Abstract
Although several risk factors exist for acute coronary syndrome (ACS) no biomarkers for survival or risk of re-infarction have been validated. Previously, reduced serum concentrations of anti-ß1AR Ab have been implicated in poorer ACS outcomes. This study further evaluates the prognostic implications of anti-ß1AR-Ab levels at the time of ACS onset. Serum anti-ß1AR Ab concentrations were measured in randomly selected patients from within the PLATO cohort. Stratification was performed according to ACS event: ST-elevation myocardial infarct (STEMI) vs. non-ST elevation myocardial infarct (NSTEMI). Antibody concentrations at ACS presentation were compared to 12-month all-cause and cardiovascular mortality, as well as 12-month re-infarction. Sub-analysis, stratifying for age and the correlation between antibody concentration and conventional cardiac risk-factors was subsequently performed. Serum anti-ß1AR Ab concentrations were measured in 400/799 (50%) STEMI patients and 399 NSTEMI patients. Increasing anti-ß1AR Ab concentrations were associated with STEMI (p = 0.001). Across all ACS patients, no associations between anti-ß1AR Ab concentration and either all-cause cardiovascular death or myocardial re-infarction (p = 0.14) were evident. However among STEMI patients ≤60 years with anti-ß1AR Ab concentration <median higher rates of re-infarction were observed, compared to those with anti-ß1AR Ab concentrations > median (14/198 (7.1%) vs. 2/190 (1.1%)); p = 0.01). Similarly, the same sub-group demonstrated greater risk of cardiovascular death in year 1, including re-infarction and stroke (22/198 (11.1%) vs. 10/190 (5.3%); p = 0.017). ACS Patients ≤60 years, exhibiting lower concentrations of ß1AR Ab carry a greater risk for early re-infarction and cardiovascular death. Large, prospective studies quantitatively assessing the prognostic relevance of Anti-ß1AR Ab levels should be considered.
Similar content being viewed by others
Introduction
Coronary heart disease (CHD) remains a leading cause of death in developed countries1. Numerous risk factors for the incidence of CHD and acute coronary syndrome (ACS) have been well-validated, including hyperlipidemia, arterial hypertension, diabetes, smoking and family history of CHD2. Biomarkers for the prognosis post-ACS have been suggested, such as Growth Differentiation Factor-15 (GDF-15) or N-terminal pro-hormone brain natriuretic peptide (NT-proBNP)3, but currently no biomarkers stratifying for risk of re-infarction have been identified.
Recently, a group of antibodies against various G protein coupled receptors including Beta1 adrenergic receptors (ß1AR) were identified in healthy individuals, which is altered by age, sex and certain diseases. These antibodies exhibit effects on the receptor as novel ligands and are important for immune cell homeostasis. Both increased as well as decreased ab concentrations were associated with diseases and disease symptoms4.
Beta1AR is known to be expressed on endothelial cells, cardiomyocytes and fibroblasts. Existing data suggests that cardiac function may be influenced by regulating ß1AR5,6. ß1AR affects both myocardial contractility7 and heart rate regulation8. Current literature suggests that autoantibodies against ß1AR (ß1AR Ab) may influence the development of cardiomyopathy9, and in particular some evidence of prognostic benefit exists for ß1AR Ab in ischemic cardiomyopathy10.
The immunological mechanisms of ß1AR Ab however remain unclear, with conflicting reports of both protective or negative effects on ß1AR as well as agonistic and antagonistic functions of ß1AR10,11,12. It would appear that the function of antibodies against ß1AR may depends upon the exact binding site and clinical status at the time of measurement, as for example different effects of ß1AR Ab were described in healthy individuals compared to patients with cardiomyopathy13.
We previously demonstrated that significantly reduced anti ß1AR Ab concentrations were evident in patients with STEMI compared to other forms of ACS and healthy controls in a combined cohort of 212 ACS patients14.
