaf growth stage, 1 µL of the previous radiolabelled solution (1.67 kBq μL−1, 100,000 dpm, imazamox concentration of 40 g ai ha−1) was added to the nutrient solution (20 mL). At 24 and 96 HAT, the plants were carefully removed from the container. The roots were washed with deionized water, and the plants were separated into shoots and roots. Three plants per time and biotype were used. Plant tissue samples, washes and nutrient solutions were prepared to quantify their radioactivity by LSS as in the previous section. The recovery was estimated from the radioactive values as follows:
The root translocation of imazamox was also visualized using a phosphor imager (Cyclon, Perkin-Elmer, Packard Bioscience BV) as described for the assays with foliar application, using another group of three plants per biotype and time.
Data analysis
Outliers of percentage data of the response-dose assays (whole plants and ALS enzymatic activity) were identified and removed based on the internally studentized residual method with α = 0.05. Then, the amount of herbicide causing a reduction in fresh weight compared to the untreated control (ED50), mortality (LD50) or ALS activity reduction by 50% (I50) was calculated by submitting the percentage data to a non-linear regression analysis using a logistic model of three parameters (four parameters (Y = c + {(d − c)/[1 + (x/g)b]}, where c and d are the upper and lower asymptotic limits, b is the slope, g is the ED50 or I50, and x is the herbicide concentration)63. Regression analyses were performed in SigmaPlot 10.0 software (Systat Software Inc.) with the R program. The resistance factor (RF) was calculated as FR = R/S by using the corresponding ED50, LD50 or I50 values of the R and S E. heterophylla biotypes.
Negative control values of the enzymatic activity were submitted to analysis of variance (ANOVA) to search for differences in the initial concentration of the ALS (basal activity) between both biotypes in a completely randomized scheme (2 × 6). Data from foliar retention were submitted to ANOVA in a completely randomized design. Data from the foliar absorption and translocation and from accumulation and distribution applied to the roots (for each plant section) assays, as well as metabolism of 14C-imazamox, were analysed as factorial schemes (biotypes by time points) using ANOVA. Model assumptions of normal distribution of errors and homogeneous variance were graphically inspected for all tests. Values of P < 0.05 were considered statistically significant and multiple mean comparisons were performed using the Tukey’s test at the 5%. Statistical analyses were conducted with the Statistix 9.0 software (Analytical Software, Tallahassee) for basal activity, foliar retention and absorption and translocation assays. The STATGRAPHICS Plus program (v 4.0) was used for data processing of imazamox metabolism.
References
Duarte, A. P., Silva, A. C. & Deuber, R. Plantas infestantes em lavouras de milho safrinha, sob diferentes manejos, no Médio Paranapanema. Planta Daninha 25, 285–291 (2007).
Tanveer, A., Khaliq, A., Javaid, M. M., Chaudhry, M. N. & Awan, I. Implications of weeds of genus Euphorbia for crop production: A review. Planta Daninha 31, 723–731 (2013).
Wilson, A. K. Euphorbia heterophylla: a Review of Distribution, Importance and Control. Trop. Pest Manag. 27, 32–38 (1981).
Braz, A. et al. Weed emergence in commom bean and wheat crops after cultivation of coverage plants. Planta daninha 24, 621–628 (2006).
Galon, L., Forte, C. T., Reichert Júnior, F. W., Trevisol, R. & Perin, G. F. Competitive interaction between commom black bean cultivars and Euphorbia heterophylla. Pesqui. Agropecuária Trop. 48, 254–260 (2018).
Heap, I. The International Survey of Herbicide Resistant Weeds. Annual Report Internet. Available at, www.weedscience.org, (Accessed: 25th October 2018) (2018).
Smith, S. & Krings, A. Euphorbia heterophylla (Euphorbiaceae) in South Carolina, North Carolina, and Virginia, USA. J. Bot. Res. Inst. Texas 12, 369–372 (2018).
Soltero-Díaz, L., Pérez-Domínguez, J. F. & Valencia-Botín, A. J. Herbicide evaluation to control weeds in irrigated chickpea (Cicer arietinum L.) at the region Ciénega of Chapala, México. Rev. UDO Agrícola 4, 831–836 (2009).
Chachalis, D. Wild poinsettia (Euphorbia heterophylla): an emerging weed in cotton and processing tomato in Greece. Hell. Plant Prot. J. 8, 27–32 (2015).
