Abstract
The role of antibiotics (AB) in semen extenders as a potential contribution to the global antimicrobial resistance threat is emerging. Here, we establish an AB-free hypothermic preservation strategy for boar semen and investigate its impact on sperm function, microbial load and fertility after artificial insemination (AI). Spermatozoa (12 boars) preserved in AB-free AndroStar Premium extender at 5 °C maintained high motility, membrane integrity, and a low DNA-fragmentation index throughout 72 h storage and results did not significantly differ from controls stored at 17 °C in extender containing AB (p = 0.072). Likewise, kinetic response of spermatoza to the capacitation stimulus bicarbonate during 180 min incubation in Tyrode’s medium did not differ from 17 °C-controls. In a competitive sperm oviduct binding assay, binding indices did not differ between semen stored for 72 h AB-free at 5 °C and 17 °C-controls (n = 6 boars). Bacterial load < 103 CFU/ml after 72 h was measured in 88.9% of samples stored at 5 °C AB-free compared to 97.2% in 17 °C-controls (n = 36 semen pools, 23 boars). Fertility traits of 817 females did not differ significantly between the two semen groups (p > 0.05). In conclusion, a hypothermic semen preservation strategy is presented which offers antibiotic-free storage of boar semen doses.
Similar content being viewed by others
Introduction
Artificial insemination (AI) in pigs is a highly efficient assisted reproductive technology used in more than 90% of sows in the primary pork producing countries. The main benefit of AI in domestic animal species is seen in the prevention of transmission of venereal diseases and an accelerated genetic progress1. Semen from healthy, fertile boars and similarly from other species contains bacteria stemming from natural colonization in the male tract and from the environment. The majority of contaminants are Gram-negative bacteria, belonging to the Enterobacteriaceae family2. Storage of boar spermatozoa at a relatively high temperature (16 to 18 °C) in a nutrient-rich preservation medium favors bacteria growth in semen doses. Storage at lower temperature, however, is contraindicated by the pronounced cold shock sensitivity of boar spermatozoa which has been associated with a high content of polyunsaturated fatty acids and a low sterol to phospholipid ratio of sperm membranes3. Accordingly, frozen semen is not routinely used in commercial farms for market pig production. To prevent disease transmission in sows through insemination and harmful effects of bacteriospermia on sperm quality4,5, the addition of antibiotics to semen extenders is mandatory in the European Union and commonly used in other countries6. As antimicrobial resistance poses a serious global threat of growing concern to human health and economics7,8, awareness increases that antimicrobials added to semen extenders may represent a route for dissemination of antimicrobials and antimicrobial resistant bacteria into the environment9. In particular, the natural back-flow of 70 to 90% of the semen dose volume (30–80 ml) from the sow’s genital tract for a period of two hours after insemination10,11 leads to a consistent entry of antibiotics and bacteria into liquid manure causing a subsequent risk of entry in the human food chain12,13. Moreover, the permanent use of various antibiotics and disinfectants in AI centers favors the generation of multidrug-resistant bacteria2,14, often belonging to the main nosocomial pathogens15.
As part of the global antimicrobial resistance defense strategy, alternative strategies for bacterial control in extended boar semen are being studied. Antibiotic-free storage of semen doses would be a primary choice. Physical reduction of bacterial contamination before semen storage can be achieved by low density colloid centrifugation16 or microfiltration17 but is still limited for practicable use due to the low laboratory efficiency. Bacteriostasis by hypothermic semen storage could be another option if chilling-induced thermotropic phase transitions of membrane lipids leading to structural and metabolic disruption of sperm function18 could be minimized. In an earlier study, a threshold for storage temperature of 12 °C was reported before motility and fertility were affected19. Accordingly, earlier attempts to preserve boar semen at 4 or 5 °C resulted in decreased motility, membrane integrity, mitochondrial activity and chromatin stability20,21,22,23. Preliminary studies using commercial membrane protecting semen extenders AndroStar Plus24 or Androstar Premium extender25 (Minitüb, Tiefenbach, Germany) indicate that low-temperature storage in absence of antibiotics could be an option for boar semen preservation if maintenance of sperm quality and control of bacterial growth is achieved. Recent research demonstrated that cell alterations in preserved boar semen can be diminished by isothermic one–step dilution at 30–33 °C followed by slow cooling26, a minimum sperm concentration of 15 × 106/ml27, use of modern commercial semen extenders28 and motionless semen storage29.
With consideration of the above, we hypothesize that implementation of all semen processing steps at their currently known optimal level offers the opportunity to antibiotic-free hypothermic storage of boar semen without compromising fertility. A holistic approach is used which includes the screening of bacteria counts in semen extender media, the use of sensitive in vitro and ex vivo measures to assess the sublethal alterations in sperm function, and ultimately a highly standardized in vivo fertilization test under field conditions. Overall, the goal of the current study is to investigate the possibility of using low temperature storage of boar semen in absence of antibiotics.
Material and Methods
Reagents and media
Chemicals were of analytical grade and, unless otherwise stated, purchased from Sigma-Aldrich (Steinheim, Germany). Fluorochromes were obtained from Life Technologies (Darmstadt, Germany), and semen extender media were obtained from Minitüb (Tiefenbach, Germany).
Temperature dependence of bacterial growth in semen extender
In a pre-experimental screening, the influence of temperature on bacterial growth during culture at 5 °C and 17 °C was examined. Bacterial counts (CFU/ml) were evaluated during a storage period for 72 h in either BTS semen extender consisting of 205 mM glucose, 20.4 mM Na3C6H5O7, 10.0 mM KCL, 15 mM NaHCO3, 3.36 mM EDTA or culture medium consisting of 85.5 mM NaCl, 39 mM NaOH in 1000 µl aqua dest. supplemented with 10 g peptone and 10 g meat extract. Typical mesophilic bacteria isolated from boar semen, Pseudomonas aeruginosa, Serratia marcescens, Klebsiella oxytoca and Burkholderia species were evaluated. Sterile-filtered extender and culture media were inoculated with 3 × 103 CFU/ml of single bacteria strains. After aerobic culture in blood agar for 48 h at 37 °C, colony forming units (CFU) per ml were counted.