To validate these findings, a large scale assessment utilising the PLATO cohort was performed, paying particular attention to the role of ß1AR Ab regarding the incidence of re-infarction and cardiovascular death in the first year post index ACS event. Given recent evidence that age may influence autoantibodies against G protein coupled receptors regarding antibody levels and function has been shown recently4. Earlier studies with autoantibodies in atherosclerosis and cardiovascular disease have been performed in younger CHD patients, below the age of 60 years, as differences regarding to the patients age in their quantity for example in MAZ-ab have been present14,15,16. For these reasons we were interested as well, if ß1AR Ab may have a different meaning in younger patients.
Methods
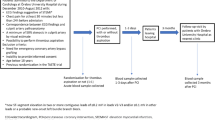
Serum samples from the Platelet Inhibition and Patient Outcomes (PLATO) trial, involving over 18.000 patients with ACS, were analyzed. Inclusion criteria consisted of hospitalization with ischemic symptoms within the previous 24 hours for ≥10 minutes, ≥18 years of age and not being pregnant. Minimum of two of the following criteria were mandatory:
-
i.
Compatible ECG changes (ST segment depression, transient ST-elevation ≥1 mm in two or more contiguous leads or new left bundle branch block)
-
ii.
Raised biomarkers (Troponin I/T or CK/MB)
-
iii.
Coronary angiography PCI planned.
and one of the following:
-
i.
Age ≥60 years
-
ii.
Previous MI or coronary by-pass, or known ischemic heart disease with ≥50% stenosis in ≥2 vessels
-
iii.
Previous ischemic stroke, physician-confirmed transient ischemic attack, carotid stenosis (≥50%) or revascularization
-
iv.
Diabetes mellitus
-
v.
Peripheral vascular disease
-
vi.
Chronic renal dysfunction.
Comprehensive details are included in the original publication of the PLATO trial17.
For the current analysis, STEMI and NSTEMI patients were selected chronologically, based on earliest recruitment to maximize follow-up data completeness. Anti-ß1AR Ab concentrations were measured in sera from all patients and compared to the primary outcome according to the same diagnostic criteria within the first year, with patient 1-year survival - both all-cause and cardiovascular-related - being the secondary outcome measures. Further sub-analysis, correlating individual antibody concentrations to documented cardiac risk factors, as well as sub-group stratification for patient age and ACS phenotype were additionally performed.
The current study was approved by the institutional ethics committees of the Medical School Hannover as well as the national and institutional regulatory authorities and ethics committees in Uppsala, Sweden. The methods were carried out in accordance with good clinical practice guidelines. All patients provided written informed consent before entering the PLATO trial.
Detection of anti-β1AR Ab using ELISA
The commercially available CE-certified ELISA kit (CellTrend GmbH, Luckenwalde, Germany) for quantifying anti-β1AR Ab was used. ELISA Validation was performed according to the FDA’s “Guidance for industry: Bioanalytical method validation”18. Using native membrane extracts from cell lines overexpressing human β1AR to measure IgG antibodies against the transmembrane β1AR in its assumed physiological conformation19,20. Tests were performed with microtiter polystyrene plates with 96 wells, which were coated with human ß1AR overexpressing extracts from transfected Chinese Hamster Ovary cells. To maintain the conformational epitopes of the receptor we added 1 mM calcium chloride to each buffer and incubated duplicate samples of a 1:100 dilution at 48 °C for 2 hours. After washing, an incubation followed for 1 hour with a 1:20.000 dilution of horseradish peroxidase-labelled goat anti-human IgG (Jackson, Bar Harbor, ME, USA) for detection. Plates were incubated with a human monoclonal antibody against ß1AR (anti-ß1AR mab), to be able to obtain a standard curve (Fig. SD3). We generated a standard curve by standardizing antibody concentrations. For example (a) 6250 ng/ml anti-ß1AR mab for standard point 1, (b) 3125 ng/ml anti-ß1AR mab for standard point 2, (c) 1563 ng/ml anti-ß1AR mab for standard point 3, (d) 781 ng/ml anti-ß1AR mab for standard point 4 (e) 391 ng/ml anti-ß1AR mab for standard point 5 and (f) 195 ng/ml anti-ß1AR mab for standard point 6. Finally, the optical density was measured. All standard points were performed in duplicates. The threshold for anti-ß1AR mab detection was set at 100 ng/ml. Analysis of all patient probes, sera and plasma samples, was performed according to the described protocol by individuals who had no information regarding the patients’ characteristics.
