Abstract
Seasonal variations have recently been described in biomarkers, cell types, and gene expression associated with the immune system, but so far no studies have been conducted among women in the peripartum period. It is of note that pregnancy complications and outcomes, as well as autoimmune diseases, have also been reported to exhibit seasonal fluctuations. We report here a clear-cut seasonal pattern of 23 inflammatory markers, analysed using proximity-extension assay technology, in pregnant women. The inflammatory markers generally peaked in the spring and had a trough in the autumn. During the postpartum period we found seasonality in one inflammatory marker, namely monocyte chemotactic protein 4 (MCP-4). Our findings suggest that seasonal variations in peripheral inflammatory markers are only observed during pregnancy. The results of this study could be valuable to professionals working within the field of immunology-related areas, and provide insight for the understanding of obstetric complications.
Similar content being viewed by others
Introduction
The interest in how the change of seasons affects disease and well-being dates back to ancient Greece1. In the present time, seasonal variations are suggested in pregnancy complications and in outcomes such as preterm birth and preeclampsia2, conditions that have also been associated with altered immunity3,4. Spontaneous preterm birth has been reported to occur more often during summer months5, but no seasonality has been observed among induced preterm births. Some studies report a second peak of preterm births during winter6, while gestational diabetes and gestational hypertension are more common during the warm months of spring and summer2,7,8. Although current data are contradictory, women giving birth in the last three months of the year have been reported to be more likely to develop postpartum depressive symptoms9,10. Autoimmune disease activity is influenced by seasonally changing environmental factors and several conditions with immunological and inflammatory components in their aetiology, including multiple sclerosis, systemic lupus erythematosus, psoriasis, and rheumatoid arthritis, display seasonal patterns11.
From an immunological perspective, pregnancy is a rather distinct condition as semi-allogeneic tissues are being developed in the woman’s body without stimulating a detrimental immune response against the foetus, while still maintaining a barrier against pathogens. Several mechanisms allowing the immunologically and genetically foreign foetus to survive to term have been suggested12, and a key role of maternal regulatory T lymphocytes (Treg) in suppressing immune response against the foetus has been described13. Furthermore, during pregnancy, there are three immunological phases which are characterised based on the macrophage milieu14. Macrophages are monocyte-derived plastic cells, which orchestrate the immune response15 and can shift from an M1 state with antigen-presenting capacity and a T cell response skewed toward the more pro-inflammatory T helper type 1 (Th1), to an M2 state associated with immunosuppressive qualities and T helper type 2 (Th2) immune response16,17. Early pregnancy has been suggested to be dominated by an M1 phase, as pro-inflammatory cytokines play an important role in the implantation and placentation16,18. In the second trimester, as the placenta is fully developed, an anti-inflammatory M2 phase follows, allowing rapid foetal growth and which may counteract preterm contractions16. This phase continues into the third trimester, but then studies have reported a last pro-inflammatory M1 phase just prior to parturition, suggested to aid in cervix ripening, uterine contractions, and placenta expulsion19,20,21. During the postpartum period, a rapid reversal of the pregnancy-associated immunological alterations occurs. Specifically, studies report a shift towards the Th1 direction and a reversal in the cytokine pattern in the first weeks following childbirth22,23, often resulting in the onset or exacerbation of various autoimmune diseases in the postpartum period23. The regulatory mechanisms of these adaptive changes remain partly unknown. The implication of sex steroid hormones such as human chorionic gonadotropin, oestriol, eostradiol, and progesterone, which modulate the number of Treg cells has been suggested24,25.
Preterm birth has been associated with elevated levels of pro-inflammatory cytokines, such as interleukin (IL)-6, IL-1β and tumor necrosis factor (TNF)-α26, which is supported by results indicating an M1-like polarisation of the decidua during spontaneous preterm birth27. Similarly, there is evidence of augmented inflammation in the pathophysiology of preeclampsia, involving TNF-α and interferon (IFN)-γ28. In women with gestational diabetes, inflammatory markers such as IL-6, IL-10, C-reactive protein, and TNF-α have been reported elevated both in the third trimester and six months postpartum29. Interestingly, although major depressive disorder in the general population has been associated with elevated levels of pro-inflammatory cytokines30, evidence is contradictory regarding peripartum depressive symptoms with both higher and lower levels of inflammatory markers reported in pregnancy31,32,33. Significant differences in cytokine levels between pregnant Hispanic and African American women, also points to the importance of considering ethnicity and setting when planning studies33.
Seasonal variations have recently been described in biomarkers, cell types, and gene expression associated with the immune system34,35,36,37,38. A seasonal expression of more than 4000 protein-coding mRNAs in white blood cells and adipose tissue has been reported, with the winter dominated by a pro-inflammatory transcriptomic profile34. Interestingly, the seasonal pattern was inverted between the Northern and Southern hemispheres34. Liu and Taioli35 reported an increased pro-inflammatory profile in winter–spring, compared with summer–autumn, with elevated levels of neutrophils, C-reactive protein, and white blood cells. The production of TNF-α, IL-1β, and IL-6 has been reported to peak in summer38. In a European sample, the analysis of full blood count data revealed seasonal patterns in lymphocytes, monocytes, neutrophils, and erythrocytes34. Lymphocytes had a pattern with a trough in the spring and a peak in the autumn, while the latter three cell types followed a reversed pattern.
Considering the significant alterations characterising the maternal immune system during pregnancy and the postpartum period, and taking into account the previously described immunological and clinical impact of seasons, it would be of interest to specifically investigate possible fluctuation in the levels of inflammatory markers during gestation and after childbirth. We hypothesise that, during pregnancy, any potential seasonal alterations in inflammatory markers may be attenuated by the vast alterations in the immune system and the large interpersonal variation. On the contrary, in the postpartum period, a seasonal pattern may be present, as the immune system rapidly normalises to a non-pregnant state. To date, no such studies exist in the literature.
Thus, the aim of this study was to investigate the occurrence of seasonal variation in peripheral levels of inflammatory markers during late pregnancy and the early postpartum period.