In vitro trials: Sperm quality in stored semen samples
Animals, semen processing and storage
All procedures that involved animals were carried out in accordance with guidelines and regulations according to the European Commission Directive for Pig Welfare and were approved by the institutional animal welfare committee of the University of Veterinary Medicine Hannover. Entire ejaculates were collected by the gloved-hand method from twelve fertile, sexually mature boars (1.5 to 6 yr of age) of different breeds (Piétrain, German Landrace, Large White, Duroc). Normospermic ejaculates were extended isothermically (37 °C) on split-sample basis in order to prepare four split semen sample groups according to extender type and storage temperature using: (1) the short-term extender Beltsville Thawing Solution30 with antibiotics (AB; gentamicin sulphate (0.25 g/L), storage at 17 °C (17 °C, BTS); (2) BTS extender with AB, storage at 5 °C (5 °C, BTS); (3) the long-term extender Androstar Premium (APrem) with antibiotics (gentamicin sulphate (0.25 g/L), storage at 17 C° (17 °C, APrem); or (4) APrem without antibiotics, storage at 5 C° (5 °C, APrem). Aliquots of samples diluted in BTS were used as positive control for 17 °C semen storage and as negative control for 5 °C storage. Final sperm concentration in plastic semen tubes (90 ml) was 20 × 106 sperm /ml. A subset of samples extended in BTS and samples of APrem with AB were maintained at 22 °C for 90 min and then stored at 17 °C in the dark. A further subset of samples extended in BTS and samples extended APrem without AB were placed in a cardboard box with a lid together with isothermic (28 °C) water–filled tubes. Boxes with 35 tubes were subsequently kept for 6 h at 22 °C and then stored in a refrigerator at 5 °C in the dark.
Computer-assisted semen analysis
Sperm kinematics was objectively evaluated after 24 h, 72 h, and 144 h with the CASA system AndroVision, Version 1.1 (Minitüb, Tiefenbach, Germany), using pre-warmed four-chamber slides (Leja, Nieuw Vennep, NL) with a chamber depth of 20 µm. For each sample, six successive fields in the central axis of a chamber were recorded at a rate of 30 frames per 0.5 s for each field using a 100 × magnification. At least 500 spermatozoa per sample were analyzed. The sperm kinematic variables recorded were the overall percentage of motile spermatozoa, curve line velocity (VCL µm/sec), beat cross frequency (BCF, Hz) and amplitude of lateral head displacement (ALH, µm). Spermatozoa were evaluated as “motile” by the AndroVision software algorithm when their ALH was higher than 1.0 µm and their VCL was higher than 24.0 µm/sec.
Flow cytometry
Sperm membrane integrity was analyzed at 24 h, 72 h, and 144 h with a Galaxy Flow cytometer (DAKO, Hamburg, Germany). A 5 µl subsample of diluted semen was transferred to 995 µl of HEPES-buffered saline medium (HBS: 137 mM NaCl, 20 mM HEPES, 10 mM Glucose, 2.5 mM KOH, 3 mg/ml BSA (Cohn’s fraction V), pH 7.4 at 20 °C, 300 ± 5 mOsmol/kg) including propidium iodide (PI; final concentration 5 µg/mL), fluorescein-isothiocyanate-conjugated peanut agglutinin (PNA-FITC; final concentration 3.0 µg/mL), and Hoechst 33342 (H-342, final concentration 0.75 µg/mL). After incubation at 25 °C in the dark for 15 min, 10,000 events were analyzed by ‘FloMax’ software (v2.4, Partec, Münster, Germany). Fluorochromes were excited at 488 nm (argon ion laser, 20 mW) and at approx. 365 nm (mercury lamp). Signals were detected using a 537.5/22.5 nm filter (PNA-FITC), a 630 nm LP filter (PI), and a 455/10 nm filter (H-342). Membrane intact spermatozoa were those with intact plasma membranes and acrosome (Hoechst-positive, PI negative and PNA-FITC negative).
The DNA fragmentation index (DFI) of spermatozoa was assessed using the sperm chromatin structure assay (SCSA31) in semen samples stored for 72 h. The assay tests for the resistance of spermatozoa to acid denaturation in situ. Snap-frozen extended semen aliquots were thawed and 10 μl were added to 490 μl TNE buffer (0.15 M NaCl, 0.01 M TRIS-HCL, 1 mM disodium EDTA, pH 1.2). Then, an aliquot of 200 μl was taken and 400 μL acid solution (0.08 M HCL, 0.15 M NaCl, 0.1% Triton X-100, pH 1.2) was added and samples were vortexed for 30 s in the dark. To stop the denaturation reaction, 1.2 mL acridine orange (Polysciences, Warrington, PA, USA) staining solution (0.15 M NaCl, 0.0037 M citric acid, 0.126 M Na2HPO2, 0.0011 M disodium EDTA, pH 6.0; containing 6 μg mL-1 acridine orange) was added. Samples were placed on ice for 3 min and then 10,000 cells were analyzed with an average flow rate of 200–300/sec using a FACScan flow cytometer (Becton-Dickinson, Heidelberg, Germany) with a 488-nm laser for excitation, and 530/30-nm band pass and 650-nm long pass filters for detecting green and red fluorescence, respectively. The DNA fragmentation index (DFI) was determined as described by Evenson et al.32.