Statistical analyses
We used R version 3.3.2 (http://www.r-project.org) for all data analyses.
The concentrations of anti-β1AR Ab have been analyzed in groups separated by age (≤60/>60 years) and ACS entity. Continuous variables were tested using either the Kruskal-Wallis rank sum test (>2 groups) or the Wilcoxon rank sum test (2 groups). Categorical variables were evaluated using Fisher’s exact test. Kaplan-Meier survival curves were constructed with patients being stratified according to anti-ß1AR Ab concentrations ≤ median and >median, ACS phenotype (STEMI vs. NSTEMI) and (≤60 vs. >60 years). Patient outcomes and anti-ß1AR Ab were analyzed within Cox proportional hazards models, fitted to the different outcomes using either anti-ß1AR Ab and STEMI/NSTEMI anti-ß1AR Ab and age group (≤60/>60 years) and the respective interactions between them as variables.
To assess the relationship between anti-ß1AR Ab and conventional cardiovascular biomarkers the time of initial ACS presentation, scatterplots were constructed and given the continuous nature of both variables, Spearman rank correlations were performed.
Results
Cohort study
Patients demographics
Anti-ß1AR Ab concentrations were measured in sera of 399 NSTEMI and 400 STEMI patients enrolled in the PLATO ACS cohort. Patients were then stratified according to anti-ß1AR Ab concentration being either greater than or less than or equal to the median concentration. Sub-group baseline characteristics are summarized in Table 1. Whilst the majority of patients in both groups were male, no significance difference in gender (69.5% vs. 69.6%; p = 0.939) or age (median 61 [52.8–70.0] vs. 61 [53.5–70.0] years; p = 0.961) was evident between groups.
With regards to past medical history, a significantly higher incidence of heart failure was identified in patients with higher anti-ß1AR Ab concentrations was observed (2% vs. 5.3%; p = 0.014). Other than statin treatment (84.8% vs. 91.0%; p = 0.009), no significant differences in standard prophylactic treatments across all drug classes were identified within the cohort.
Anti-ß1AR Ab, ACS phenotype and rate of re-infarction
Comparing anti-ß1AR Ab levels between ACS phenotypes, a significantly higher proportion of STEMI patients exhibited concentrations of anti-ß1AR Ab above the median value (60.4% vs. 39.8%; p < 0.001). Those exhibiting lower anti-ß1AR Ab concentrations tended to have poorer cardiovascular outcomes in the subsequent demonstrated a higher incidence of cardiovascular deaths in the ensuing 12 months (n = 16, p = 0.067), although this failed to achieve significance. Likewise, no significant differences in the incidence of either stroke or myocardial re-infarction were observed within the entire cohort (p = 0.06), with sub-group analyses in patients < 60 years (n = 388) revealing that low anti-ß1AR Ab concentrations were associated with the incidence of re-infarction within 12 months (p = 0.010, Fig. 1). Considering all cardiovascular events collectively across these patients, patients with lower anti-β1AR Ab concentrations were associated with higher rates of adverse outcomes (p = 0.017, Fig. 2A).
Kaplan-Meier curves for spontaneous MI over and under median ß1-AdrR-ab concentrations. Kaplan-Meier curves are stratified according to age groups. Number of events: all patients 48 (A), patients ≤60 years 16 (B) and patients >60 years 32 (C). ß1-AR ab below median was associated with a higher incidence of re-infarction in younger patients, particularly within the first 8 weeks. No differences were observed in patients >60 years (C) or all patients (A). P-values calculated using Cox-proportional hazards Score-Test.
Kaplan-Meier curve summarizing all-cause cardiovascular mortality in the first 12 months after the index acute coronary syndrome with respect to ß1-AR ab level at original presentation for patients ≤60 years (A) and all patients (B). Once again, lower ß1-AR ab levels were associated with a higher incidence of fatal events in patients aged ≤60 years. In older patients, no differences were observed (p = 0.961, data suppl.). Number of events: 22 (A) und 69 (B). P-values calculated using Cox-proportional hazards Score-Test.