Methods
Subjects
The current study was undertaken as part of the BASIC project, an on-going population-based study at Uppsala University Hospital, Sweden. The primary aim of the study is to investigate correlates of affective symptoms during pregnancy and after childbirth. Women who register for the routine ultrasound examination at the hospital around gestational week 17 were asked to participate. Exclusion criteria were age less than 18 years, not being able to adequately communicate in Swedish, protected identity, blood borne infectious diseases, and non-viable pregnancies. The BASIC study mainly collects data through web surveys sent out at the time of consent (around gestational week 17), at gestational weeks 32, as well as at 6 weeks, 6 months, and 12 months postpartum. The surveys contain, inter alia, the Edinburgh Postnatal Depression Scale (EPDS)39. The EPDS is a validated, self-administered questionnaire aimed to study depressive symptoms during pregnancy and the postpartum40,41. The medical records were used to retrieve information such as date of childbirth, newborn sex, and data on several obstetric variables.
The current cross-sectional study included participants who were invited to the research laboratory of the Department of Obstetrics and Gynaecology at approximately gestational week 38 and 8 weeks postpartum. The overall aim of this sub-study was to more thoroughly assess a selection of the participants, who were invited based on their EPDS score at gestational week 32 and/or 6 weeks postpartum (participation at both time-points was not mandatory). Participants scoring ≥12 and ≤6, and those on selective serotonin reuptake inhibitors (SSRI) medication were targeted. Two-hundred-twenty-one pregnant and 192 postpartum women participated. A non-fasting blood sample was collected, the Mini International Neuropsychiatric Interview (M.I.N.I.) was conducted, and the participants filled out the EPDS.
Adding to the pregnancy samples collected at the research laboratory, participants who underwent an elective caesarean section (n = 117) during the years 2010–2014 were also specifically asked for participation in order to increase the number of fasting pregnancy samples. These participants were asked to fill out the EPDS and provide a fasting blood sample prior to the surgical procedure. This resulted in a total of 338 (221 + 117) blood samples collected from pregnant women.
Ethical considerations
The study protocol has been approved by the Regional Ethical Review Board in Uppsala, Sweden (Dnr 2009/171) and the study was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participants when consenting to participate in the BASIC study, as well as when participating in the sub-study or undergoing elective caesarean section, prior to any testing.
Sample collection and analytic procedure
Coded plasma samples were kept at room temperature for a maximum of one hour before being centrifuged for ten minutes in 1500 RCF (relative centrifugal force). The plasma was separated and stored at −70 °C before being sent to the Clinical Biomarker Facility at SciLife Lab, Uppsala, Sweden, for analysis. None of the samples used in this study had previously been thawed.
The samples were analysed for 92 protein biomarkers using Proseek Multiplex Inflammation I panel (Olink Bioscience, Uppsala, Sweden), which is based on the proximity extension assay (PEA) technology42. Limit of detection (LOD) was determined for each biomarker based on the mean value of triplicate negative controls analysed in each run. Data were presented in unit Normalized Protein Expression (NPX), obtained through normalising Cq-values against extension control, interplate control, and a correction factor. The NPX values correspond to relative quantification between samples and are presented on log2 scale. A list of the 92 inflammation markers analysed in the panel with corresponding UniProt identities is reported elsewhere43. The same batch of reagents was used for all samples, with cases and controls evenly distributed within the plates. Further details on the methodology have been described by Bränn et al.31.
Seventeen pregnancy samples and three postpartum samples were excluded due to technical reasons or missing data on exposure variables, which resulted in 321 samples analysed from pregnant women and 189 from postpartum women.
Study variables
Inflammation summary variable
A summary variable was constructed in order to capture a particular woman’s overall level of immune system activation. This was done by combining information from all inflammatory markers with a detectable NPX value for more than 75% of the blood samples (pregnancy samples, n = 70; postpartum samples, n = 66), separately for the pregnancy and postpartum samples, and is a new approach used in a previous study by our research group31. Firstly, the NPX-value of every inflammatory marker was transformed to a Z-score according to the formula (value - mean)/SD. Secondly, a mean of those Z-scores was calculated for each participating woman. Finally, the resulting mean was transformed to a final Z-score, representing a summary inflammatory profile, the inflammation summary variable, for each woman.
Exposure variables and related variables
In order to address the main exposure variable, seasonality, a Lomb-Scargle Periodogram44 was used to identify if there was any periodicity in the data. The periodicity was investigated using day of sampling and the inflammation summary variable. Thereafter, sine and cosine functions were constructed as a measurements of seasonality based on a method by Stolwijk, Straatman, and Zielhuis45, using day of sampling. The sin and cosine function below were integrated in the regression models for both time periods:
-
sin(2*pi*day of sampling/366)
-
cos(2*pi*day of sampling/366)
Trigonometric relationships could then be used to transform the estimated coefficients into more intuitive measures such as amplitude, horizontal shift, and day of peak/trough.
Most of the background characteristics derived from the web surveys and were self-reported. At gestational week 17, the participants were asked about their height and weight, highest educational attainment, depression history, allergies, and whether the pregnancy was planned. Data on previous depression were derived from the question “Have you ever suffered from depression?”, with yes/no as alternative answers, combined with a question on whether the participant had ever visited a psychologist or psychiatrist. Information on allergies was derived from the question “Have you ever had any of these diseases before you became pregnant?”, where allergy was one alternative answer. Lastly, planned pregnancy derived from the question “Was the pregnancy planned”?, with yes/no as alternative answers. At gestational week 32, the participants were asked about current employment, hours of sleep per night, and they filled out the EPDS. At six weeks postpartum, data was collected on hours of sleep per night, breastfeeding, baby problems, perceived help with baby care by their partner, and stressful life events. Stressful life events were assessed using the Rosengren Scale46. In addition, the participants were asked to fill out the EPDS.
Outcome variables
The inflammation summary variable and the individual inflammatory markers were treated as outcome variables. A complete list of analysed inflammatory markers can be found in Supplementary TablesS1, S2. Due to a potential technical problem with the assessment of BDNF, reported by Olink Bioscience, this marker was excluded from the statistical analyses. Only inflammatory markers with a detectable NPX value for more than 75% of the blood samples were included. A complete list of excluded inflammatory markers is reported in Supplementary TableS3. Samples that had NPX values below LOD were replaced by LOD/sqrt(2)47. The values were expressed as log2(NPX).