Gelmini, G. A. et al. Resistence of Euphorbia heterophylla L. to ALS-inhibiting herbicides in soybean. Sci. Agric. 62, 452–457 (2005).
Trezzi, M. et al. Quick test of foliar immersion of Euphorbia heterophylla to confirm resistance to PPO and ALS-inhibiting herbicides. Planta Daninha 29, 901–912 (2011).
Vargas, L. et al. Response of Euphorbia heterophylla biotypes to glyphosate rates. Planta Daninha 29, 1121–1128 (2011).
Vargas, L. et al. Management practices x Euphorbia heterophylla resistance to ALS-inhibitors and tolerance to glyphosate in Rio Grande do Sul. Planta Daninha 31, 427–432 (2013).
Xavier, E. et al. Acetolactate synthase activity in Euphorbia heterophylla resistant to ALS- and protox- inhibiting herbicides. Planta Daninha 31, 867–874 (2013).
Shaner, D. L. & O’Connor, S. L. The Imidazolinone Herbicides. (CRC Press), https://doi.org/10.1201/9780203709993 (1991).
Ghanizadeh, H. & Harrington, K. C. Non-target Site Mechanisms of Resistance to Herbicides. CRC. Crit. Rev. Plant Sci. 36, 24–34 (2017).
Ghanizadeh, H. & Harrington, K. C. Perspectives on non-target site mechanisms of herbicide resistance in weedy plant species using evolutionary physiology. AoB Plants 9, plx035 (2017).
Anderson, J. A., Matthiesen, L. & Hegstad, J. Resistance to an imidazolinone herbicide is conferred by a gene on chromosome 6DL in the wheat line cv. 9804. Weed Sci. 52, 83–90 (2004).
Park, K. W. & Mallory-Smith, C. A. Physiological and molecular basis for ALS inhibitor resistance in Bromus tectorum biotypes. Weed Res. 44, 71–77 (2004).
Zheng, D., Patzoldt, W. L. & Tranel, P. J. Association of the W574L ALS substitution with resistance to cloransulam and imazamox in common ragweed (Ambrosia artemisiifolia). Weed Sci. 53, 424–430 (2005).
Riar, D. S. et al. Physiological and Molecular Basis of Acetolactate Synthase-Inhibiting Herbicide Resistance in Barnyardgrass (Echinochloa crus-galli). J. Agric. Food Chem. 61, 278–289 (2013).
Rojano-Delgado, A. M., Priego-Capote, F., Luque de Castro, M. D. & De Prado, R. Mechanism of imazamox resistance of the Clearfield® wheat cultivar for better weed control. Agron. Sustain. Dev. 35, 639–648 (2015).
Domínguez-Mendez, R. et al. Multiple mechanisms are involved in new imazamox-resistant varieties of durum and soft wheat. Sci. Rep. 7, 14839 (2017).
Rey-Caballero, J., Menéndez, J., Osuna, M. D., Salas, M. & Torra, J. Target-site and non-target-site resistance mechanisms to ALS inhibiting herbicides in Papaver rhoeas. Pestic. Biochem. Physiol. 138, 57–65 (2017).
Christoffers, M. J., Nandula, V. K., Howatt, K. A. & Wehking, T. R. Target-site resistance to acetolactate synthase inhibitors in wild mustard (Sinapis arvensis). Weed Sci. 54, 191–197 (2006).
Jimenez, F. et al. Physiological, biochemical and molecular characterization of an induced mutation conferring imidazolinone resistance in wheat. Physiol. Plant. 158, 2–10 (2016).
Kaloumenos, N. S., Chatzilazaridou, S. L., Mylona, P. V., Polidoros, A. N. & Eleftherohorinos, I. G. Target-site mutation associated with cross-resistance to ALS-inhibiting herbicides in late watergrass (Echinochloa oryzicola Vasing.). Pest Manag. Sci. 69, 865–873 (2013).
Green, J. M. Review of Glyphosate and Als-inhibiting Herbicide Crop Resistance and Resistant Weed Management. Weed Technol. 21, 547–558 (2007).
Yu, Q. & Powles, S. B. Resistance to AHAS inhibitor herbicides: current understanding. Pest Manag. Sci. 70, 1340–1350 (2014).
Goggin, D. E., Cawthray, G. R. & Powles, S. B. 2,4-D resistance in wild radish: reduced herbicide translocation via inhibition of cellular transport. J. Exp. Bot. 67, 3223–3235 (2016).