In relation to spontaneous MI, patients with anti-ß1AR Ab concentrations ≤ median values appeared to incur a significantly higher number of events (n = 16; p = 0.010, Fig. 1) among younger patients ≤60 years. In older patients, spontaneous MI rates appeared largely independent of antibody concentration (n = 32; p = 0.186), Fig. 1C.
Anti-ß1AR Ab and conventional clinical risk factors
Between the stratified anti-ß1AR Ab concentration groups, no differences in the incidence or distribution of conventional risk factors across all patients were observed. Weak trends suggesting an association between lower anti-ß1AR Ab concentrations and increased incidence of diabetes mellitus (23.8% vs. 18.0%; p = 0.055) and dyslipidemia (45.5% vs. 39.1%; p = 0.073) were observed, levels (p = 0.055 and p = 0.073) in both groups were evident but neither achieved outright significance. Statin use was associated with higher anti-ß1AR Ab levels (p = 0.009).
Among patients ≤60 years, those exhibiting lower anti-ß1AR Ab concentrations had higher rates of diabetes mellitus (20.2 vs. 11.6%; p = 0.026) and were less likely to be receiving statins at study inclusion (83.8 vs. 91.6%; p = 0.021). Otherwise no differences in clinical risk factors in younger patients correlated with anti-ß1AR Ab concentrations (Table SD7).
Anti-ß1AR Ab and ACS biomarkers
Within the stratified anti-ß1AR Ab groups, the only serum biomarker demonstrating an inter-group difference was C-reactive protein, which was higher among patients with higher anti-ß1AR Ab concentrations (2.7 [1.2–6.2]mg/L vs. 3.5 [1.4–8.4]mg/L; p = 0.018). Further analysis, quantitively comparing the actual measured anti-ß1AR Ab concentrations with the respective serum biomarkers using the Spearman Rank Correlation test however confirmed that anti-ß1AR Ab did not correlate with any of the available biomarkers, apolipoprotein A1 and B, Cystatin, Troponin I and T, NT-proBNP, GDF-15, GFR and IL-6 (all coefficients Rs < ± 0.10; Supplemental Data Sheet, Fig. SD1 and Table SD8).
Discussion
Anti-ß1AR Ab levels in ACS
Across the entire cohort, empirical cumulative distribution functions of anti-ß1AR Ab concentrations revealed significantly higher values in STEMI than NSTEMI (p < 0.001), which retained significance following stratification for age > or ≤60 years (both p < 0.001; see Supplementary Data Sheet, Fig. SD2).
Overall, anti-ß1AR Ab concentrations across all 799 ACS patients failed to show any meaningful associations to either survival or re-infarction in the first year. Subgroup analyses using both unadjusted and adjusted Cox Regression Models demonstrated that younger patients with anti-ß1AR Ab concentrations ≤ median were less likely to remain free of either re-infarction (HR 0.14 [0.03–0.63]; p = 0.01) or any cardiovascular events (HR 0.30 [0.11–0.80]; p = 0.017), impacting inherently on event-free survival (Fig. 1). These findings corroborate to an extent earlier data, which suggested a worse outcome for ACS patients with low anti-ß1AR Ab levels14.
Furthermore, the current study associates lower Ab concentration with increased rates of early re-infarction, similar to trends seen in particular ACS sub-groups in our previous paper. To our knowledge, no biomarkers are routinely used in risk stratification for re-infarction, whilst only a few biomarkers such as GDF-15 or NT-proBNP are of proven prognostic relevance after ACS3,21, particularly in combination22. Future studies are needed, to explore if anti-ß1AR Ab could provide additional insight in such cases.