Adjustments for oversampling
As previously mentioned, participants were more likely to be invited to the sub-study if they had an EPDS ≥12 (both time points) or were on treatment with SSRIs (pregnancy). Similarly, this study includes more elective caesarean sections than the general population, which were also oversampled based on depressive symptoms. Hence, in order to avoid bias, the models constructed for the pregnancy sample were adjusted for dichotomised EPDS, treatment with SSRIs, and elective caesarean sections. Similarly, the postpartum models were adjusted for dichotomised EPDS at 6 weeks postpartum.
Statistical analyses
Exclusion criteria and adjustments
Decisions regarding further adjustments and exclusions were made based on the inflammation summary variable as outcome.
A confounder must be associated with both the exposure and the outcome; the following covariates were considered as potentially being associated with inflammation: time of sampling, body mass index (BMI), parity, planned pregnancy, and employment status at gestational week 32. Three linear regression models with the different covariates were created to determine if any of these covariates should be included in the final model. To assess time trends, the models also included year of sampling.
Model 1: oversampled variables + sine and cosine functions + year of sampling
Model 2: variables from model 1 and covariates with p < 0.2 (employment and planned pregnancy)
Model 3: variables from model 2 and all other possible covariates
The 10% change-in-estimator method was applied, meaning that if the covariates in Model 2 and 3 did not change the β coefficient of the sine and cosine functions from Model 1 by more than 10%, Model 1 was considered the final model48.
Once the final model was determined, we were concerned about women with preterm labour (n = 1), preeclampsia (n = 4), twin births (n = 5), inflammatory or rheumatoid disease (n = 6), smoking (n = 8), on-going or recently completed treatment with antibiotics, antivirals, or immunoglobulins (n = 8). Our concern was that these variables might be confounders or effect modifiers, however, due to their small numbers we would be unable to adjust for them appropriately. We therefore ran two models 1) including and 2) excluding these women, to see if the estimator was changed by more than 10%, i.e. to see if these women materially impacted our model estimates.
Decisions regarding adjustments and exclusions were based on the pregnancy inflammation summary variable. Based on the results from the 10% change-in-estimator method, the linear regression models were not adjusted nor were any participants excluded, hence, Model 1 was used.
Linear regression and bonferroni correction
For each individual inflammatory marker and the inflammation summary variable, a linear regression model was run. For each model the “seasonality” was assessed by performing a likelihood ratio test on the cos/sin variables simultaneously. These p-values were then corrected for multiple testing by using the Bonferroni correction.
To obtain more intuitive measures, we applied trigonometric relationships to the coefficients of the sine and cosine functions to calculate the amplitude (mean to extreme absolute difference of NPX), as well as when the peak and the trough occurred45.
β1 = coefficient sine curve
β 2 = coefficient cosine curve
T = time period = 366 days
“If β1/β2 > 0, then t > 0 and indicates the first extreme; the other extreme value is found at t + T/2. If β1/β2 ≤ 0; the extreme values are found at t + T/2 and at t + T. If β1> 0, the first extreme is a maximum and the second a minimum; if β ≤ 0 it is the other way around45”. As the inflammatory markers were expressed on log2 scale, to obtain the expected relative difference between trough and peak, the following calculation was applied for each inflammatory marker: 100*(2(2*amplitude)−1).
Sub-analysis
As the focus of the BASIC study is peripartum mood and women with EPDS ≥12 were targeted when recruiting for the sub-study, there was an overrepresentation of participants suffering from depressive symptoms. This was the reason for adjusting for EPDS in the above mentioned analyses, but as a further step, a sub-analysis was conducted, including solely participants identified as not having depressive symptoms. The inclusion criteria were EPDS at blood sampling <13 (pregnancy) or <12 (postpartum), a negative outcome on the depression section of the M.I.N.I., as well as not reporting treatment with SSRIs, giving a sample size of 226 pregnancy samples and 131 postpartum samples. The cut-offs used for the EDPS score have been validated in a Swedish population40,41. The M.I.N.I. is a structured interview with the depression diagnosis corresponding to the DSM-V criteria for a depressive episode49. This interview was conducted at the research laboratory by researchers.
The statistical analyses were conducted in SPSS version 24 (IBM Corp, Armonk, NY) and R version 3.450. Graphs were created in R. Statistical significance was set to p-value of <0.05, if not stated otherwise.
Results
Background characteristics
The background characteristics of the participants are listed in Table 1 and flowcharts of the participants can be seen in Figs 1 and 2. Briefly, for both pregnancy and postpartum, the median age was 32.0 years (interquartile range (IQR): 29.0–35.0). The median BMI was 23.3 kg/m2 (IQR: 21.3–26.1) in pregnancy and 23.0 (IQR: 21.1–25.3) postpartum. Most participants had a college or university degree and were working or studying at gestational week 32. Approximately 25% reported to have some type of allergy. In the postpartum group, 74% of the participants gave birth vaginally, and 75% breastfed exclusively. Lastly, in both groups, approximately one-third of the participants had depressive symptoms. Ten percent were on treatment with SSRIs at gestational week 32.
Periodicity test
The periodicity test (Lomb-Scargle) revealed a peak in the inflammation summary variable occurring annually (approximately every 366 days) in the pregnant study group (Supplementary Fig. S1). There was no seasonality in the postpartum inflammation summary variable (Supplementary Fig. S2).
Main analysis
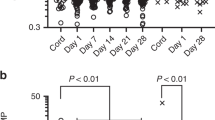
After Bonferroni correction, 23 inflammatory markers were found to follow a seasonal pattern during pregnancy (Table 2, Fig. 3). The peaks occurred between days 61 and 124 (March–May), while the day of trough varied from day 244 to 307 (August–November). The expected relative difference of inflammation between trough and peak varied between 16.7–78.6%. Of note, all inflammatory markers had peaks in spring and troughs in autumn. The inflammatory markers with statistically strongest seasonal associations were vascular endothelial growth factor A (VEGF-A) (p <0.001), macrophage colony-stimulating factor (CSF-1) (p <0.001), osteoprotegerin (OPG) (p <0.001), CUB domain-containing protein 1 (CDCP1) (p <0.001), and tumor necrosis factor receptor superfamily member 9 (TNFRSF9) (p <0.001). As an example of the raw data, scatter plots of VEGF-A and CSF-1 are shown in Figs 4 and 5. The inflammatory markers with the largest seasonal variation were STAM-binding protein (STAM-BP), axin-1 (AXIN1), and SIR2-like protein (SIRT2), with an expected relative difference of inflammation between trough and peak of 64.9%, 68.5%, and 78.6%, respectively.