The response to capacitating conditions was evaluated by measurement of intracellular calcium as described by Henning et al.33 in semen samples (n = 6) stored for 72 h. 4 mL of extended semen were incubated with 4 µL of Fluo-3/AM stock solution (final concentration: 1 µM) for 30 min at RT in the dark. Aliquots of Fluo-3-loaded spermatozoa were incubated at a concentration of 1.5–2.0 × 105/mL in two variations of Tyrode´s medium. The capacitating medium Tyrode A consisted of 96 mM NaCl, 20 mM HEPES, 5 mM glucose, 3.1 mM KCl, 2 mM CaCl2, 0.4 mM MgSO4, 0.3 mM KH2PO4, 100 µg/mL gentamycin sulphate (SERVA, Heidelberg, Germany), 20 µg/mL phenol red, 1.0 mM sodium pyruvate, 21.7 mM sodium lactate, 3 mg/mL bovine serum albumin (Cohn’s fraction V, fatty acid free), 15 mM NaHCO3 and 2 mM CaCl2. Additionally, non-capacitating control medium Tyrode C was used to study the specific effect of the capacitating conditions. The control medium was lacking the capacitation inducer bicarbonate. Both media were adjusted to a pH of 7.40 at 38 °C and an osmolality of 300 mOsmol/kg. Propidium iodide (final concentration: 2.5 µg/mL) was added before the beginning of incubation in the Tyrode’s media. Samples were assessed after 3, 60, 90, 120 and 180 min of incubation at 38 °C under CO2 (Tyrode) or under air (Control). After 150 min, Ca2+ ionophore A23187 was added to induce cytosolic calcium influx as a positive control. The destabilization of spermatozoa under capacitating and non-capacitating conditions was evaluated by flow cytometric measurement of the live sperm population with low intracellular calcium (H-342 positive, PI-negative, Fluo-3 negative).
Ex vivo trial: Sperm-oviduct binding
The sperm’s capacity to form the female sperm reservoir was determined using a recently established competitive oviduct binding assay34. Single ejaculates from six boars were diluted with APrem to 50 × 106 sperm/ml. Diluted semen was split into two aliquots and sperm in these subsamples were labelled either with MitoTracker Red FM or MitoTracker Green FM (200 nM) for 15 min at 38 °C in the dark. To remove excessive dye, samples were centrifuged through 20% Percoll. Percoll-saline working solutions (300 ± 5 mOsmol/kg, pH 7.40 ± 0.05) were prepared by diluting Percoll (GE Healthcare, Munich, Germany) with a HEPES-buffered saline solution (HBS; 137 mM NaCl, 20 mM HEPES, 10 mM Glucose, 2.5 mM KOH, pH 7.60 ± 0.05 at room temperature, 300 ± 5 mOsmol/kg) as described previously35. Samples were centrifuged for 10 min at 300 g followed by 10 min at 750 g. Sperm concentration in the pellet was adjusted to 20 × 106 sperm/mL with isothermic extender supplemented with 10% (v/v) fresh autologous seminal plasma. Semen was then stored at 5 °C and 17 °C for 72 h, respectively. Oviduct explants were collected from slaughtered sows (parity ≥1). Explants were prepared as described previously34,36. Each semen sample was tested with four explants from two sows. Semen samples were pre-incubated at 38 °C in a water bath and then co-incubated for 15 min at 38 °C under 5% CO2 in a cross-over design with explants. Red-tagged sperm from 5 °C storage were tested against green-tagged sperm stored at 17 °C on the same explant. Next, the opposite combination was tested with another explant from the same sow. The average sperm binding index for samples stored at 5 °C and 17 °C and the average ratio of bound spermatzoa were calculated.
The average number of bound spermatozoa per 1 mm2 was defined as binding index (BI) as described previously34,36:
where E1 is the explant from Sow no. 1, A1, A2 and A3 are the areas of Location no. 1, 2, and 3, respectively, and N1, N2 and N3 are number of sperm bound on Location no. 1, 2, and 3, respectively. The binding index was calculated separately for each color.
In vivo trial: microbiology of stored semen samples and fertility post-AI
A field insemination trial was performed at a pig farm located in Rancagua, Chile, to assess the fertilizing capacity of antibiotic-free semen stored at 5 °C compared to semen doses routinely stored at 17 °C with the AB gentamicin sulphate (0.25 g/L). Thirtysix semen pools from 23 mature boars (PIC 337) each consisting of normospermic ejaculates from three boars were split sample-diluted with APrem extender with and without AB. Sperm concentration and volume in semen doses were prepared according the routine use in the breeding organization. After dilution, semen doses were transported within 20 min to the sow farm. Aliquots of diluted semen samples were kept at the laboratory and subjected to the same cooling and storage conditions as samples on the farm. Samples with AB were stored at 17 °C and samples without AB were kept for 6 h at 22–23 °C and then stored at 5 °C in the dark. Directly after dilution (0 h) and at 24 h and 72 h of storage, bacteria counts (colony forming units/mL) were performed after aerobic culture in blood agar (Labser Laboratory, Rancagua, Chile). Estrus was monitored twice daily in presence of a teaser boar. Sows were inseminated two to three times per estrus with the first insemination performed 15–20 h after first detection of estrus. Weaned multiparous sows (parity 1 to 6, n = 666) were post-cervically inseminated using semen doses with 2.2 × 109 spermatozoa in 50 mL stored for 24 to 72 h. Gilts (parity 0) were transcervically inseminated using semen doses with 4 × 109 spermatozoa in 90 mL. Animals were randomly assigned to the two insemination groups according to their entrance in estrus after weaning. Insemination data of 829 sows and gilts (PIC Camborough) were collected over a period of two months.