In contrast to previous data on anti-ß1AR Ab in ACS, which suggested that lower concentrations were found in STEMI compared to NSTEMI, both of which were markedly reduced compared to healthy controls14, the current study revealed higher concentrations in STEMI patients compared to NSTEMI. This may reflect, that STEMI is not automatically associated with greater injury or poorer worse outcome, as multiple additional confounders may have reasonably influenced mortality and prognosis23,24,25. STEMI patients are younger (FAST MI study 63.5 ± 13.8 (STEMI) vs. 68.1 ± 13.5 (NSTEMI)) and have a more transmural muscle damage, whilst NSTEMI patients are older, have more comorbidities and commonly a greater severity of coronary vascular disease. Therefore, NSTEMI patients have a lower early mortality but higher risk for long-term mortality compared to STEMI patients26.
Conceivably, our results regarding anti-ß1AR Ab concentrations in different ACS phenotypes may merely reflect demographic differences within the cohorts rather than any direct effect of anti-ß1AR Ab. It should be reiterated that the inclusion criteria for the PLATO trial excluded patients <60 years without known vascular comorbidities. Our original cohort was by contrast composed of particularly young ACS patients (NSTEMI median age 57 (48–61) years and STEMI 52(41–58) years) independent of comorbidities or known cardiac risk factors.
The results go along with the finding that anti-ß1AR Ab levels are independent of troponin or CK-values, suggesting no correlation to myocardial muscle damage.
Due to the lack of knowledge about the exact function of anti-ß1AR Ab, there are several possible explanations for these results. One could be a worse response to betablocker therapy. Nagatomo et al. have shown that patients with heart failure and evidence of anti-ß1AR Ab responded significantly better to betablocker therapy than patients with no increased anti-ß1AR Ab concentrations. Patients positive for anti-ß1AR Ab had for example a greater improvement in ejection fraction, greater changes of the left ventricular end-diastolic dimension and tended to have a better reduction of pro-BNP27.
A possible effect of betablocker therapy in our cohort is difficult to analyze as betablocker were taken in both groups, STEMI and NSTEMI, up to 80% of the patients before the ACS and nearly 100% after ACS. Even the following thesis is highly speculative, it could well be, that in earlier analyses patients with low anti-ß1AR Ab levels just did worse because they did not respond to ß-blocker treatment.
Another explanation for our results could be a stronger agonistic effect of anti-ß1AR Ab due to higher expression of ß1AR during and after an ACS28. This up-regulation of ß1AR may lead to a high binding of anti-ß1AR Ab and therefore falling levels in serum in ACS patients in general14. Suggesting a protective or blocking role of anti-ß1AR Ab on the receptors, the natural ligand adrenalin could stimulate the receptor stronger leading to vasoconstriction and worse outcome.
Statin use was associated with higher ab levels. Based on the role of statins in the prevention of infarction, high ab levels could be more beneficial.
Risk factors and routinely used ACS biomarker
Based upon comparisons to conventional cardiac risk factors, no significant correlations to anti-ß1AR concentrations were observed. This may further supports the hypothesis of an additional autoimmune component to cardiovascular disease. Further research looking at the immunological aspects in RF-naïve ACS patients is needed. Whilst previous work has shown contradictory results regarding dyslipidemia14, we can show only trends towards a correlation with lower levels of anti-ß1AR Ab (p = 0.073).
It has to be noted, that the specific function of anti-ß1AR Ab may rely greatly on its binding antigen to the receptor, which could partially explain the contradicting results regarding the effect of anti-ß1AR Ab29.
Although levels for CRP were significantly higher in patients with high anti-ß1AR Ab 2.7 (1.2–6.2) mg/l versus 3.5 (1.4–8.4) mg/l (p = 0.018), values for IL-6 were not different, suggesting IL-6-independent associations for this observation.
Anti-ß1AR Ab levels in congestive heart disease
Our results compliment the association of anti-ß1AR Ab with chronic heart failure, showing a significant increase of anti-ß1AR Ab in congestive heart failure (p = 0.014). Several anti-ß1AR Ab associated diseases are known to lead to chronic heart failure, such as dilated cardiomyopathy30, Chagas disease31 and atrial fibrillation32,33.
Limitations
The aim of the current analysis was to validate the findings of our previous single center study in a large, well-characterized cohort. Despite the larger numbers of patients involved, this approach also introduced a number of further limitations to the current analysis.