Graph of inflammatory markers exhibiting statistically significant seasonal variation after Bonferroni correction. The top graph illustrates 23 significant inflammatory markers found in the total sample. The bottom graph illustrates 11 inflammatory markers in pregnant women without depressive symptoms, as well as one postpartum marker.
One inflammatory marker, monocyte chemotactic protein 4 (MCP-4), was found to exhibit a seasonal pattern in the postpartum group (p = 0.045). MCP-4 peaked on day 194 (July) and had its trough on day 11 (January), with an expected relative difference of inflammation between trough and peak of 37.3% (Table 2, Fig. 3). Results from the linear regression for all inflammatory markers are reported in Supplementary Tables S1, S2.
Sub-analysis
The sub-analysis included 226 pregnancy samples and 131 postpartum samples from participants without depressive symptoms. After Bonferroni correction, 11 inflammatory markers were significant in pregnancy samples (Fig. 3, Table 3). Among the postpartum samples, neurotrophin-3 (NT-3) had a peak on day 120, a trough on day 303, with an expected relative difference between trough and peak of 36.4% (p = 0.033) (Fig. 3, Table 3). Information on the seasonal pattern of all inflammatory markers is presented in Supplementary Tables S4, S5.
Discussion
The aim of this study was to investigate if there are seasonal variations in levels of peripheral inflammatory markers during late pregnancy and the early postpartum period. During pregnancy, an annually recurring pattern was found for 23 inflammatory markers and in the constructed inflammation summary variable. For the majority of the markers, the peak occurred in March–April and the trough in September–October. In the postpartum period, only one marker had a statistically significant seasonal pattern after Bonferroni correction.
The sub-analysis, which only included participants without depressive symptoms, revealed a seasonal pattern in 11 inflammatory markers, including one postpartum marker. Nine of the inflammatory markers from the pregnancy samples were among the 23 markers identified as having significant seasonality in the main analysis. There were small differences with regard to peak, trough, and the expected relative difference between trough and peak, when the results were compared with the main analysis. The reduction in number of inflammatory markers displaying seasonality could be the result of reduced power in detecting differences, as the sample sizes of these sub-groups (pregnancy and postpartum) were around 30% smaller. The similarities in results between the main and sub-analysis indicate that the seasonality was not driven by the inclusion of women with depressive symptoms. The low sample size of participants with depressive symptoms in this study would make the result of an analysis of seasonality in this subgroup dubious.
VEGF-A, OPG, and CSF-1 were found among the markers with the strongest statistical significance among pregnancy samples. VEGF-A is a growth factor and vasodilator involved in angiogenesis during pregnancy51, and inhibiting its effect has been associated with pregnancy complications such as gestational hypertension and preeclampsia52. Preeclampsia occurrence has been suggested to be increased among childbirths during the coldest months of the year2. It is theoretically plausible that seasonality of preeclampsia could be associated to the seasonal pattern of VEGF-A shown in the current study, as this marker has its trough in the end of September. OPG is a tumour necrosis factor decoy receptor that has been reported to vary in maternal plasma and serum concentrations throughout pregnancy, possibly having a role in the regulation of bone turnover during pregnancy53. Vitamin D has been reported as a bone resorption stimulating factor, which inhibits the production of OPG54. In Northern Europe, vitamin D levels are generally high in late summer and reach their minimum after winter55. Hence, seasonally varying levels of vitamin D may to some extent underlie the patterns observed in this marker. CSF-1, a chemokine with anti-inflammatory properties, has been suggested to play a role in shaping decidual macrophages in early pregnancy, and thus having a key role in supporting a tolerant immune milieu56. Levels of CSF-1 have been reported to increase in late pregnancy57. SIRT2 was the marker with the largest expected relative difference of inflammation between trough and peak. SIRT2 is an enzyme involved in stress response, and a reduction in SIRT2 has been reported in both preeclampsia58 and in pregnant women who later develop postpartum depressive symptoms31. Whether the seasonal pattern of SIRT2 could be implicated in the varying incidence of preeclampsia throughout the year remains to be investigated. No other studies on seasonality in this marker have been identified. The only inflammatory marker that displayed an annually recurring pattern among the postpartum samples was MCP-4, having its peak in the summer. MCP-4 is a chemokine that plays a role in the immune recruitment of monocytes and T lymphocytes59, and has been associated with allergic respiratory disease trough activation of histamine release60. MCP-4 also displayed significant variations in the pregnancy sample, with an earlier peak in spring. Regarding the seasonality identified in postpartum NT-3 levels, after the removal of participants with depressive symptoms, it could be speculated that women with depressive symptoms have lower seasonal fluctuations in comparison with healthy women. Theoretically, this could originate from a reduced capacity of the immune system of individuals with depressive symptoms to adapt or respond to outside factors. Further assessment of women with peripartum depressive symptoms would add knowledge on the potential association between seasonal peripartum depressive symptoms and fluctuations in inflammatory markers.
The fact that seasonality was only identified in pregnancy and not in the postpartum period contradicts our hypothesis and could be explained by sizable and rapid changes in the immune system after childbirth, as well as other factors such as wound healing, which is likely to affect the immune system to a larger degree than season. In mouse models, VEGF-A has been reported to dramatically drop postpartum following the rapid reduction in progesterone levels51. Similarly, at 12 weeks postpartum, OPG has been reported to not differ significantly from pre-conception levels61. Of note, in a study based on the same data, 15 out of the 23 inflammatory markers identified as having a significant seasonal variation among pregnancy samples in the present study, were significantly lower around 8 weeks postpartum compared with levels at gestational week 3862. In parallel, it has to be noted that there were more samples available for analysis from pregnant women, and that the significant rise in levels of most inflammatory markers during pregnancy might have rendered seasonal differences during pregnancy easier to detect.