Statistical analysis
For data analysis, the Statistical Analysis System for Windows SAS, version 9.4 (SAS Institute Inc., Cary, North Carolina, USA) was used. Depending on the experiment, different (mixed) models were fit to the data: Sperm quality data were analysed with a two-way repeated measures ANOVAs with fixed and random effects. By considering ‘temperature-extender’ as one between-subjects factor with four levels, repeated measures were only present in two dimensions (temperature-extender treatment and time). An interaction term of treatment x time was included in the model to test whether the treatment effect varied according to time points. Various covariance structures were tested for the residuals. According to the Akaike’s and Bayesian’s information criteria a constant correlation between time points (CS) was selected for the former model of motility and membrane integrity. A general unstructured (UN) covariance model was assumed for nonspecific correlation among measurements in the second model of live, low-calcium sperm. A by subject random intercept was added to the models to account for the correlation between observations from the same individuals. If significant effects were observed, post hoc comparison tests regarding the interesting combinations of fixed factors were performed with Bonferroni correction for multiple comparisons. By means of a one-way ANOVA for correlated samples, repeated measurements in explants and in temperature were considered for the analysis the sperm-oviduct binding experiment using a CS covariance structure. Since the within-subjects factor ‘temperature’ contained only two levels, no adjustment for multiplicity was needed. Categorical variables were compared using Chi-square test for comparisons between treatment groups. CFU were allocated to the three categories, CFU = 0, 0 < CFU/ml < 1000 and ≥1000 CFU/ml, and evaluated using the chi-square test or Fisher’s exact test, as appropriate. For dichotomous endpoints (i.e. Non-Return rate and farrowing rate), logistic regression was used with temperature and parity as linear predictors. Due to large sample sizes, count data (i.e. number of offspring in total and number of live offspring) could be evaluated with a two-way ANOVA of aforementioned factors. In general, a p-value of less than 0.05 was considered statistically significant. Data are shown as mean ± standard error of the mean (SEM).
Results
Temperature dependence of bacterial growth in semen extender
Growth of test bacteria was screened during storage at 5 °C and 17 °C in extender medium to determine whether a semen storage temperature of 5 °C would limit bacteria growth. Culture medium was used as control medium. After 72°h at 5 °C, bacterial counts remained constant or declined 2- to 20-fold. In contrast, the bacterial population increased at 17 °C storage up to x108 in culture medium and x105 in BTS, respectively (Supplementary SFig. 1).
In vitro trials: Sperm quality in stored semen samples
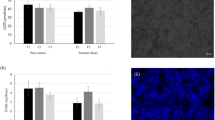
All effects on sperm motility were statistically significant (temperature-extender treatment: p < 0.001, time: p < 0.001) as well as the interaction effect (p = 0.033), indicating that the treatment has a different effect in storage time points (Supplementary STable 1). Sperm motility did not significantly decline during storage in samples stored in APrem at 17 °C or at 5 °C (Fig. 1A). At all storage time points, sperm motility in 5 °C, APrem was higher than 80% on average (Supplementary STable 2). A statistically significant difference could not be shown between 5 °C/APrem and BTS, 17 °C (p > 0.05). At 144 h, samples in 17 °C, APrem displayed the highest values for sperm motility (88.8 ± 0.9%) and samples in 5 °C/BTS showed the lowest values for this trait throughout storage. The percentage of motile spermatozoa in 5 °C, BTS significantly declined to below threshold values after 144 h of storage compared to 24 h (66.7 ± 2.7 vs. 75.3 ± 2.5; p < 0.001). In the analysis of membrane integrity, temperature-extender treatment showed a significant main effect (p < 0.001) whereas storage time did not show a significant difference (p = 0.238, see Supplementary STable 1). No significant treatment x time interaction was observed (p = 0.272). At 72 h of storage, the proportion of membrane intact spermatozoa did not exhibit a significant difference between 5 °C, APrem (89.2 ± 14.2%) and semen samples stored at 17 °C, BTS (90.3 ± 0.5%) or 17 °C, APrem (92.1 ± 0.5%; Fig. 1B). The percentage of membrane intact spermatozoa in 5 °C, BTS samples (82.8 ± 1.9) was significantly different compared to the other three groups (p < 0.001 for all three comparisons).
Sperm quality of split semen samples (n = 12 boars) assigned to four groups: (1) BTS extender with antibiotics, storage at 17 °C (17 °C, BTS), (2) BTS extender with antibiotics (AB), storage at 5 °C (5 °C, BTS), (3) AndroStar Premium with AB, storage at 17 C° (17 °C, APrem), (4) APrem without AB, storage at 5 C° (5 °C, APrem). (A) Total motile spermatozoa (%; means ± SEM) after 24, 72, and 144 h of storage. (B) Plasma membrane and acrosome intact (PI-negative and FITC-PNA negative) spermatozoa (%; means ± SEM) after 72 h of storage. (C) DNA fragmentation index (DFI %; means ± SEM) after 72 h of storage. *Values of 5 °C, BTS differ from all other semen groups at a given storage time (p < 0.05).
The DNA fragmentation index was less than 3% on average in all treatments at 72 h. The effect of treatment was significant (p = 0.048), but did not show significant differences between groups in the post-hoc analysis (Fig. 1C).
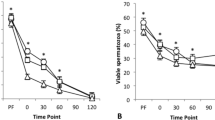
Kinetic profiles of calcium influx during incubation in capacitating and non-capacitating media were evaluated to assess the ability of the spermatozoa to respond to capacitation signals. The decrease of the proportion of live low-calcium sperm population (PI-neg/Ca2+-neg) is indicative of ongoing destabilization of sperm membranes and is thus a sensitive method to assess storage-induced sublethal effects on sperm function27,30. In non-capacitating control medium Tyrode C, both treatment and storage time were statistically significant (temperature-extender treatment: p < 0.001, storage time: p < 0.001, Supplementary STable 3). The interaction effect was non-significant (p = 0.081). The proportion of live low-calcium spermatozoa was significantly lower at all incubation time points in 5 °C, BTS compared to all other treatment groups (p < 0.001 for all comparisons; Fig. 2A). There was a significant main effect for treatment and storage time in capacitating Tyrode A medium (temperature-extender treatment: p < 0.001, storage time: p < 0.001) and no significant interaction effect (p = 0.118, Supplementary STable 3). The proportion of live low-calcium spermatozoa decreased in all sample groups after 60 min compared to 3 min. The 5 °C, BTS samples yielded the lowest values on average compared to all other sample groups at all time points (Fig. 2B). There was no significant difference between 17 °C, BTS, 17 °C, APrem and 5 °C, APrem at any time of incubation (p > 0.05, Supplementary STable 4). As positive control, calcium-influx was inducible after the addition of Ca2+ ionophore A23187 in all samples.