The existing results suggested that the relevance of anti-ß1AR Ab in ACS was primarily in patients below the age of 60 years. Whilst the PLATO cohort included many such patients, there is an inherent inclusion bias against younger patients with no comorbidity. It is also unclear, what if any influence a previous cardiac event prior to the index event would have on antibody concentration. In this respect, limiting analysis to first presentation only may have been prudent.
Although the number of patients analyzed was high (n = 799), numbers of re-infarction, cardiovascular death and all-cause death were comparably low, hampering statistical analysis of subgroups.
Similarly, the cohort lacks a suitably matched healthy control group, we were not able to assess if the significance of anti-ß1AR Ab concentrations actually relates to the presence of an ACS or not.
The lack of understanding about the exact function of anti-ß1AR Ab is another main limitation. Therefore, the interpretation of our results remains speculative. Functional tests and longitudinal measurements are needed to further explore the functional influence and effect of anti-ß1AR Ab.
Conclusion
Lowered titers of anti-ß1AR Ab in serum of ACS patients may be associated with higher risk for re-infarction or worse prognosis. These results have shown the greatest significance in patients below the age of 60 years. In this subgroup low anti-ß1AR Ab concentrations were associated with re-infarction (p = 0.01) and all cardiovascular events (p = 0.017) within 12 months after the index event. An association between low anti-ß1AR Ab and ACS compared to healthy controls and patients with atherosclerosis as well as a possible negative prognostic factor for survival and re-infarction has been shown recently14.
Larger cohorts are needed to finally confirm our findings. Furthermore functional analyses are necessary to understand the effect of anti-ß1AR Ab during and after ACS.
References
Laslett, L. J. et al. The worldwide environment of cardiovascular disease: prevalence, diagnosis, therapy, and policy issues: a report from the American College of Cardiology. J Am Coll Cardiol 60, S1–49, https://doi.org/10.1016/j.jacc.2012.11.002 (2012).
Yusuf, S. et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 364, 937–952, https://doi.org/10.1016/S0140-6736(04)17018-9 (2004).
Wollert, K. C. et al. Prognostic value of growth-differentiation factor-15 in patients with non-ST-elevation acute coronary syndrome. Circulation 115, 962–971, https://doi.org/10.1161/CIRCULATIONAHA.106.650846 (2007).
Cabral-Marques, O. et al. GPCR-specific autoantibody signatures are associated with physiological and pathological immune homeostasis. Nat Commun 9, 5224, https://doi.org/10.1038/s41467-018-07598-9 (2018).
Capote, L. A., Mendez Perez, R. & Lymperopoulos, A. GPCR signaling and cardiac function. Eur J Pharmacol 763, 143–148, https://doi.org/10.1016/j.ejphar.2015.05.019 (2015).
Vicco, M. H. et al. beta1-selective adrenoceptor antagonists increase plasma levels of anti-p2beta antibodies and decrease cardiac involvement in chronic progressive Chagas heart disease. Can J Cardiol 30, 332–337, https://doi.org/10.1016/j.cjca.2013.09.017 (2014).
Lymperopoulos, A. & Bathgate, A. Arrestins in the cardiovascular system. Prog Mol Biol Transl Sci 118, 297–334, https://doi.org/10.1016/B978-0-12-394440-5.00012-7 (2013).
Bathgate-Siryk, A. et al. Negative impact of beta-arrestin-1 on post-myocardial infarction heart failure via cardiac and adrenal-dependent neurohormonal mechanisms. Hypertension 63, 404–412, https://doi.org/10.1161/HYPERTENSIONAHA.113.02043 (2014).
Caforio, A. L. et al. Clinical implications of anti-heart autoantibodies in myocarditis and dilated cardiomyopathy. Autoimmunity 41, 35–45, https://doi.org/10.1080/08916930701619235 (2008).
Nagatomo, Y. et al. Myocardial Recovery in Patients With Systolic Heart Failure and Autoantibodies Against beta1-Adrenergic Receptors. J Am Coll Cardiol 69, 968–977, https://doi.org/10.1016/j.jacc.2016.11.067 (2017).