Previous studies have reported on seasonal variations in levels of IL-6 and TNF-α38. In the current study, TNF-α was excluded based on the fact that too many samples were under LOD. These two markers are of particular interest to study with regard to preterm birth and preeclampsia. Prior to the Bonferroni correction, IL-6 was significantly different (p = 0.001) in pregnancy, exhibiting a similar seasonal pattern as the final significant inflammatory markers. IL-6 has been associated with preterm birth, a condition reported to increase during the summer months, although a winter peak also have been identified5. In this study, samples from premature births were too few to be studied and compared with term births, in terms of seasonal patterns.
Considering that gene expression related to the immune system has been reported to have both summer and winter peaks34, it is interesting that all inflammatory markers in the current study exhibited only a spring peak. Nevertheless, the same peak and trough pattern as in the current study has been reported in total white blood cell count, monocytes, and neutrophils34. Discrepancies in peaks and troughs between studies conducted on gene expression and those investigating circulating markers could be due to post-translational processes38.
The peak and trough pattern identified in the current study could be attributed to sunlight, presence of pollen, or diet. Relating back to vitamin D, the production of which is dependent on sunlight, a potential immunomodulatory role of this vitamin in the pathogenesis of preeclampsia has been described63. Vitamin D deficiency has also been implicated in the increased risk of immune-mediated diseases when being born during certain seasons64,65,66. Elevated levels of vitamin D in the summer have been associated with a reduced capacity of certain T cells to produce pro-inflammatory cytokines such as IL-2, IL-17, and IFN-γ67. A decrease in secretion of TNF and IL-6 is also associated with increased vitamin D levels68. With regards to the results of the current study, it is interesting to consider that the spring peak and autumn trough identified in inflammatory markers might emerge from a seasonal pattern in vitamin D. The spring peak could also represent remnants of an infection having occurred during winter. Furthermore, the spring peak also corresponds quite well with the start of the pollen season in Sweden, which triggers an inflammatory reaction in sensitive individuals69,70. As previously mentioned, around 25% of the participants in the current study reported to have some type of allergy. Immune related seasonal variations have been noted in human gut microbiota. In this case, variations in diet are proposed to be the mechanism behind the seasonal pattern71. Variation of the gut microbiota is a possible mechanism for changes in inflammatory status72. In the current sub-study there was no information on diet available.
The growing literature73,74,75 on the negative health aspects of being born in a specific season is interesting to consider from the point of possible epigenetic programming, and the possible role of seasonal variation in the immune system of a pregnant woman. A review of seasonal birth studies regarding different neurological diseases showed the most consistent pattern for epilepsy with an excess of births in winter, while MS, amyotrophic lateral sclerosis, and possibly Parkinson’s disease seem to be more common in spring births75. Studies of cerebral palsy are not conclusive, although there are suggestions that there may be an excess of summer births75. Similarly, the birth of patients with schizophrenia, recurrent depressive disorder, and bipolar affective disorder has been reported to be significantly higher in certain seasons74.
Whether a seasonal patterns in inflammatory markers can drive the proposed seasonality of some pregnancy complications warrants further investigations and data should preferably be derived from the same country, or countries with similar environmental cues. Even if no clear-cut evidence for the association between these seasonal patterns will be found, it can be hypothesized that it is not the seasonality of the inflammatory markers per se that results in pregnancy complications. Perhaps, among women with unfavourable levels of inflammatory markers, a seasonal peak or trough may result in a shift from a normal pregnancy to a complicated one. From an evolutionary perspective, seasonality has been crucial in generating biodiversity and has given rise to physiological adaptations and behaviour76. Thus, it could also be speculated that a seasonal pattern in inflammatory markers is a sign of an adaptive immune response and that problems occur when the system does not adapt to seasonal (or other) variations.
A major strength of the current study is its novelty, investigating seasonal variations in inflammatory markers in the peripartum period, adding to the literature on the seasonality of the immune system. Furthermore, it reports on a large number of inflammatory markers and includes a fairly large number of participants. The statistical methods, using sine and cosine functions can be seen as more robust in comparison with a regression model only using season, many times defined as quarters of the year, as exposure. Using sine and cosine functions pin-points the fluctuations with greater precision. In addition, in comparison with a number of other statistical tests for investigating seasonality, it allows for adjustments of confounders, if appropriate. A limitation pertains to the blood sampling taking place at the convenience of the participant; the time of sampling during the day was therefore not standardised. Nevertheless, the analyses for adjustments did not suggest that time of sampling had a significant impact on the inflammation summary variable. Furthermore, we acknowledge the underrepresentation of summer samples. A major difficulty when assessing women in the peripartum period is the dynamic changes in several bodily parameters. Multiple samples at different time-points in pregnancy and the postpartum period from the same woman would have been preferable, to facilitate taking into consideration intrapersonal variations throughout the peripartum period. However, this was not possible within the scope of this study. With regards to seasonality, it would have been ideal to follow the participants also in consecutive pregnancies, in order to distinguish between pregnancy-related and seasonality related changes.
As also discussed by Dopico et al.34 in their study on seasonal gene expression, understanding seasonality in inflammatory markers may be of clinical relevance as it could result in inter- and intra-person variability of the immune profile depending on when during the year the blood samples are collected.
In conclusion, seasonality in peripheral inflammatory markers was common in late pregnancy but not in the postpartum period. This study provides insight for the understanding of pregnancy complications including prematurity, preeclampsia, and peripartum depression where inflammation and alterations of the immune system could be of importance. Larger studies, using extensive inflammatory panels need to be conducted in order to replicate these results and provide further insight into the effect of season on the immune system in the peripartum setting. These results might also have important practical implications, and researchers might in the future need to consider and adjust for season when designing studies or interpreting results on inflammatory markers in the different fields of peripartum research.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Hippocrates. Aphorisms. In: Jones WHS, trans-ed. Hippocrates. Vol 4. Vol. 4 (Harvard University Press, 1931).
Beltran, A. J., Wu, J. & Laurent, O. Associations of meteorology with adverse pregnancy outcomes: a systematic review of preeclampsia, preterm birth and birth weight. Int. J. Environ. Res. Public Health 11, 91–172, https://doi.org/10.3390/ijerph110100091 (2013).
Faas, M. M., Spaans, F. & De Vos, P. Monocytes and Macrophages in Pregnancy and Pre-Eclampsia. Front. Immunol. 5, 298, https://doi.org/10.3389/fimmu.2014.00298 (2014).