(A,B) In vitro response of boar semen samples to capacitating conditions during incubation at 38 °C under 5% CO2 in Tyrode A medium with bicarbonate (B) and to control conditions during incubation at 38 °C in Tyrode C medium without bicarbonate (A) as measured by changes in the population of live, low calcium (Propidium iodide negative, Fluo-3 negative) spermatozoa. Semen samples stored for 72 h (n = 6 boars) were assigned to four groups: (1) BTS extender with antibiotics (AB), storage at 17 °C (17 °C, BTS), (2) BTS extender with AB, storage at 5 °C (5 °C, BTS), (3) AndroStar Premium with AB, storage at 17 C° (17 °C, APrem), (4) APrem without AB, storage at 5 C° (5 °C, APrem). ♦: Value after the addition of Ca2+-ionophore A23187 in all sample groups. *Values of 5 °C, BTS differ from all other semen groups at a given storage time (p < 0.05). Values declined (p < 0.01) in all semen groups after 60 min of incubation in Tyrode A. (C,D) Example of flow cytometry analyses illustrating the shift towards a sperm population with higher fluorescence of the calcium probe Fluo 3 and a concomitant increase of dead spermatozoa (propidium iodide positive) after 60 min incubation in Tyrode A (D); this shift is not observed in Tyrode C (C). Non-sperm particles were excluded.
Ex vivo trial: Sperm-oviduct binding
The binding indices resulting from competitive sperm binding to oviductal explant did not significantly differ between 5 °C and 17 °C storage temperature in semen samples extended in APrem (p = 0.072; Fig. 3A). There was a tendency for a skewed sperm binding ratio in favor of samples stored at 17 °C (55.3% bound sperm in samples stored at 17 °C samples vs. 44.7% in samples stored at 5 °C; Fig. 3B).
(A) Box-whisker plots showing the outcome of competitive sperm binding to oviductal explants of semen samples stored for 72 h either at 5 °C without antibiotics or 17 °C with antibiotics in Androstar Premium (APrem). The number of bound spermatozoa per mm2 oviductal surface (binding index) in the two semen groups is illustrated as Box-whisker plots. Values did not indicate statistical significance (p = 0.07). (A) The numeric proportion (binding ratio) in the competitive oviduct explant assay is presented as circle-chart (B). Values did not indicate statistical significance (p = 0.07). Differential staining was done in spermatozoa taken from the same semen sample and stored either at 5 °C or at 17 °C.
In vivo trial: Microbiology of stored semen samples and fertility post-AI
Microbiology results
Bacterial counts of semen samples extended in APrem and used for the in vivo trial are presented in Fig. 4. Immediately after dilution, 80.6% of the semen pools (0 h) had <103 CFU/ml. After 24 h and 72 h semen storage, the majority of samples contained <103 CFU/ml regardless of presence or absence of AB (with AB/17 °C: 100% and 97.2%; without AB/5 °C: 94.4% and 88.9%, respectively). Maximum bacterial counts in samples with AB/17 °C was 1 × 104 (72 h), and in samples without AB/5 °C it was 7.6 × 103 (24 h). The relation between categorized CFU/ml and storage time was not significant in either temperature group (5 °C: p = 0.445; 17 °C: p = 0.198).
Frequency distribution of semen pools (n = 36) in three classed of bacterial counts (colony forming units (CFU)/ml) initially after dilution in antibiotic (AB)-free Androstar Premium extender (0 h), and after 24 h and 72 h of storage either at 5 °C without AB ( ) or at 17 °C with AB (
) or at 17 °C with AB ( ). Maximum values: 5 °C without AB: 7.6 × 103 (24 h); 17 °C with AB: 1 × 104 (72 h).*Values differ (P < 0.01).
). Maximum values: 5 °C without AB: 7.6 × 103 (24 h); 17 °C with AB: 1 × 104 (72 h).*Values differ (P < 0.01).
Fertility results
Twelve inseminated animals, i.e. eight from the group inseminated with AB/17 °C and five animals from the group inseminated without AB/5 °C, were excluded due to extra-genital illness or death. Parity of females was similar in the two insemination groups (Supplementary STable 5). The percentage of sows versus gilts (parity) did not differ by treatment group (p = 0.21). Semen doses from all 36 pools maintained motility values for 72 h above the threshold of 70°% and were used for AI. Fertility results of 817 females are summarized in Table 1. The fertility traits Non-Return rate, farrowing rate and litter size (total born piglets and live piglets) were all at a high level and did not significantly differ between the animals inseminated with extended semen either containing AB and stored at 17 °C or without AB and stored at 5 °C (p > 0.05, Table 1). Farrowing rates and average numbers of total born piglets of gilts inseminated with AB/17 °C (n = 71) were 94.1% and 14.48 ± 3.35, and of gilts inseminated without AB/5 °C (n = 87) they were 93.1% and 15.04 ± 2.53, respectively.
Discussion
The limitation of antibiotic use in semen extenders and the concomitant guarantee of microbial safety and high fertilizing capacity of the preserved spermatozoa presents a major challenge in porcine assisted reproduction1. The present study provides evidence that boar spermatozoa can be hypothermically stored in an antibiotic-free environment without compromising fertility. The experimental designs were based on two challenges associated with the low-temperature storage and the absence of AB: i). prevention of sperm damage and malfunction due to chilling injury and ii). inhibition of bacterial growth leading to sperm damage and/or affecting the female reproductive tract.