Dan Hess, F. Herbicide Absorption and Translocation and their Relationship to Plant Tolerances and Susceptibility. In Weed Physiology (ed. Stephen O. Duke) 191–214 (CRC Press), https://doi.org/10.1201/9781351077736-8 (1985).
Rojano-Delgado, A. M., Priego-Capote, F., De Prado, R. & de Castro, M. D. L. Ultrasound-assisted Extraction with LC-TOF/MS Identification and LC-UV Determination of Imazamox and its Metabolites in Leaves of Wheat. Plants. Phytochem. Anal. 25, 357–363 (2014).
Jugulam, M., DiMeo, N., Veldhuis, L. J., Walsh, M. & Hall, J. C. Investigation of MCPA (4-Chloro-2-ethylphenoxyacetate) Resistance in Wild Radish (Raphanus raphanistrum L.). J. Agric. Food Chem. 61, 12516–12521 (2013).
Breccia, G. et al. Contribution of non-target-site resistance in imidazolinone-resistant Imisun sunflower. Bragantia 76, 536–542 (2017).
Christoffoleti, P. J., Westra, P. & Moore, F. Growth analysis of sulfonylurea-resistant and -susceptible kochia (Kochia scoparia). Weed Sci. 45, 691–695 (1997).
Tardif, F. J., Rajcan, I. & Costea, M. A mutation in the herbicide target site acetohydroxyacid synthase produces morphological and structural alterations and reduces fitness in Amaranthus powellii. New Phytol. 169, 251–264 (2006).
Yu, Q. et al. Resistance evaluation for herbicide resistance-endowing acetolactate synthase (ALS) gene mutations using Raphanus raphanistrum populations homozygous for specific ALS mutations. Weed Res. 52, 178–186 (2012).
Tan, S., Evans, R. & Singh, B. Herbicidal inhibitors of amino acid biosynthesis and herbicide-tolerant crops. Amino Acids 30, 195–204 (2006).
Tranel, P. J., Wright, T. R. & Heap, I. M. Mutations in herbicide-resistant weeds to ALS inhibitors. (2018).
Patzoldt, W. L. & Tranel, P. J. Multiple ALS Mutations Confer Herbicide Resistance in Waterhemp (Amaranthus tuberculatus). Weed Sci. 55, 421–428 (2007).
McNaughton, K. E., Letarte, J., Lee, E. A. & Tardif, F. J. Mutations in ALS confer herbicide resistance in redroot pigweed (Amaranthus retroflexus) and Powell amaranth (Amaranthus powellii). Weed Sci. 53, 17–22 (2005).
Han, H. et al. A novel amino acid substitution Ala-122-Tyr in ALS confers high-level and broad resistance across ALS-inhibiting herbicides. Pest Manag. Sci. 68, 1164–1170 (2012).
Laplante, J., Rajcan, I. & Tardif, F. J. Multiple allelic forms of acetohydroxyacid synthase are responsible for herbicide resistance in Setaria viridis. Theor. Appl. Genet. 119, 577–585 (2009).
Domínguez-Mendez, R. et al. Stacked traits conferring multiple resistance to imazamox and glufosinate in soft wheat. Pest Manag. Sci., https://doi.org/10.1002/ps.5159 (2018).
Chachalis, D., Reddy, K. N., Elmore, C. D. & Steele, M. L. Herbicide efficacy, leaf structure, and spray droplet contact angle among Ipomoea species and smallflower morningglory. Weed Sci. 49, 628–634 (2001).
Feng, P. C. C. et al. Investigations into glyphosate-resistant horseweed (Conyza canadensis): retention, uptake, translocation, and metabolism. Weed Sci. 52, 498–505 (2004).
González-Torralva, F. et al. Differential Susceptibility to Glyphosate among the Conyza Weed Species in Spain. J. Agric. Food Chem. 58, 4361–4366 (2010).
Yao, C., Myung, K., Wang, N. & Johnson, A. Spray Retention of Crop Protection Agrochemicals on the Plant Surface. In Retention, Uptake, and Translocation of Agrochemicals in Plants (eds Kyung, M., Norbert M. & Satchivi Coleen K. K.) 1–22, https://doi.org/10.1021/bk-2014-1171.ch001 (2014).
Délye, C. Unravelling the genetic bases of non-target-site-based resistance (NTSR) to herbicides: a major challenge for weed science in the forthcoming decade. Pest Manag. Sci. 69, 176–187 (2013).