Cappelletti, M., Della Bella, S., Ferrazzi, E., Mavilio, D. & Divanovic, S. Inflammation and preterm birth. J. Leukoc. Biol. 99, 67–78, https://doi.org/10.1189/jlb.3MR0615-272RR (2016).
Walfisch, A., Kabakov, E., Friger, M. & Sheiner, E. Trends, seasonality and effect of ambient temperature on preterm delivery. J. Matern. Fetal Neonatal Med. 30, 2483–2487, https://doi.org/10.1080/14767058.2016.1253063 (2017).
Baroutis, G., Mousiolis, A., Hoffman, D. & Antsaklis, A. Preterm birth seasonality in Greece: an epidemiological study. J. Matern. Fetal Neonatal Med. 25, 1406–1412, https://doi.org/10.3109/14767058.2011.636103 (2012).
Verburg, P. E. et al. Seasonality of hypertensive disorders of pregnancy – A South Australian population study. Pregnancy Hypertens 12, 118–123, https://doi.org/10.1016/j.preghy.2018.04.006 (2018).
Chiefari, E. et al. Impact of Seasonality on Gestational Diabetes Mellitus. Endocr. Metab. Immune Disord. Drug Targets 17, 246–252, https://doi.org/10.2174/1871530317666170808155526 (2017).
Henriksson, H. E., Sylven, S. M., Kallak, T. K., Papadopoulos, F. C. & Skalkidou, A. Seasonal patterns in self-reported peripartum depressive symptoms. Eur. Psychiatry 43, 99–108, https://doi.org/10.1016/j.eurpsy.2017.03.001 (2017).
Sylvén, S. M. et al. Seasonality patterns in postpartum depression. Am. J. Obstet. Gynecol. 204, 413.e411–413.e416, https://doi.org/10.1016/j.ajog.2011.01.022 (2011).
Watad, A. et al. Seasonality and autoimmune diseases: The contribution of the four seasons to the mosaic of autoimmunity. J. Autoimmun. 82, 13–30, https://doi.org/10.1016/j.jaut.2017.06.001 (2017).
Poole, J. A. & Claman, H. N. Immunology of pregnancy. Implications for the mother. Clin. Rev. Allergy Immunol. 26, 161–170, https://doi.org/10.1385/CRIAI:26:3:161 (2004).
Aluvihare, V. R., Kallikourdis, M. & Betz, A. G. Regulatory T cells mediate maternal tolerance to the fetus. Nat. Immunol. 5, 266–271, https://doi.org/10.1038/ni1037 (2004).
Mor, G. & Cardenas, I. The immune system in pregnancy: a unique complexity. Am. J. Reprod. Immunol. 63, 425–433, https://doi.org/10.1111/j.1600-0897.2010.00836.x (2010).
Germolec, D. R., Frawley, R. P. & Evans, E. Markers of inflammation. Methods Mol. Biol. 598, 53–73, https://doi.org/10.1007/978-1-60761-401-2_5 (2010).
Brown, M. B., von Chamier, M., Allam, A. B. & Reyes, L. M1/M2 macrophage polarity in normal and complicated pregnancy. Front. Immunol. 5, 606, https://doi.org/10.3389/fimmu.2014.00606 (2014).
Mills, C. D., Kincaid, K., Alt, J. M., Heilman, M. J. & Hill, A. M. M-1/M-2 macrophages and the Th1/Th2 paradigm. J. Immunol. 164, 6166–6173 (2000).
Mor, G., Cardenas, I., Abrahams, V. & Guller, S. Inflammation and pregnancy: the role of the immune system at the implantation site. Ann. N. Y. Acad. Sci. 1221, 80–87, https://doi.org/10.1111/j.1749-6632.2010.05938.x (2011).
Shynlova, O., Tsui, P., Dorogin, A. & Lye, S. J. Monocyte chemoattractant protein-1 (CCL-2) integrates mechanical and endocrine signals that mediate term and preterm labor. J. Immunol. 181, 1470–1479 (2008).
Dubicke, A. et al. Pro-inflammatory and anti-inflammatory cytokines in human preterm and term cervical ripening. J. Reprod. Immunol. 84, 176–185, https://doi.org/10.1016/j.jri.2009.12.004 (2010).
Hamilton, S. et al. Macrophages infiltrate the human and rat decidua during term and preterm labor: evidence that decidual inflammation precedes labor. Biol. Reprod. 86, 39, https://doi.org/10.1095/biolreprod.111.095505 (2012).
Singh, N. & Perfect, J. R. Immune reconstitution syndrome and exacerbation of infections after pregnancy. Clin. Infect. Dis. 45, 1192–1199, https://doi.org/10.1086/522182 (2007).
Elenkov, I. J. et al. IL-12, TNF-alpha, and hormonal changes during late pregnancy and early postpartum: implications for autoimmune disease activity during these times. J. Clin. Endocrinol. Metab. 86, 4933–4938, https://doi.org/10.1210/jcem.86.10.7905 (2001).
La Rocca, C., Carbone, F., Longobardi, S. & Matarese, G. The immunology of pregnancy: regulatory T cells control maternal immune tolerance toward the fetus. Immunol Lett 162, 41–48, https://doi.org/10.1016/j.imlet.2014.06.013 (2014).
Polanczyk, M. J. et al. Cutting edge: estrogen drives expansion of the CD4+CD25+ regulatory T cell compartment. J. Immunol. 173, 2227–2230, https://doi.org/10.4049/jimmunol.173.4.2227 (2004).
Lyon, D. et al. Integrated review of cytokines in maternal, cord, and newborn blood: part I–associations with preterm birth. Biol. Res. Nurs. 11, 371–376, https://doi.org/10.1177/1099800409344620 (2010).
Xu, Y. et al. An M1-like macrophage polarization in decidual tissue during spontaneous preterm labor that is attenuated by rosiglitazone treatment(). J. Immunol. 196, 2476–2491, https://doi.org/10.4049/jimmunol.1502055 (2016).
Xie, C., Yao, M. Z., Liu, J. B. & Xiong, L. K. A meta-analysis of tumor necrosis factor-alpha, interleukin-6, and interleukin-10 in preeclampsia. Cytokine 56, 550–559, https://doi.org/10.1016/j.cyto.2011.09.021 (2011).