Until now, evidence for the successful application of hypothermic storage of boar semen at supra-zero temperature in large-scale field inseminations is lacking. Cooling induced thermotropic phase transitions of sperm membrane lipids lead to changes in membrane structure, disturbed ion transports and eventually to structural and metabolic disruption of cell function18,28,37. The extent of chilling injury can be modified by sperm handling during collection, processing, transport and storage38. Considering recently identified semen-processing associated effectors on sperm physiology, data from the present study indicate that boar spermatozoa stored hypothermically at 5 °C maintain motility, membrane and DNA integrity, sperm binding to oviductal epithelium, the response to capacitation stimuli, and ultimately in vivo fertility. Essential functional sperm attributes were monitored in several in vitro and ex vivo assays. Potential damage to these attributes would otherwise remain undetected in an in vivo fertility trial due to sperm numbers in the AI doses above threshold values, or dominating farm or female influences39. From the results obtained here, it has been demonstrated that 5 °C-storage of boar semen without compromising fertility is practical.
Remarkably, even in BTS-stored samples used as negative control, conventional sperm quality traits were maintained above thresholds for semen use in AI indicating the importance of careful semen processing management. Particularly a slow cooling achieved by holding times between 17 and 26 °C up to 24 h protects spermatozoa against chilling injury presumably by uptake of membrane stabilizing seminal plasma components40,41. The role of long-term semen extenders becomes obvious when comparing in vitro sperm data of samples in APrem with the standard short-term extender BTS. Recipes of most commercially marketed semen extenders are not published. However, long-term extenders may contain membrane stabilizers and/or capacitation inhibitors which may prolong shelf life in vitro42. Cell stabilizing ingredients, such as antioxidants, might hinder the responsiveness of surviving cells to capacitation signals in the female tract38. Using a previously established sensitive assay for kinetic assessment of capacitation-associated sperm functionality33, we demonstrated that spermatozoa stored in APrem maintain their reactivity to the capacitation stimulant bicarbonate. Noteworthy, destabilization as measured by the progressive loss of live spermatozoa with low calcium occurred slower in APrem compared to BTS controls suggesting a longer survival time in the oviduct.
The binding of spermatozoa to oviductal cells forms a reservoir43 which is mediated by carbohydrates on the oviductal cell apical membranes and lectin-like molecules on the sperm surface44,45. Several studies have shown that oviductal epithelial cells select boar spermatozoa according to their functional membrane-mediated characteristics36,46,47. The slightly reduced binding capacity of spermatozoa stored at 5° observed in the present study may be attributed to chilling-induced membrane lipid alterations. It is suggested that the lower binding capacity can be compensated by a higher sperm number in the semen dose48. In the present study, the sperm DNA integrity was not affected by the cold storage, thus compensating effect could become effective. In any case, efficiency of semen use remains high compared to frozen semen where at least a two-fold higher sperm number per semen dose and more inseminations per estrus compared to 17 °C liquid semen storage are required to achieve satisfying fertility outcomes. Noteworthy, in the present in vivo trial, sperm numbers per semen dose were equal to the routine farm insemination using semen stored at 17 °C in the presence of AB. As the most relevant finding of this study, fertility of semen stored at 5 °C in absence of antibiotics was not compromised compared to split-semen samples which were conventionally stored at 17 °C in the presence of antibiotics.
Efficient bacteriostasis of four bacteria strains commonly detected in boar ejaculates was achieved in the absence of antibiotics at a 5 °C storage temperature. Bacterial counts in AB-free stored semen was below 104 CFU/ml in all semen samples, and in the majority (88.9%) below 103 CFU/ml. Noteworthy, the highest bacterial count was found in a sample stored with AB at 17 °C, reflecting a typical situation of antibiotic resistance. Logically, depending on temperature tolerance for bacterial multiplication, 17 °C storage bears a higher risk for increased bacterial growth in situations of AB-inefficacy compared to 5 °C AB-free storage. Several studies reported no adverse effects of mesophilic aerobic bacteria on boar sperm quality or fertility if bacteria load is between x103 and x107 CFU/ml4,5,49,50,51. Consequently, there is no rationale for complete elimination of bacteria in pig semen doses. Considering the high numeric exposure to bacteria at natural mating when high semen volumes (200–500 ml) containing up to 109 CFU/ml14,49 are transmitted into the female tract, a physiological immunogenic role of commensal bacteria in ejaculates on the uterine milieu deserves to be considered. There are a few specific bacteria that are of concern for causing infectious diseases or loss of reproductive performance in sow herds, including brucellosis, chlamydophilosis and leptospirosis52. Regular diagnostic serological testing for brucellosis in boar studs is effective for early detection of this pathogen; therefore, the risk of its occurrence in extended semen is low. The prevalence of chlamydia and leptospira pathogens in the swine population is largely unknown and may differ among global regions. Strict hygiene measures can greatly aid in controlling the introduction of these pathogens into a boar stud52,53. Nonetheless, the efficacy of low temperature semen storage on the growth of specific pathogens should be considered in future studies.
In conclusion, the present study provides strong evidence that hypothermic storage of boar semen at 5 °C allows the elimination of antibiotics in extender used to prepare boar semen doses without affecting fertility. This semen processing strategy can be easily implemented in artificial insemination centers for further evaluation of this novel concept under different farm conditions. Overall, antibiotic-free hypothermic semen storage contributes to bioeconomy in pig AI with a special emphasize on the global antimicrobial defense strategy.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Waberski, D., Riesenbeck, A., Schulze, M., Weitze, K. F. & Johnson, L. Application of preserved boar semen for artificial insemination: past, present and future challenges. Theriogenology 137, 2–7 (2019).
Althouse, G. C. & Lu, K. G. Bacteriospermia in extended porcine semen. Theriogenology 63, 573–584 (2005).
Parks, J. E. & Lynch, D. V. Lipid composition and thermotropic phase behavior of boar, bull, stallion, and rooster sperm membranes. Cryobiology 29, 255–266 (1992).
Maroto Martín, L. O. et al. Bacterial contamination of boar semen affects the litter size. Anim Reprod Sci 120, 95–104 (2010).