Michitte, P., De Prado, R., Espinoza, N., Pedro Ruiz-Santaella, J. & Gauvrit, C. Mechanisms of Resistance to Glyphosate in a Ryegrass (Lolium multiflorum) Biotype from Chile. Weed Sci. 55, 435–440 (2007).
Rosario, J. M., Cruz-Hipolito, H., Smeda, R. J. & De Prado, R. White mustard (Sinapis alba) resistance to ALS-inhibiting herbicides and alternative herbicides for control in Spain. Eur. J. Agron. 35, 57–62 (2011).
Steinmaus, S. Herbicide Translocation and Metabolism. In 2012 CWSS Proceedings, 26–29 (2012).
Markus, C., Pecinka, A., Karan, R., Barney, J. N. & Merotto, A. Epigenetic regulation - contribution to herbicide resistance in weeds? Pest Manag. Sci. 74, 275–281 (2018).
Dang, H. T. et al. Reduced translocation in 2,4-D-resistant oriental mustard populations (Sisymbrium orientale L.) from Australia. Pest Manag. Sci. 74, 1524–1532 (2018).
Rey-Caballero, J. et al. Unravelling the resistance mechanisms to 2,4-D (2,4-dichlorophenoxyacetic acid) in corn poppy (Papaver rhoeas). Pestic. Biochem. Physiol. 133, 67–72 (2016).
Torra, J. et al. Enhanced 2,4-D Metabolism in Two Resistant Papaver rhoeas Populations from Spain. Front. Plant Sci. 8, 1584 (2017).
Délye, C., Duhoux, A., Pernin, F., Riggins, C. W. & Tranel, P. J. Molecular Mechanisms of Herbicide Resistance. Weed Sci. 63, 91–115 (2015).
Margaritopoulou, T. et al. Involvement of Epigenetic Mechanisms in Herbicide Resistance: The Case of Conyza canadensis. Agriculture 8, 17 (2018).
Dyer, W. E. Stress-induced evolution of herbicide resistance and related pleiotropic effects. Pest Manag. Sci. 74, 1759–1768 (2018).
Turnbull, G. C. & Stephenson, G. R. Translocation of Clopyralid and 2,4-D in Canada Thistle (Cirsium arvense). Weed Sci. 33, 143–147 (1985).
Bukun, B. et al. Aminopyralid and Clopyralid Absorption and Translocation in Canada Thistle (Cirsium arvense). Weed Sci. 57, 10–15 (2009).
Palma-Bautista, C. et al. First Case of Conyza canadensis from Hungary with Multiple Resistance to Glyphosate and Flazasulfuron. Agronomy 8, 157 (2018).
Seefeldt, S. S., Jensen, J. E. & Fuerst, E. P. Log-Logistic Analysis of Herbicide Dose-Response Relationships. Weed Technol. 9, 218–227 (1995).
Shaner, D. L., Anderson, P. C. & Stidham, M. A. Imidazolinones: potent inhibitors of acetohydroxyacid synthase. Plant Physiol. 76, 545–546 (1984).
Hatami, Z. M. et al. Multiple Mechanisms Increase Levels of Resistance in Rapistrum rugosum to ALS Herbicides. Front. Plant Sci. 7, 169 (2016).
Bradford, M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 (1976).
Cruz-Hipolito, H. E. et al. Resistance mechanism to tribenuron methyl in white mustard (Sinapis alba) from southern Spain. Weed Sci. 61, 341–347 (2013).
Acknowledgements
This work was funded by the AGL2016-78944-R (Spain) project and Asociación de Agroquímicos y Medioambiente. The authors are grateful for the technical collaboration of Antonio Jesús García.
Author information
Authors and Affiliations
Contributions
E.A. and R.D.: Idea and designed the experiments; A.R.-D., R.A.-d.l.C., E.A. and R.D.: Performed the research; A.R.-D., J.d.P., C.P.-B., R.A.-d.l.C., J.T., E.A. and R.D.: Interpretation and analysis of results (of raw data); A.R.-D., J.d.P., C.P.-B., R.A.-d.l.C., J.T., E.A. and R.D.: Wrote and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rojano-Delgado, A.M., Portugal, J.M., Palma-Bautista, C. et al. Target site as the main mechanism of resistance to imazamox in a Euphorbia heterophylla biotype. Sci Rep 9, 15423 (2019). https://doi.org/10.1038/s41598-019-51682-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-51682-z
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.