Nagatomo, Y. et al. Autoantibodies Specifically Against beta1 Adrenergic Receptors and Adverse Clinical Outcome in Patients With Chronic Systolic Heart Failure in the beta-Blocker Era: The Importance of Immunoglobulin G3 Subclass. J Card Fail 22, 417–422, https://doi.org/10.1016/j.cardfail.2016.03.005 (2016).
Nussinovitch, U. & Shoenfeld, Y. The clinical significance of anti-beta-1 adrenergic receptor autoantibodies in cardiac disease. Clin Rev Allergy Immunol 44, 75–83, https://doi.org/10.1007/s12016-010-8228-9 (2013).
Bornholz, B. et al. Impact of human autoantibodies on beta1-adrenergic receptor conformation, activity, and internalization. Cardiovasc Res 97, 472–480, https://doi.org/10.1093/cvr/cvs350 (2013).
Ernst, D. et al. Beta-1-Adrenergic Receptor Antibodies in Acute Coronary Syndrome: Is Less Sometimes More? Front Cardiovasc Med 5, 170, https://doi.org/10.3389/fcvm.2018.00170 (2018).
Ernst, D. et al. Antibodies against MYC-Associated Zinc Finger Protein: An Independent Marker in Acute Coronary Syndrome? Front Immunol 8, 1595, https://doi.org/10.3389/fimmu.2017.01595 (2017).
Ernst, D. et al. Anti-MYC-associated zinc finger protein antibodies are associated with inflammatory atherosclerotic lesions on 18F-fluorodeoxyglucose positron emission tomography. Atherosclerosis 259, 12–19, https://doi.org/10.1016/j.atherosclerosis.2017.02.010 (2017).
James, S. et al. Comparison of ticagrelor, the first reversible oral P2Y(12) receptor antagonist, with clopidogrel in patients with acute coronary syndromes: Rationale, design, and baseline characteristics of the PLATelet inhibition and patient Outcomes (PLATO) trial. Am Heart J 157, 599–605, https://doi.org/10.1016/j.ahj.2009.01.003 (2009).
Booth, B. et al. Workshop report: Crystal City V–quantitative bioanalytical method validation and implementation: the 2013 revised FDA guidance. AAPS J 17, 277–288, https://doi.org/10.1208/s12248-014-9696-2 (2015).
Bornholz, B. et al. Detection of DCM-associated beta1-adrenergic receptor autoantibodies requires functional readouts or native human beta1-receptors as targets. Int J Cardiol 202, 728–730, https://doi.org/10.1016/j.ijcard.2015.10.068 (2016).
Bornholz, B. et al. A standardised FACS assay based on native, receptor transfected cells for the clinical diagnosis and monitoring of beta1-adrenergic receptor autoantibodies in human heart disease. Clin Chem Lab Med 54, 683–691, https://doi.org/10.1515/cclm-2015-0603 (2016).
Shapiro, B. P., Chen, H. H., Burnett, J. C. Jr. & Redfield, M. M. Use of plasma brain natriuretic peptide concentration to aid in the diagnosis of heart failure. Mayo Clin Proc 78, 481–486, https://doi.org/10.4065/78.4.481 (2003).
Klingenberg, R. et al. Improved risk stratification of patients with acute coronary syndromes using a combination of hsTnT, NT-proBNP and hsCRP with the GRACE score. Eur Heart J Acute Cardiovasc Care 7, 129–138, https://doi.org/10.1177/2048872616684678 (2018).
Chan, M. Y. et al. Long-term mortality of patients undergoing cardiac catheterization for ST-elevation and non-ST-elevation myocardial infarction. Circulation 119, 3110–3117, https://doi.org/10.1161/CIRCULATIONAHA.108.799981 (2009).
Steg, P. G. et al. Baseline characteristics, management practices, and in-hospital outcomes of patients hospitalized with acute coronary syndromes in the Global Registry of Acute Coronary Events (GRACE). Am J Cardiol 90, 358–363 (2002).