Úbeda, J. L. et al. Adverse effects of members of the Enterobacteriaceae family on boar sperm quality. Theriogenology 80, 565–570 (2013).
Council Directive, European Union, 92/65/EEC (1992), https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex:31992L0065; accessed Jan18, 2019.
WHO list of critically important antimicrobials 5th edition, Advisory Group on Integrated Surveillance of Antimicrobial Resistance (AGISAR), October 2016, http://who.int/foodsafety/publications/antimicrobials-fifth/en/, accessed 12 December 2018.
Aslam, B. et al. Antibiotic resistance: a rundown of a global crisis. Infect Drug Resist. 11, 1645–1658 (2018).
EMA and EFSA Joint Scientific Opinion on measures to reduce the need to use antimicrobial agents in animal husbandry in the European Union, and the resulting impacts on food safety (RONAFA). EFSA Journal 2017;15(1):4666, https://www.efsa.europa.eu/en/efsajournal/pub/4666. accessed Jan 18, 2019.
Steverink, D. W., Soede, N. M., Bouwman, E. G. & Kemp, B. Semen backflow after insemination and its effect on fertilisation results in sows. Anim Reprod Sci 54, 109–119 (1998).
Araújo, E. B. et al. Reproductive performance of sows submitted to intrauterine insemination. Revista Brasileira de Zootecnia 38, 1460–1467 (2009).
Verraes, C. et al. Antimicrobial resistance in the food chain: a review. Int J Environ Res Public Health 10, 2643–2669 (2013).
Jechalke, S., Heuer, H., Siemens, J., Amelung, W. & Smalla, K. Fate and effects of veterinary antibiotics in soil. Trends Microbiol. 22, 536–545 (2014).
Schulze, M., Ammon, C., Rüdiger, K., Jung, M. & Grobbel, M. Analysis of hygienic critical control points in boar semen production. Theriogenology 83, 430–437 (2015).
Peleg, A. Y. & Hooper, D. C. Hospital-acquired infections due to Gram-negative bacteria. N Engl J Med. 362, 1804–1813 (2010).
Morrell, J. M. et al. Removal of bacteria from boar semen using a low-density colloid. Theriogenology 126, 272–278 (2019).
Barone, F., Ventrella, D., Zannoni, A., Forni, M. & Bacci, M. L. Can microfiltered seminal plasma preserve the morphofunctional characteristics of porcine spermatozoa in the absence of antibiotics? A preliminary study. Reprod Domest Anim. 51, 604–10 (2016).
Drobnis, E. Z. et al. Cold shock damage is due to lipid phase transitions in cell membranes: a demonstration using sperm as a model. J Exp Zool 265, 432–437 (1993).
Althouse, G. C., Wilson, M. E., Kuster, C. E. & Parsley, M. Characterization of lower temperature storage limitations of fresh-extended porcine semen. Theriogenology 50, 535–543 (1998).
Zou, C. X. & Yang, Z. M. Evaluation on sperm quality of freshly ejaculated boar semen during in vitro storage under different temperatures. Theriogenology 53, 1477–88 (2000).
Foote, R. H. Within-herd use of boar semen at 5 degrees C, with a note on electronic monitoring of oestrus. Reprod Domest Anim 37, 61–63 (2002).
Fraser, L. & Strzezek, J. The use of comet assay to assess DNA integrity of boar spermatozoa following liquid preservation at 5 degrees C and 16 degrees C. Folia Histochem Cytobiol. 42, 49–55 (2004).
Dziekońska, A. & Strzezek, J. Boar variability affects sperm metabolism activity in liquid stored semen at 5 degrees C. Pol J Vet Sci. 14, 21–27 (2011).
Menezes, Tila de Alcantara Avaliação da temperatura de armazenamento e uso de antimicrobianos na qualidade de doses seminais de suínos. Universidade Federal do Rio Grande do Sul. Faculdade de Veterinária. Programa de Pós-Graduação em Ciências Veterinárias, http://hdl.handle.net/10183/179696, accessed Jul 16, 2019.
Grossfeld, R., Peralta, W., Weitze, K.-F. & Waberski, D. (2016) Antibiotic-free hypothermic storage of boar semen in Androstar +5 extender results in similar fertility results compared to semen stored at 17 °C in extender with antibiotic content. Animal Reproduction Science 169, 125 (Abstract) (2018).
Schulze, M. et al. Impact of different dilution techniques on boar sperm quality and sperm distribution of the extended ejaculate. Anim Reprod Sci. 182, 138–145 (2017).
Luther, A.-M. & Waberski, D. In vitro aging of boar spermatozoa: role of sperm proximity and seminal plasma. Andrology 7, 382–390 (2019).
Schmid, S., Henning, H., Petrunkina, A. M., Weitze, K. F. & Waberski, D. Response to capacitating stimuli indicates extender-related differences in boar sperm function. J Anim Sci. 91:5018–5025 (2013).
Schulze, M., Rüdiger, K. & Waberski, D. Rotation of boar semen doses during storage affects sperm quality. Reprod Domest Anim. 50, 684–687 (2015).
Johnson, L. A., Aalbers, J. G. & Grooten, H. J. G. Artificial insemination of swine: fecundity of boar semen stored in Beltsville TS (BTS), modified modena (MM), or MR-A and inseminated on one, three and four days after collection. Zuchthygiene 23, 49–55 (1988).
Evenson, D. P., Darzynkiewicz, Z. & Melamed, M. R. Relation of mammalian sperm chromatin heterogeneity to fertility. Science 210, 1131–1133 (1980).
Evenson, D. P., Larson, K. L. & Jost, L. K. Sperm chromatin structure assay: its clinical use for detecting sperm DNA fragmentation in male infertility and comparisons with other techniques. Journal of Andrology 23, 25–43 (2002).