Dalfra, M. G. et al. Elevations of inflammatory cytokines during and after pregnancy in gestational diabetes. J. Endocrinol. Invest. 32, 289–290, https://doi.org/10.1007/bf03346470 (2009).
Dowlati, Y. et al. A meta-analysis of cytokines in major depression. Biol. Psychiatry 67, 446–457, https://doi.org/10.1016/j.biopsych.2009.09.033 (2010).
Brann, E. et al. Inflammatory markers in late pregnancy in association with postpartum depression-A nested case-control study. Psychoneuroendocrinology 79, 146–159, https://doi.org/10.1016/j.psyneuen.2017.02.029 (2017).
Edvinsson, A. et al. Lower inflammatory markers in women with antenatal depression brings the M1/M2 balance into focus from a new direction. Psychoneuroendocrinology 80, 15–25, https://doi.org/10.1016/j.psyneuen.2017.02.027 (2017).
Osborne, L. M. et al. Innate immune activation and depressive and anxious symptoms across the peripartum: An exploratory study. Psychoneuroendocrinology 99, 80–86, https://doi.org/10.1016/j.psyneuen.2018.08.038 (2018).
Dopico, X. C. et al. Widespread seasonal gene expression reveals annual differences in human immunity and physiology. Nat Commun 6, 7000, https://doi.org/10.1038/ncomms8000 (2015).
Liu, B. & Taioli, E. Seasonal variations of complete blood count and inflammatory biomarkers in the US population - Analysis of NHANES data. PLoS One 10, e0142382, https://doi.org/10.1371/journal.pone.0142382 (2015).
Goldinger, A. et al. Seasonal effects on gene expression. PLoS One 10, e0126995, https://doi.org/10.1371/journal.pone.0126995 (2015).
De Jong, S. et al. Seasonal changes in gene expression represent cell-type composition in whole blood. Hum. Mol. Genet. 23, 2721–2728, https://doi.org/10.1093/hmg/ddt665 (2014).
Ter Horst, R. et al. Host and environmental factors influencing individual human cytokine responses. Cell 167, 1111–1124.e1113, https://doi.org/10.1016/j.cell.2016.10.018 (2016).
Cox, J. L., Holden, J. M. & Sagovsky, R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br. J. Psychiatry 150, 782–786 (1987).
Wickberg, B. & Hwang, C. P. The Edinburgh Postnatal Depression Scale: validation on a Swedish community sample. Acta Psychiatr. Scand. 94, 181–184 (1996).
Rubertsson, C., Börjesson, K., Berglund, A., Josefsson, A. & Sydsjö, G. The Swedish validation of Edinburgh Postnatal Depression Scale (EPDS) during pregnancy. Nord J Psychiatry 65, 414–418, https://doi.org/10.3109/08039488.2011.590606 (2011).
Assarsson, E. et al. Homogenous 96-plex PEA immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PLoS One 9, e95192, https://doi.org/10.1371/journal.pone.0095192 (2014).
Larsson, A. et al. The body mass index (BMI) is significantly correlated with levels of cytokines and chemokines in cerebrospinal fluid. Cytokine 76, 514–518, https://doi.org/10.1016/j.cyto.2015.07.010 (2015).
Ruf, T. The Lomb-Scargle periodogram in biological rhythm research: Analysis of incomplete and unequally spaced time-series. Biol. Rhythm Res. 30, 178–201, https://doi.org/10.1076/brhm.30.2.178.1422 (1999).
Stolwijk, A. M., Straatman, H. & Zielhuis, G. A. Studying seasonality by using sine and cosine functions in regression analysis. J. Epidemiol. Community Health 53, 235–238 (1999).
Rosengren, A., Orth-Gomer, K., Wedel, H. & Wilhelmsen, L. Stressful life events, social support, and mortality in men born in 1933. BMJ 307, 1102–1105, https://doi.org/10.1136/bmj.307.6912.1102 (1993).
Centers for Disease Control and Prevention (CDC) & National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey, NHANES 2011–2012, Data Documentation, Codebook, and Frequencies, https://wwwn.cdc.gov/nchs/nhanes/2011-2012/PP_G.htm (2013).
Bliss, R., Weinberg, J., Webster, T. & Vieira, V. Determining the probability distribution and evaluating sensitivity and false positive rate of a confounder detection method applied to logistic regression. J. Biomet. Biostat. 3, 142–142, https://doi.org/10.4172/2155-6180.1000142 (2012).
Sheehan, D. V. et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 59 Suppl 20, 22–33;quiz 34–57 (1998).
R: A language and environment for statistical computing (R Foundation for Statistical Computing, Vienna, Austria, 2017).
Kim, M. et al. VEGF-A regulated by progesterone governs uterine angiogenesis and vascular remodelling during pregnancy. EMBO Mol. Med. 5, 1415–1430, https://doi.org/10.1002/emmm.201302618 (2013).
Valdes, G. et al. Vasodilator factors in the systemic and local adaptations to pregnancy. Reprod. Biol. Endocrinol. 7, 79, https://doi.org/10.1186/1477-7827-7-79 (2009).
Hong, J. S. et al. Maternal plasma osteoprotegerin concentration in normal pregnancy. Am. J. Obstet. Gynecol. 193, 1011–1015, https://doi.org/10.1016/j.ajog.2005.06.051 (2005).
Nakamichi, Y., Udagawa, N., Suda, T. & Takahashi, N. Mechanisms involved in bone resorption regulated by vitamin D. J. Steroid Biochem. Mol. Biol. 177, 70–76, https://doi.org/10.1016/j.jsbmb.2017.11.005 (2018).
Klingberg, E., Oleröd, G., Konar, J., Petzold, M. & Hammarsten, O. Seasonal variations in serum 25-hydroxy vitamin D levels in a Swedish cohort. Endocrine 49, 800–808, https://doi.org/10.1007/s12020-015-0548-3 (2015).
Svensson, J. et al. Macrophages at the fetal-maternal interface express markers of alternative activation and are induced by M-CSF and IL-10. J. Immunol. 187, 3671–3682, https://doi.org/10.4049/jimmunol.1100130 (2011).