Hasdai, D. et al. A prospective survey of the characteristics, treatments and outcomes of patients with acute coronary syndromes in Europe and the Mediterranean basin; the Euro Heart Survey of Acute Coronary Syndromes (Euro Heart Survey ACS). Eur Heart J 23, 1190–1201 (2002).
Puymirat, E. et al. Acute Myocardial Infarction: Changes in Patient Characteristics, Management, and 6-Month Outcomes Over a Period of 20 Years in the FAST-MI Program (French Registry of Acute ST-Elevation or Non-ST-Elevation Myocardial Infarction) 1995 to 2015. Circulation 136, 1908–1919, https://doi.org/10.1161/CIRCULATIONAHA.117.030798 (2017).
Nagatomo, Y. et al. A pilot study on the role of autoantibody targeting the beta1-adrenergic receptor in the response to beta-blocker therapy for congestive heart failure. J Card Fail 15, 224–232, https://doi.org/10.1016/j.cardfail.2008.10.027 (2009).
Ihl-Vahl, R., Marquetant, R., Bremerich, J. & Strasser, R. H. Regulation of beta-adrenergic receptors in acute myocardial ischemia: subtype-selective increase of mRNA specific for beta 1-adrenergic receptors. J Mol Cell Cardiol 27, 437–452 (1995).
Wallukat, G. The beta-adrenergic receptors. Herz 27, 683–690, https://doi.org/10.1007/s00059-002-2434-z (2002).
Jahns, R. et al. Autoantibodies activating human beta1-adrenergic receptors are associated with reduced cardiac function in chronic heart failure. Circulation 99, 649–654 (1999).
Wallukat, G. et al. Distinct patterns of autoantibodies against G-protein-coupled receptors in Chagas’ cardiomyopathy and megacolon. Their potential impact for early risk assessment in asymptomatic Chagas’ patients. J Am Coll Cardiol 55, 463–468, https://doi.org/10.1016/j.jacc.2009.06.064 (2010).
Wallukat, G. & Schimke, I. Agonistic autoantibodies directed against G-protein-coupled receptors and their relationship to cardiovascular diseases. Semin Immunopathol 36, 351–363, https://doi.org/10.1007/s00281-014-0425-9 (2014).
Stavrakis, S. et al. Activating autoantibodies to the beta-1 adrenergic and m2 muscarinic receptors facilitate atrial fibrillation in patients with Graves’ hyperthyroidism. J Am Coll Cardiol 54, 1309–1316, https://doi.org/10.1016/j.jacc.2009.07.015 (2009).
Acknowledgements
The authors like to thank the laboratory staff of CellTrend for performing the ELISA tests. Diana Ernst is supported by a Government Grant Ellen-Schmidt 2018 of Hannover Medical School, Germany. Gerrit Ahrenstorf and Alexandra Jablonka were funded by the Young Academy Clinician/Scientist program of Hannover Medical School, Germany. The project was supported by KFO 250 of the German research foundation. Laboratory staff of CellTrend performed ELISA testing.
Author information
Authors and Affiliations
Contributions
All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation. Contribution to the manuscript. D.E. conception and design of the study, acquisition and interpretation of data, drafting the article, and final approval of the version to be submitted. G.A., A.J., G.S. and R.E.S. interpretation of data, revising critically for important intellectual content and final approval of the version to be submitted. H.H. acquisition of data, revising critically for important intellectual content and final approval of the version to be submitted. J.W. analyses, interpretation of data, revising critically for important intellectual content and final approval of the version to be submitted. L.W., G.R. and T.W. conception and design of the study, interpretation of data, revising critically for important intellectual content and final approval of the version to be submitted.
Corresponding author
Ethics declarations
Competing Interests
H.H. is the owner of CellTrend. CellTrend had no influence in data analysis. All other authors declare no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ernst, D., Westerbergh, J., Sogkas, G. et al. Lowered anti-beta1 adrenergic receptor antibody concentrations may have prognostic significance in acute coronary syndrome. Sci Rep 9, 14552 (2019). https://doi.org/10.1038/s41598-019-51125-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-51125-9
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.