Henning, H., Petrunkina, A. M., Harrison, R. A. & Waberski, D. Bivalent response to long-term storage in liquid-preserved boar semen: a flow cytometric analysis. Cytometry A 81, 576–587 (2012).
Henning, H. H. W., Batz-Schott, J., Grünther, B., Le Thi, X. & Waberski, D. Fluorescent labelling of boar spermatozoa for quantitative studies on competitive sperm-oviduct binding. Reprod Fertil Develop, https://doi.org/10.1071/RD19081. [Epub ahead of print] (2019 May 10).
Vincent, R. & Nadeau, D. Adjustment of the osmolality of Percoll for the isopycnic separation of cells and cell organelles. Anal Biochem 141, 322–328 (1984).
Petrunkina, A. M., Gehlhaar, R., Drommer, W., Waberski, D. & Töpfer-Petersen, E. Selective sperm binding to pig oviductal epithelium in vitro. Reproduction 121, 889–896 (2001).
De Leeuw, F. E., Chen, H. C., Colenbrander, B. & Verkleij, A. J. Cold induced ultrastructural changes in bull and boar sperm plasma membranes. Cryobiology 27, 171–183 (1990).
Leahy, T. & Gadella, B. M. Sperm surface changes and physiological consequences induced by sperm handling and storage. Reproduction 142, 759–778 (2011).
Amann, R. P., Saacke, R. G., Barbato, G. F. & Waberski, D. Measuring male-to-male differences in fertility or effects of semen treatments. Annu Rev Anim Biosci. 6, 255–286 (2018).
Pursel, V. G., Schulman, L. L. & Johnson, L. A. Effect of holding time on storage of boar spermatozoa at 5C. J Anim Sci 37, 785–789 (1973).
Casas, I. & Althouse, G. C. The protective effect of a 17 °C holding time on boar sperm plasma membrane fluidity after exposure to 5 °C. Cryobiology 66, 69–75 (2013).
Gadea, J. Review: Semen extenders used in the artificial insemination of swine. Spanish Journal of Agricultural Research 1, 17–27 (2003).
Hunter, R. Sperm transport and reservoirs in the pig oviduct in relation to the time of ovulation. J Reprod Fertil 63, 109–117 (1981).
Wagner, A. et al. Carbohydrate-based interactions of oviductal sperm reservoir formation-studies in the pig. Mol. Reprod. Dev. 61, 249–257 (2002).
Kadirvel, G. et al. Porcine sperm bind to specific 6-sialylated biantennary glycans to form the oviduct reservoir. Biol. Reprod. 87(Article147), 1–11 (2012).
Fazeli, A., Duncan, A., Watson, P. & Holt, W. Sperm-oviduct interaction: induction of capacitation and preferential binding of uncapacitated spermatozoa to oviductal epithelial cells in porcine species. Biol Reprod 60, 879–86 (1990).
López-Úbeda, R., García-Vázquez, F. A., Gadea, J. & Matás, C. Oviductal epithelial cells selected boar sperm according to their functional characteristics. Asian J Androl. 19, 396–403 (2017).
Saacke, R. G. Sperm morphology: its relevance to compensable and uncompensable traits in semen. Theriogenology 70, 473–478 (2008).
Althouse, G. C., Kuster, C. E., Clark, S. G. & Weisiger, R. M. Field investigations of bacterial contaminants and their effects on extended porcine semen. Theriogenology 53, 1167–7116 (2000).
Bussalleu, E. et al. Effects of different concentrations of enterotoxigenic and verotoxigenic E. coli on boar sperm quality. Anim Reprod Sci. 127, 176–82 (2011).
Pinart, E., Domènech, E., Bussalleu, E., Yeste, M. & Bonet, S. A comparative study of the effects of Escherichia coli and Clostridium perfringens upon boar semen preserved in liquid storage. Anim Reprod Sci 177, 65–78 (2017).
Althouse, G. C. & Rossow, K. The potential risk of infectious disease dissemination via artificial insemination in swine. Reprod Domest Anim. 46(Suppl 2), 64–67 (2011).
Strutzberg-Minder, K., Tschentscher, A., Beyerbach, M., Homuth, M. & Kreienbrock, L. Passive surveillance of Leptospira infection in swine in Germany. Porcine Health Manag. 4, 10 (2018).
Acknowledgements
This study was supported by Association of Bioeconomy Research (FBF e.V.) and by the Rentenbank - Germany’s development agency for agribusiness (AMIKOS 823 600). The authors thank Minitüb (Tiefenbach, Germany) for donation of the extender media. The publication of this study was supported by Deutsche Forschungsgemeinschaft and University of Veterinary Medicine Hannover, Foundation, within the funding programme Open Access Publishing.
Author information
Authors and Affiliations
Contributions
D.W., W.P., H.H. and K.F.W. designed the study, B.G., A.L. and H.J. conducted the experiments. C.V. and A.L. performed the statistical analysis. D.W., A.L. and C.V. wrote a draft of the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Waberski, D., Luther, AM., Grünther, B. et al. Sperm function in vitro and fertility after antibiotic-free, hypothermic storage of liquid preserved boar semen. Sci Rep 9, 14748 (2019). https://doi.org/10.1038/s41598-019-51319-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-51319-1
This article is cited by
-
Bacteriospermia and its antimicrobial resistance in relation to boar sperm quality during short-term storage with or without antibiotics in a tropical environment
Porcine Health Management (2023)
-
N-thiocarboxyanhydrides, amino acid-derived enzyme-activated H2S donors, enhance sperm mitochondrial activity in presence and absence of oxidative stress
BMC Veterinary Research (2023)
-
Validation of the volumetric flow cytometry for bovine sperm concentration
Scientific Reports (2023)
-
In vitro performance and in vivo fertility of antibiotic-free preserved boar semen stored at 5 °C
Journal of Animal Science and Biotechnology (2021)
-
Relevance of Leptospira in boar and for the development of alternative antimicrobial concepts in boar semen preservation
Porcine Health Management (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.