Hayashi, M., Hombo, Y., Shibazaki, M., Nakajima, A. & Inaba, N. Elevation of macrophage colony-stimulating factor in amniotic fluid at late stage of normal pregnancy. Am. J. Reprod. Immunol. 55, 226–231, https://doi.org/10.1111/j.1600-0897.2005.00352.x (2006).
Hannan, N. J. et al. Key players of the necroptosis pathway RIPK1 and SIRT2 are altered in placenta from preeclampsia and fetal growth restriction. Placenta 51, 1–9, https://doi.org/10.1016/j.placenta.2017.01.002 (2017).
Uguccioni, M. et al. Monocyte chemotactic protein 4 (MCP-4), a novel structural and functional analogue of MCP-3 and eotaxin. J. Exp. Med. 183, 2379–2384 (1996).
Stellato, C. et al. Production of the novel C-C chemokine MCP-4 by airway cells and comparison of its biological activity to other C-C chemokines. J. Clin. Invest. 99, 926–936, https://doi.org/10.1172/jci119257 (1997).
Naylor, K. E. et al. Serum osteoprotegerin as a determinant of bone metabolism in a longitudinal study of human pregnancy and lactation. J. Clin. Endocrinol. Metab. 88, 5361–5365, https://doi.org/10.1210/jc.2003-030486 (2003).
Brann, E., Edvinsson, A., Rostedt Punga, A., Sundstrom-Poromaa, I. & Skalkidou, A. Inflammatory and anti-inflammatory markers in plasma: from late pregnancy to early postpartum. Sci. Rep. 9, 1863, https://doi.org/10.1038/s41598-018-38304-w (2019).
Smith, T. A., Kirkpatrick, D. R., Kovilam, O. & Agrawal, D. K. Immunomodulatory role of vitamin D in the pathogenesis of preeclampsia. Expert Rev. Clin. Immunol. 11, 1055–1063, https://doi.org/10.1586/1744666x.2015.1056780 (2015).
Harvey, L., Burne, T. H. J., McGrath, J. J. & Eyles, D. W. Developmental vitamin D3 deficiency induces alterations in immune organ morphology and function in adult offspring. J. Steroid Biochem. Mol. Biol. 121, 239–242, https://doi.org/10.1016/j.jsbmb.2010.03.050 (2010).
Ramagopalan, S. V. et al. A ChIP-seq defined genome-wide map of vitamin D receptor binding: associations with disease and evolution. Genome Res. 20, 1352–1360, https://doi.org/10.1101/gr.107920.110 (2010).
Holmes, V. A., Barnes, M. S., Alexander, H. D., McFaul, P. & Wallace, J. M. Vitamin D deficiency and insufficiency in pregnant women: a longitudinal study. Br. J. Nutr. 102, 876–881, https://doi.org/10.1017/s0007114509297236 (2009).
Khoo, A. L. et al. Seasonal variation in vitamin D(3) levels is paralleled by changes in the peripheral blood human T cell compartment. PLoS One 7, e29250, https://doi.org/10.1371/journal.pone.0029250 (2012).
Ojaimi, S. et al. Vitamin D deficiency impacts on expression of toll-like receptor-2 and cytokine profile: a pilot study. J. Transl. Med. 11, 176, https://doi.org/10.1186/1479-5876-11-176 (2013).
Naturhistoriska riksmuseet. Pollenkalendern, http://pollenrapporten.se/pollenkalender.4.5dae555f13d5eaab60014b.html (2016).
Galli, S. J., Tsai, M. & Piliponsky, A. M. The development of allergic inflammation. Nature 454, 445–454, https://doi.org/10.1038/nature07204 (2008).
Davenport, E. R. et al. Seasonal Variation in Human Gut Microbiome Composition. PLoS One 9, e90731, https://doi.org/10.1371/journal.pone.0090731 (2014).
Geuking, M. B., Köller, Y., Rupp, S. & McCoy, K. D. The interplay between the gut microbiota and the immune system. Gut Microbes 5, 411–418, https://doi.org/10.4161/gmic.29330 (2014).
Disanto, G. et al. Month of birth, vitamin D and risk of immune-mediated disease: a case control study. BMC Med. 10, 69, https://doi.org/10.1186/1741-7015-10-69 (2012).
Disanto, G. et al. Seasonal distribution of psychiatric births in England. PLoS One 7, e34866, https://doi.org/10.1371/journal.pone.0034866 (2012).
Torrey, E. F., Miller, J., Rawlings, R. & Yolken, R. H. Seasonal Birth Patterns of Neurological Disorders. Neuroepidemiology 19, 177–185 (2000).
Williams, C. M. et al. Understanding evolutionary impacts of seasonality: An introduction to the symposium. Integr. Comp. Biol. 57, 921–933, https://doi.org/10.1093/icb/icx122 (2017).
Acknowledgements
This study was funded by the Swedish Research Council to A.S. (Project No. 523-2014-2342) and I.S.P. (Project No. 521-2013-2339), the Swedish Society of Medicine to A.S. (Project No. SLS-250581), the Marianne and Marcus Wallenberg Foundation to A.S. (Project No. MMW2011.0115) and the Naeslund scholarship (Norrlands nation) to H.E.H. The funding sources had no involvement in the study design, the collection, analysis, and interpretation of data, the writing of the report, or the decision to submit the article for publication. The authors would like to thank all women who participated in this study as well as Åsa Edvinsson, Lena Moby, Natasa Kollia, and Pepita Knuutila for their valuable help in the administration of this study. We would also like to express our gratitude to the Clinical biomarker facility at SciLifeLab, Uppsala, Sweden, for providing assistance with the PEA. Open access funding provided by Uppsala University.
Author information
Authors and Affiliations
Contributions
A.S. had the idea for the study. H.E.H., R.A.W., S.I.I., E.F., F.C.P., I.S.P. and A.S. designed the study. H.E.H. and R.A.W. performed the statistical analyses. H.E.H. wrote the manuscript with contributions from R.A.W., S.I.I., E.F., F.C.P., I.S.P., and A.S. All authors reviewed and contributed to the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Henriksson, H.E., White, R.A., Iliadis, S.I. et al. Spring peaks and autumn troughs identified in peripheral inflammatory markers during the peripartum period. Sci Rep 9, 15328 (2019). https://doi.org/10.1038/s41598-019-51527-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-51527-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.